Abstract
An in vivo assay using the cytochrome P450 (P450) suicide inhibitor 1-aminobenzotriazole (ABT) and 24 hour food intake was developed to determine the relative importance of P450s in two populations of Neotoma lepida with respect to biotransformation of plant secondary compounds (PSCs) in the animals’ natural diets. The efficacy of ABT as a P450 inhibitor was first validated using hypnotic state assays with and without pretreatment with ABT. Pretreatment with 100mg/kg ABT by gavage increased hexobarbital sleep times 3–4 fold in both populations, showing effective inhibition of P450s in woodrats. Next, the Great Basin population was fed a terpene-rich juniper diet, and the Mojave population was fed a phenolic-rich creosote diet, with rabbit chow serving as the control diet in each group. Treatment with ABT inhibited food intake in the Great Basin population fed the juniper diet to a greater extent (35%) than the Great Basin population fed the control diet (19%) or the Mojave population fed the creosote diet (16%). The food intake of the Mojave population fed the control diet was not significantly inhibited by ABT. The findings suggest that the biotransformation of terpenes in juniper relies more heavily on P450s than that of phenolics in creosote. This assay provides an inexpensive and non-invasive method to explore the relative importance of P450s in the biotransformation strategies of wild mammalian herbivores.
Introduction
Mammalian herbivores are thought to have evolved efficient mechanisms for dealing with the potentially toxic plant secondary compounds (PSCs) in their diet (Freeland et al. 1974; McLean et al. 1997; Boyle et al. 1999; McLean et al. 2001; Marsh et al. 2006). Herbivores can reduce ingestion of PSCs through diet switching (Wiggins et al. 2006a; Wiggins et al. 2006b; Torregrossa et al. 2009b) or caching (Dearing 1997; Torregrossa et al. 2009a), but once consumed, any toxic PSCs must be metabolized and excreted efficiently to avoid toxic effects. Herbivores rely on a suite of biotransformation enzymes to metabolize absorbed PSCs (Sorensen et al. 2006).
The biotransformation enzymes of mammals can be separated into two different groups: Phase I or functionalization enzymes and Phase II or conjugation enzymes. Cytochromes P450 (P450s) are a superfamily of enzymes that carry out >75% of Phase I biotransformation (Williams et al. 2004, Guengerich 2008). Substrate oxidation generally renders the compound more reactive to conjugation by Phase II enzymes (Klaassen et al. 2003, Williams et al. 2004). Thousands of P450 enzymes have been sequenced (Nelson 2011). The number of functional P450 enzymes within an individual species varies from 57 in humans to over a hundred in the house mouse (Mus musculus) with only 34 possible orthologs between these two species (Nelson et al. 2004; Feyereisen 2011). While much is known about P450s in humans and model species such as the rat, mouse and dog (Halpert 1995, Domanski and Halpert 2001, Scott and Halpert 2005, Martignoni et al. 2006, Graham and Lake 2008), little is known about the role of P450s in wild mammalian herbivores. Many techniques used to understand the role of P450s in model species such as cloning, sequencing, functional assays, and X-ray crystallography require specialized equipment and knowledge. These approaches may not be easily adopted by ecologists interested in plant-mammal interactions (Balani et al. 2005; Sorensen et al. 2006). However, there are some pharmacological techniques, such as the use of P450 inhibitors, that are easily applicable to non-model systems and may provide excellent insights into the role of biotransformation in an animal’s ability to consume plants with potentially toxic PSCs (Fontana et al. 2005). Taking advantage of such a pharmacological technique, we developed a whole animal assay using the P450 suicide inhibitor, 1-aminobenzotriazole (ABT) to investigate the relative importance of P450s to woodrats (genus Neotoma) consuming two plants with disparate PSC profiles.
The desert woodrat (Neotoma lepida) represents an ideal choice for studies of P450s in a wild species. The woodrat is widely distributed in the western United States in both the Great Basin and Mojave deserts and has distinct diets in each of these habitats. The Great Basin desert is dominated by juniper, pinyon pine and sagebrush, while the Mojave Desert is dominated by creosote bush and Joshua tree. Juniper (Juniperus osteosperma) is a common element in the diet of populations of N. lepida in the Great Basin, which can ingest a diet of ca. 50% juniper in the laboratory (Magnanou et al. 2009). Juniper is currently not present in the Mojave, because it was replaced by creosote bush (Larrea tridentata) ca. 17,000 years ago during a natural climatic event (Devender 1977; Van Devender et al. 1979). Creosote bush dominates the diet of the population of N. lepida in the Mojave and can comprise up to 75% of the diet (Cameron and Rainey 1972). Importantly, the largest proportion of PSCs in juniper is terpenes, while phenolics predominate in creosote bush (Adams et al. 1983, Hyder et al. 2002). Both terpenes and phenolics are metabolized by and can induce P450 enzymes in woodrats, as measured by enzyme activity as well as gene and protein expression (Lamb et al. 2004; Haley et al. 2007; Skopec et al. 2007; Haley et al. 2008; Magnanou et al. 2009). There is evidence that woodrats that feed on creosote may rely more heavily on Phase II mechanisms for biotransforming the phenolics present in their diet (Mangione et al. 2000; Lamb et al. 2004; Haley et al. 2008; Magnanou et al. 2009), whereas juniper feeders may rely more heavily on Phase I mechanisms in the metabolism of dietary terpenes (Haley et al. 2007; Skopec et al. 2007).
We wanted to determine whether ABT would be a useful tool for “pharm-ecologists” studying biotransformation processes in wild-caught animals. ABT has been used in a number of different species, including guinea pigs, lab rats, mice, and pigs, in both in vivo and in vitro studies to investigate the P450-mediated metabolism of specific drugs (Woodcroft et al. 1997; Balani et al. 2004; Balani et al. 2005; Fontana et al. 2005). First, we confirmed that ABT is an effective inhibitor of P450s in woodrats by using hypnotic state assays, which are a relatively non-invasive way to measure in vivo activity of biotransformation enzymes (Sasaki 1994; Kim et al. 2005) and have been successfully used in woodrats (Dearing et al. 2006). After confirmation of ABTs effectiveness in woodrats, we then conducted feeding trials with control diets and PSC-amended diets in conjunction with ABT treatment to determine whether inhibition of food intake is a sensitive measure of the importance of P450 enzymes in biotransforming PSCs. While previous studies have given insight into how the two populations of N. lepdia may be altering biotransformation when consuming creosote and/or juniper, the approach did not allow for repeated measures as they were lethal endpoint studies (Lamb et al. 2004; Haley et al. 2008; Magnanou et al. 2009). In vivo assays such as the one described in this study allow for relatively small numbers of animals to be used for multiple treatments, while still garnering physiological and pharmacological insight into how Neotoma sp., and presumably other wild herbivores are biotransforming the PSCs in their diets.
