Abstract
Endothelins are 21-amino acid peptides involved in vascular homeostasis. Three types of peptide have been identified, with endothelin-1 (ET-1) being the most potent vasoconstrictor currently known. Two endothelin receptor sub-types are found in various tissues, including the brain, heart, blood vessel, lung, and placenta. The ETA-receptor is associated with vasoconstriction in vascular smooth muscle. Conversely, the ETB-receptor can elicit a vasoconstrictor effect in vascular smooth muscle and a vasodilator effect via its action in endothelial cells. Both receptors play a key role in maintaining circulatory homeostasis and vascular function. Changes in ET-1 expression are found in various disease states, and overexpression of ET-1 is observed in hypertension and preeclampsia in pregnancy. Placental localization of ET-1 implies a key role in regulating the uteroplacental circulation. Additionally, ET-1 is important in the fetal circulation and is involved in the pulmonary circulation and closure of the ductus arteriosus after birth, as well as fetal growth constriction in utero. ET receptor antagonists and nitric oxide donors may provide therapeutic potential in treating conditions associated with overexpression of ET and hypertension.
Keywords: Endothelin-1, placenta, fetal circulation, preeclampsia, hypertension
1. ENDOTHELINS AND ENDOTHELIN RECEPTORS
Endothelin (ET) is a 21-amino acid peptide involved in regulating vascular homeostasis. The endothelium-derived peptide was originally isolated from porcine aortic endothelial cells in 1988 [1]. This original peptide was identified as endothelin-1 with the subsequent discoveries of two other isoforms, endothelin-2 (ET-2) and endothelin-3 (ET-3) [2]. Endothelin-1 (ET-1) is currently the most potent vasoconstrictor known [1, 3, 4] and is mainly secreted from vascular endothelial cells [5]. The ET-2 peptide differs from ET-1 by two amino acids whereas the ET-3 peptide differs by six amino acids [6].
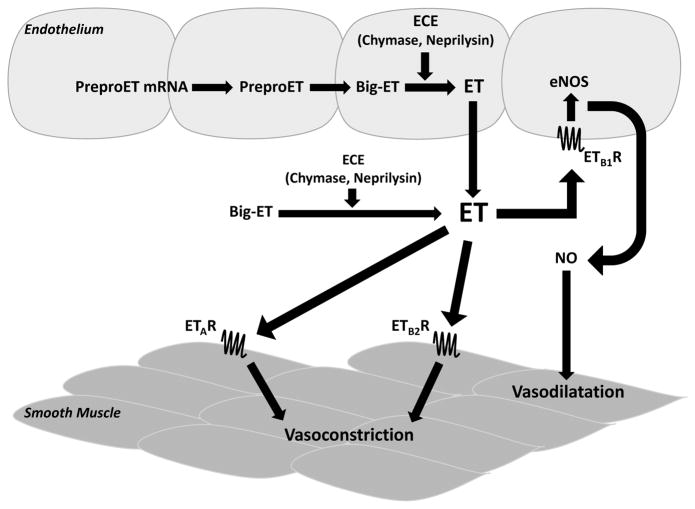
As shown in (Fig. 1), the endothelin synthesis pathway begins with the transcription of pre-proendothelin mRNA that is then translated into pre-proendothelin, a 212-residue peptide [5, 7]. A short secretory sequence is cleaved before furin-like proteases process the prepro-ET into the 38 to 39-residue peptide, big-endothelin [5, 7]. A number of endothelin converting enzyme isoforms (ECE-1, ECE-2, ECE-3) cleave the big-ET into the functional 21-residue peptide, endothelin [5, 7, 8]. ECE-1 is mainly found in endothelial cells and ECE-2 is often found in neurons. These two converting enzymes have a higher affinity for big ET-1 versus big ET-2 or big ET-3 [6]. In addition to ECE, it has been shown that chymase and neprilysin can convert big-ET-1 into its active form ET-1 [9]. Furthermore, matrix metallo- proteinase-2 (MMP-2, gelatinase A) can also convert human big ET-1 (1–38) to ET-1 (1–32), which preferentially acts on ETBR [10, 11].
Fig. 1. Endothelin Sythesis and Action Pathway.
ET: endothelin; ECE: endothelin-converting enzyme; NOS: nitric oxide synthase; NO: nitric oxide; ETAR: endothelin receptor A; ETB1R: endothelin receptor B subtype 1; ETB2R: endothelin receptor B subtype 2
The ET peptides are strong vasoactive substances that can bind one of two types of G-protein coupled receptors, the ETA- or ETB-receptor. The ETA-receptor (ETAR) is found in vascular smooth muscle [12] and therefore is associated with the vasoconstrictor effects of ET. It has the highest binding affinity for ET-1 followed by ET-2 and then ET-3 [4, 13–15]. All three isoforms have equal binding affinity for the ETB-receptor (ETBR), which is involved in both vasoconstriction and vasodilatation effects [4, 13–16]. Due to the ETBR’s involvement in constriction and dilatation, it has been categorized into two subtypes, ETB1 and ETB2 [6, 17]. The ETB1 subtype is mainly found in endothelial cells [15] regulating the vasodilator effect and ETB2 is found predominantly in vascular smooth muscle cells [14] exerting vasoconstrictor effects along with ETAR. The ETBR is also involved in the clearance of endothelins from tissues [18].
ET signaling acts via autocrine and paracrine secretion as opposed to acting as an endocrine hormone [5, 19, 20]. It has been well established that ET acts as an autacoid [18]. The endothelial cell is the main site of ET-1 production [2]. ET-2 is secreted in endothelial cells, heart and kidney [5], while ET-3 is not produced in endothelial cells [21] but rather in the endocrine, gastrointestinal, and central nervous systems [5]. Endothelin is important in normal physiological processes, especially during growth and development. The general effect of ET is an increase in both blood pressure and vascular tone [5, 22], as well as reducing cardiac output and heart rate [22]. The most potent vasoconstrictor, ET-1, has been implicated in various diseases such as asthma, essential and pulmonary hypertension, cardiac failure and uterine dysfunction. ET-1 can act as a growth factor and play a key role in tissue development and differentiation and induce proliferation and vascular smooth muscle cell growth [23]. An upregulation of ET-1 [24, 25] and ETAR and a downregulation of ETBR were observed in the myocardium of human end-stage heart failure patients [25]. There are many stimuli for the secretion of ET-1 including: vasoactive hormones, growth factors, hypoxia, shear stress, ischemia, lipoproteins, free radicals, endotoxin and cyclosporine [8]. Endothelium-derived NO, vasodilators, natriuretic peptides, heparin and prostaglandins, can inhibit ET-1 production [5].
2. ROLE OF ET IN THE UTEROPLACENTAL CIRCULATION
2.1. Placental Localization of ET
ET-1-like immunoreactivity is localized in human placentas. It has been observed that ET is involved in constricting fetoplacental vessels and is distributed throughout the vascular endothelium of the placenta. Additionally, the ETBR is highly localized in placental tissues [18].
ET-1-like immunoreactivity was found in endothelial cells lining small to medium sized arteries and veins of the placenta as well as the capillaries. Focal staining was also observed in the medium and large arteries and veins. Altogether, this study suggests that overexpression of ET-1 may be involved in diseases associated with excessive constriction of fetoplacental vessels [18]. Barros et al. compared ET-1 expression localization in the human placenta of both normal and preeclamptic pregnancies [26]. ET-1 localization was identified in the endothelium of the blood vessels. The seventh week of gestation was the earliest stage that ET-1 expression was observed. Within the normal placenta group, there was no obvious difference in ET-1 expression between the 7th and 22nd week of gestation. ET-1 immunoreactivity was found in normal placental villi from all gestational ages studied, including: 7, 9, 12, 16, 19, 22, 29 to 36, and 38 to 40 weeks. There was intense distribution of ET-1 in the syncytiotrophoblast layer of the chorionic villi for all gestational ages. During the first and second trimester placentas, ET was also localized in the cytotrophoblasts. The extravillous cytotrophoblasts (EVT) in second and third trimester as well as term placentas stained positive with variable intensity of ET-1 expression [26].
Trophoblasts are essential in the normal development of the placenta providing adequate nutrients to the fetus. The process of trophoblast invasion is a key step in the implantation and formation of the placenta. It has been suggested that trophoblasts may serve as an extravascular source for growth factors necessary for cell proliferation and differentiation within the placenta [26]. ET-1 binding sites and immunoreactivity were observed in cultured trophoblasts. After five days of culture, the trophoblasts mostly differentiated into syncytiotrophoblasts with high amounts of ET-1 and ET-2 [27]. Thus ET localization to these cells may indicate the importance of the potent vasoactive agents in placental involvement.
It has been shown that amniotic cells secrete ET leading to high levels of this peptide in the amniotic fluid [28, 29]. However there are no ET-receptors present in amniotic cells [30]. Both ETAR and ETBR have been observed in the placenta to regulate constriction of placental arteries [31], and also present in the chorion laeve and decidua vera at high concentrations [30], suggesting that the amnion may secrete the ET which then acts on the receptors found in the chorion laeve and decidua vera to induce its effects. Production of ET-1 in the placenta supports the mechanism of both autocrine and paracrine secretion. Within the placental villi, the endothelium and muscle cells are closely associated implying a paracrine mode of secretion [27]. The presence of receptors on endothelial cells that secrete ET themselves indicates autocrine function.
ECE-1, the enzyme that converts ET-1 into its active form, has been localized to specific areas of the placenta corresponding with ET-1 distribution. The five cell populations with ECE-1 localization include: (1) endothelial cells lining the maternal basal plate blood vessels, (2) intermediate trophoblasts, (3) endothelial cells lining the chorionic villous blood vessels, (4) chorionic villous stromal cells, and (5) chorionic villous trophoblasts [32].
2.2. ET and Preeclampsia
Changes in ET expression levels have been seen in many disease states [5, 33, 34]. Endothelin is found in increased levels during hypertension [35] as well as in preeclampsia [36–38]. Pregnancy-induced hypertension after mid-gestation is known as preeclampsia (PE). This condition is characterized by increased blood pressure, a systolic blood pressure over 140 mmHg or a diastolic blood pressure over 90 mmHg. Additionally, proteinuria is a key characteristic at more than 300 mg per 24-hour [39]. The cause of this disease is most likely due to an imbalance of vasoactive agents, thus supporting its vascular nature [40]. The potent vasoconstrictor, ET-1, appears to be key in the disruption of vascular homeostasis during PE.
