Abstract
Background
Several epidemiological studies have investigated the associations of methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms with hypertension (H) or hypertension in pregnancy (HIP). However, the results were controversial. We therefore performed a comprehensive meta-analysis to provide empirical evidences on the associations.
Methodologies
The English and Chinese databases were systematically searched to identify relevant studies. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the associations. Meta-regression, subgroup analysis, sensitivity analysis, cumulative meta-analysis and assessment of publication bias were performed in our study.
Principal Findings
A total of 114 studies with 15411 cases and 21970 controls were included, 111 studies with 15094 cases and 21633 controls for the C677T polymorphism and 21 with 2533 cases and 2976 controls for the A1298C polymorphism. Overall, the C677T polymorphism was significantly associated with H and HIP (H & HIP: OR = 1.26, 95% CI = 1.17–1.34; H: OR = 1.36, 95% CI = 1.20–1.53; HIP: OR = 1.21, 95% CI = 1.08–1.32). Stratified analysis by ethnicity revealed a significant association among East Asians and Caucasians, but not among Latinos, Black Africans, and Indians and Sri Lankans. In the stratified analyses according to source of controls, genotyping method, sample size and study quality, significant associations were observed in all the subgroups, with the exception of population based subgroup in H studies and large sample size and “others” genotyping method subgroups in HIP studies. For the A1298C polymorphism, no significant association was observed either in overall or subgroup analysis under all genetic models.
Conclusions
This meta-analysis suggests that the MTHFR C677T rather than A1298C polymorphism may be associated with H & HIP, especially among East Asians and Caucasians.
Introduction
Hypertension (H), whose prevalence has dramatically increased in recent years, is a major risk factor for many disorders including stroke, cardiovascular diseases and renal failure and ultimately increases mortality worldwide [1]. The development of H is influenced by genetic, environmental, demographic factors and their interactions [2]. Current evidences suggest that 30–50% of variation of blood pressure levels could be attributed to genetic factors [3]. Therefore, identification of H susceptibility genes will help clarify the pathogenesis of the disease and provide new therapeutic and preventive strategies [3]. In the last decade, exhaustive efforts have been devoted to unraveling the genetic underpinning of H, and hundreds of genes and polymorphisms have been hypothesized to be involved in the pathogenesis of the disease [4]–[6]. Among them, C677T and A1298C polymorphisms in methylenetetrahydrofolate reductase (MTHFR) gene have been assessed as potential candidates.
MTHFR is an enzyme that catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methytetrahydrofolate, the carbon donor for the remethylation of homocysteine (Hcy) to methionine [7]. The MTHFR gene is localized on chromosome 1 at 1p36.6 [8]. The C677T polymorphism is a C to T transition at base pair 677 resulting an alanine to valine substitution, and the A1298C polymorphism is an A to C transition at base pair 1298 leading to a glutamate to alanine substitution. The prevalence of the two polymorphisms varies in different geographical regions and ethnic groups [9], [10]. The variant genotypes of them have been confirmed to reduce enzyme activity and decrease folate levels, and subsequently result in hyperhomocysteinemia (HHcy) [11], [12]. HHcy has been linked to H and hypertension in pregnancy (HIP) because it may induce arteriolar constriction, renal dysfunction and increased sodium reabsorption, and also increase arterial stiffness and oxidative stress [13]–[15]. Therefore, the MTHFR C677T and A1298C polymorphisms as common genetic causes for HHcy are expected to be associated with hypertension and hypertension in pregnancy (H & HIP).
Numerous epidemiological studies were conducted in recent years to evaluate the associations between the MTHFR C677T and A1298C polymorphisms and H & HIP. However, the results were conflicting or inconclusive, presumably due to small sample size in each published study, various genetic backgrounds and possible selection bias. Meta-analysis is a widely used statistical method in medical research, especially for a topic being extensively studied while controversial results are being reported. Two meta-analyses, one by Qian et al. [16], the other by Niu et al. [17], were performed in 2007 and 2011, respectively, to investigate the associations of the C677T polymorphism with H & HIP and significant results were reported. However, Niu et al.’s [17] meta-analysis only included studies in the analysis of Chinese population. Additionally, new epidemiological studies have recently been conducted to estimate the associations of the MTHFR C677T and A1298C polymorphisms with H and/or HIP in different populations and provide new evidences that were not included in these previous meta-analyses. Moreover, both meta-analyses did not address the associations of the A1298C polymorphism with H and/or HIP. To provide a more comprehensive assessment of the associations of the MTHFR C677T and A1298C polymorphisms with H & HIP in worldwide populations, we carried out a meta-analysis of all eligible studies.
Materials and Methods
Search Strategy and Inclusion Criteria
All studies reporting the relationships of the MTHFR C677T and A1298C polymorphisms with H or HIP published before December 10, 2013 were identified by computerized searches in databases including Pubmed, Embase, ISI Web of Science, China Biological Medicine Database (CBM), Wanfang, China National Knowledge Infrastructure (CNKI), and Chongqing VIP Chinese Science and Technology Periodical Database (VIP). The search strategies were based on combinations of the following key words: (“methylenetetrahydrofolate reductase” or “MTHFR”) and (“hypertension” or “hypertension in pregnancy” or “pregnancy induced hypertension” or “preeclampsia” or “eclampsia” or “gestational hypertension”) and (“gene” or “allele” or “genotype” or “mutation” or “variant” or “variation” or “polymorphism”). The reference lists of retrieved articles were also hand searched for additional articles.
Qualified studies had to meet the following criteria: (1) evaluation of the MTHFR C677T and/or A1298C polymorphisms and H or HIP; (2) hypertensive patients were diagnosed according to the criteria of SBP≥140 mmHg or DBP≥90 mmHg and the controls were healthy individuals; (3) case-control or cohort study, regardless of sample size, using a hospital based or a population based design; (4) sufficient published data for estimating the Odds Ratio (OR) and 95% confidence interval (CI); (5) for duplicate publication, the most recent or largest study was selected.
Data Extraction
Two reviewers (Boyi Yang and Shujun Fan) independently extracted the following information from each included study: the first author’s name, publication year, sample size, source of controls, ethnicity, genotyping method, matching variables of controls with cases, H type (H vs. HIP), age, gender proportion, and counts of alleles and genotypes in both cases and controls.
