Abstract
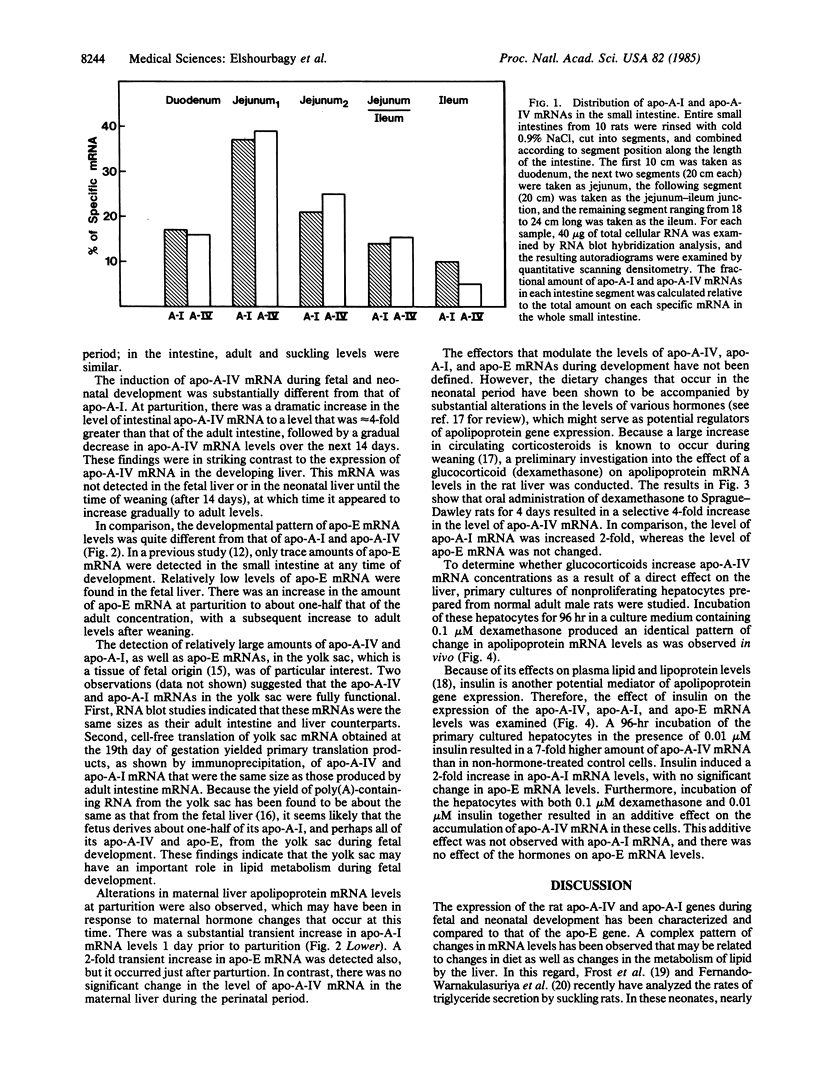
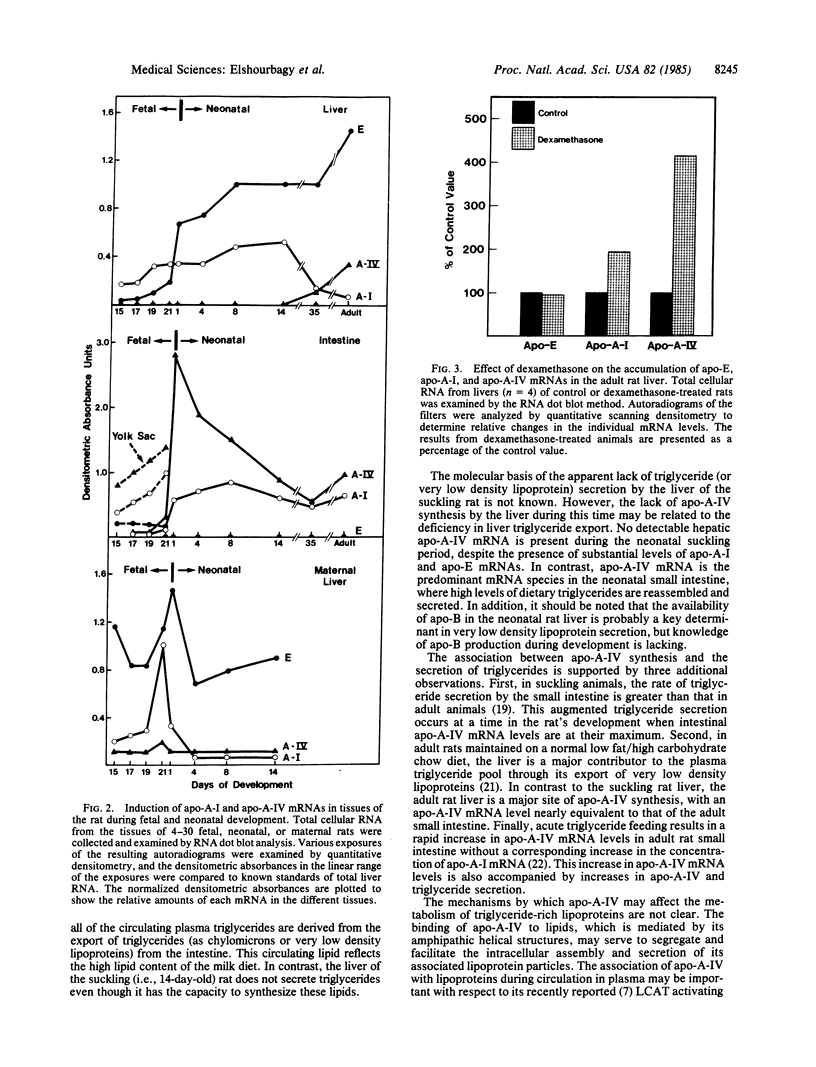
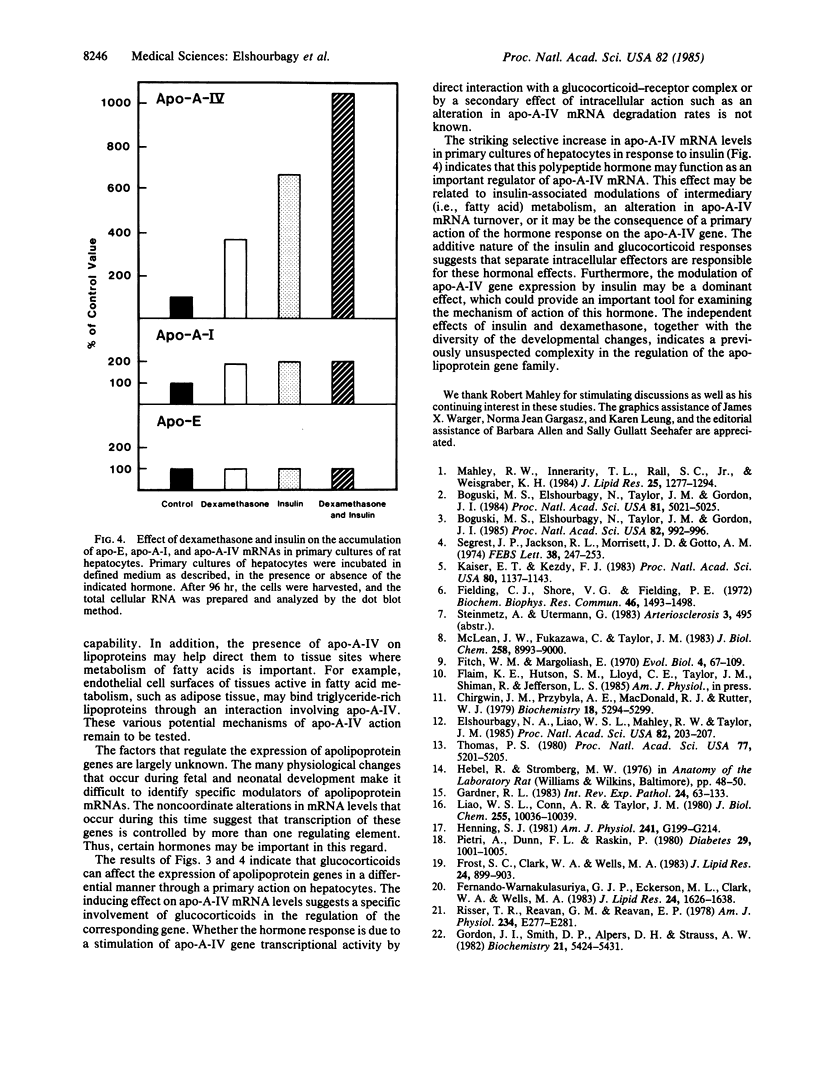
Rat apolipoproteins (apo-) A-IV and A-I share many structural similarities, the most notable of which is a domain of repeated docosapeptides with amphipathic helical potential. Although the genes for apo-A-IV and apo-A-I probably diverged from a common ancestor, these proteins seem to have developed different functions in their evolution. In the present study, cloned cDNAs were used to characterize the expression of apo-A-IV and apo-A-I mRNAs in a wide variety of adult rat tissues, as well as in small intestine and liver obtained from fetal, suckling, and weanling animals; comparisons were made to the expression of apo-E mRNA. The apo-A-IV and apo-A-I mRNAs were most abundant in adult small intestine and liver, with trace amounts detected in other tissues. Substantial amounts of these mRNAs were detected in the yolk sac, suggesting that this fetal tissue plays an important role in lipid metabolism during gestation. Noncoordinate accumulation of apo-A-IV and apo-A-I mRNAs was observed within and between the liver and small intestine during neonatal development. The apo-A-IV mRNA levels in the developing small intestine and liver appeared to correlate with their triglyceride secretion rates, suggesting that this protein plays an important role in the metabolism of triglyceride-rich lipoproteins. When dexamethasone (0.1 microM), insulin (0.01 microM), or insulin and dexamethasone together were incubated with primary cultures of nonproliferating adult rat hepatocytes, apo-A-IV mRNA levels were 4-, 7-, and 11-fold higher, respectively, than in non-hormone-treated control hepatocytes. Hormone administration resulted in a 2-fold greater amount of apo-A-I mRNA in each case, with no significant change in the level of apo-E mRNA. The overall results suggest that these structurally related apolipoproteins are regulated in substantially different ways.