Materials and Methods
Study System
Neotoma lepida were trapped from the Great Basin and Mojave Deserts using Sherman live traps and transported to the University of Utah, Department of Biology’s animal facility. Great Basin N. lepida were trapped near White Rocks in Tooele County, Utah (40°19’N, 112°54’W), and Mojave N. lepida were trapped near Beaver Dam (Lytle Ranch) in Washington County, Utah (37°07′N, 114°00′W). All animals were screened for Sin Nombre virus before experimentation (Dearing et al. 1998). Woodrats were housed in individual cages (48cm × 27 cm × 20 cm) with pine shavings on a 12L: 12D cycle at 28°C and with a relative humidity of 15%. The woodrats were fed high-fiber rabbit chow (Harlan Teklad formula 2031) and water ad libitum. All experimental procedures involving woodrats were approved by the University of Utah’s Institutional Animal Care and Use Committee protocol number 10-01013.
Hypnotic state assays
A total of six Great Basin N. lepida (4 males and 2 females) and four Mojave N. lepida (2 males and 2 females) were used in the hypnotic state assays. All animals were fed rabbit chow prior to the assays. Animals were randomly assigned to be pretreated by gavage with either 100 mg/kg of ABT in a vehicle of 2% dimethyl sulfoxide (DMSO) in saline solution or the vehicle alone (2% DMSO in saline). The ABT was dissolved in DMSO and placed in saline for a final concentration of 10 mg ABT/ml. Woodrats have a gag reflex, and therefore animals were anesthetized by the open drop method using isoflurane (Hanusch et al. 2007) prior to gavage. Animals received either the saline or ABT gavage two hours prior to the start of the hypnotic state assay as recommended by Balani et al., (2002; 2004). Hexobarbital (100mg/kg) was injected into the intraperitoneal (IP) cavity, and the length of the resulting hypnotic state was measured as the duration of time after injection during which an animal is unable to right itself twice within 30 seconds of being placed on its back (Dearing et. al. 2006). In the second round of hypnotic assays, the pretreatment was switched: animals previously receiving ABT were pretreated with only the vehicle and vice versa. We have found that repeating hypnotic state assays in the same individual sooner than six weeks leads to decreased sleep times (unpublished observation), since hexobarbital has the longer-term consequence of inducing the P450s that metabolize it (Groen et al. 1996; Murayama et al. 1996; Lewis et al. 1997). We, therefore, used a wash-out period of at least 6 weeks between each hypnotic state assay. If time in captivity needs to be minimized it is possible to complete the hypnotic state assays in a non-repeated measures design where half the animals receive just hexobarbital and the other half are pre-treated with ABT prior to hexobarbital administration. However, because of large interindividual variation in sleep times, larger sample sizes may be required.
To determine whether there were differences in hexobarbital sleep times in the hypnotic state assays within and between populations, repeated measures analysis of variance (ANOVA) was used with treatment (ABT or saline) and population (Great Basin or Mojave) as independent factors and sleep time as the dependent variable. Each woodrat’s sleep time when pretreated with ABT was divided by their saline pretreated sleep time. Student’s t-test was then used to determine any difference between the two populations in the relative increase in sleep time caused by ABT.
Feeding trials
Feeding trials were conducted to evaluate the effect of ABT on food intake in animals on control versus toxic diets. The general approach was to present woodrats with a diet containing levels of PSCs that approached their maximum tolerable dose but low enough that dry matter intake (DMI) was not significantly impacted. The rationale behind this approach was that at this dose, P450 biotransformation would be close to its maximum and subsequent inhibition of P450s should reduce intake. No change in DMI would indicate that P450s were not critical in the biotransformation process.
Great Basin woodrats (n=6, 4 males and 2 females) were fed the control diet (ground high fiber rabbit chow, Teklad 2031), or a toxic diet that was 50% ground rabbit chow and 50% juniper on a dry matter basis. A ground diet was used to prevent sorting by the woodrats. A previous laboratory tolerance trial revealed that a 50% juniper diet is the maximum concentration on which the Great Basin N. lepida can maintain body mass (Magnanou et al. 2009). The juniper used in the dietary treatments (Juniperus osteosperma) was collected from trees at woodrat trapping sites and kept frozen at −20 °C until use. Foliage was used in this trial instead of PSC extracts because the terpenes in the juniper are volatile and cannot be maintained in a chemical extract. The juniper was ground on dry ice until passed through a 1.0 mm screen. The diets were made daily to minimize volatilization of the terpenes. Juniper foliage used in the diets had an average water weight of 52%; therefore we added water to the control diet of ground rabbit chow such that the water content was also 52%. Total terpene concentration of J. monosperma is ~ 0.55% dry weight with alpha-pinene accounting for >50% of the terpene content (Adams et al. 1983).
The Mojave woodrats (n=7; 3 males and 4 females) were fed the control diet or a toxic diet that was 4% creosote resin in ground rabbit chow, on a dry matter basis. Creosote resin was used instead of foliage as in the juniper diet, because the viscous nature of the resin on the leaves makes it difficult to grind the whole leaves, and the resin is stable (Mangione et al. 2000) and contains the majority of PSCs (Mabry et al. 1977). A laboratory tolerance trial found that 4% creosote resin is the maximum concentration of creosote on which the Mojave N. lepida can maintain DMI levels similar to DMI for control diet (data not shown). Creosote bush leaves were collected from woodrat trapping sites and kept frozen at −20°C until use. Resin was extracted and creosote diets prepared as inMagnanou et al. (2009). The control diet and 4% creosote diet presented to the Mojave N. lepida were 98% dry matter.