Quality Assessment
Two authors (Boyi Yang and Xueyuan Zhi) independently assessed the quality of the included studies according to Newcastle Ottawa Scale (NOS) (www.ohri. ca/programs/clinical_epidemiology/oxford.asp). This scale consists of three parts relating to selection, comparability and ascertainment of exposure. A maximum nine scores could be given to the highest quality studies. A score of five or more was regarded as “high quality”; otherwise, the study was regarded as “low quality”.
Statistical Analysis
All statistic tests performed in this study were two tailed and P<0.05 was taken as statistically significant, unless otherwise stated. Statistic analyses were performed using STATA package version 11.0 program (Stata corp, College Station, TX). Hardy-Weinberg equilibrium (HWE) in controls was calculated again in our meta-analysis. The chi-square goodness of fit was used to test deviation from HWE.
Crude ORs with corresponding 95% CIs were calculated to estimate the strength of the associations of the MTHFR C677T and A1298C polymorphisms with H and/or HIP. The significance of the pooled OR was determined by the Z test. Pooled frequency analysis was carried out using the method suggested by Thakkinstian [18]. The overall pooled ORs were calculated using allele contrast model, dominant model and recessive model. Moreover, comparisons of OR1 (AA vs. aa), OR2 (Aa vs. aa) and OR3 (AA vs. Aa) were explored with A as the risk allele. The above pairwise differences were used to determine the most appropriate genetic model. If OR1 = OR3 ≠ 1 and OR2 = 1, then a recessive model is selected. If OR1 = OR2 ≠ 1 and OR3 = 1, then a dominant model is selected. If OR2 = 1/OR3 ≠ 1 and OR1 = 1, then a complete overdominant model is selected. If OR1> OR2>1 and OR1> OR3>1 (or OR1< OR2<1 and OR1< OR3<1), then a codominant model is selected [19]. Additionally, if some genotypes were very rare or could not be identified in either case or control group in some studies, a recessive or dominant model is selected to combine rare homozygous and heterozygous [20].
Between-study heterogeneity was calculated by Cochran’s Chi-square based Q-test [21]. Simultaneously, it was also detected using the I 2 statistic (I 2 = 0–25% represents no heterogeneity; I 2 = 25–50% represents moderate heterogeneity; I 2 = 50–75% represents large heterogeneity; I 2 = 75–100% represents extreme heterogeneity) [22]. If the between-study heterogeneity was statistically significant (P<0.10 for Q-test or I2>50%), the Dersimonian and Laird random effects model was used; otherwise, the Mantel Haenszel method fixed effects model was applied [23]. Subgroup analysis based on ethnicity (East Asians, Caucasians, Latinos, Indians and Sri Lankans, Black Africans), source of controls (population based vs. hospital based), genotyping method (polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) vs. “others”), sample size (studies with ≥ median number of participants vs. studies with < median number) and study quality (high quality vs. low quality), respectively, were also performed under the most appropriate genetic model. Furthermore, meta-regression was employed to explore potential sources of heterogeneity including publication date, ethnicity, genotyping method, source of controls, study quality and sample size [24]. To explore the dynamic trends as studies accumulated over time, cumulative meta-analysis was performed by date of publication [25]. Sensitivity analysis was also conducted to examine the influence of excluding each study or some specific studies on the overall estimate [25]. Finally, potential publication bias was assessed using funnel plot and Egger’s regression test [26].
Results
Study Characteristics
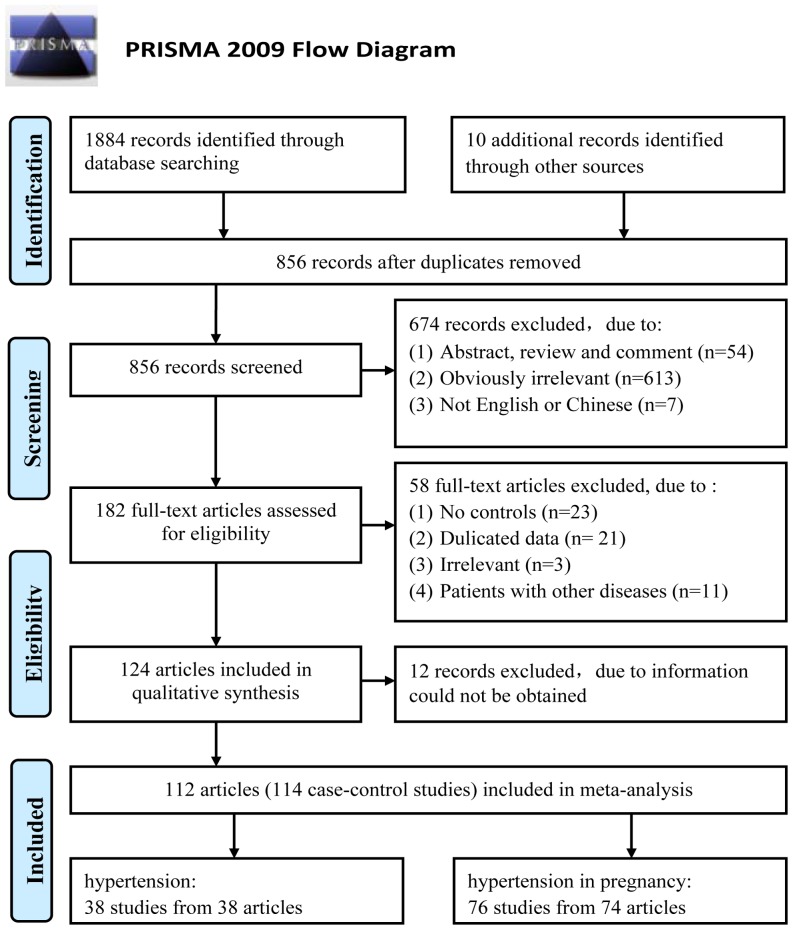
The combined search yielded 1884 articles. After the removal of overlapping articles and those did not meet our inclusion criteria, a total of 112 articles [27]–[138] including 114 studies with 15411 cases and 21970 controls were finally included in the meta-analysis (Figure 1). One hunderd and eleven studies dealt with C677T. The sample sizes ranged from 39 to 2104 with a median of 225. Twenty one studies dealt with A1298C. The sample sizes ranged from 58 to 754 with a median of 170. The main characteristics of the included studies are presented in Table S1. Among all studies, 42 studies were performed among East Asians [27], [28], [33], [37], [42], [43], [48], [50], [54], [56], [57], [60], [65], [73], [75], [78], [79], [81], [86]–[90], [94]–[97], [102], [103], [105]–[107], [115]–[118], [122], [124], [131], [132], [134], [136], 54 among Caucasians [29], [31], [32], [34], [35], [38]–[41], [44]–[47], [49], [51]–[53], [55], [59], [63], [64], [66], [67], [69]–[72], [74], [76], [77], [80], [82], [84], [92], [93], [98]–[101], [109], [110], [112]–[114], [119]–[121], [125], [126], [130], [133], [135], [137], 10 among Latinos [58], [61], [68], [83], [91], [104], [108], [127], [128], [138], five among Indians and Sri Lankans [53], [85], [111], [123], [129] and three among Black Africans [30], [36], [62]. Thirty eight studies focused on H [28], [37], [45], [54], [55], [64]–[66], [75], [78], [80], [81], [85], [87], [88], [90], [92], [95], [98], [99], [102], [105], [106], [114]–[118], [134], [119], [120], [122], [124], [128], [131], [132], [136], [137] and 76 studies focused on HIP [27], [29]–[36], [38], [39]–[44], [46]–[53], [56]–[63], [67]–[74], [76], [77], [79], [82]–[84], [86], [89], [91], [93], [94], [96], [97], [100], [101], [103], [104], [107]–[113], [121], [123], [125]–[127], [129], [130], [133], [135], [138]. The sources of controls were hospital based in 91 studies and were population based in 23 studies. PCR-RFLP was the most commonly used genotyping method in these included studies. Genotype and allele frequencies, HWE and NOS scale information are presented in Table S2 and Table S3. Of the total 114 studies, 20 different studies [27], [35], [40], [45], [73], [79], [85], [87], [94], [96], [105], [107], [109], [110], [115], [116], [122], [129], [135] showed significant deviations from HWE (18 studies concerned C677T and two studies concerned A1298C). Thirteen studies only reported combined genotypes (CC+CT, CT+TT, AC+CC), thus HWE could not be evaluated (12 studies concerned C677T [29], [41], [49], [51], [67], [69], [71], [77], [100], [101], [114], [119] and one study concerned A1298C [119]). According to NOS scale, there were 100 studies with high quality and 14 with low quality.
Figure 1. Flow diagram of study selection process in this meta-analysis.
Frequency of Risk Allele in the Control Population
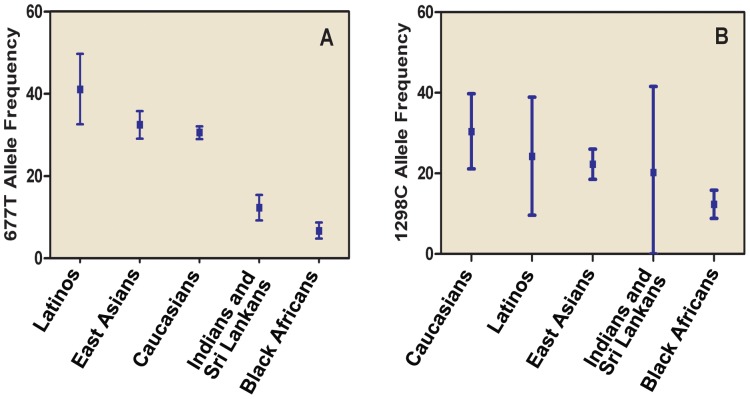
Figure 2 shows the pooled frequencies of the 677T and 1298C alleles in the control populations stratified by ethnicity. The frequencies of the 677T allele varied among ethnicities: the pooled 677T allele frequency was highest among Latinos (41.5%, 95% CI = 34.0–49.0%), followed by East Asians (33.0%, 95% CI = 29.7–36.3%), Caucasians (30.1%, 95% CI = 28.5–31.6%), Indians and Sri Lankans (12.3%, 95% CI = 9.2–15.4%) and Black Africans (6.7%, 95% CI = 4.8–8.7%). The pooled 1298C allele frequencies also showed heterogeneity among different ethnicities: high among Caucasians (30.4%, 95% CI = 21.1–39.8%), intermediate among Latinos (24.2%, 95% CI = 9.6–38.9%), East Asians (22.3%, 95% CI = 18.5–26.0%) and Indians and Sri Lankans (20.2%, 95%CI = 0–41.6%), and low among Black Africans (12.3%, 95% CI = 8.8–15.8%).
Figure 2. Pooled frequencies of the MTHFR 677T allele and 1298C allele among controls stratified by ethnicity.
Quantitative Synthesis and Heterogeneity Analysis
Association of MTHFR C677T polymorphism with H & HIP
We firstly pooled all the studies (111 studies with 15094 cases and 21633 controls) involving both H and HIP to estimate the associations between the diseases and the MTHFR C677T polymorphism. Table 1 summarizes the ORs with corresponding 95% CIs for the relationships of the polymorphism with H & HIP in homozygous codominant, heterozygous codominant, dominant, recessive and allele contrast genetic models (Figure S1–S5). The dominant model was determined according to the principle of genetic model selection [19], [20]. The summary results indicated a significant association between the MTHFR C677T polymorphism and H & HIP. For the dominant model, the pooled OR using random effects model was 1.26 (95% CI = 1.17–1.34) (Table 1 and Figure S3). Subgroup analysis for ethnicity indicated that the polymorphism was associated with H & HIP among East Asians and Caucasians, but not among Latinos, Black Africans, and Indians and Sri Lankans. Additionally, when stratified analyses were conducted according to source of controls, genotyping method, sample size and study quality, the polymorphism was significantly associated with H & HIP in all the subgroups (Table 2). Significant heterogeneity was observed, thus a meta-regression was performed subsequently to explore the heterogeneity sources. The results of meta-regression indicated that ethnicity had a statistical significance (P = 0.043), while the H type (P = 0.829), year of publication (P = 0.293), source of controls (P = 0.400), genotyping method (P = 0.439) and sample size (P = 0.579) had no statistical significance.