There are two stages of preeclampsia. In the first, the vascular remodeling is disrupted leading to placental hypoxia, which then allows for the release of various factors [41, 42]. The release of factors leads to an imbalance in which vasoconstrictors, such as ET, are favored [41], resulting in vessel constriction. The symptomatic phase is the second stage [42] where the release of these vasoactive factors during the first stage causes maternal endothelial dysfunction to occur [41, 42].
Trophoblasts invade the vessels via the endothelial lining therefore causing vascular remodeling in the placenta. Normally under these conditions, the low-capacitance and high-resistance vessels convert into high-capacitance and low-resistance vessels. This remodeling is key to facilitate adequate blood flow through the placenta to the fetus [42]. Oxygen tension is a key element in the regulation of cytotrophoblast differentiation. When the cells are in hypoxic conditions, they are only able to carry out the first phase of normal differentiation. A lack of trophoblast invasion can lead to shallow placentation and potential diseases, such as preeclampsia [43]. In preeclampsia, the vascular remodeling is greatly disrupted. The spiral arteries do not undergo this conversion and remain at the low-capacitance, high-resistance state thus leading to insufficient blood flow to the placenta and consequently the fetus [42, 44].
Increased levels of the anti-angiogenic proteins soluble fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng) have been associated with preeclampsia [45, 46] as well as with increased circulating levels of ET-1 [45]. Preeclampsia is also associated with high levels of sE-selection and sVCAM-1 (soluble vascular cell adhesion molecule 1) in maternal circulation, and has been correlated with low NO synthesis in fetal endothelium [47]. Altered levels of angiogenic factors associated with preeclampsia may disrupt fetal vascular development and function.
Preeclamptic women are at an increased risk for developing cardiovascular (CV) disease later in life [48–50]. Women with preeclamptic pregnancies were found years later to have increased blood pressure compared to women who had a normotensive pregnancy [51] as well as a higher risk for ischemic heart disease [52]. There is also evidence to support the idea that the infants of preeclamptic women have a greater risk for preeclampsia [53, 54] as well as CV disease later in life [41]. Fetal undernutrition can result from poor blood flow caused by the disruption of vascular remodeling and the malformation of blood vessels. Poor nutrition in the mother can lead to an increased risk of CV disease in offspring [41, 55]. David Barker has established the idea of developmental programming of CV disease in which the development of the fetus and intrauterine environment are key participants in the risk factor of developing CV disease during adult life [56]. Therefore poor fetal development due to preeclampsia may influence the newborn’s predisposition to adult-onset CV disease.
In severe preeclamptic placentas with preterm pregnancies, the ET-1 expression was weak or nonexistent in syncytiotrophoblasts and endothelial cells of the blood vessels. However in less severe PE, many placentas had positive staining similar to that of normal pregnancies. The binding of ET-1 to the placenta was lower in PE, most likely due to fewer receptors. In PE, the binding of ET-1 to trophoblast membranes was higher in preterm pregnancies compared to normal pregnancies [26]. The expression was sparse in the syncytiotrophoblasts between the 29th and 32nd weeks of gestation however it became more variable at the 33rd week of gestation. Syncytiotrophoblasts stained positive during the 35th and 36th week of gestation. The fetal vessels found in the placental villi were more intensely stained for ET-1. The EVT demonstrated strong ET-1 staining in preeclamptic placentas during all gestational ages. However there was no clear difference between normal and preeclamptic placentas in regards to ET-1 expression in the EVT [26]. Overall, the staining pattern differences between the placentas from normal and preeclamptic patients were consistent between 29 to 32 weeks and 33 to 36 weeks [26].
Several studies have shown increased ET-1 expression levels in preeclamptic women [40, 57–59]. ET-1 concentration in amniotic fluid of women who later developed PE was seen as significantly increased compared to that of controls [60]. One study showed that the addition of exogenous ET-1 to both normotensive and preeclamptic trophoblasts lead to an upregulation of ET-1 mRNA [40]. The same study showed decreased expression of inducible nitric oxide synthase (iNOS) and increased expression of endothelial NOS (eNOS) in preeclamptic trophoblasts. This indicates that ET-1 may cause increased expression of itself as well as altering expression levels of the NOS isoforms in the trophoblasts for both preeclamptic and normal cells [40]. Preeclamptic patients had significantly increased ET-1 expression in the amnion, chorion laeve, placental plate chorion and fetal placenta compared to normotensive placentas [61]. This is contrary to another study that showed no significant difference in ET-1 concentrations between preeclamptic and normotensive placentas [62]. The high expression levels of ET-1 in preeclamptic women can be potentially due to an upregulation in ECE activity [63]. ECE cleaves big ET into the mature and active ET form, which can exert its vasoactive effects via the ET-receptors.
Hypoxia is a strong inducer of ET expression via hypoxia-inducible factor 1-alpha (HIF-1α) [64]. HIF-1α is expressed in a low-oxygen environment such as the placenta. This factor is key in placental development and has been seen to be upregulated in preeclamptic placentas [65, 66]. Abnormal trophoblast invasion and uterine spiral artery remodeling result in high levels of HIF-1α. This increased expression arrests differentiation of the trophoblasts, causing them to remain in an immature state [67]. Studies in pregnant mice suggest that increased HIF-1α expression induces preeclampsia as well as intrauterine growth restriction [68]. Thus hypoxia and its relatedness to ET expression may be indicative of poor uteroplacental circulation and vascular function leading to the disease.
3. ROLE OF ET IN THE FETAL CIRCULATION
3.1. ET and Pulmonary Circulation
In the fetus, the pulmonary artery demonstrated greater pressure than the systemic artery [69]. Fetal pulmonary vascular resistance is higher than that of newborn and adult [70, 71]. In a concentration-dependent manner, fetal pulmonary resistance arteries and veins are constricted by ET-1 [72]. Together these findings indicate that greater vasoconstriction occurs in the pulmonary circulation than the systemic circulation leading to the observed increased pressure in the pulmonary system in the fetus.
The lung development occurs in five stages characterized by histological differences. These stages include embryonic (1–7 weeks of gestation), pseudoglandular (7–17 weeks of gestation), canalicular (17–27 weeks of gestation), saccular (28–36 weeks of gestation), and alveolar (36 week of gestation until term) [73]. In a study by Levy et al., ET-1 expression in the human lung is higher in the saccular and alveolar stages compared to the canalicular stage. The expression of the ET-receptors in the lung was also analyzed showing a constant strong expression of the ETA-receptor throughout gestation. However the ETB-receptor was expressed weakly in the canalicular stage but the expression was increased greatly during the saccular and alveolar stages [74].
ET-1 plays a key role in maintaining high basal pulmonary vascular resistance by acting mainly on ETAR in both pulmonary arteries and veins in the fetus [75–78]. Fetal pulmonary endothelial cells express ECE-1 that produces the majority of ET-1 in fetal lungs. The expression of ECE-1 is developmentally regulated and falls just prior to birth, resulting in a fall in ET-1 in fetal pulmonary vasculature [79]. It appears that the greater activity of ETAR that mediates vasoconstriction than that of ETBR that induces vasodilatation in fetal pulmonary vasculature favors vasoconstriction in normal fetal lungs [75, 76]. The expression of ETBR in pulmonary endothelial cells increases at the end of gestation and after birth, which, combined with the decreased ET-1 expression just prior to birth may play an important role in perinatal pulmonary vasodilatation [76, 79, 80]. Thus, the abnormal increase in pulmonary ET-1 concentrations, progressive ETAR-mediated vasoconstriction, and the loss of ETBR-mediated vasodilatation may contribute to the pathophysiology of persistent pulmonary hypertension in the newborn [16, 70, 77, 81–83].
3.2. ET and Ductus Arteriosus
The ductus arteriosus (DA) of the fetus is a muscular artery consisting of vascular smooth muscle cell layers with elastin separating them [84]. The DA is a shunt from the pulmonary artery to the aortic arch. It plays an important functional role in the fetus by shunting the blood away from the lungs and directly to the aortic arch to be distributed throughout the body. Since the blood is coming across the placenta from the mother where it has been oxygenated, the majority of the blood travels through the DA and thus bypasses the lungs. This also prevents the heart and lungs from having to perform unnecessary work.
At birth, the lungs take on the role of oxygenating the blood; at this point the DA is no longer necessary and begins to close. The changes involved in birth, mainly the lungs’ exposure to air, are necessary for the remodeling and closure of the DA [84]. There are two stages of the postnatal closure. The first stage occurs within a few hours after birth and is instigated by smooth muscle contraction in the DA. The second stage of DA closure is more permanent, characterized by intimal thickening and loss of smooth muscle cells, which can occur within several days to weeks depending on the species [85, 86].
Blood oxygen tension increases at the onset of respiration to facilitate DA closure [85]. The role of vascular smooth muscle cells (VSMCs) in regulating the closure of the DA is essential. The VSMCs begin contracting with the increase in oxygen tension [84]. A major regulating factor in the closure of the DA involves the decrease in the levels of prostaglandin E2 (PGE2) [87], the most potent vasodilator in the DA [88]. The VSMCs sense the change in oxygen as well as the change in PGE2 levels, causing the DA to close off [87].
In the lamb fetus, ET-1’s ability to constrict the DA progressively increases throughout gestation [89]. Ductal smooth muscle development may be regulated by ET-1 [87]. The active ET-1 peptide can bind ETA-receptors in VSMCs of the DA to induce vasoconstriction [87, 89, 90]. ETA-receptors have been suggested to regulate the postnatal DA closure of rats since rats with defects in the ETA-receptors suffered from suffocation and death [91]. Although several studies have demonstrated that ET-1 is the key vasoconstrictor involvement in the contractile response of the DA to oxygen [92–95], it has also been reported that ET-1 is not necessarily responsible for the closure of the DA. Thus, pharmacological blockade or knockout of ETAR did not prevent postnatal DA closure [92, 96].