All diets were presented for three days to allow for full induction of P450 enzymes, and on the fourth day animals were gavaged with 100 mg/kg ABT two hours prior to the presentation of the food. This design allowed us to compare each animal’s diet intake (control or toxic before ABT treatment and after). Each diet was then presented for an additional two days. Body weight and food intake were measured each day. A washout period of two weeks took place between the control and toxic diet treatments. We used a cross-over design; animals fed the toxic diet in the first trial were fed the control diet in the second, and vice versa. During the washout period animals were fed rabbit chow pellets ad libitum.
Great Basin and Mojave animals were not fed both toxic diets, i.e. Great Basin animals were not fed creosote and Mojave animals were not fed juniper. While a fully factorial design might have allowed direct comparisons between the two populations on all diets, feeding Great Basin animals an equivalent dose of creosote is not possible, and feeding Mojave animals an equivalent dose of juniper is not ecologically relevant. Previous studies have shown that Great Basin animals differ greatly in their tolerance to and biotransformation of creosote when compared with the Mojave animals; Great Basin animals are not able to maintain body mass on diets containing more than 2% creosote (Mangione et al. 2000, Haley et al. 2008, Magnanou et al. 2009). Although both species have evolutionary experience with juniper, it is no longer present in the Mojave, having been replaced by creosote bush ca. 17,000 years ago during a natural climatic event (Devender 1977; Van Devender et al. 1979). We chose these two populations of woodrats because they differ in diet and in the biotransformation of the disparate PSCs found in juniper versus creosote. This experimental design allowed us to test whether the inhibition of food intake by the P450 inhibitor ABT was a sensitive enough method to determine the relative role of P450’s in the biotransformation of PSCs.
Wet diets, such as the control and 50% juniper diet presented to the Great Basin N. lepidaare known to cause prolonged hyperphagia in lab rats (Ramirez 1991) and chickens (Yasar et al. 2000). Also in previous laboratory experiments with woodrats the DMI of juniper diets and their respective “wet” control diets is higher than that of creosote diets and their respective “dry” control diets (Haley et al. 2007; Haley et al. 2008). Therefore DMIs were compared only within a population. Paired t-tests were used to determine whether there were differences in body mass and DMI between control and toxic diets. A repeated measures ANOVA with day and diet (control or toxic) as the independent variables and DMI as the dependent variable was used to determine a difference in DMI on days 3, 4 and 5 of diets being offered. Day 3 represented DMI the 24 hours prior to ABT treatment, Day 4 represented DMI the 24 hours post ABT treatment and Day 5 represented DMI 25 to 48 hours post ABT treatment. Post-hoc Bonferroni adjusted pairwise comparisons were used to analyze differences between individual means.
Percent inhibition of DMI by ABT was calculated as the difference in DMI of each individual in the 24 hours directly preceding ABT treatment (Day 3) and the DMI in the 24 hours directly after ABT treatment (Day 4) divided by the DMI of Day 3 and multiplied by 100. A repeated measures ANOVA with diet (control or toxic) and population (Great Basin or Mojave) as independent factors and percent inhibition of DMI by ABT was used to determine whether there were difference between diets and populations in the percent inhibition of DMI by ABT. Post-hoc Bonferroni adjusted pairwise comparisons were used to analyze differences between individual means and to determine whether percent inhibition of DMI by ABT was significantly different from zero for each treatment.
Results
Hypnotic state assays
ABT increased the hexobarbital sleep times in both populations of woodrats 3–4 fold (F1, 8 = 95.24 p<0.001, Figure 1). However, the Mojave woodrats slept 52% longer than the Great Basin woodrats when pretreated with ABT, leading to a significant population effect (F1, 8 = 5.914 p=0.041) and a trend for a treatment by population interaction (F1, 8=4.481 p=0.067). There was no difference in the Great Basin and Mojave woodrats hexobarbital sleep times when pretreated with saline and no significant difference between the populations in the relative increase in hexobarbital sleep time after pretreatment with ABT (3.4 ± 0.12 fold for Great Basin lepida and 4.11± 0.99 fold for Mojave lepida, t=−0.706, df=8, p=0.5).
Figure 1.
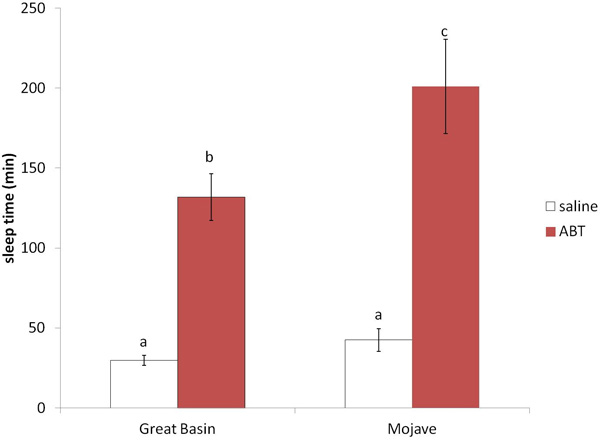
Influence of P450 inhibitor ABT on hexobarbital-induced sleep times in woodrats. Great Basin (n=6) and Mojave (n=4) woodrats were pretreated with saline or 100mg/kg ABT two hours prior to IP injection of 100mg/kg hexobarbital. Means ± SE shown, letter a, b and c denote means that are significantly different (p<0.05) and means with the same letter are not significantly different.
Feeding trial results
There was no difference in the body mass of Great Basin woodrats when presented with control or 50% juniper (toxic) diets (t=0.5, df=5 and p=0.6, Table 1). There was also no difference in the DMI of control and 50% juniper diet (F1,10=2.70, p=0.13, Table 1). Day but not diet was a significant predictor of DMI (F2,20=12.37 p<0.001 for day, F1,10=2.69 p=0.132 for diet). DMIs for both the control and 50% juniper diet on day 4 after animals were gavaged with ABT were significantly lower than DMIs for either day 3 and/or 5 (Table 1).
Table 1.