Table 1. Summarized ORs with 95% CIs for the associations of MTHFR polymorphisms with H and HIP.
| Polymorphism | Genetic model | n | Statistical model | OR (95% CI) | P z | I2 (%) | P h | P e | |
| C677T | |||||||||
| Allele contrast | H & HIP | 99 | Random | 1.23 (1.16–1.31) | <0.001 | 56.0 | <0.001 | 0.280 | |
| H | 34 | Random | 1.30 (1.18–1.43) | <0.001 | 64.1 | <0.001 | 0.816 | ||
| HIP | 65 | Random | 1.19 (1.10–1.29) | <0.001 | 48.7 | <0.001 | 0.149 | ||
| Homozygous | H& HIP | 99 | Random | 1.47 (1.30–1.66) | <0.001 | 41.5 | <0.001 | 0.362 | |
| codominant | H | 34 | Random | 1.63 (1.34–1.98) | <0.001 | 54.1 | <0.001 | 0.497 | |
| HIP | 65 | Random | 1.37(1.18–1.58) | <0.001 | 31.0 | 0.011 | 0.495 | ||
| Heterozygous | H & HIP | 99 | Random | 1.18 (1.10–1.27) | <0.001 | 38.4 | <0.001 | 0.059 | |
| codominant | H | 34 | Random | 1.25 (1.11–1.40) | <0.001 | 43.1 | 0.005 | 0.979 | |
| HIP | 65 | Random | 1.14 (1.03–1.26) | 0.009 | 34.3 | 0.004 | 0.052 | ||
| Dominant | H & HIP | 101 | Random | 1.26 (1.17–1.34) | <0.001 | 48.2 | <0.001 | 0.711 | |
| H | 35 | Random | 1.36 (1.20–1.53) | <0.001 | 55.0 | <0.001 | 0.918 | ||
| HIP | 66 | Random | 1.19 (1.08–1.32) | <0.001 | 41.0 | <0.001 | 0.651 | ||
| Recessive | H & HIP | 109 | Random | 1.37 (1.23–1.52) | <0.001 | 43.7 | <0.001 | 0.072 | |
| H | 35 | Random | 1.43 (1.21–1.68) | <0.001 | 45.6 | 0.002 | 0.123 | ||
| HIP | 74 | Random | 1.34 (1.16–1.53) | <0.001 | 43.5 | <0.001 | 0.118 | ||
| A1298C | |||||||||
| Allele contrast | H & HIP | 20 | Fixed | 1.01 (0.92–1.11) | 0.791 | 29.2 | 0.108 | 0.112 | |
| H | 7 | Random | 1.05 (0.79–1.39) | 0.733 | 67.6 | 0.005 | 0.614 | ||
| HIP | 13 | Fixed | 1.01 (0.90–1.14) | 0.824 | 0.0 | 0.760 | 0.315 | ||
| Homozygous | H & HIP | 20 | Fixed | 1.06 (0.85–1.32) | 0.630 | 0.0 | 0.696 | 0.348 | |
| codominant | H | 7 | Fixed | 1.08 (0.78–1.50) | 0.649 | 0.0 | 0.658 | 0.735 | |
| HIP | 13 | Fixed | 1.04 (0.77–1.40) | 0.816 | 0.0 | 0.506 | 0.716 | ||
| Heterozygous | H & HIP | 20 | Fixed | 0.99 (0.84–1.17) | 0.928 | 35.4 | 0.060 | 0.818 | |
| codominant | H | 7 | Random | 0.96 (0.65–1.44) | 0.854 | 71.0 | 0.002 | 0.708 | |
| HIP | 13 | Fixed | 1.01 (0.86–1.19) | 0.918 | 0.0 | 0.760 | 0.716 | ||
| Dominant | H & HIP | 21 | Fixed | 1.06 (0.90–1.26) | 0.474 | 45.3 | 0.013 | 0.643 | |
| H | 8 | Random | 1.10(0.75–1.61) | 0.637 | 77.2 | <0.001 | 0.941 | ||
| HIP | 13 | Fixed | 1.01 (0.87–1.18) | 0.906 | 0.0 | 0.092 | 0.219 | ||
| Recessive | H & HIP | 20 | Fixed | 1.10 (0.89–1.36) | 0.392 | 0.0 | 0.709 | 0.621 | |
| H | 7 | Fixed | 1.15 (0.84–1.57) | 0.393 | 0.0 | 0.780 | 0.866 | ||
| HIP | 13 | Fixed | 1.06 (0.79–1.41) | 0.712 | 0.0 | 0.453 | 0.528 |
Abbreviation: MTHFR, methylenetetrahydrofolate reductase; H, hypertension; HIP, hypertension in pregnancy; OR, odds ratio; CI, confidence interval; P z, P value for association test; P h, P value for heterogeneity test; P e, P value for publication bias test; n, the number of studies.
Table 2. Stratified analysis of the associations of MTHFR C677T polymorphism with H and HIP under dominant model.