Patency of the DA, in which the shunt does not fully close, is seen in many pre-term and term infants. Both the closure and the patency of the DA are regulated by vasoactive factors. Vasoconstrictors are mainly responsible for the closure while vasodilators maintain patency. The mechanism in which DA constriction occurs in the fetus is not fully known however the mechanism of vasodilatation has been better identified. In the pre-term infant, the capacity to respond to PGE2 remains in addition to an increased production of nitric oxide (NO). The contribution of these two vasodilators allows the DA to remain open after birth [86].
4. ROLE OF ET IN FETAL GROWTH RESTRICTION
Restriction of fetal nutrient supply can result in insufficient growth. The placenta is imperative in the transfer of blood, oxygen, and other necessary nutrients from the mother to the fetus. Therefore placental formation is essential for adequate fetal development.
Intrauterine growth restriction (IUGR) is characterized by low birth weight, below the 10th percentile [97]. However there is a distinction between those with growth restriction and those who are simply small-for-gestational age but not actually growth restricted. IUGR is often associated with preeclampsia, occurring in about 25% of PE cases [98]. Endothelial dysfunction and abnormal placentation are characteristics common to both PE and IUGR [99]. While some preeclamptic women give birth to infants with low birth weights, others do not have this problem, indicating an independent effect of fetal growth restriction in preeclampsia [60]. The foremost idea surrounding the development of PE and IUGR is resultant placental ischemia due to defects in spiral artery remodeling and trophoblast invasion [100].
It has been shown that severe IUGR resulted in rats exposed to chronic hypoxia compared to rats in normoxic conditions [101]. The rats with IUGR had both lower fetal weights as well as lower placental weights by 20% and 11% respectively, compared to controls [101]. HIF-1α is suggested to induce IUGR when overexpressed in pregnant mice. Both fetal and maternal blood vessels were abnormally constricted in pregnant mice with HIF-1α overexpression [68], suggesting that upregulation of HIF-1α restricts adequate blood flow to the fetus leading to poor development. However there was no observation of abnormal trophoblast invasion in HIF-1α overexpression pregnant mice, which is a characteristic of preeclampsia [68].
Plasma levels of ET-1 in both the mother and fetus are significantly increased in patients with IUGR. Additionally, ET-1 levels are higher in pregnancies with both IUGR and PE versus those without PE as well as normal controls. A negative correlation between plasma ET-1 levels and birth weight has been established in IUGR patients with and without PE [102]. A major stimulus of ET-1 expression is hypoxia. Chronic hypoxia is shown to upregulate ET-1 circulating levels in rats [103]. Thus ET-1 may play a role in hypoxia-induced IUGR. The increased vasoconstriction of the placenta restricts blood supply to the fetus leading to growth restriction. ET-1 is involved in uteroplacental blood supply regulation under pathological conditions by inhibition of NOS [104]. Activation of NOS leads to the formation of nitric oxide (NO), a vasodilator substance that counteracts the vasoconstrictor effects of ET-1. NOS is critical in the placenta in order to maintain sufficient perfusion to the fetus. Fetal growth restriction in the rat can result from chronic NOS inhibition [104]. Increased levels of ET-1 are found when NOS is inhibited, leading to hypertension during pregnancy and poor perfusion of the uteroplacental bed [104]. In placentas with fetal growth restriction, there was found to be a decrease in iNOS in syncytiotrophoblasts compared to controls. However, iNOS staining was increased in endothelial cells of growth-restricted placentas. These results suggest that differential local production of iNOS may be greatly involved in the fetal-placental communication [105].
Abnormal ET-1 levels found in IUGR fetuses may adversely affect the heart development. ET-1 expresses in the fetal heart and plays an important role in regulating cardiomyocyte differentiation during the heart development [106–110]. ECE-1 knockout mice show defects in the developing heart. While ECE-2 null mice develop normally, ECE-1(−/−) and ECE-2(−/−) double null mice exhibit abnormal atrioventricular valve formation that is not seen in ECE-1 single knockout fetuses [110]. Several studies have demonstrated that ET-1 induces hypertrophy of cardiomyocytes [111–115] and promotes cardiac fibrosis [116–118]. ET-1-induced cardiomyocyte hypertrophy is mainly mediated by activating ETAR and by promoting overexpression of VEGF. While both ET-1 and ET-3 can induce the synthesis of type I and III collagens in the heart via the activation of both ETAR and ETBR, ET-1, but not ET-3, may reduce collagenase activity mainly through ETAR. In a rat model of IUGR induced by maternal hypoxia, the fetal heart was found to have decreased cardiomyocyte proliferation and increased transition of mononucleated cells to binucleated cells with larger cell sizes [119, 120]. In rat heart development, the transition of proliferative and hyperplasic growth of mononucleated cells to hypertrophic growth of binucleated cells and terminal differentiation of cardiomyocytes take place within the first two weeks after birth [121]. The premature exit of the cell cycle with a larger number of terminally differentiated cardiomyocytes in the fetal heart and early morphologic indication of cardiomyocyte hypertrophy found in IUGR fetuses resulted in fewer but larger cardiomyocytes in offspring [122, 123]. Additionally, the IUGR fetal heart showed abnormal expression patterns of MMPs and an increase in collagen deposition [119]. The functional impact of these changes has been demonstrated in the adult heart in which a sustained reduction of MMP-2 and enhanced collagen accumulation were found along with left ventricular hypertrophy and stiffening, diastolic dysfunction, and increased ischemic injury [122–127]. Whether and to what extent the increased ET-1 in IUGR fetuses contribute to the premature transition of proliferative mononucleated cells to terminally differentiated binucleated cells and programming of increased risk of cardiac fibrosis present intriguing questions that warrant further investigation.
5. THERAPEUTIC POTENTIAL OF ET RECEPTOR INHIBITORS
Numerous studies have shown that ET receptor antagonists [37, 128] and nitric oxide [33, 129] can inhibit vasoconstriction induced by high levels of ET expression. It has been suggested that ECE-1 may be a useful target for treating hypertensive diseases due to its key role in converting ET-1 into its active form [32, 130]. In spontaneously hypertensive rats that underwent treatment with BQ123, an ETAR antagonist, or BQ788, an ETBR antagonist, VSMC proliferation was attenuated [131]. BQ123 inhibited an increase in intracellular calcium concentration due to high levels of ET-1. The ETAR antagonist in combination with BQ788 was even more efficient in inhibiting intracellular calcium increase due to ET-1 [132]. Nitric oxide can inhibit endothelial ET-1 expression and inhibition of nitric oxide synthesis lead to increased expression of ET-1 [133]. ET antagonists may be beneficial in treating cardiac and vascular diseases that involve vasoconstriction and hypertension [5, 131, 134].
5.1. Preeclampsia
The pathophysiology of preeclampsia appears to have a common pathway in which endothelin is involved [135]. This indicates that ET antagonism may have potential in treating preeclampsia [135–137]. Experimentally, hypertension during pregnancy has been reduced using ET antagonists, mainly ETA receptor antagonists. The ETAR acted on by ET-1 seems clearly involved in preeclampsia [138] due to its induction of vasoconstriction. However, complete knockout of the ETA receptor in mice leads to cranial and cardiac defects. Blocking the receptor showed windows before and after mid-gestation in which similar birth defects were observed [139]. Therefore use of ETAR antagonists as treatment for preeclampsia may be harmful to the developing fetus.
However, ET-1 plasma levels are typically highest during the late stage of preeclampsia, thus not necessarily involved in its initiation but rather its progression [140]. As of yet, ETAR antagonists have not been administered in late gestation to determine their efficacy. More severe forms of PE may have potential to be treated via an ETA receptor antagonist, especially during late gestation [42]. In a rat model of preeclampsia, induced by reduction in uterine placental pressure (RUPP), the values for uterine artery resistive index (UARI) and the mean arterial pressure (MAP) were increased compared to controls. However when the RUPP dams were pretreated with an ETAR antagonist both the MAP and UARI values were attenuated, with no significant effect on the normal rats’ blood pressure [141]. Angiotensin receptor-activating autoantibodies (AT1-AAs) are present in preeclamptic women where they can bind and activate AT1 receptors [142, 143]. High levels of AT1-AA lead to activation of the ET system in preeclamptic women. This hypertension was attenuated by administration of ETAR antagonists [144, 145]. Hypoxia is a stimulus for ET production thus there is a higher incidence of preeclampsia in high altitude. Treatment by moving to a lower altitude, promoting vasodilators, or using ET antagonists may be beneficial for high altitude preeclampsia [146].
In preeclamptic women, the use of isosorbide dinitrate (ISDN) improved both maternal blood circulation and fetoplacental circulation. ISDN is a nitrate that donates NO and thus acts as a relaxant by causing vasodilatation [147]. Both ET-receptor antagonists and NO donors may provide therapeutic potential in the treatment of preeclampsia.
5.2. Pulmonary Arterial Hypertension (PAH)
PAH is a serious and fatal condition in which ET-receptor antagonists have been examined for treatment efficacy. Bosentan is a dual ET-receptor antagonist that is currently used to treat PAH patients who are class III or IV [148]. This drug is a sulfonamide-based competitive drug for both ETA and ETB receptors, with a slightly higher affinity for the ETA receptor over the ETB receptor [149]. Treatment of rats with PAH using vasoactive intestinal peptide (VIP) almost completely prevented PAH pathology. More so, the combination treatment of VIP with bosentan entirely reversed the PAH pathology in these rats [150]. Hepatocellular injury is the major toxicity associated with bosentan treatment [151, 152].
Sitaxsentan, also a sulfonamide drug, is a selective ETA receptor antagonist that is currently only available in EU and Canada and is used for the treatment of WHO class III PAH [153]. Ambrisentan is the second ET receptor antagonist that is FDA-approved to treat PAH patients who are WHO class II or III. Patients who have failed the treatment with bosentan or sitaxsentan due to liver problems have been better able to tolerate ambrisentan [153, 154]. This propionic acid-based drug has high specificity for the ETA receptor over the ETB receptor. Efficacy of ambrisentan was seen in the form of increased exercise capacity and delay of clinical worsening of PAH [153]. Eisenmenger syndrome (ES), known for its association with congenital heart disease, is the most severe form of PAH. Studies using either the dual-receptor antagonist bosentan or the ETAR antagonist sitaxsentan have shown improvement in treating ES [155]. In ES patients with congenital heart defects who were treated with ETR antagonists, improvement in 6-minute walk distance, oxygen saturation, and pulmonary artery pressures was observed [156]. Macitentan, is a dual ETR antagonist that is currently undergoing clinical trials. It is designed to target tissues with overexpression of ET receptors and antagonize the binding of ET-1. More data on macitentan must be collected to determine its efficacy in treating pulmonary hypertension [157–159].
