Abstract
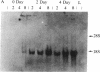
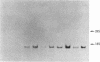
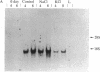
The regulation of neuropeptide gene expression has been investigated by using rat adrenal medullae grown in explant culture. After 3 days in culture the (now denervated) explants exhibited a 10-fold increase in leucine-enkephalin (leu-EK) content. Inhibition of protein synthesis with cycloheximide completely blocked the rise, whereas inhibition of RNA synthesis with actinomycin D or alpha-amanitin inhibited the increase by 50%. Inhibition of DNA synthesis with 1-beta-D-arabinofuranosylcytosine (cytosine arabinoside) had no discernible effect. To determine whether the rise in leu-EK was associated with an increase in specific mRNA coding for the opiate peptide precursor, blot hybridization analysis was performed. A single species of preproenkephalin mRNA was detected after various culture periods. The amount of mRNA increased 34-fold after 2 days in culture and 74-fold after 4 days. Consequently, the rise in mRNA levels preceded the increase in the amount of leu-EK. Depolarization of the adrenal medullae with either elevated potassium or veratridine, which prevents leu-EK accumulation, inhibited the increase in the amount of preproenkephalin mRNA. Moreover, the effect of veratridine was blocked by tetrodotoxin, suggesting that transmembrane sodium ion influx affects the increase in the amount of message. Our studies suggest that elevation of leu-EK in explanted (denervated) medullae is associated with increased amounts of mRNA coding for the peptide precursor and that these processes can be regulated by depolarization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black I. B., Chikaraishi D. M., Lewis E. J. Trans-synaptic increase in RNA coding for tyrosine hydroxylase in a rat sympathetic ganglion. Brain Res. 1985 Jul 22;339(1):151–153. doi: 10.1016/0006-8993(85)90635-3. [DOI] [PubMed] [Google Scholar]
- Bönisch H., Otten U., Thoenen H. The role of sodium influx mediated by nicotinic receptors as an initial event in trans-synaptic induction of tyrosine hydroxylase in adrenergic neurons. Naunyn Schmiedebergs Arch Pharmacol. 1980 Sep;313(3):199–203. doi: 10.1007/BF00505734. [DOI] [PubMed] [Google Scholar]
- Chambon P. Eukaryotic nuclear RNA polymerases. Annu Rev Biochem. 1975;44:613–638. doi: 10.1146/annurev.bi.44.070175.003145. [DOI] [PubMed] [Google Scholar]
- Comb M., Seeburg P. H., Adelman J., Eiden L., Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982 Feb 25;295(5851):663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Fleminger G., Lahm H. W., Udenfriend S. Changes in rat adrenal catecholamines and proenkephalin metabolism after denervation. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3587–3590. doi: 10.1073/pnas.81.11.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govoni S., Hanbauer I., Hexum T. D., Yang H. Y., Kelly G. D., Costa E. In vivo characterization of the mechanisms that secrete enkephalin-like peptides stored in dog adrenal medulla. Neuropharmacology. 1981 Jul;20(7):639–645. doi: 10.1016/0028-3908(81)90110-6. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Perlman R. L. Activation of tyrosine hydroxylase in the superior cervical ganglion by nicotinic and muscarinic agonists. J Neurochem. 1984 Aug;43(2):546–552. doi: 10.1111/j.1471-4159.1984.tb00933.x. [DOI] [PubMed] [Google Scholar]
- Howells R. D., Kilpatrick D. L., Bhatt R., Monahan J. J., Poonian M., Udenfriend S. Molecular cloning and sequence determination of rat preproenkephalin cDNA: sensitive probe for studying transcriptional changes in rat tissues. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7651–7655. doi: 10.1073/pnas.81.23.7651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Johansson O., Ljungdahl A., Lundberg J. M., Schultzberg M. Peptidergic neurones. Nature. 1980 Apr 10;284(5756):515–521. doi: 10.1038/284515a0. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Fleminger G., Udenfriend S. Denervation of rat adrenal glands markedly increases preproenkephalin mRNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7221–7223. doi: 10.1073/pnas.81.22.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGamma E. F., Adler J. E., Black I. B. Impulse activity differentially regulates [Leu]enkephalin and catecholamine characters in the adrenal medulla. Science. 1984 Jun 8;224(4653):1102–1104. doi: 10.1126/science.6144183. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Dean D. M., Whelan L. G., Udenfriend S., Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981 Jan 22;289(5795):317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Mallet J., Faucon Biguet N., Buda M., Lamouroux A., Samolyk D. Detection and regulation of the tyrosine hydroxylase mRNA levels in rat adrenal medulla and brain tissues. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):305–308. doi: 10.1101/sqb.1983.048.01.033. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Trans-synaptic enzyme induction. Life Sci. 1974 Jan 16;14(2):223–235. doi: 10.1016/0024-3205(74)90052-6. [DOI] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. P., Chang K. J., Viveros O. H. Proportional secretion of opioid peptides and catecholamines from adrenal chromaffin cells in culture. J Neurosci. 1982 Aug;2(8):1150–1156. doi: 10.1523/JNEUROSCI.02-08-01150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa K., Williams C., Sabol S. L. Rat brain preproenkephalin mRNA. cDNA cloning, primary structure, and distribution in the central nervous system. J Biol Chem. 1984 Nov 25;259(22):14301–14308. [PubMed] [Google Scholar]