| H & HIP | H | HIP | |||||||
| Subgroup analysis | n | OR (95% CI) | P h (I2%) | n | OR (95% CI) | P h (I2%) | n | OR (95% CI) | P h (I2%) |
| All HWE | 81 | 1.23 (1.13–1.34) | <0.001 (44.9) | 28 | 1.35 (1.17–1.54) | <0.001 (51.0) | 53 | 1.16 (1.05–1.28) | 0.003 (38.7) |
| Ethnicity | |||||||||
| East Asians | 40 | 1.46 (1.29–1.66) | <0.001 (55.7) | 23 | 1.38 (1.19–1.60) | <0.001 (55.5) | 17 | 1.64 (1.28–2.10) | 0.002 (57.6) |
| Caucasians | 43 | 1.18 (1.07–1.29) | 0.116 (21.0) | 10 | 1.29 (1.01–1.63) | 0.004 (62.5) | 33 | 1.15 (1.05–1.26) | 0.648 (0.00) |
| Latinos | 10 | 1.09 (0.86–1.39) | 0.098 (38.9) | 1 | 1.43 (0.81–2.51) | – | 9 | 1.06 (0.82–1.37) | 0.094(41.0) |
| Indians and | 5 | 0.93 (0.56–1.56) | 0.004 (74.4) | 1 | 1.71 (1.00–2.91) | – | 4 | 0.78 (0.48–1.29) | 0.039 (64.2) |
| Sri Lankans | |||||||||
| Black Africans | 3 | 1.22 (0.89–1.67) | 0.513 (0.00) | 0 | – | – | 3 | 1.22 (0.89–1.67) | 0.513 (0.0) |
| Source of controls | |||||||||
| Hospital based | 80 | 1.30 (1.18–1.43) | <0.001 (51.0) | 23 | 1.51 (1.30–1.75) | 0.003 (50.5) | 57 | 1.20 (1.07–1.35) | <0.001 (46.8) |
| Population based | 21 | 1.19 (1.06–1.34) | 0.048 (36.4) | 12 | 1.15 (0.95–1.40) | 0.005 (59.3) | 9 | 1.22 (1.08–1.42) | 0.808 (0.0) |
| Genotyping methods | |||||||||
| PCR-RFLP | 86 | 1.28 (1.18–1.40) | <0.001 (50.4) | 31 | 1.36 (1.20–1.54) | <0.001 (57.6) | 55 | 1.23 (1.10–1.38) | <0.001 (44.5) |
| Others | 15 | 1.16 (1.02–1.32) | 0.122 (30.9) | 4 | 1.40 (1.01–1.96) | 0.213 (33.2) | 11 | 1.08(0.93–1.26) | 0.262 (19.1) |
| Sample size | |||||||||
| Large (≥225) | 51 | 1.17 (1.07–1.27) | <0.001 (54.0) | 25 | 1.30 (1.14–1.48) | <0.001 (58.6) | 26 | 1.05 (0.94–1.17) | 0.033 (36.7) |
| Small (<225) | 50 | 1.46 (1.28–1.67) | 0.014 (33.2) | 10 | 1.63 (1.31–2.04) | 0.154 (33.0) | 40 | 1.41 (1.21–1.63) | 0.025 (32.8) |
| Study quality | |||||||||
| High (≥5 scores) | 89 | 1.25 (1.15–1.36) | <0.001 (49.2) | 31 | 1.34 (1.18–1.52) | <0.001 (55.9) | 58 | 1.19 (1.07–1.32) | <0.001 (42.8) |
| Low (<5 scores) | 12 | 1.34 (1.06–1.70) | 0.063 (41.8) | 4 | 1.58 (1.03–2.42) | 0.086 (54.6) | 8 | 1.23 (1.01–1.64) | 0.135 (36.8) |
Abbreviation: MTHFR, methylenetetrahydrofolate reductase; HWE, Hardy-Weinberg equilibrium; H, hypertension; HIP, hypertension in pregnancy; OR, odds ratio; CI, confidence interval; P h, P value for heterogeneity test; n, the number of studies; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Association of MTHFR A1298C polymorphism with H & HIP
Twenty one studies with 2533 cases and 2976 controls on the relationship between the A1298C polymorphism and H & HIP were included in the meta-analysis. The dominant model was determined according to the principle of genetic model selection [19], [20]. No significant relationship was observed between the MTHFR A1298C polymorphism and H & HIP under all genetic models (Table 1 and Figure S6–S10). For the dominant model, the overall pooled OR using random effects model was 1.06 (95% CI = 0.90–1.26) (Table 1 and Figure S8). Similarly, stratified analyses based on ethnicity, source of controls, genotyping method, sample size and study quality did not reveal any significant association of the polymorphism with H & HIP (Table 3). Significant heterogeneity was observed, and meta-regression analysis was performed to explore the sources of heterogeneity. However, the H type (P = 0.155), year of publication (P = 0.351), ethnicity (P = 0.411), source of controls (P = 0.906), genotyping method (P = 0.197) and sample size (P = 0.850) were not the sources of heterogeneity.
Table 3. Stratified analysis of the associations of MTHFR A1298C polymorphism with H and HIP under dominant model.
| H & HIP | H | HIP | |||||||
| Subgroup analysis | n | OR (95% CI) | P h (I2%) | n | OR (95% CI) | P h (I2%) | n | OR (95% CI) | P h (I2%) |
| All HWE | 18 | 0.96 (0.85–1.09) | 0.946 (0.0) | 5 | 0.88 (0.72–1.08) | 0.698 (0.0) | 13 | 1.01 (0.87–1.18) | 0.903 (0.00) |
| Ethnicity | |||||||||
| East Asians | 5 | 0.91 (0.69–1.19) | 0.789 (0.00) | 2 | 0.79 (0.56–1.12) | 0.740 (0.0) | 3 | 1.12(0.73–1.71) | 0.995 (0.0) |
| Caucasians | 12 | 1.00 (0.85–1.25) | 0.041 (45.9) | 5 | 1.02 (0.64–1.64) | 0.003 (74.8) | 7 | 0.91 (0.74–1.13) | 0.727 (0.0) |
| Latinos | 1 | 1.25 (0.44–3.56) | – | 0 | – | – | 1 | 1.25 (0.44–3.56) | – |
| Indians and | 2 | 1.79 (0.72–4.45) | 0.011 (84.6) | 1 | 2.91 (1.64–5.15) | – | 1 | 1.15 (0.75–1.76) | – |
| Sri Lankans | |||||||||
| Black Africans | 1 | 1.11 (0.78–1.59) | – | 0 | – | – | 1 | 1.11 (0.78–1.59) | – |
| Source of controls | |||||||||
| Hospital based | 14 | 1.16 (0.98–1.36) | 0.396 (5.0) | 3 | 1.34 (0.59–3.00) | 0.006 (80.2) | 11 | 1.09 (0.91–1.30) | 0.996 (0.00) |
| Population based | 7 | 0.93 (0.79–1.10) | 0.003 (69.4) | 5 | 0.98 (0.62–1.55) | <0.001 (77.4) | 2 | 0.79 (0.57–1.08) | 0.516 (0.00) |
| Genotyping methods | |||||||||
| PCR-RFLP | 18 | 1.03 (0.87–1.21) | 0.065 (36.0) | 7 | 1.00 (0.67–1.47) | <0.001 (72.5) | 11 | 1.03 (0.87–1.21) | 0.926 (0.00) |
| Others | 3 | 1.25 (0.67–2.33) | 0.026 (72.5) | 1 | 1.98 (1.28–3.08) | – | 2 | 0.88 (0.57–1.37) | 0.396(0.00) |
| Sample size | |||||||||
| Large (≥170) | 10 | 1.10 (0.86–1.40) | <0.001 (69.6) | 5 | 1.26 (0.78–2.03) | <0.001 (83.2) | 5 | 0.97 (0.80–1.17) | 0.331 (13.0) |
| Small (<170) | 11 | 1.02 (0.81–1.29) | 0.735 (0.0) | 3 | 0.81 (0.39–1.07) | 0.070 (62.5) | 8 | 1.11 (0.84–1.47) | 1.000 (0.00) |
| Study quality | |||||||||
| High (≥5 scores) | 19 | 1.09 (0.92–1.29) | 0.030 (41.6) | 7 | 1.22 (0.84–1.79) | <0.001 (75.8) | 12 | 0.99 (0.84–1.17) | 0.928 (0.00) |
| Low (<5 scores) | 2 | 0.72 (0.24–2.16) | 0.023 (80.6) | 1 | 0.39 (0.17–0.89) | – | 1 | 1.20 (0.72–2.00) | – |
Abbreviation: MTHFR, methylenetetrahydrofolate reductase; HWE, Hardy-Weinberg equilibrium; H, hypertension; HIP, hypertension in pregnancy; OR, odds ratio; CI, confidence interval; P h, P value for heterogeneity test; n, the number of studies; PCR-RFLP, polymerase chain reaction-restriction fragment length polymorphism.