It is still unclear at this point as to whether selective or nonselective ET receptor antagonists are more effective in treating PAH. As of current, both selective and nonselective antagonists show efficacy in the PAH treatment by reducing pulmonary artery pressure [4, 160]. Therefore combination treatment appears to be a logical next step for reducing this hypertension.
5.3. Ductus Arteriosus
The NSAID (non-steroidal anti-inflammatory drug), indomethacin, has strong tocolytic properties and inhibits the production of prostaglandins, a key vasodilator. Ibuprofen is a second NSAID used for DA closure with a similar efficacy rate to that of indomethacin [161, 162]. The use of indomethacin suppresses vasodilatation and thus leads to the closure of the fetal DA prematurely while still in utero. ETA receptor antagonists have been tested in pregnant rats with indomethacin administration to prevent premature closure of the DA in utero. The results showed that DA closure was prevented with co-administration of the ETAR antagonist. This suggests the potential for the treatment with ETA receptor antagonists to keep the fetal DA open during the administration of tocolysis-causing NSAIDs [91]. Both prostaglandins and NO are involved in patency of the DA in pre-term newborns. Treatment using a NOS-inhibitor and indomethacin facilitates DA closure more effectively than indomethacin alone, suggesting that combined NO inhibition may provide a better therapeutic treatment of DA patency in premature newborns [163]. DA contraction was attenuated by treatment with both BQ123 and phosphoramindon, an inhibitor of big ET-1 conversion to active ET-1 [164, 165], suggesting a role for ETR antagonists in the treatment of patent DA.
6. CONCLUSION
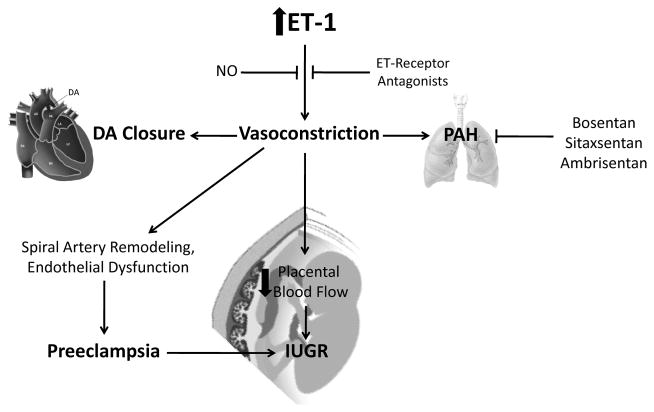
Since its discovery in 1988, many aspects of endothelin’s physiological function have been identified. However there is more work to be done to fully elucidate its role in disease states. Endothelin-1 is the most potent vasoconstrictor currently known and subsequently is implicated to be involved in hypertensive conditions including preeclampsia and pulmonary arterial hypertension. An overexpression of ET-1 is observed in preeclamptic patients and patients with PAH, as shown in (Fig. 2).
Fig. 2. Role of Endothelin in the Uteroplacental and Fetal Circulation.
ET: endothelin; NO: nitric oxide; DA ductus arteriosus; PAH: pulmonary arterial hypertension; IUGR: intrauterine growth restriction.
Furthermore, the vasoconstrictor peptide is involved in the uteroplacental circulation and fetal development (see Fig. 2). The ductus arteriosus is a necessary shunt for the fetal circulation, however at birth its closure is crucial. ET-1 is believed to be involved in the constrictive response to oxygen and the closure of DA after birth. High levels of ET-1 have been observed in fetal growth constriction for both the mother and the fetus. The upregulation of ET-1 in the fetus restricts proper growth and development. Overall more studies on endothelin’s role and its full effects on the uteroplacental circulation and fetal development are needed.
Some therapeutic agents of ET receptor antagonists have been developed, mainly for the treatment of PAH. Several studies have suggested the potential of ET receptor antagonists in reducing high levels of vasoconstriction in other hypertensive conditions as well. Nitric oxide, a vasodilator that counteracts ET-1 effects, may also be a target for treatment. Several studies using a NO donor were able to reduce high levels of vasoconstriction implying its therapeutic potential.
There are critical windows of development in which organs and their systems are most sensitive to their environment, and adverse conditions may cause them to adapt. These critical periods typically occur during phases of rapid cell division and thus the fetus compensates by slowing cellular division [166] which leads to poor growth. For most organisms, the most critical point occurs in utero during the fetal development [55]. Placental development is a key in determining if the fetus will have adequate nutrient and blood supply available. Abnormal placental development may cause the fetus to undergo compensatory growth that can lead to additional health problems later in life. Endothelin’s role in maintaining vascular homeostasis appears to be an important factor in influencing the fetal development. Further studies into the role of ET within the developing fetus should be a priority in clarifying its effects on in utero life as well as disease predisposition later in life.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL82779 (LZ), HL83966 (LZ), HL89012 (LZ), HL110125 (LZ), and HD31226 (LZ). We apologize to those authors whose excellent studies covered by the scope of this review were unable to be cited due to space restriction.
Footnotes
Send Orders for Reprints to reprints@benthamscience.net
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
References
- 1.Yanagisawa M, Inoue A, Ishikawa T, et al. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci USA. 1988;85(18):6964–7. doi: 10.1073/pnas.85.18.6964. Epub 1988/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inoue A, Yanagisawa M, Kimura S, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989;86(8):2863–7. doi: 10.1073/pnas.86.8.2863. Epub 1989/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–5. doi: 10.1038/332411a0. Epub 1988/03/31. [DOI] [PubMed] [Google Scholar]
- 4.Kawanabe Y, Nauli SM. Endothelin. Cellular and molecular life sciences : CMLS. 2011;68(2):195–203. doi: 10.1007/s00018-010-0518-0. Epub 2010/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agapitov AV, Haynes WG. Role of endothelin in cardiovascular disease. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2002;3(1):1–15. doi: 10.3317/jraas.2002.001. Epub 2002/05/02. [DOI] [PubMed] [Google Scholar]
- 6.Kedzierski RM, Yanagisawa M. Endothelin system: the double-edged sword in health and disease. Ann Rev Pharmacol Toxicol. 2001;41:851–76. doi: 10.1146/annurev.pharmtox.41.1.851. Epub 2001/03/27. [DOI] [PubMed] [Google Scholar]
- 7.Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Can J Physiol Pharmacol. 2008;86(8):485–98. doi: 10.1139/Y08-059. Epub 2008/09/02. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90(4):1291–335. doi: 10.1152/physrev.00032.2009. Epub 2010/10/21. [DOI] [PubMed] [Google Scholar]
- 9.Simard E, Jin D, Takai S, Miyazaki M, Brochu I, D’Orleans-Juste P. Chymase-dependent conversion of Big endothelin-1 in the mouse in vivo. J Pharmacol Exp Therapeut. 2009;328(2):540–8. doi: 10.1124/jpet.108.142992. Epub 2008/11/07. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85(10):906–11. doi: 10.1161/01.res.85.10.906. Epub 1999/11/13. [DOI] [PubMed] [Google Scholar]
- 11.Jeyabalan A, Novak J, Danielson LA, Kerchner LJ, Opett SL, Conrad KP. Essential role for vascular gelatinase activity in relaxin-induced renal vasodilation, hyperfiltration, and reduced myogenic reactivity of small arteries. Circ Res. 2003;93(12):1249–57. doi: 10.1161/01.RES.0000104086.43830.6C. Epub 2003/11/01. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda K, Nakao K, Hiroshi A, et al. Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett. 1991;287(1–2):23–6. doi: 10.1016/0014-5793(91)80007-p. Epub 1991/08/05. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa M. The endothelin system. A new target for therapeutic intervention. Circulation. 1994;89(3):1320–2. doi: 10.1161/01.cir.89.3.1320. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 14.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348(6303):730–2. doi: 10.1038/348730a0. Epub 1990/12/20. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T, Yanagisawa M, Takuwa Y, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348(6303):732–5. doi: 10.1038/348732a0. Epub 1990/12/20. [DOI] [PubMed] [Google Scholar]
- 16.Perreault T, Coceani F. Endothelin in the perinatal circulation. Can J Physiol Pharmacol. 2003;81(6):644–53. doi: 10.1139/y03-013. Epub 2003/07/04. [DOI] [PubMed] [Google Scholar]
- 17.Warner TD, Allcock GH, Corder R, Vane JR. Use of the endothelin antagonists BQ-123 and PD 142893 to reveal three endothelin receptors mediating smooth muscle contraction and the release of EDRF. Brit J Pharmacol. 1993;110(2):777–82. doi: 10.1111/j.1476-5381.1993.tb13879.x. Epub 1993/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilkes BM, Susin M, Mento PF. Localization of endothelin-1-like immunoreactivity in human placenta. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1993;41(4):535–41. doi: 10.1177/41.4.8450193. Epub 1993/04/01. [DOI] [PubMed] [Google Scholar]
- 19.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacological reviews. 1994;46(3):325–415. Epub 1994/09/01. [PubMed] [Google Scholar]
- 20.Wagner OF, Christ G, Wojta J, et al. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267(23):16066–8. Epub 1992/08/15. [PubMed] [Google Scholar]