Mean body mass and dry matter intake (DMI) of the Great Basin woodrats fed their respective control and toxic diets (data represented as means ± SE)
| Diet | Control Rabbit Chow (n=6) |
Toxic 50% juniper (n=6) |
|---|---|---|
| Body mass (g) | 127.0±16.3 | 125.5±17.3 |
| DMI Day 3 (g/day) | 14.5±1.1a | 14.1±1.1a |
| DMI Day 4 ABT treatment (g/day) | 12.3±1.0b | 10.0±1.0c |
| DMI Day 5 (g/day) | 13.8±0.8a,b | 12.7±1.1a,b |
denote DMIs that are significantly different (p<0.05) both within and between rows or columns as determined by Bonferroni adjusted pairwise comparisons. Means with the same letter are not significantly different.
The Mojave woodrats also showed no significant differences in body mass (t=0, df=6, p=1) or DMI (t=1.9, df=6, p=0.1) when fed the control or 4% creosote (toxic) diet. Day but not diet was also a significant predictor of DMI (F2,24=14.91 p<0.001 for day and F1,12=0.69 p=0.42 for diet). DMI for the 4% creosote diet on day 4 after animals were gavaged with ABT were significantly lower than DMIs for either day 3 and/or 5 (Table 2), however there was no difference in the DMI of the control diet on day 4 compared to day 3 and/or 5.
Table 2.
Mean body mass and dry matter intake (DMI) of the Mojave woodrats fed their respective control and toxic diets (data represented as means±SE)
| Diet | Control Rabbit Chow (n=7) |
Toxic 4% creosote (n=7) |
|---|---|---|
| Body mass (g) | 114.3±7.5 | 114.3±7.8 |
| DMI Day 3 (g/day) | 9.3±0.7a | 8.1±0.5a |
| DMI Day 4 ABT treatment (g/day) | 8.1±0.7a,b | 6.9±0.4b |
| DMI Day 5 (g/day) | 9.1±0.6a | 8.8±0.5a |
denote DMIs that are significantly different (p<0.05) both within and between rows and columns determined by Bonferroni adjusted pairwise comparisons. Means with the same letter are not significantly different.
Inhibitory effect of ABT
The inhibitory effect of ABT on DMI was both population (F1,11=4.786, p=0.05) and diet (F1,11=4.952, p=0.048) dependent. The P450 inhibitor ABT inhibited the DMI of the 50% juniper diet to a greater extent than either control diets or the 4% creosote diet (Figure 2). The inhibitory effect of ABT on DMI did not differ among the control diets in both populations or the 4% creosote diet in the Mojave animals. However, the percent inhibition of DMI by ABT was significantly different from zero for the Great Basin animals fed the control diet and the Mojave animals fed the 4% creosote diet (p<0.05), while the percent inhibition of DMI by ABT for the Mojave animals fed the control diet was not significantly different from zero (p>0.05).
Figure 2.
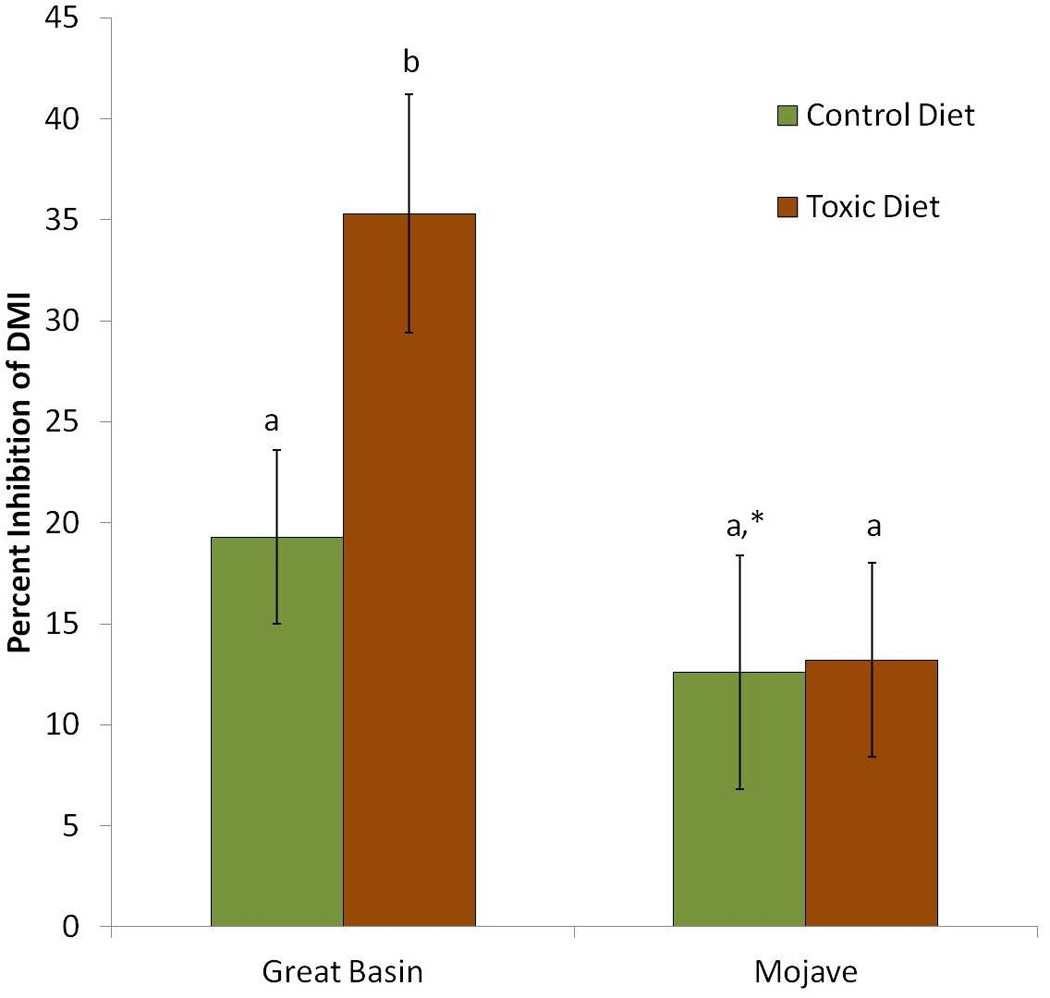
Influence of the P450 inhibitor ABT on dry matter intake (DMI). Woodrats were treated with ABT 100mg/kg 2 hours prior to food presentation. Percent inhibition was calculated as the difference between DMIs 24 hours prior to ABT treatment and DMI 24 hours post ABT treatment. Control diets were ground rabbit chow. The toxic diet of Great Basin woodrats (n=6) was 50% juniper. The toxic diet of Mojave woodrats (n=7) was 4% creosote resin added to control diet. Means ± SE shown, letters a, b denote means that are significantly different from one another (p<0.05) and means with the same letter are not significantly different.