Association of MTHFR C677T polymorphism with H
Thirty six studies with 6584 cases and 6760 controls reporting the relationship between the MTHFR C677T polymorphism and H were included in our meta-analysis. The results of overall pooled analyses under five genetic models are listed in Table 1. The dominant model was determined according to the principle of genetic model selection [19], [20]. The summary results indicated that the polymorphism was significantly associated with H. For the dominant model, the overall pooled OR using random effects model was 1.36 (95% CI = 1.20–1.53). Table 2 summarizes the results of stratified analyses under dominant genetic model. As stratified analysis by ethnicity, significant associations were found among East Asians and Caucasians, but not among Latinos, Black Africans, and Indians and Sri Lankans. Stratified analysis by source of controls showed significant association in hospital based studies, but not in population based studies. When stratified analyses were conducted based on genotyping method, sample size and study quality, significant associations were found in all the subgroups. Meta-regression was performed to find the sources of heterogeneity. However, the year of publication (P = 0.191), ethnicity (P = 0.953), source of controls (P = 0.066), genotyping method (P = 0.734) and sample size (P = 0.551) were not the sources of heterogeneity.
Association of MTHFR A1298C polymorphism with H
We identified eight studies with 1196 cases and 1213 controls investigating the relationship of the polymorphism with H. The results of overall pooled analyses under five genetic models are listed in Table 1. The dominant model was determined according to the principle of genetic model selection [19], [20]. In the overall comparison, the polymorphism was not significantly with H in any of the genetic model. For the dominant model, the overall pooled OR using random effects model was 1.10 (95% CI = 0.75–1.61) (Table 1). As stratified analyses by ethnicity, genotyping method and study quality, significant associations were found in Indians and Sri Lankans and “others” genotyping method studies, whereas a significant negative association was found in low quality studies (Table 3). Notably, each of these associations was based on only one study; therefore, the results should be interpreted with great caution (Table 3). Although significant heterogeneity existed, a meta-regression analysis was not performed due to the limited number of the studies (<10) included in this group.
Association of MTHFR C677T polymorphism with HIP
Seventy five studies with 8510 cases and 14873 controls on the relationship between the MTHFR C677T polymorphism and HIP were included in the meta-analysis. The results of overall pooled analyses under five genetic models are presented in Table 1. The dominant model was determined according to the principle of genetic model selection [19], [20]. The summary results indicated that the polymorphism was significantly associated with HIP. For the dominant model, the overall pooled OR using random effects model was 1.19 (95% CI = 1.08–1.32) (Table 1). Results from subgroup analysis based on ethnicity indicated that the C677T polymorphism was associated with HIP among East Asians and Caucasians. However, no significant associations were found among Latinos, Black Africans, and Indians and Sri Lankans. As stratified analyses by source of controls, genotyping method, sample size and study quality, significant associations were found in all the subgroups, with the exception of large sample size subgroup and “others” genotyping method subgroup (Table 2). To explore the sources of heterogeneity, a meta-regression was performed, and the results showed that ethnicity had a statistical significance (P = 0.004) while the year of publication (P = 0.240), source of controls (P = 0.290), genotyping method (P = 0.476) and sample size (P = 0.713) had no statistical significance.
Association of MTHFR A1298C polymorphism with HIP
Thirteen studies with 1337 cases and 1763 controls on the relationship of the MTHFR A1298C polymorphism with HIP were included in the meta-analysis. The summary results of overall pooled analysis under five genetic models are showed in Table 1. The dominant model was determined according to the principle of genetic model selection [19], [20]. The summary results indicated that the polymorphism was not significantly associated with HIP. For the dominant model, the pooled OR using fixed effects model was 1.01 (95% CI = 0.87–1.18) (Table 1). Similarly, in the stratified analyses by ethnicity, source of controls, genotyping method, sample size and study quality, no significant association was found in all the subgroups (Table 3).
Cumulative Meta-analysis
Cumulative meta-analyses were performed using a dominant model for the MTHFR C677T and A1298C polymorphisms. Regarding to C677T, a trend of a more significant association was consistently observed with a narrowing of the 95% CI as information accumulated by year (Figure S11). However, for A1298C, as studies were published, the association of the polymorphism with H & HIP was statistically non-significant (Figure S12).
Sensitivity Analysis
Sensitivity analysis was performed to confirm the stability and liability of the meta-analysis by sequentially omitting individual eligible studies. When any single study was excluded, the corresponding ORs were not materially changed (data were not shown), indicating the stability of our results. Additionally, we excluded the studies that genotype distribution in the controls deviating from HWE, and the corresponding pooled ORs were not significantly changed (Table 2 and Table 3).