- 21.Howard PG, Plumpton C, Davenport AP. Anatomical localization and pharmacological activity of mature endothelins and their precursors in human vascular tissue. J Hypertens. 1992;10(11):1379–86. doi: 10.1097/00004872-199211000-00010. Epub 1992/11/01. [DOI] [PubMed] [Google Scholar]
- 22.Kelly JJ, Whitworth JA. Endothelin-1 as a mediator in cardiovascular disease. Clin Exp Pharmacol Physiol. 1999;26(2):158–61. doi: 10.1046/j.1440-1681.1999.03011.x. Epub 1999/03/05. [DOI] [PubMed] [Google Scholar]
- 23.Goldie RG. Endothelins in health and disease: an overview. Clin Exp Pharmacol Physiol. 1999;26(2):145–8. doi: 10.1046/j.1440-1681.1999.03014.x. Epub 1999/03/05. [DOI] [PubMed] [Google Scholar]
- 24.Ponicke K, Vogelsang M, Heinroth M, et al. Endothelin receptors in the failing and nonfailing human heart. Circulation. 1998;97(8):744–51. doi: 10.1161/01.cir.97.8.744. Epub 1998/03/14. [DOI] [PubMed] [Google Scholar]
- 25.Zolk O, Quattek J, Sitzler G, et al. Expression of endothelin-1, endothelin-converting enzyme, and endothelin receptors in chronic heart failure. Circulation. 1999;99(16):2118–23. doi: 10.1161/01.cir.99.16.2118. Epub 1999/04/27. [DOI] [PubMed] [Google Scholar]
- 26.Barros JS, Bairos VA, Baptista MG, Fagulha JO. Immunocytochemical localization of endothelin-1 in human placenta from normal and preeclamptic pregnancies. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2001;20(1):125–37. doi: 10.1081/PRG-100104179. Epub 2002/06/05. [DOI] [PubMed] [Google Scholar]
- 27.Ferre F, Mondon F, Mignot TM, et al. Endothelin-1 binding sites and immunoreactivity in the cultured human placental trophoblast: evidence for an autocrine and paracrine role for endothelin-1. J Cardiovasc Pharmacol. 1993;22 (Suppl 8):S214–8. doi: 10.1097/00005344-199322008-00058. Epub 1993/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Casey ML, Word RA, MacDonald PC. Endothelin-1 gene expression and regulation of endothelin mRNA and protein biosynthesis in avascular human amnion. Potential source of amniotic fluid endothelin. J Biol Chem. 1991;266(9):5762–8. Epub 1991/03/25. [PubMed] [Google Scholar]
- 29.Hasegawa M, Sagawa N, Ihara Y, et al. Concentrations of endothelin-1 in human amniotic fluid at various stages of pregnancy. J Cardiovasc Pharmacol. 1991;7(17 Suppl):S440–2. doi: 10.1097/00005344-199100177-00126. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 30.Hasegawa M, Sagawa N, Itoh H, et al. Endothelin receptors in the human amnion, chorion laeve, decidua vera and placenta. Reproduction, fertility, and development. 1995;7(6):1585–9. doi: 10.1071/rd9951585. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 31.Sand AE, Ostlund E, Andersson E, Fried G. Endothelin-induced contractions in placental arteries is mediated by both ETA- and ETB-receptors. Acta Physiol Scand. 1998;163(3):227–34. doi: 10.1046/j.1365-201x.1998.00368.x. Epub 1998/08/26. [DOI] [PubMed] [Google Scholar]
- 32.Ahmad Z, Reznik SE. Immunohistochemical localization of ECE-1 in the human placenta. Placenta. 2000;21(2–3):226–33. doi: 10.1053/plac.1999.0454. Epub 2000/03/29. [DOI] [PubMed] [Google Scholar]
- 33.Gan XT, Chakrabarti S, Karmazyn M. Increased endothelin-1 and endothelin receptor expression in myocytes of ischemic and reperfused rat hearts and ventricular myocytes exposed to ischemic conditions and its inhibition by nitric oxide generation. Can J Physiol Pharmacol. 2003;81(2):105–13. doi: 10.1139/y03-030. Epub 2003/04/25. [DOI] [PubMed] [Google Scholar]
- 34.Wieczorek I, Haynes WG, Webb DJ, Ludlam CA, Fox KA. Raised plasma endothelin in unstable angina and non-Q wave myocardial infarction: relation to cardiovascular outcome. Brit Heart J. 1994;72(5):436–41. doi: 10.1136/hrt.72.5.436. Epub 1994/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander BT, Rinewalt AN, Cockrell KL, Massey MB, Bennett WA, Granger JP. Endothelin type a receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37(2 Part 2):485–9. doi: 10.1161/01.hyp.37.2.485. Epub 2001/03/07. [DOI] [PubMed] [Google Scholar]
- 36.Tanbe AF, Khalil RA. Circulating and Vascular Bioactive Factors during Hypertension in Pregnancy. Curr Bioact Comp. 2010;6(1):60–75. doi: 10.2174/157340710790711737. Epub 2010/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dechanet C, Fort A, Barbero-Camps E, Dechaud H, Richard S, Virsolvy A. Endothelin-dependent vasoconstriction in human uterine artery: application to preeclampsia. PloS one. 2011;6(1):e16540. doi: 10.1371/journal.pone.0016540. Epub 2011/02/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. American journal of physiology Heart and circulatory physiology. 2008;294(2):H541–50. doi: 10.1152/ajpheart.01113.2007. Epub 2007/12/07. [DOI] [PubMed] [Google Scholar]
- 39.Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension. 2003;41(3):437–45. doi: 10.1161/01.HYP.0000054981.03589.E9. Epub 2003/03/08. [DOI] [PubMed] [Google Scholar]
- 40.Napolitano M, Miceli F, Calce A, Vacca A, Gulino A, Apa R, et al. Expression and relationship between endothelin-1 messenger ribonucleic acid (mRNA) and inducible/endothelial nitric oxide synthase mRNA isoforms from normal and preeclamptic placentas. J Clin Endocrinol Metab. 2000;85(6):2318–23. doi: 10.1210/jcem.85.6.6623. Epub 2000/06/14. [DOI] [PubMed] [Google Scholar]
- 41.Anderson CM. Preeclampsia: exposing future cardiovascular risk in mothers and their children. Journal of obstetric, gynecologic, and neonatal nursing : JOGNN / NAACOG. 2007;36(1):3–8. doi: 10.1111/j.1552-6909.2006.00115.x. Epub 2007/01/24. [DOI] [PubMed] [Google Scholar]
- 42.George EM, Granger JP. Endothelin: key mediator of hypertension in preeclampsia. Am J Hypertens. 2011;24(9):964–9. doi: 10.1038/ajh.2011.99. Epub 2011/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Investig. 1996;97(2):540–50. doi: 10.1172/JCI118447. Epub 1996/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27(9–10):939–58. doi: 10.1016/j.placenta.2005.12.006. Epub 2006/02/24. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal PK, Chandel N, Jain V, Jha V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26(4):236–41. doi: 10.1038/jhh.2011.29. Epub 2011/04/01. [DOI] [PubMed] [Google Scholar]
- 46.Masuyama H, Nakatsukasa H, Takamoto N, Hiramatsu Y. Correlation between soluble endoglin, vascular endothelial growth factor receptor-1, and adipocytokines in preeclampsia. J Clin Endocrinol Metab. 2007;92(7):2672–9. doi: 10.1210/jc.2006-2349. Epub 2007/04/12. [DOI] [PubMed] [Google Scholar]
- 47.Veas CJ, Aguilera VC, Munoz IJ, et al. Fetal endothelium dysfunction is associated with circulating maternal levels of sE-selectin, sVCAM1, and sFlt-1 during pre-eclampsia. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(11):1371–7. doi: 10.3109/14767058.2011.556204. Epub 2011/03/09. [DOI] [PubMed] [Google Scholar]
- 48.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ (Clinical Research ed) 2001;323(7323):1213–7. doi: 10.1136/bmj.323.7323.1213. Epub 2001/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangos GJ. Cardiovascular disease following pre-eclampsia: understanding the mechanisms. J Hypertens. 2006;24(4):639–41. doi: 10.1097/01.hjh.0000217844.57466.85. Epub 2006/03/15. [DOI] [PubMed] [Google Scholar]
- 50.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ (Clinical Research ed) 2003;326(7394):845. doi: 10.1136/bmj.326.7394.845. Epub 2003/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin R, Gorostidi M, Portal CG, Sanchez M, Sanchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2000;19(2):199–209. doi: 10.1081/prg-100100136. Epub 2000/07/06. [DOI] [PubMed] [Google Scholar]
- 52.Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129,290 births. Lancet. 2001;357(9273):2002–6. doi: 10.1016/S0140-6736(00)05112-6. Epub 2001/07/05. [DOI] [PubMed] [Google Scholar]
- 53.Esplin MS, Fausett MB, Fraser A, et al. Paternal and maternal components of the predisposition to preeclampsia. New Engl J Med. 2001;344(12):867–72. doi: 10.1056/NEJM200103223441201. Epub 2001/03/22. [DOI] [PubMed] [Google Scholar]
- 54.Skjaerven R, Vatten LJ, Wilcox AJ, Ronning T, Irgens LM, Lie RT. Recurrence of pre-eclampsia across generations: exploring fetal and maternal genetic components in a population based cohort. BMJ (Clinical research ed) 2005;331(7521):877. doi: 10.1136/bmj.38555.462685.8F. Epub 2005/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barker DJ. The developmental origins of chronic adult disease. Acta paediatrica (Oslo, Norway : 1992) Supplement. 2004;93(446):26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. Epub 2005/02/11. [DOI] [PubMed] [Google Scholar]
- 56.Barker DJ. Fetal origins of coronary heart disease. BMJ (Clinical research ed) 1995;311(6998):171–4. doi: 10.1136/bmj.311.6998.171. Epub 1995/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernardi F, Constantino L, Machado R, Petronilho F, Dal-Pizzol F. Plasma nitric oxide, endothelin-1, arginase and superoxide dismutase in pre-eclamptic women. J Obstet Gynaecol Res. 2008;34(6):957–63. doi: 10.1111/j.1447-0756.2008.00860.x. Epub 2008/11/18. [DOI] [PubMed] [Google Scholar]
