Abstract
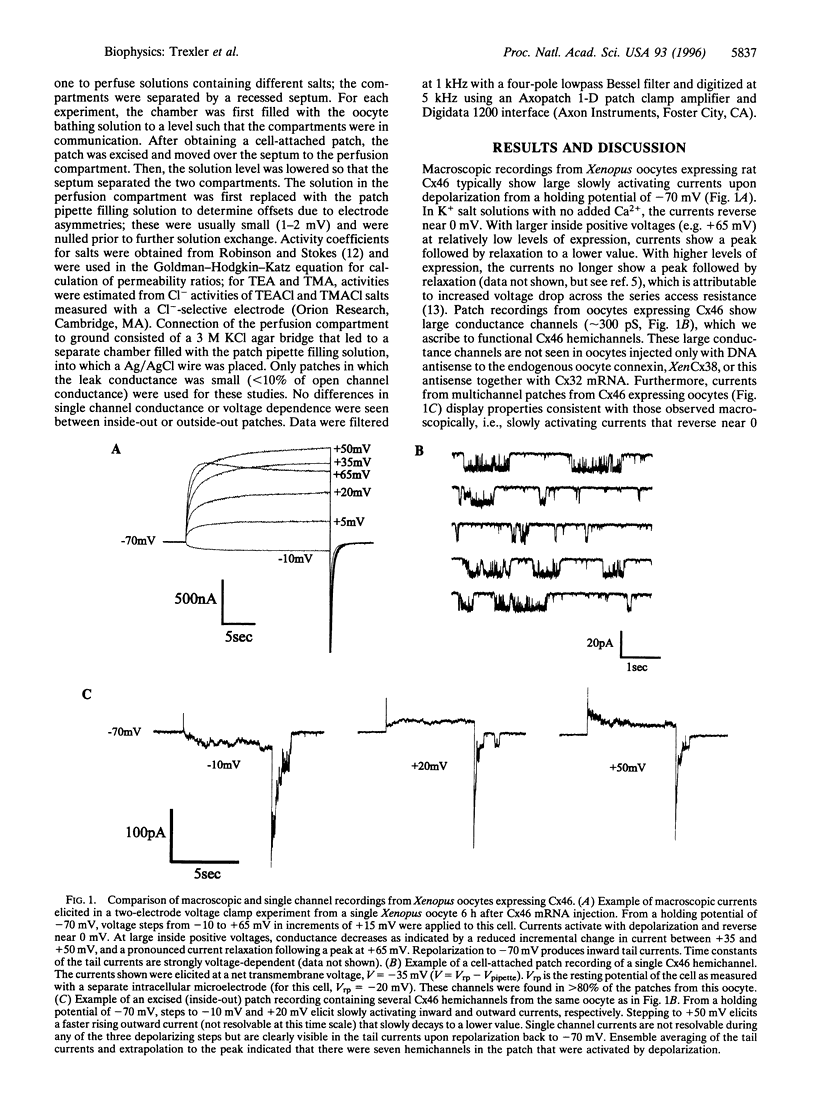
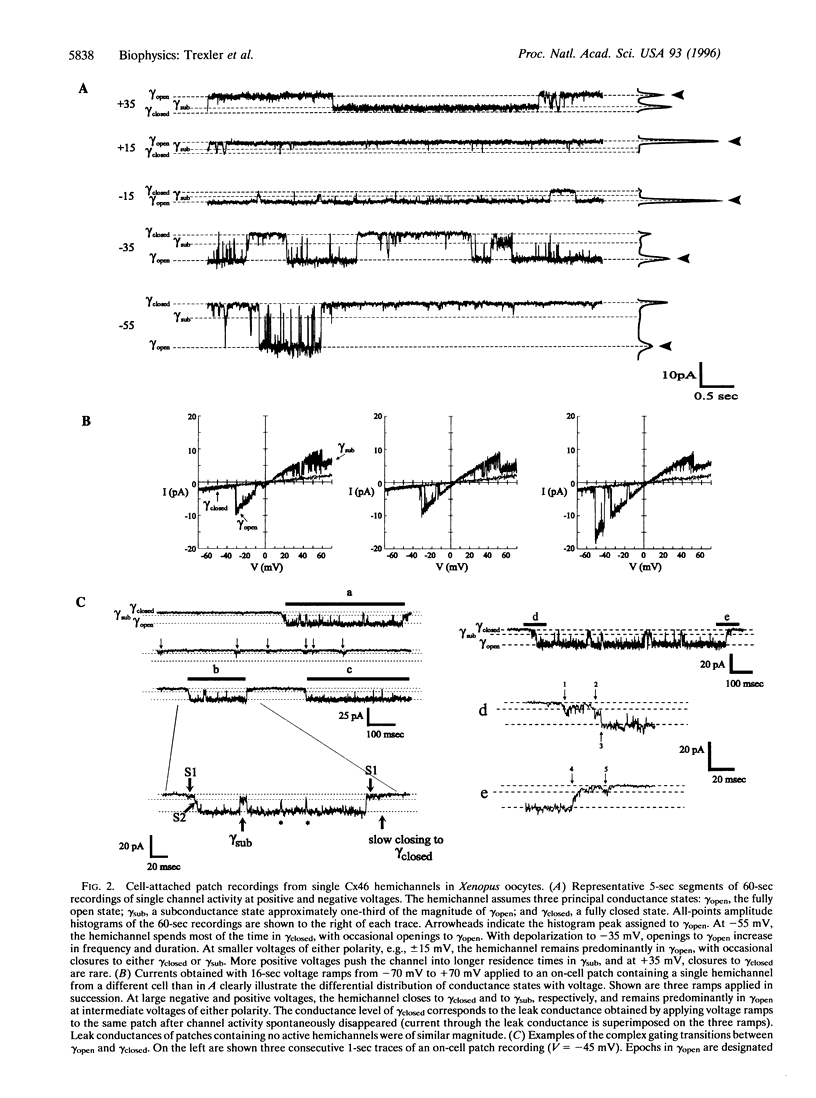
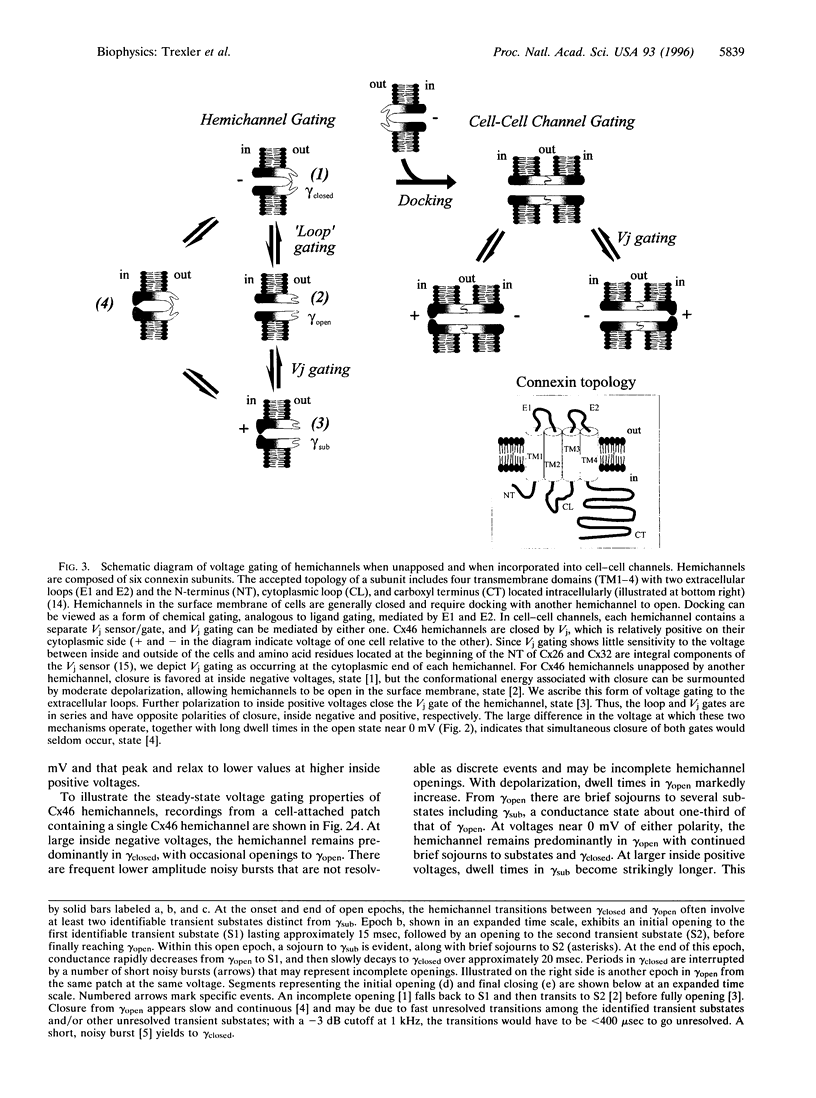
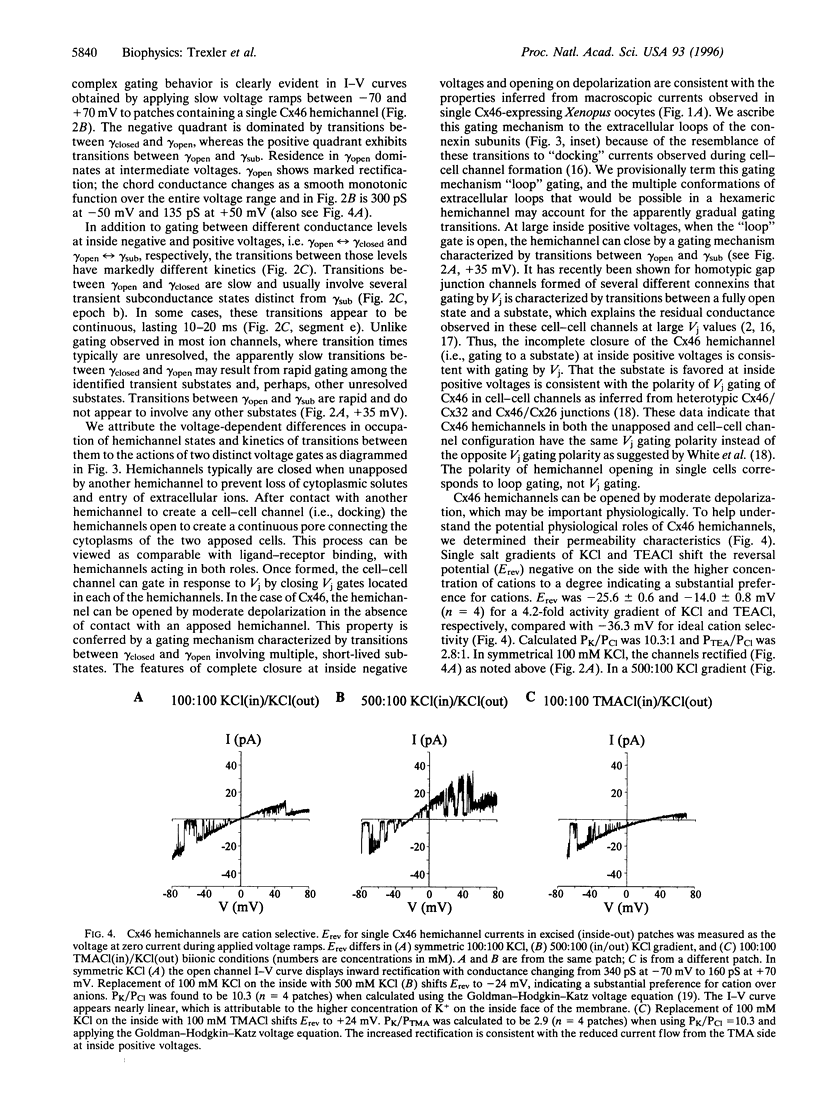
Gap junction channels are formed by members of the connexin gene family and mediate direct intercellular communication through linked hemichannels (connexons) from each of two adjacent cells. While for most connexins, the hemichannels appear to require an apposing hemichannel to open, macroscopic currents obtained from Xenopus oocytes expressing rat Cx46 suggested that some hemichannels can be readily opened by membrane depolarization [Paul, D. L., Ebihara, L., Takemoto, L. J., Swenson, K. I. & Goodenough, D. A. (1991), J. Cell Biol. 115, 1077-1089]. Here we demonstrate by single channel recording that hemichannels comprised of rat Cx46 exhibit complex voltage gating consistent with there being two distinct gating mechanisms. One mechanism partially closes Cx46 hemichannels from a fully open state, gammaopen, to a substate, gammasub, about one-third of the conductance of gammaopen; these transitions occur when the cell is depolarized to inside positive voltages, consistent with gating by transjunctional voltage in Cx46 gap junctions. The other gating mechanism closes Cx46 hemichannels to a fully closed state, gammaclosed, on hyperpolarization to inside negative voltages and has unusual characteristics; transitions between gammaclosed and gammaopen appear slow (10-20 ms), often involving several transient substates distinct from gammasub. The polarity of