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Comparative analysis of repeated sequences in rat apolipoproteins A-I, A-IV, and E. Proc Natl Acad Sci U S A. 1985 Feb;82(4):992–996. doi: 10.1073/pnas.82.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguski M. S., Elshourbagy N., Taylor J. M., Gordon J. I. Rat apolipoprotein A-IV contains 13 tandem repetitions of a 22-amino acid segment with amphipathic helical potential. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5021–5025. doi: 10.1073/pnas.81.16.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando-Warnakulasuriya G. J., Eckerson M. L., Clark W. A., Wells M. A. Lipoprotein metabolism in the suckling rat: characterization of plasma and lymphatic lipoproteins. J Lipid Res. 1983 Dec;24(12):1626–1638. [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- Frost S. C., Clark W. A., Wells M. A. Studies on fat digestion, absorption, and transport in the suckling rat. IV. In vivo rates of triacylglycerol secretion by intestine and liver. J Lipid Res. 1983 Jul;24(7):899–903. [PubMed] [Google Scholar]
- Gardner R. L. Origin and differentiation of extraembryonic tissues in the mouse. Int Rev Exp Pathol. 1983;24:63–133. [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Alpers D. H., Strauss A. W. Cloning of a complementary deoxyribonucleic acid encoding a portion of rat intestinal preapolipoprotein AIV messenger ribonucleic acid. Biochemistry. 1982 Oct 26;21(22):5424–5431. doi: 10.1021/bi00265a007. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Postnatal development: coordination of feeding, digestion, and metabolism. Am J Physiol. 1981 Sep;241(3):G199–G214. doi: 10.1152/ajpgi.1981.241.3.G199. [DOI] [PubMed] [Google Scholar]