- 58.Florijn KW, Derkx FH, Visser W, et al. Plasma immunoreactive endothelin-1 in pregnant women with and without pre-eclampsia. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S446–8. doi: 10.1097/00005344-199100177-00128. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 59.Ihara Y, Sagawa N, Hasegawa M, et al. Concentrations of endothelin-1 in maternal and umbilical cord blood at various stages of pregnancy. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S443–5. doi: 10.1097/00005344-199100177-00127. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 60.Wang CN, Chang SD, Peng HH, et al. Change in amniotic fluid levels of multiple anti-angiogenic proteins before development of preeclampsia and intrauterine growth restriction. J Clin Endocrinol Metab. 2010;95(3):1431–41. doi: 10.1210/jc.2009-1954. Epub 2010/01/19. [DOI] [PubMed] [Google Scholar]
- 61.Singh HJ, Rahman A, Larmie ET, Nila A. Endothelin-l in feto-placental tissues from normotensive pregnant women and women with pre-eclampsia. Acta Obstet Gynecol Scand. 2001;80(2):99–103. doi: 10.1034/j.1600-0412.2001.080002099.x. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 62.Benigni A, Orisio S, Gaspari F, Frusca T, Amuso G, Remuzzi G. Evidence against a pathogenetic role for endothelin in pre-eclampsia. Brit J Obstet Gynaecol. 1992;99(10):798–802. doi: 10.1111/j.1471-0528.1992.tb14409.x. Epub 1992/10/01. [DOI] [PubMed] [Google Scholar]
- 63.Ajne G, Wolff K, Fyhrquist F, Carlstrom K, Hemsen-Mortberg A, Nisell H. Endothelin converting enzyme (ECE) activity in normal pregnancy and preeclampsia. Hypertension in pregnancy : official journal of the International Society for the Study of Hypertension in Pregnancy. 2003;22(3):215–24. doi: 10.1081/PRG-120024025. Epub 2003/10/24. [DOI] [PubMed] [Google Scholar]
- 64.Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP J Biol Chem. 2001;276(16):12645–53. doi: 10.1074/jbc.M011344200. Epub 2001/03/30. [DOI] [PubMed] [Google Scholar]
- 65.Caniggia I, Winter JL. Adriana and Luisa Castellucci Award lecture 2001. Hypoxia inducible factor-1: oxygen regulation of trophoblast differentiation in normal and pre-eclamptic pregnancies--a review. Placenta. 2002;23 (Suppl A):S47–57. doi: 10.1053/plac.2002.0815. Epub 2002/04/30. [DOI] [PubMed] [Google Scholar]
- 66.Rajakumar A, Brandon HM, Daftary A, Ness R, Conrad KP. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25(10):763–9. doi: 10.1016/j.placenta.2004.02.011. Epub 2004/09/29. [DOI] [PubMed] [Google Scholar]
- 67.Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Investig. 2000;105(5):577–87. doi: 10.1172/JCI8316. Epub 2000/03/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tal R, Shaish A, Barshack I, et al. Effects of hypoxia-inducible factor-1 alpha overexpression in pregnant mice: possible implications for preeclampsia and intrauterine growth restriction. Am J Pathol. 2010;177(6):2950–62. doi: 10.2353/ajpath.2010.090800. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morin FC, 3rd, Egan EA. Pulmonary hemodynamics in fetal lambs during development at normal and increased oxygen tension. J Appl Physiol (Bethesda, Md : 1985) 1992;73(1):213–8. doi: 10.1152/jappl.1992.73.1.213. Epub 1992/07/01. [DOI] [PubMed] [Google Scholar]
- 70.Ivy DD, Parker TA, Ziegler JW, et al. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Investig. 1997;99(6):1179–86. doi: 10.1172/JCI119274. Epub 1997/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rasanen J, Wood DC, Debbs RH, Cohen J, Weiner S, Huhta JC. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97(3):257–62. doi: 10.1161/01.cir.97.3.257. Epub 1998/02/14. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Coceani F. Isolated pulmonary resistance vessels from fetal lambs. Contractile behavior and responses to indomethacin and endothelin-1. Circ Res. 1992;71(2):320–30. doi: 10.1161/01.res.71.2.320. Epub 1992/08/01. [DOI] [PubMed] [Google Scholar]
- 73.Hislop A. Developmental biology of the pulmonary circulation. Paediatric respiratory reviews. 2005;6(1):35–43. doi: 10.1016/j.prrv.2004.11.009. Epub 2005/02/09. [DOI] [PubMed] [Google Scholar]
- 74.Levy M, Maurey C, Chailley-Heu B, Martinovic J, Jaubert F, Israel-Biet D. Developmental changes in endothelial vasoactive and angiogenic growth factors in the human perinatal lung. Pediatr Res. 2005;57(2):248–53. doi: 10.1203/01.PDR.0000148280.86670.7B. Epub 2004/12/22. [DOI] [PubMed] [Google Scholar]
- 75.Ivy DD, Kinsella JP, Abman SH. Physiologic characterization of endothelin A and B receptor activity in the ovine fetal pulmonary circulation. J Clin Investig. 1994;93(5):2141–8. doi: 10.1172/JCI117210. Epub 1994/05/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ivy DD, le Cras TD, Parker TA, et al. Developmental changes in endothelin expression and activity in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L785–93. doi: 10.1152/ajplung.2000.278.4.L785. Epub 2000/04/06. [DOI] [PubMed] [Google Scholar]
- 77.Ivy DD, Ziegler JW, Dubus MF, Fox JJ, Kinsella JP, Abman SH. Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr Res. 1996;39(3):435–42. doi: 10.1203/00006450-199603000-00010. Epub 1996/03/01. [DOI] [PubMed] [Google Scholar]
- 78.Schindler MB, Hislop AA, Haworth SG. Postnatal changes in pulmonary vein responses to endothelin-1 in the normal and chronically hypoxic lung. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1273–9. doi: 10.1152/ajplung.00173.2006. Epub 2007/01/30. [DOI] [PubMed] [Google Scholar]
- 79.Ivy D, Tanzawa K, Abman SH. Ontogeny of endothelin-converting enzyme gene expression and protein content in the ovine fetal lung. Biol Neonate. 2002;81(2):139–44. doi: 10.1159/000047199. Epub 2002/02/15. [DOI] [PubMed] [Google Scholar]
- 80.Levy M, Maurey C, Dinh-Xuan AT, Vouhe P, Israel-Biet D. Developmental expression of vasoactive and growth factors in human lung. Role in pulmonary vascular resistance adaptation at birth. Pediatr Res. 2005;57(5 Pt 2):21R–5R. doi: 10.1203/01.PDR.0000159575.58834.8D. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 81.Endo A, Ayusawa M, Minato M, Takada M, Takahashi S, Harada K. Endogenous nitric oxide and endothelin-1 in persistent pulmonary hypertension of the newborn. Eur J Pediatr. 2001;160(4):217–22. doi: 10.1007/pl00008431. Epub 2001/04/25. [DOI] [PubMed] [Google Scholar]
- 82.Ivy DD, Le Cras TD, Horan MP, Abman SH. Increased lung preproET-1 and decreased ETB-receptor gene expression in fetal pulmonary hypertension. Am J Physiol. 1998;274(4 Pt 1):L535–41. doi: 10.1152/ajplung.1998.274.4.L535. Epub 1998/05/12. [DOI] [PubMed] [Google Scholar]
- 83.Kumar P, Kazzi NJ, Shankaran S. Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. Am J Perinatol. 1996;13(6):335–41. doi: 10.1055/s-2007-994352. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 84.Bokenkamp R, DeRuiter MC, van Munsteren C, Gittenberger-de Groot AC. Insights into the pathogenesis and genetic background of patency of the ductus arteriosus. Neonatology. 2010;98(1):6–17. doi: 10.1159/000262481. Epub 2009/12/04. [DOI] [PubMed] [Google Scholar]
- 85.Heymann MA, Rudolph AM. Control of the ductus arteriosus. Physiol Rev. 1975;55(1):62–78. doi: 10.1152/physrev.1975.55.1.62. Epub 1975/01/01. [DOI] [PubMed] [Google Scholar]
- 86.Clyman RI. Mechanisms regulating the ductus arteriosus. Biol Neonate. 2006;89(4):330–5. doi: 10.1159/000092870. Epub 2006/06/14. [DOI] [PubMed] [Google Scholar]
- 87.Ivey KN, Sutcliffe D, Richardson J, Clyman RI, Garcia JA, Srivastava D. Transcriptional regulation during development of the ductus arteriosus. Circ Res. 2008;103(4):388–95. doi: 10.1161/CIRCRESAHA.108.180661. Epub 2008/07/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Clyman RI, Mauray F, Roman C, Rudolph AM. PGE2 is a more potent vasodilator of the lamb ductus arteriosus than is either PGI2 or 6 keto PGF1alpha. Prostaglandins. 1978;16(2):259–64. doi: 10.1016/0090-6980(78)90028-x. Epub 1978/08/01. [DOI] [PubMed] [Google Scholar]
- 89.Coceani F, Kelsey L, Seidlitz E. The response of the lamb ductus arteriosus to endothelin: developmental changes and influence of light. Life Sci. 2002;71(10):1209–17. doi: 10.1016/s0024-3205(02)01822-2. Epub 2002/07/04. [DOI] [PubMed] [Google Scholar]
- 90.Momma K, Nakanishi T, Imamura S. Inhibition of in vivo constriction of fetal ductus arteriosus by endothelin receptor blockade in rats. Pediatr Res. 2003;53(3):479–85. doi: 10.1203/01.PDR.0000049516.70216.2E. Epub 2003/02/22. [DOI] [PubMed] [Google Scholar]
- 91.Taniguchi T, Muramatsu I. Pharmacological knockout of endothelin ET(A) receptors. Life Sci. 2003;74(2–3):405–9. doi: 10.1016/j.lfs.2003.09.027. Epub 2003/11/11. [DOI] [PubMed] [Google Scholar]
- 92.Coceani F, Liu Y, Seidlitz E, Kelsey L, Kuwaki T, Ackerley C, et al. Endothelin A receptor is necessary for O(2) constriction but not closure of ductus arteriosus. Am J Physiol. 1999;277(4 Pt 2):H1521–31. doi: 10.1152/ajpheart.1999.277.4.H1521. Epub 1999/10/12. [DOI] [PubMed] [Google Scholar]
- 93.Costa M, Barogi S, Socci ND, et al. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genomics. 2006;25(2):250–62. doi: 10.1152/physiolgenomics.00231.2005. Epub 2006/01/19. [DOI] [PubMed] [Google Scholar]