. * denotes mean that was not significantly different from zero as determined by Bonferroni corrected t-tests (p>0.05).
Discussion
We found that ABT was an effective inhibitor of P450s in N. lepida and that it had disparate effects on the two populations consuming PSCs with which they have ecological experience. The 3–4 fold increase in hexobarbital sleep times in the woodrats treated with ABT can be compared with prior studies with other inhibitors and species. Koul et al., (2000) found that a doubling of hexobarbital sleep time in mice by pretreatment with a P450 inhibitor (a piperine analog) was analogous to a 50% inhibition of CYP1A and CYP2B activity in liver microsomes. Zvi and Kaplawski (1980) found that a 2.3 fold increase in hexobarbital sleep times in rats due to heat exposure was analogous to a 67% reduction in the metabolic clearance time of antipyrine, which is metabolized by P450 enzymes. Based on this work on other species, we infer that the 3.7 fold increase in hexobarbital sleep time in the woodrats potentially decreases P450 activity to about 25%.
The shorter sleep time of the Great Basin than Mojave woodrats after ABT pretreatment has interesting implications for population level difference in P450 enzymes and suggests that the P450 enzymes in the Great Basin animals have a higher capacity for metabolizing hexobarbital-like compounds and/or the Great Basin animals have a higher dependence on P450 enzymes to metabolize hexobarbital-like compounds due to reduced activities of conjugation pathways for hexobarbital. This higher capacity could arise from a greater concentration of P450 enzymes, a bias towards those that metabolize hexobarbital, or unique variants of P450s with faster turnover rates for hexobarbital. The same study however does not support the idea that Great Basin animals have higher overall P450 levels asHaley et al. (2008) found significantly lower total P450 concentrations in Great Basin woodrats compared with Mojave animals. More recent evidence revealed a divergence in the prevalence of different CYP2B isoforms between these two populations of N. lepida (Malenke et al. 2012), and isoforms of CYP2B are known to metabolize hexobarbital (Jori et al. 1970, Waxman et al. 1992, Lewis et al. 1997). It is possible that the Great Basin animals express a version of CYP2B that is more effective at metabolizing hexobarbital than the Mojave animals, and that this difference is likely driven by their different dietary habits (Malenke et al. 2012). Alternatively, perhaps Great Basin animals depend more on P450 enzymes to metabolize hexobarbital than the Mojave animals due to having a reduced amount of conjugation pathways for hexobarbital. Previous work has shown that the Great Basin animals have lower activity of the conjugation enzyme glutathione-S-transferase compared to the Mojave animals on both a control and creosote diet (Haley et al. 2008), and there is evidence that glutathione conjugation is a method for hexobarbital biotransformation in laboratory rats (Takenoshita and Toki 2004).
Even though the two populations consumed different amounts of their respective diets, there was no difference in DMI within population between control and toxic diets on Day 3 of the feeding trials. The amount of plant components added to each diet was chosen to simulate the maximum amount of juniper and creosote the animals would consume in the wild or show tolerance for in the lab (Cameron et al. 1972; Meyer et al. 1989; Mangione et al. 2000; Magnanou et al. 2009). The fact that body mass and DMI were maintained on the respective toxic diets prior to ABT treatment shows that each population was acclimated to the relevant PSCs.
ABT caused a decrease in DMI for 24 hours in Great Basin woodrats consuming both the control and toxic diets. The Mojave woodrats had lower DMI 24 hours after ABT treatment when consuming the toxic diet but not when consuming the control diet. Both Great Basin and Mojave woodrats recovered DMI by 48 hours after ABT treatment, as evidenced by the fact that Day 5 DMI’s are not different from the respective Day 3 DMIs. The recovery of food intake so soon after treatment with an irreversibly binding inhibitor of P450s illustrates how rapidly animals are able to produce new P450s and also the appropriate time course for such a study.
Pretreatment with ABT decreased the DMIs of all diets except for the control diet presented to the Mojave woodrats, indicating that P450s are integral to biotransforming dietary constituents. While the control diet of high fiber rabbit chow is considered our “nontoxic” diet, it includes plant material such as alfalfa, soybeans, oats, wheat and corn. These contain low levels of primarily phenolic PSCs that may become feeding deterrents to the Great Basin woodrats when P450s are inhibited by the ABT treatment. Based on previous studies, as well as the current study it seems that Great Basin animals may be relying more heavily on P450 pathways in general, whereas and the Mojave animals may be more reliant on conjugation pathways that were not inhibited by the ABT treatment (Haley et al. 2008, Magnanou et al. 2009).
Interestingly ABT did not inhibit the DMI of animals on 4% creosote resin diets to a greater extent than the control diet in the Mojave woodrats. The DMI’s of both diets were inhibited ~13% after treatment with ABT. In contrast, ABT inhibited DMI of woodrats on the 50% juniper diet to a greater extent than the control diet in the Great Basin woodrats. We interpret this toxin and population difference to mean that, as hypothesized, P450s are more important in biotransforming the suite of PSCs present in juniper compared with creosote.
It is conceivable that if fed creosote levels below their tolerance, the Mojave woodrats would have excess P450 capacity to biotransform the PSCs in creosote and therefore not need to decrease DMI. However, creosote resin has been shown to be effective in reducing food intake at levels near to or even lower than 4% in other populations of woodrats (Mangione et al. 2000, Sorensen et al. 2005, Torregrossa et al. 2012). The variation in effective dose is a function of evolutionary history with creosote resin (Mangione et al. 2000) and it is possible that the population used in this study, which has a long history with creosote, had not yet ingested a maximum dose.