activation and kinetics of this latter form of gating indicate that it is the mechanism by which these hemichannels open in the cell surface membrane when unapposed by another hemichannel. Cx46 hemichannels display a substantial preference for cations over anions, yet have a large unitary conductance (approximately 300 pS) and a relatively large pore as inferred from permeability to tetraethylammonium (approximately 8.5 angstroms diameter). These hemichannels open at physiological voltages and could induce substantial cation fluxes in cells expressing Cx46.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Zheng X., Sogin M. L. The connexins and their family tree. Soc Gen Physiol Ser. 1994;49:223–233. [PubMed] [Google Scholar]
- Bukauskas F. F., Elfgang C., Willecke K., Weingart R. Heterotypic gap junction channels (connexin26-connexin32) violate the paradigm of unitary conductance. Pflugers Arch. 1995 Apr;429(6):870–872. doi: 10.1007/BF00374812. [DOI] [PubMed] [Google Scholar]
- Bukauskas F. F., Weingart R. Voltage-dependent gating of single gap junction channels in an insect cell line. Biophys J. 1994 Aug;67(2):613–625. doi: 10.1016/S0006-3495(94)80521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries S. H., Schwartz E. A. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992 Jan;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Berthoud V. M., Beyer E. C. Distinct behavior of connexin56 and connexin46 gap junctional channels can be predicted from the behavior of their hemi-gap-junctional channels. Biophys J. 1995 