Publication Bias
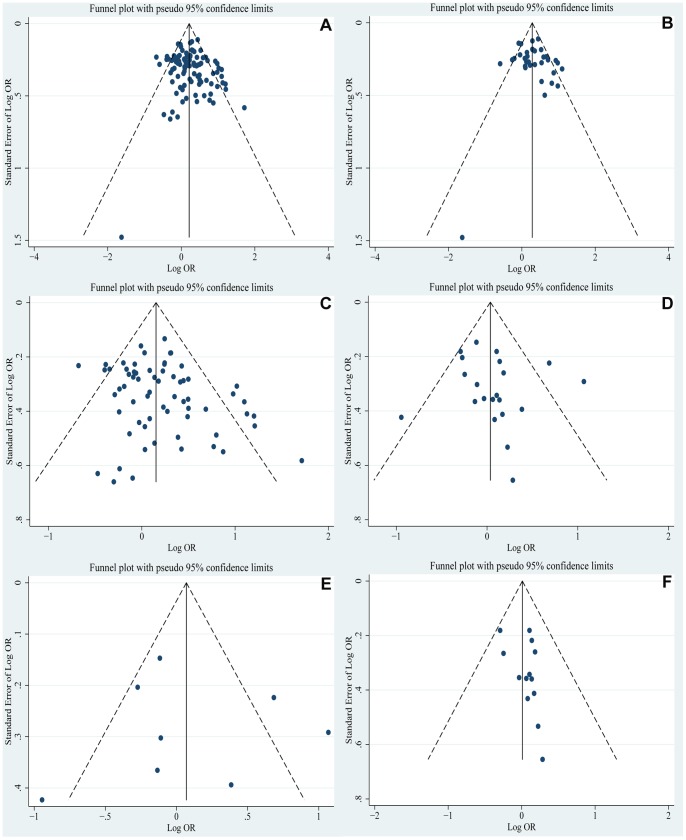
Funnel plot and Egger’s linear regression were performed to assess the publication bias of the included studies. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (Figure 3). The results of Egger’s test also showed that there was no strong statistical evidence of publication bias (Table 1).
Figure 3. Funnel plot analysis on the detection of publication bias in the meta-analysis of the associations between MTHFR polymorphisms and H & HIP (A: C677T and H & HIP; B: C677T and H; C: C677T and HIP; D: A1298C and H & HIP; E: A1298C and H; F: A1298C and HIP).
Discussion
The present meta-analysis involved 111 studies with 15094 cases and 21633 controls that investigated the C677T polymorphism and 21 studies with 2533 cases and 2976 controls investigated the A1298C polymorphism. Overall, our meta-analytical results provided evidences that the MTHFR C677T polymorphism was associated with both H and HIP (H & HIP: OR = 1.26, 95% CI = 1.17–1.34; H: OR = 1.36, 95% CI = 1.20–1.53; HIP: OR = 1.19, 95% CI = 1.08–1.32). However, no association was detected between the MTHFR A1298C polymorphism and H & HIP (H & HIP: OR = 1.06, 95% CI = 0.90–1.26; H: OR = 1.10, 95% CI = 0.75–1.61; HIP: OR = 1.01, 95% CI = 0.87–1.18). Sensitivity analysis and cumulative meta-analysis further strengthened the validity of these results.
In recent years several meta-analyses have been done to investigate the associations of the MTHFR C677T polymorphism with H and/or HIP, and our findings were largely in line with these published meta-analyses [16], [17], [139]–[141]. Intuitively, our study is seemingly superfluous, but it enjoyed apparent superiority over these previous meta-analyses in terms of the following aspects: first, we performed literature searches from seven electronic databases including PubMed, Embase, Web of Science, CNKI, Wanfang, CBM and VIP, while these previous meta-analyses only searched part of the aforementioned databases, thus our more comprehensive search can ensure as many studies as possible and minimize selection bias; second, our study inspected not only H but HIP and included approximately 6 times as many participants as Niu et al. [17], Qian et al. [16] and Kosmas et al.’s [139] meta-analyses, and 23 more studies than the most recent meta-analysis by Wang et al. [141]; finally, besides stratified analyses, we further performed meta-regression and cumulative meta-analysis to investigate potential sources of heterogeneity and study stability respectively. Based on the above advantages, our study can provide a more precise estimation of associations between the C677T polymorphism and H & HIP.
After subgroup analysis according to ethnicity, the results indicated that the MTHFR C677T polymorphism was associated with H & HIP among East Asians and Caucasians, but not among Latinos, Black Africans, and Indians and Sri Lankans. Several factors may contribute to the phenomenon that the C677T polymorphism was associated with H & HIP in one population and the association was nil for another population. Above all, different genetic backgrounds may attribute to the discrepancy, since the 677T allele distributions vary among Latinos, East Asians, Caucasians, Black Africans, and Indians and Sri Lankans, with a prevalence of 41.1%, 32.5%, 30.6%, 12.3% and 6.7%, respectively. Another explanation may be that different populations live with multiple life styles and environmental factors, some of which may affect disease development [2]. Other factors such as selection bias and different matching criteria should also be considered. Additionally, relative small sample sizes for Latinos, Black Africans, and Indians and Sri Lankans limited us to detect stable effects in these populations. Therefore, additional studies are warranted to validate possible ethnic differences in the associations of the C677T polymorphism with H & HIP, especially among Latinos, Black Africans, and Indians and Sri Lankans. When stratifying by source of controls and sample size, significant associations were observed in almost all the subgroups, with the exception of population based subgroup in H association studies and large sample size subgroup in HIP association studies. In addition, hospital based and small sample size studies seem to have stronger associations than population based and large sample size studies. Hospital based studies are prone to produce unreliable results because controls from hospital based studies are less representative of the general population, especially when the polymorphism under investigation are expected to be related to disorders that the hospital based controls may have [142], [143]. Small sample with limited participants is often accompanied with selection biases, and lacks sufficient power to support or deny an association [144]. It is therefore speculated that our meta-analysis might overestimate the magnitude of association between the polymorphism and H & HIP in the overall effect estimates. Although this may not influence the final conclusions, further large scale and well designed population based studies are warranted to explore the associations reliably. Stratified analysis by genotyping method suggested significant associations in both PCR-RFLP and “others” genotyping method studies, except among those HIP association studies taking “others” as genotyping method. PCR-RFLP is the most commonly used method for genotyping MTHFR in this meta-analysis because of its relative simplicity. Although it is reported that other genotyping methods (Taqman, Mass Array and gene chip) may provide high sensitivity and accuracy in SNP genotyping under optimized condition [145], [146], [147], only 12 of total 114 studies included in our meta-analysis employed these genotyping methods. Thus the discrepancies should be concerned with great caution, and the sensitivity and specificity of those genotyping techniques should be further explored to seek out the optimal approaches that could minimize the genotyping errors.