Our results do not exclude the possibility that P450s play some role in biotransforming the PSCs in creosote but do indicate that P450s are relatively more important for biotransforming the PSCs in juniper. Previous studies have shown that Phase II or conjugation pathways may be more important for Mojave woodrats when feeding on creosote, especially glucuronidation and glutathione conjugation (Mangione et al 2000, Haley et al 2008, Lamb et al. 2001, and Manganou et al. 2009). The importance of these pathways is consistent with what is known about the biotransformation of nordihydroguaiaretic acid (NDGA, a major component of creosote resin) in mice and humans (Lu et al. 2010, Lambert et al. 2002, Lambert et al. 2004). The observation that ABT did not decrease the DMI of the control diet in Mojave woodrats but did in Great Basin woodrats may mean that Mojave woodrats are relying more heavily on conjugation pathways than P450 metabolism in general.
The juniper presented to the Great Basin woodrats is an evergreen that contains high levels of PSCs (8–10% dry matter) with terpenes being the major class of PSC (5% dry matter) and condensed tannins and other phenolics found in smaller quantities (Adams et al. 1981; Adams et al. 1983; Adams 2000; Utsumi et al. 2009; Adams 2011). Previous studies have found the upregulation of a number of P450s in other woodrat species consuming juniper diets (Haley et al. 2007; Skopec et al. 2007), andMagnanou et al. (2009) found that both Great Basin and Mojave woodrats had higher gene expression of CYP3A, CYP2B, CYP2D, CYP2C and P450 oxidoreductase when fed a 50% juniper diet compared with a 2% creosote diet. Other mammalian herbivores, such as the koala (Phascolarctos cinereus), greater glider (Petauroides volans) and ringtail possum (Pseudocheirus peregrines) that consume high levels of terpenes also seem to rely heavily on P450-mediated biotransformation (Boyle et al. 1999; Boyle et al. 2000; Boyle et al. 2001). Therefore the greater inhibition of DMI by ABT in woodrats on the 50% juniper diet compared than the 4% creosote diet is consistent with previous results in this and other mammalian systems.
In conclusion we have developed an effective in vivonon-lethal assay to assess the role of P450s in the biotransformation of PSCs by mammalian herbivores. We demonstrated the P450 suicide inhibitor ABT was effective at inhibiting P450s in woodrats, and that food intake is an easy and sensitive method for detecting differences in the relative importance of P450s in biotransforming disparate PSC mixtures. This assay represents a relatively cheap and noninvasive new tool for the “pharm-ecologist’s” toolbox. We propose that the use of ABT in other wild caught mammalian herbivores would help scientists narrow their focus on which pathways may be involved in the biotransformation of the PSCs present in the in animals natural diet.
Acknowledgements
The authors would like to acknowledge the adept technical assistance of Ethan King. Funding was provided by NSF IOS 0817527 to M.D. Dearing and the NIH grant ES003619 to J.R. Halpert.
Literature Cited
- Adams RP. The serrate leaf margined Juniperus (Section Sabina) of the western hemisphere: systematics and evolution based on leaf essential oils and Random Amplified Polymorphic DNAs (RAPDs) Biochem Syst and Ecol. 2000;28:975–990. doi: 10.1016/s0305-1978(00)00022-3. [DOI] [PubMed] [Google Scholar]
- Adams RP. Junipers of the world: The genus Juniperus. Bloomington, IN: Trafford Publishing; 2011. [Google Scholar]
- Adams RP, Von Rudloff E, Hogge L. Chemosystematic studies of the western North American junipers based on their volatile oils. Biochem Syst and Ecol. 1983;11:189–193. [Google Scholar]
- Balani SK, Li P, Nguyen J, Cardoza K, Zeng H, Mu DX, Wu JT, Gan LS, Lee FW. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in guinea pigs and mice using serial sampling. Drug Metab Dispos. 2004;32:1092–1095. doi: 10.1124/dmd.104.000349. [DOI] [PubMed] [Google Scholar]
- Balani SK, Miwa GT, Gan LS, Wu JT, Lee FW. Strategy of utilizing in vitro and in vivo ADME tools for lead optimization and drug candidate selection. Curr Top Med Chem. 2005;5:1033–1038. doi: 10.2174/156802605774297038. [DOI] [PubMed] [Google Scholar]
- Balani SK, Zhu T, Yang TJ, Liu Z, He B, Lee FW. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in rats, dogs, and monkeys. Drug Metab Dispos. 2002;30:1059–1062. doi: 10.1124/dmd.30.10.1059. [DOI] [PubMed] [Google Scholar]
- Boyle R, McLean S, Foley W, Davies NW, Peacock EJ, Moore B. Metabolites of dietary 1, 8-cineole in the male koala (Phascolarctos cinereus) Comp Biochem Phys C. 2001;129:385–395. doi: 10.1016/s1532-0456(01)00214-9. [DOI] [PubMed] [Google Scholar]
- Boyle R, McLean S, Foley WJ, Davies NW. Comparative metabolism of dietary terpene, p-cymene, in generalist and specialist folivorous marsupials. J Chem Eco. 1999;25:2109–2126. [Google Scholar]
- Boyle R, McLean S, Foley WJ, Moore BD, Davies NW, Brandon S. Fate of the dietary terpene, p-cymene, in the male koala. J Chem Eco. 2000;26:1095–1111. [Google Scholar]
- Cameron GN, Rainey DG. Habitat utilization by Neotoma lepida in the Mohave desert. J Mammal. 1972:251–266. [Google Scholar]
- Dearing MD, Skopec MM, Bastiani M. Detoxification rates of wild herbivorous woodrats (Neotoma) Comp Biochem Phys A. 2006;145:419–422. doi: 10.1016/j.cbpa.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Dearing MD. The function of haypiles of pikas (Ochotona princeps) J Mammal. 1997;78:1156–1163. [Google Scholar]