- 94.Baragatti B, Ciofini E, Scebba F, et al. Cytochrome P-450 3A13 and endothelin jointly mediate ductus arteriosus constriction to oxygen in mice. Am J Physiol Heart Circ Physiol. 2011;300(3):H892–901. doi: 10.1152/ajpheart.00907.2010. Epub 2011/01/05. [DOI] [PubMed] [Google Scholar]
- 95.Agren P, Cogolludo AL, Kessels CG, et al. Ontogeny of chicken ductus arteriosus response to oxygen and vasoconstrictors. American Journal of physiology Regulatory, Integrative and Comparative Physiology. 2007;292(1):R485–96. doi: 10.1152/ajpregu.00204.2006. Epub 2006/08/19. [DOI] [PubMed] [Google Scholar]
- 96.Fineman JR, Takahashi Y, Roman C, Clyman RI. Endothelin-receptor blockade does not alter closure of the ductus arteriosus. Am J Physiol. 1998;275(5 Pt 2):H1620–6. doi: 10.1152/ajpheart.1998.275.5.H1620. Epub 1998/11/14. [DOI] [PubMed] [Google Scholar]
- 97.Cetin I, Foidart JM, Miozzo M, et al. Fetal growth restriction: a workshop report. Placenta. 2004;25(8–9):753–7. doi: 10.1016/j.placenta.2004.02.004. Epub 2004/09/29. [DOI] [PubMed] [Google Scholar]
- 98.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. Epub 2005/03/01. [DOI] [PubMed] [Google Scholar]
- 99.Ness RB, Sibai BM. Shared and disparate components of the pathophysiologies of fetal growth restriction and preeclampsia. Am J Obstet Gynecol. 2006;195(1):40–9. doi: 10.1016/j.ajog.2005.07.049. Epub 2006/07/04. [DOI] [PubMed] [Google Scholar]
- 100.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science (New York, NY) 2005;308(5728):1592–4. doi: 10.1126/science.1111726. Epub 2005/06/11. [DOI] [PubMed] [Google Scholar]
- 101.Thaete LG, Neerhof MG, Caplan MS. Endothelin receptor A antagonism prevents hypoxia-induced intrauterine growth restriction in the rat. Am J Obstet Gynecol. 1997;176(1 Pt 1):73–6. doi: 10.1016/s0002-9378(97)80014-2. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 102.Nezar MA, el-Baky AM, Soliman OA, Abdel-Hady HA, Hammad AM, Al-Haggar MS. Endothelin-1 and leptin as markers of intrauterine growth restriction. Ind J Pediatr. 2009;76(5):485–8. doi: 10.1007/s12098-009-0079-0. Epub 2009/04/25. [DOI] [PubMed] [Google Scholar]
- 103.Li H, Chen SJ, Chen YF, et al. Enhanced endothelin-1 and endothelin receptor gene expression in chronic hypoxia. Journal of applied physiology (Bethesda, Md : 1985) 1994;77(3):1451–9. doi: 10.1152/jappl.1994.77.3.1451. Epub 1994/09/01. [DOI] [PubMed] [Google Scholar]
- 104.Thaete LG, Kushner DM, Dewey ER, Neerhof MG. Endothelin and the regulation of uteroplacental perfusion in nitric oxide synthase inhibition-induced fetal growth restriction. Placenta. 2005;26(2–3):242–50. doi: 10.1016/j.placenta.2004.06.003. Epub 2005/02/15. [DOI] [PubMed] [Google Scholar]
- 105.Giannubilo SR, Menegazzi M, Tedeschi E, Bezzeccheri V, Suzuki H, Tranquilli AL. Doppler analysis and placental nitric oxide synthase expression during fetal growth restriction. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(9):617–22. doi: 10.1080/14767050802214774. Epub 2008/10/02. [DOI] [PubMed] [Google Scholar]
- 106.Brand M, Kempf H, Paul M, Corvol P, Gasc JM. Expression of endothelins in human cardiogenesis. Journal of molecular medicine (Berlin, Germany) 2002;80(11):715–23. doi: 10.1007/s00109-002-0379-6. Epub 2002/11/19. [DOI] [PubMed] [Google Scholar]
- 107.Chen M, Lin YQ, Xie SL, Wang JF. Mitogen-activated protein kinase in endothelin-1-induced cardiac differentiation of mouse embryonic stem cells. J Cell Biochem. 2010;111(6):1619–28. doi: 10.1002/jcb.22895. Epub 2010/11/06. [DOI] [PubMed] [Google Scholar]
- 108.Patel R, Kos L. Endothelin-1 and Neuregulin-1 convert embryonic cardiomyocytes into cells of the conduction system in the mouse. Developmental dynamics : an official publication of the American Association of Anatomists. 2005;233(1):20–8. doi: 10.1002/dvdy.20284. Epub 2005/03/11. [DOI] [PubMed] [Google Scholar]
- 109.Wharton J, Rutherford RA, Gordon L, et al. Localization of endothelin binding sites and endothelin-like immunoreactivity in human fetal heart. J Cardiovasc Pharmacol. 1991;17 (Suppl 7):S378–84. doi: 10.1097/00005344-199100177-00107. Epub 1991/01/01. [DOI] [PubMed] [Google Scholar]
- 110.Yanagisawa H, Hammer RE, Richardson JA, et al. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Investig. 2000;105(10):1373–82. doi: 10.1172/JCI7447. Epub 2000/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hauselmann SP, Rosc-Schluter BI, Lorenz V, et al. beta1-Integrin is up-regulated via Rac1-dependent reactive oxygen species as part of the hypertrophic cardiomyocyte response. Free Radic Biol Med. 2011;51(3):609–18. doi: 10.1016/j.freeradbiomed.2011.05.007. Epub 2011/05/31. [DOI] [PubMed] [Google Scholar]
- 112.Komati H, Maharsy W, Beauregard J, Hayek S, Nemer M. ZFP260 is an inducer of cardiac hypertrophy and a nuclear mediator of endothelin-1 signaling. J Biol Chem. 2011;286(2):1508–16. doi: 10.1074/jbc.M110.162966. Epub 2010/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Majumdar P, Chen S, George B, Sen S, Karmazyn M, Chakrabarti S. Leptin and endothelin-1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes/metabolism research and reviews. 2009;25(5):452–63. doi: 10.1002/dmrr.964. Epub 2009/04/25. [DOI] [PubMed] [Google Scholar]
- 114.Shimojo N, Jesmin S, Zaedi S, et al. Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293(1):H474–81. doi: 10.1152/ajpheart.00922.2006. Epub 2007/03/21. [DOI] [PubMed] [Google Scholar]
- 115.Tamamori M, Ito H, Adachi S, Akimoto H, Marumo F, Hiroe M. Endothelin-3 induces hypertrophy of cardiomyocytes by the endogenous endothelin-1-mediated mechanism. J Clin Investig. 1996;97(2):366–72. doi: 10.1172/JCI118424. Epub 1996/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alvarez D, Briassouli P, Clancy RM, et al. A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem. 2011;286(35):30444–54. doi: 10.1074/jbc.M111.263657. Epub 2011/07/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guarda E, Katwa LC, Myers PR, Tyagi SC, Weber KT. Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res. 1993;27(12):2130–4. doi: 10.1093/cvr/27.12.2130. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 118.Widyantoro B, Emoto N, Nakayama K, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121(22):2407–18. doi: 10.1161/CIRCULATIONAHA.110.938217. Epub 2010/05/26. [DOI] [PubMed] [Google Scholar]
- 119.Tong W, Xue Q, Li Y, Zhang L. Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. Am J Physiol Heart Circ Physiol. 2011;301(5):H2113–21. doi: 10.1152/ajpheart.00356.2011. Epub 2011/08/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285(3):H983–90. doi: 10.1152/ajpheart.00005.2003. Epub 2003/05/17. [DOI] [PubMed] [Google Scholar]
- 121.Louey S, Thornburg KL. The prenatal environment and later cardiovascular disease. Early Hum Dev. 2005;81(9):745–51. doi: 10.1016/j.earlhumdev.2005.07.001. Epub 2005/09/03. [DOI] [PubMed] [Google Scholar]
- 122.Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol. 2004;286(5):H1712–9. doi: 10.1152/ajpheart.00898.2003. Epub 2004/01/13. [DOI] [PubMed] [Google Scholar]
- 123.Xu Y, Williams SJ, O’Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB journal : official publication of the Federation of Am Soc Expe Biol. 2006;20(8):1251–3. doi: 10.1096/fj.05-4917fje. Epub 2006/04/25. [DOI] [PubMed] [Google Scholar]
- 124.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10(5):265–74. doi: 10.1016/s1071-5576(03)00074-1. Epub 2003/07/11. [DOI] [PubMed] [Google Scholar]
- 125.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ Res. 2010;107(3):365–73. doi: 10.1161/CIRCRESAHA.110.221259. Epub 2010/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89(2):300–8. doi: 10.1093/cvr/cvq303. Epub 2010/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Therapeut. 2009;330(2):624–32. doi: 10.1124/jpet.109.153239. Epub 2009/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Belaidi E, Joyeux-Faure M, Ribuot C, Launois SH, Levy P, Godin-Ribuot D. Major role for hypoxia inducible factor-1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J Am Coll Cardiol. 2009;53(15):1309–17. doi: 10.1016/j.jacc.2008.12.050. Epub 2009/04/11. [DOI] [PubMed] [Google Scholar]
- 129.Hedegaard ER, Stankevicius E, Simonsen U, Frobert O. Non-endothelial endothelin counteracts hypoxic vasodilation in porcine large coronary arteries. BMC Physiol. 2011;11(1):8. doi: 10.1186/1472-6793-11-8. Epub 2011/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Haynes WG, Webb DJ. Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens. 1998;16(8):1081–98. doi: 10.1097/00004872-199816080-00001. Epub 1998/10/30. [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Levesque LO, Anand-Srivastava MB. Epidermal growth factor receptor transactivation by endogenous vasoactive peptides contributes to hyperproliferation of vascular smooth muscle cells of SHR. Am J Physiol Heart Circ Physiol. 2010;299(6):H1959–67. doi: 10.1152/ajpheart.00526.2010. Epub 2010/09/21. [DOI] [PubMed] [Google Scholar]