- Kaiser E. T., Kézdy F. J. Secondary structures of proteins and peptides in amphiphilic environments. (A review). Proc Natl Acad Sci U S A. 1983 Feb;80(4):1137–1143. doi: 10.1073/pnas.80.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W. S., Conn A. R., Taylor J. M. Changes in rat alpha 1-fetoprotein and albumin mRNA levels during fetal and neonatal development. J Biol Chem. 1980 Nov 10;255(21):10036–10039. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Rall S. C., Jr, Weisgraber K. H. Plasma lipoproteins: apolipoprotein structure and function. J Lipid Res. 1984 Dec 1;25(12):1277–1294. [PubMed] [Google Scholar]
- McLean J. W., Fukazawa C., Taylor J. M. Rat apolipoprotein E mRNA. Cloning and sequencing of double-stranded cDNA. J Biol Chem. 1983 Jul 25;258(14):8993–9000. [PubMed] [Google Scholar]
- Pietri A., Dunn F. L., Raskin P. The effect of improved diabetic control on plasma lipid and lipoprotein levels: a comparison of conventional therapy and continuous subcutaneous insulin infusion. Diabetes. 1980 Dec;29(12):1001–1005. doi: 10.2337/diab.29.12.1001. [DOI] [PubMed] [Google Scholar]
- Risser T. R., Reaven G. M., Reaven E. P. Intestinal contribution to secretion of very low density lipoproteins into plasma. Am J Physiol. 1978 Mar;234(3):E277–E281. doi: 10.1152/ajpendo.1978.234.3.E277. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Morrisett J. D., Gotto A. M., Jr A molecular theory of lipid-protein interactions in the plasma lipoproteins. FEBS Lett. 1974 Jan 15;38(3):247–258. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]