May;68(5):1796–1803. doi: 10.1016/S0006-3495(95)80356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Beyer E. C., Swenson K. I., Paul D. L., Goodenough D. A. Cloning and expression of a Xenopus embryonic gap junction protein. Science. 1989 Mar 3;243(4895):1194–1195. doi: 10.1126/science.2466337. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993 Jul;102(1):59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992 Feb;3(1):49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- Malchow R. P., Qian H., Ripps H. Evidence for hemi-gap junctional channels in isolated horizontal cells of the skate retina. J Neurosci Res. 1993 Jun 15;35(3):237–245. doi: 10.1002/jnr.490350303. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Rook M. B., Fishman G. I., Spray D. C. Gap junction channels: distinct voltage-sensitive and -insensitive conductance states. Biophys J. 1994 Jul;67(1):113–119. doi: 10.1016/S0006-3495(94)80460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991 Nov;115(4):1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Armendariz E. M., Romano M. C., Luna J., Miranda C., Bennett M. V., Moreno A. P. Characterization of gap junctions between pairs of Leydig cells from mouse testis. Am J Physiol. 1994 Aug;267(2 Pt 1):C570–C580. doi: 10.1152/ajpcell.1994.267.2.C570. [DOI] [PubMed] [Google Scholar]
- Rubin J. B., Verselis V. K., Bennett M. V., Bargiello T. A. A domain substitution procedure and its use to analyze voltage dependence of homotypic gap junctions formed by connexins 26 and 32. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3820–3824. doi: 10.1073/pnas.89.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. B., Verselis V. K., Bennett M. V., Bargiello T. A. Molecular analysis of voltage dependence of heterotypic gap junctions formed by connexins 26 and 32. Biophys J. 1992 Apr;62(1):183–195. doi: 10.1016/S0006-3495(92)81804-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub O., Eckert R., Lichtenberg-Fraté H., Elfgang C., Bastide B., Scheidtmann K. H., Hülser D. F., Willecke K. Immunochemical and electrophysiological characterization of murine connexin40 and -43 in mouse tissues and transfected human cells. Eur J Cell Biol. 1994 Jun;64(1):101–112. [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Beyer E. C., Brink P. R. Selective dye and ionic permeability of gap junction channels formed by connexin45. Circ Res. 1994 Sep;75(3):483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Verselis V. K., Ginter C. S., Bargiello T. A. Opposite voltage gating polarities of two closely related connexins. Nature. 1994 Mar 24;368(6469):348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- White T. W., Bruzzone R., Goodenough D. A., Paul D. L. Voltage gating of connexins. Nature. 1994 Sep 15;371(6494):208–209. doi: 10.1038/371208a0. [DOI] [PubMed] [Google Scholar]
- Wilders R., Jongsma H. J. Limitations of the dual voltage clamp method in assaying conductance and kinetics of gap junction channels. Biophys J. 1992 Oct;63(4):942–953. doi: 10.1016/S0006-3495(92)81664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]