- Dearing MD, Mangione AM, Karasov WH, Morzunov S, Otteson E, St. Jeor S. Prevalence of hantavirus in four species of Neotoma from Arizona and Utah. J Mammal. 1998;79:1254–1259. [Google Scholar]
- Devender TRv. Holocene woodlands in the Southwestern deserts. Science. 1977;198:189–192. doi: 10.1126/science.198.4313.189. [DOI] [PubMed] [Google Scholar]
- Domanski T, Halpert J. Analysis of mammalian Cytochrome P450 structure and function by site-directed mutagenesis. Curr Drug Metab. 2001;2:117–137. doi: 10.2174/1389200013338612. [DOI] [PubMed] [Google Scholar]
- Feyereisen R. Arthropod CYPomes illustrate the tempo and mode in P450 evolution. BBA- Proteins and Proteom. 2011;1814:19–28. doi: 10.1016/j.bbapap.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Fontana E, Dansette P, Poli S. Cytochrome p450 enzymes mechanism based inhibitors: common sub-structures and reactivity. Curr Drug Metab. 2005;6:413–451. doi: 10.2174/138920005774330639. [DOI] [PubMed] [Google Scholar]
- Freeland WJ, Janzen DH. Strategies in herbivory by mammals: The role of plant secondary compounds. Am Nat. 1974;108:269–289. [Google Scholar]
- Graham MJ, Lake BG. Induction of drug metabolism: species differences and toxicological relevance. Toxicology. 2008;254:184–191. doi: 10.1016/j.tox.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Groen K, Breimer DD. [19] Antipyrine, theophylline, and hexobarbital as in vivo P450 probe drugs. Method in Enzymol. 1996;272:169–177. doi: 10.1016/s0076-6879(96)72021-9. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- Haley SL, Lamb JG, Franklin MR, Constance JE, Dearing MD. "Pharm-Ecology" of diet shifting: Biotransformation of plant secondary compounds in creosote (Larrea tridentata) by a woodrat herbivore, Neotoma lepida. Physiol Biochem Zool. 2008;81:584–593. doi: 10.1086/589951. [DOI] [PubMed] [Google Scholar]
- Haley SL, Lamb JG, Franklin MR, Constance JE, Denise Dearing M. Xenobiotic metabolism of plant secondary compounds in juniper (Juniperus monosperma) by specialist and generalist woodrat herbivores, genus Neotoma. Comp Biochem Phys C. 2007;146:552–560. doi: 10.1016/j.cbpc.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Halpert JR. Structural basis of selective cytochrome P450 inhibition. Annu Rev Pharmacol. 1995;35:29–53. doi: 10.1146/annurev.pa.35.040195.000333. [DOI] [PubMed] [Google Scholar]
- Hanusch C, Hoeger S, Beck G. Anaesthesia of small rodents during magnetic resonance imaging. Methods. 2007;43:68–78. doi: 10.1016/j.ymeth.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Hyder PW, Fredrickson E, Estell RE, Tellez M, Gibbens RP. Distribution and concentration of total phenolics, condensed tannins, and nordihydroguaiaretic acid (NDGA) in creosotebush (Larrea tridentata) Biochem Syst Ecol. 2002;30:905–912. [Google Scholar]
- Jori A, Bianchetti A, Prestini P. Relations between barbiturate brain levels and sleeping time in various experimental conditions. Biochem Pharmacol. 1970;19:2687–2694. doi: 10.1016/0006-2952(70)90094-8. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Shin WH. General pharmacology of CKD-732, a new anticancer agent: effects on central nervous, cardiovascular, and respiratory system. Biol Pharm Bull. 2005;28:217–223. doi: 10.1248/bpb.28.217. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Watkins JB. Casarett & Doull's essentials of toxicology. New York: McGraw-Hill; 2003. [Google Scholar]
- Koul S, Koul JL, Taneja SC, Dhar KL, Jamwal DS, Singh K, Reen RK, Singh J. Structure-activity relationship of piperine and its synthetic analogues for their inhibitory potentials of rat hepatic microsomal constitutive and inducible cytochrome P450 activities. Bioorgan Med Chem. 2000;8:251–268. doi: 10.1016/s0968-0896(99)00273-4. [DOI] [PubMed] [Google Scholar]
- Lamb JG, Sorensen JS, Dearing MD. Comparison of detoxification enzyme mRNAs in woodrats (Neotoma lepida) and laboratory rats. J Chem Ecol. 2001;27:845–857. doi: 10.1023/a:1010366322299. [DOI] [PubMed] [Google Scholar]
- Lambert J, Dorr R, Timmermann B. Nordihydroguaiaretic acid: a review of its numerous and varied biological activities. Pharm Biol. 2004;42:149–158. [Google Scholar]
- Lambert JD, Zhao D, Meyers RO, Kuester RK, Timmermann BN, Dorr RT. Nordihydroguaiaretic acid: hepatotoxicity and detoxification in the mouse. Toxicon. 2002;40:1701–1708. doi: 10.1016/s0041-0101(02)00203-9. [DOI] [PubMed] [Google Scholar]
- Lewis D, Lake B. Molecular modelling of mammalian CYP2B isoforms and their interaction with substrates, inhibitors and redox partners. Xenobiotica. 1997;27:443–478. doi: 10.1080/004982597240433. [DOI] [PubMed] [Google Scholar]
- Lu JM, Nurko J, Weakley SM, Jiang J, Kougias P, Lin PH, Yao Q, Chen C. Molecular mechanisms and clinical applications of nordihydroguaiaretic acid (NDGA) and its derivatives: an update. Med Sci Monit. 2010;16:RA93–R100. [PMC free article] [PubMed] [Google Scholar]
- Magnanou E, Malenke J, Dearing MD. Expression of biotransformation genes in woodrat (Neotoma) herbivores on novel and ancestral diets: identification of candidate genes responsible for dietary shifts. Mol Ecol. 2009;18:2401–2414. doi: 10.1111/j.1365-294X.2009.04171.x. [DOI] [PubMed] [Google Scholar]