- 132.Hayasaki-Kajiwara Y, Naya N, Shimamura T, Iwasaki T, Nakajima M. Endothelin generating pathway through endothelin 1–31 in human cultured bronchial smooth muscle cells. Brit J Pharmacol. 1999;127(6):1415–21. doi: 10.1038/sj.bjp.0702664. Epub 1999/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kourembanas S, McQuillan LP, Leung GK, Faller DV. Nitric oxide regulates the expression of vasoconstrictors and growth factors by vascular endothelium under both normoxia and hypoxia. J Clin Investig. 1993;92(1):99–104. doi: 10.1172/JCI116604. Epub 1993/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rautureau Y, Schiffrin EL. Endothelin in hypertension: an update. Curr Opin Nephrol Hypertens. 2012;21(2):128–36. doi: 10.1097/MNH.0b013e32834f0092. Epub 2012/01/20. [DOI] [PubMed] [Google Scholar]
- 135.George EM, Palei AC, Granger JP. Endothelin as a final common pathway in the pathophysiology of preeclampsia: therapeutic implications. Curr Opin Nephrol Hypertens. 2012;21(2):157–62. doi: 10.1097/MNH.0b013e328350094b. Epub 2012/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sabry S, Mondon F, Levy M, Ferre F, Dinh-Xuan AT. Endothelial modulation of vasoconstrictor responses to endothelin-1 in human placental stem villi small arteries. Brit J Pharmacol. 1995;115(6):1038–42. doi: 10.1111/j.1476-5381.1995.tb15915.x. Epub 1995/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sheppard SJ, Khalil RA. Risk factors and mediators of the vascular dysfunction associated with hypertension in pregnancy. Cardiovasc Hematol Disorders Drug Targets. 2010;10(1):33–52. doi: 10.2174/187152910790780096. Epub 2010/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.George EM, Granger JP. Mechanisms and potential therapies for preeclampsia. Curr Hypertens Rep. 2011;13(4):269–75. doi: 10.1007/s11906-011-0204-0. Epub 2011/04/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clouthier DE, Hosoda K, Richardson JA, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development (Cambridge, England) 1998;125(5):813–24. doi: 10.1242/dev.125.5.813. Epub 1998/05/09. [DOI] [PubMed] [Google Scholar]
- 140.LaMarca BD, Alexander BT, Gilbert JS, et al. Pathophysiology of hypertension in response to placental ischemia during pregnancy: a central role for endothelin? Gender Med. 2008;5 (Suppl A):S133–8. doi: 10.1016/j.genm.2008.03.013. Epub 2008/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tam Tam KB, George E, Cockrell K, et al. Endothelin type A receptor antagonist attenuates placental ischemia-induced hypertension and uterine vascular resistance. Am J Obstet Gynecol. 2011;204(4):330, e1–4. doi: 10.1016/j.ajog.2011.01.049. Epub 2011/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia Y, Kellems RE. Receptor-activating autoantibodies and disease: preeclampsia and beyond. Expert Rev Clin Immunol. 2011;7(5):659–74. doi: 10.1586/eci.11.56. Epub 2011/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia Y, Zhou CC, Ramin SM, Kellems RE. Angiotensin receptors, autoimmunity, and preeclampsia. J Immunol (Baltimore, Md : 1950) 2007;179(6):3391–5. doi: 10.4049/jimmunol.179.6.3391. Epub 2007/09/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.LaMarca B, Parrish M, Ray LF, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54(4):905–9. doi: 10.1161/HYPERTENSIONAHA.109.137935. Epub 2009/08/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Reslan OM, Khalil RA. Molecular and vascular targets in the pathogenesis and management of the hypertension associated with preeclampsia. Cardiovasc Hematol Agents Med Chem. 2010;8(4):204–26. doi: 10.2174/187152510792481234. Epub 2010/10/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Angerio AD. Endothelin-mediated preeclampsia at high altitude. Crit Care Nursing Quart. 2000;23(2):73–8. doi: 10.1097/00002727-200008000-00009. Epub 2002/02/21. [DOI] [PubMed] [Google Scholar]
- 147.Nakatsuka M, Takata M, Tada K, et al. A long-term transdermal nitric oxide donor improves uteroplacental circulation in women with preeclampsia. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2002;21(8):831–6. doi: 10.7863/jum.2002.21.8.831. Epub 2002/08/08. [DOI] [PubMed] [Google Scholar]
- 148.Channick RN, Sitbon O, Barst RJ, Manes A, Rubin LJ. Endothelin receptor antagonists in pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12 Suppl S):62S–7S. doi: 10.1016/j.jacc.2004.02.042. Epub 2004/06/15. [DOI] [PubMed] [Google Scholar]
- 149.Venitz J, Zack J, Gillies H, Allard M, Regnault J, Dufton C. Clinical Pharmacokinetics and Drug-Drug Interactions of Endothelin Receptor Antagonists in Pulmonary Arterial Hypertension. J Clin Pharmacol. 2011 doi: 10.1177/0091270011423662. Epub 2011/12/30. [DOI] [PubMed] [Google Scholar]
- 150.Hamidi SA, Lin RZ, Szema AM, Lyubsky S, Jiang YP, Said SI. VIP and endothelin receptor antagonist: an effective combination against experimental pulmonary arterial hypertension. Respir Res. 2011;12:141. doi: 10.1186/1465-9921-12-141. Epub 2011/10/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Agarwal R, Gomberg-Maitland M. Current therapeutics and practical management strategies for pulmonary arterial hypertension. Am Heart J. 2011;162(2):201–13. doi: 10.1016/j.ahj.2011.05.012. Epub 2011/08/13. [DOI] [PubMed] [Google Scholar]
- 152.O’Callaghan DS, Savale L, Yaici A, et al. Endothelin receptor antagonists for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2011;12(10):1585–96. doi: 10.1517/14656566.2011.564159. Epub 2011/04/21. [DOI] [PubMed] [Google Scholar]
- 153.Kingman M, Ruggiero R, Torres F. Ambrisentan, an endothelin receptor type A-selective endothelin receptor antagonist, for the treatment of pulmonary arterial hypertension. Expert Opin Pharmacother. 2009;10(11):1847–58. doi: 10.1517/14656560903061275. Epub 2009/07/16. [DOI] [PubMed] [Google Scholar]
- 154.Frampton JE. Ambrisentan. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2011;11(4):215–26. doi: 10.2165/11207340-000000000-00000. Epub 2011/06/01. [DOI] [PubMed] [Google Scholar]
- 155.Beghetti M, Galie N. Eisenmenger syndrome a clinical perspective in a new therapeutic era of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;53(9):733–40. doi: 10.1016/j.jacc.2008.11.025. Epub 2009/02/28. [DOI] [PubMed] [Google Scholar]
- 156.Mehta PK, Simpson L, Lee EK, Lyle TA, McConnell ME, Book WM. Endothelin receptor antagonists improve exercise tolerance and oxygen saturations in patients with Eisenmenger syndrome and congenital heart defects. Texas Heart Institute journal / from the Texas Heart Institute of St Luke’s Episcopal Hospital, Texas Children’s Hospital. 2008;35(3):256–61. Epub 2008/10/23. [PMC free article] [PubMed] [Google Scholar]
- 157.Iglarz M, Binkert C, Morrison K, et al. Pharmacology of macitentan, an orally active tissue-targeting dual endothelin receptor antagonist. J Pharmacol Exp Ther. 2008;327(3):736–45. doi: 10.1124/jpet.108.142976. Epub 2008/09/11. [DOI] [PubMed] [Google Scholar]
- 158.Raja SG. Macitentan, a tissue-targeting endothelin receptor antagonist for the potential oral treatment of pulmonary arterial hypertension and idiopathic pulmonary fibrosis. Current opinion in investigational drugs (London, England : 2000) 2010;11(9):1066–73. Epub 2010/08/24. [PubMed] [Google Scholar]
- 159.Oishi P, Datar SA, Fineman JR. Pediatric pulmonary arterial hypertension: current and emerging therapeutic options. Expert Opin Pharmacother. 2011;12(12):1845–64. doi: 10.1517/14656566.2011.585636. Epub 2011/05/26. [DOI] [PubMed] [Google Scholar]
- 160.Boniface S, Reynaud-Gaubert M. Endothelin receptor antagonists -- their role in pulmonary medicine. Revue des maladies respiratoires. 2011;28(8):e94–e107. doi: 10.1016/j.rmr.2009.07.001. Epub 2011/11/22. [DOI] [PubMed] [Google Scholar]
- 161.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125(5):1020–30. doi: 10.1542/peds.2009-3506. Epub 2010/04/28. [DOI] [PubMed] [Google Scholar]
- 162.Zanardo V, Vedovato S, Chiozza L, Faggian D, Favaro F, Trevisanuto D. Pharmacological closure of patent ductus arteriosus: effects on pulse pressure and on endothelin-1 and vasopressin excretion. Am J Perinatol. 2008;25(6):353–8. doi: 10.1055/s-2008-1078763. Epub 2008/05/30. [DOI] [PubMed] [Google Scholar]
- 163.Seidner SR, Chen YQ, Oprysko PR, Mauray F, Tse MM, Lin E, et al. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50(3):365–73. doi: 10.1203/00006450-200109000-00012. Epub 2001/08/24. [DOI] [PubMed] [Google Scholar]
- 164.Angerio AD, Kot PA. Closure of the ductus arteriosus--new insights for critical care. Crit Care Nursing Quart. 1998;20(4):80–5. Epub 1998/03/13. [PubMed] [Google Scholar]
- 165.Coceani F, Kelsey L, Seidlitz E. Evidence for an effector role of endothelin in closure of the ductus arteriosus at birth. Can J Physiol Pharmacol. 1992;70(7):1061–4. doi: 10.1139/y92-146. Epub 1992/07/01. [DOI] [PubMed] [Google Scholar]
- 166.Barker DJ. Fetal nutrition and cardiovascular disease in later life. Brit Med Bull. 1997;53(1):96–108. doi: 10.1093/oxfordjournals.bmb.a011609. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]