- Malenke JR, Magnanou E, Thomas K, Dearing MD. Cytochrome P450 2B diversity and dietary novelty in the herbivorous, desert woodrat (Neotoma lepida) PloS one. 2012;7:e41510. doi: 10.1371/journal.pone.0041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangione AM, Dearing MD, Karasov WH. Interpopulation differences in tolerance to creosote bush resin in desert woodrats (Neotoma lepida) Ecology. 2000;81:2067–2076. [Google Scholar]
- Marsh K, Wallis I, Andrew R, Foley W. The detoxification limitation hypothesis: Where did it come from and where is it going? J Chem Ecol. 2006;32:1247–1266. doi: 10.1007/s10886-006-9082-3. [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis MGM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- McLean S, Foley WJ. Metabolism of Eucalyptus terpenes by herbivorous marsupials. Drug Metab Rev. 1997;29:213–218. doi: 10.3109/03602539709037582. [DOI] [PubMed] [Google Scholar]
- McLean S, Pass G, Foley W, Brandon S, Davies N. Does excretion of secondary metabolites always involve a measurable metabolic cost? Fate of plant antifeedant salicin in common brushtail possum, Trichosurus vulpecula . J Chem Ecol. 2001;27:1077–1089. doi: 10.1023/a:1010303726439. [DOI] [PubMed] [Google Scholar]
- Meyer MW, Karasov WH. Antiherbivore chemistry of Larrea tridentata: effects on woodrat (Neotoma lepida) feeding and nutrition. Ecology. 1989;70:953–961. [Google Scholar]
- Murayama N, Shimada M, Yamazoe Y, Sogawa K, Nakayama K, Fujii-Kuriyama Y, Kato R. Distinct effects of phenobarbital and its N-methylated derivative on liver cytochrome P450 induction. Arch Biochem Biophys. 1996;328:184–192. doi: 10.1006/abbi.1996.0159. [DOI] [PubMed] [Google Scholar]
- Nelson DR. Progress in tracing the evolutionary paths of cytochrome P450. BBA - Proteins and Proteom. 2011;1814:14–18. doi: 10.1016/j.bbapap.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman GSM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenet Genom. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. [DOI] [PubMed] [Google Scholar]
- Ramirez I. High-fat diets stimulate transient hyperphagia whereas wet diets stimulate prolonged hyperphagia in Fischer rats. Physiol Behav. 1991;49:1223–1228. doi: 10.1016/0031-9384(91)90355-r. [DOI] [PubMed] [Google Scholar]
- Sasaki N. Effects of furazolidone on duration of righting reflex loss induced with hexobarbital and zoxazolamine in the rat. Jpn J Vet Sci. 1994;56:667–670. doi: 10.1292/jvms.56.667. [DOI] [PubMed] [Google Scholar]
- Scott EE, Halpert JR. Structures of cytochrome P450 3A4. Trends Biochem Sci. 2005;30:5–7. doi: 10.1016/j.tibs.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Skopec MM, Haley S, Dearing MD. Differential hepatic gene expression of a dietary specialist (Neotoma stephensi) and generalist (Neotoma albigula) in response to juniper (Juniperus monosperma) ingestion. Comp Biochem Physiol D. 2007;2:34–43. doi: 10.1016/j.cbd.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Sorensen JS, McLister JD, Dearing MD. Novel plant secondary metabolites impact dietary specialists more than generalists (Neotoma spp) Ecology. 2005;86:140–154. [Google Scholar]
- Sorensen JS, Skopec MM, Dearing MD. Application of pharmacological approaches to plant–mammal interactions. J Chem Ecol. 2006;32:1229–1246. doi: 10.1007/s10886-006-9086-z. [DOI] [PubMed] [Google Scholar]
- Takenoshita R, Toki S. New aspects of hexobarbital metabolism: Stereoselective metabolism, new metabolic pathway via GSH conjugation, and 3-hydroxyhexobarbital dehydrogenases. Yakugaku Zasshi. 2004;124:857. doi: 10.1248/yakushi.124.857. [DOI] [PubMed] [Google Scholar]
- Torregrossa AM, Dearing MD. Caching as a Behavioral Mechanism to Reduce Toxin Intake. J Mammal. 2009;90:803–810. [Google Scholar]
- Torregrossa AM, Dearing MD. Nutritional toxicology of mammals: regulated intake of plant secondary compounds. Funct Ecol. 2009;23:48–56. [Google Scholar]
- Utsumi SA, Cibils AF, Estell RE, Soto-Navarro SA, Van Leeuwen D. Seasonal changes in one seed juniper intake by sheep and goats in relation to dietary protein and plant secondary metabolites. Small Ruminant Res. 2009;81:152–162. [Google Scholar]
- Van Devender TR, Spaulding WG. Development of vegetation and climate in the southwestern United States. Science. 1979;204:701–710. doi: 10.1126/science.204.4394.701. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins NL, McArthur C, Davies NW. Diet switching in a generalist mammalian folivore: fundamental to maximizing intake. Oecologia. 2006;147:650–657. doi: 10.1007/s00442-005-0305-z. [DOI] [PubMed] [Google Scholar]
- Wiggins NL, McArthur C, Davies NW, McLean S. Behavioral responses of a generalist mammalian folivore to the physiological constraints of a chemically defended diet. J Chem Ecol. 2006;32:1133–1147. doi: 10.1007/s10886-006-9076-1. [DOI] [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- Woodcroft KJ, Webb CD, Yao M, Weedon AC, Bend JR. Metabolism of the Cytochrome P450 mechanism-based inhibitor N-benzyl-1-aminobenzotriazole to products that covalently bind with protein in guinea pig liver and lung microsomes: comparative study with 1-aminobenzotriazole. Chem Res Toxic. 1997;10:589–599. doi: 10.1021/tx960185g. [DOI] [PubMed] [Google Scholar]
- Yasar S, Forbes J. Enzyme supplementation of dry and wet wheat-based feeds for broiler chickens: performance and gut responses. Brit J Nutr. 2000;84:297–307. doi: 10.1017/s0007114500001574. [DOI] [PubMed] [Google Scholar]
- Zvi Z, Kaplanski J. Effects of chronic heat exposure on drug metabolism in the rat. J Pharm Pharmacol. 1980;32:368–369. doi: 10.1111/j.2042-7158.1980.tb12941.x. [DOI] [PubMed] [Google Scholar]