To the best of our knowledge, this is the first comprehensive meta-analysis to date investigating the associations between the MTHFR A1298C polymorphism and H & HIP. Overall, our meta-analytical results indicated that the A1298C polymorphism was not associated with either H or HIP. In the stratified analyses according to ethnicity, source of controls, genotyping method, sample size and study quality, no evidence of any gene-association was obtained in almost all the subgroups. Although significant associations were found in Indians and Sri Lankans, “others” genotyping method and low quality subgroups for H association studies, these results should be interpreted with great caution because only one study was included in each of these subgroups. The overall lack of the correlation may be due to relatively small sample numbers of studies and participants. Detecting a very small effect may require much larger sample sizes. Another potential explanation may be that the effect of a single polymorphism might have a limited effect on H & HIP. This is consistent with the hypothesis that H & HIP are multi-factorial conditions that result from complicated interactions between environmental and genetic factors.
Several potential limitations of the present meta-analysis should be acknowledged. Firstly, significant heterogeneity was observed in overall and subgroup analyses, especially for the MTHFR C677T polymorphism. Although several potential sources of the heterogeneity were investigated including ethnicity, year of publication, source of controls, genotyping, sample size and study quality, none of them sufficiently explain the between-study heterogeneity. These results indicated that other unmeasured characteristics in various study populations and/or inherited limitations of the included studies might partially cause the detected heterogeneity. Secondly, the sample size of the MTHFR A1298C polymorphism involved is not large enough, especially for subgroup analysis. Thus they do not have adequate power to detect the possible association for this polymorphism and the observed significant associations in some subgroup analyses may be false. For the MTHFR C677T polymorphism, the results for East Asians and Black Africans should also be interpreted with caution due to the limited sample size. Thirdly, although funnel plot and Egger’s test showed that publication bias was not evident in the present study, selection bias might have occured because only studies in English and Chinese (expect one study in Persian) were included in our meta-analysis. Finally, gene-gene, gene-environment or even the different polymorphism loci of the MTHFR gene interactions were not estimated in our study because of the insufficient information. Despite these limitations, our meta-analysis has several clear advantages: (1) including a substantial number of cases and controls (15411 cases and 21970 controls) from different studies, thus guaranteeing the statistical power of our meta-analysis and obtaining more precise estimates; (2) the quality of the studies included in this meta-analysis was sufficient according to our well-designed selection criteria; (3) no evidence of publication bias was observed, and cumulative meta-analysis and sensitivity analysis indicated that our results were statistically robust.
In conclusion, our meta-analysis provides evidences that the MTHFR C677T polymorphism is associated with H & HIP, especially among East Asians and Caucasians. However, the MTHFR A1298C polymorphism is not associated with H & HIP. Considering the limitations aforementioned, further large-scale and population based studies, especially among Black Africans and Indians and Sri Lankans, are warranted to validate the associations observed in our meta-analysis and to explore the potential gene-gene and gene-environment interactions between the polymorphisms and H & HIP.
Supporting Information
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in homozygous codominant model (TT vs. CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in heterozygous codominant model (CT vs. CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in dominant model (TT+CT vs. CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in recessive model (TT vs. CT+CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in allele contrast model (T vs. C).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphism and H & HIP in homozygous codominant model (CC vs. AA).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphism and H & HIP in heterozygous codominant model (AC vs. AA).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphis and H & HIP in dominant model (CC+AC vs. AA).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphism and H & HIP in recessive model (CC vs AC+AA).
(TIF)
Forest plot of the association between MTHFR A1298 polymorphism and H & HIP in allele contrast model (C vs A).
(TIF)
The cumulative forest plot of OR with 95% CI for MTHFR C677T polymorphism and H &HIP in dominant model.
(TIF)
The cumulative forest plot of OR with 95% CI for MTHFR A1298C polymorphism and H & HIP in dominant model.
(TIF)
Baseline characteristics of qualified studies in this meta-analysis.
(DOC)
Distribution of genotype and allele frequencies of the MTHFR C677T polymorphism.
(DOC)
Distribution of genotype and allele frequencies of the MTHFR A1298C polymorphism.
(DOC)
PRISMA checklist.
(DOC)
Funding Statement
This study was supported by a grant (No. 81072243) from the National Natural Science Foundation of China (NSFC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lawes CM, Vander HS, Rodgers A (2008) Global burden of blood-pressure-related disease, 2001. Lancet 371: 1513–1518. [DOI] [PubMed] [Google Scholar]
- 2. Staessen JA, Wang J, Bianchi G, Birkenhager WH (2003) Essential hypertension. Lancet 361: 1629–1641. [DOI] [PubMed] [Google Scholar]
- 3. Tanira MO, Al BK (2005) Genetic variations related to hypertension: a review. J Hum Hypertens 19: 7–19. [DOI] [PubMed] [Google Scholar]
- 4. Padmanabhan S, Newton-Cheh C, Dominiczak AF (2012) Genetic basis of blood pressure and hypertension. Trends Genet 28: 397–408. [DOI] [PubMed] [Google Scholar]
- 5. Levy D, Ehret GB, Rice K, Verwoert GC, Launer LJ, et al. (2009) Genome-wide association study of blood pressure and hypertension. Nat Genet 41: 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, et al. (2009) Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet 41: 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 8. Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, et al. (1994) Human methylenetetrahydrofolate reductase: isolation of cDNA, mapping and mutation identification. Nat Genet 7: 195–200. [DOI] [PubMed] [Google Scholar]
- 9. Yang B, Liu Y, Li Y, Fan S, Zhi X, et al. (2013) Geographical Distribution of MTHFR C677T, A1298C and MTRR A66G Gene Polymorphisms in China: Findings from 15357 Adults of Han Nationality. PLoS One 8: e57917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, et al. (2003) Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas worldwide. J Med Genet 40: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weisberg I, Tran P, Christensen B, Sibani S, Rozen R (1998) A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab 64: 169–172. [DOI] [PubMed] [Google Scholar]
- 12. van der Put NM, Blom HJ (2000) Neural tube defects and a disturbed folate dependent homocysteine metabolism. Eur J Obstet Gynecol Reprod Biol 92: 57–61. [DOI] [PubMed] [Google Scholar]
- 13. Sen U, Tyagi SC (2010) Homocysteine and Hypertension in Diabetes: Does PPARgamma Have a Regulatory Role?. PPAR Res 2010: 806538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stehouwer CD, van Guldener C (2003) Does homocysteine cause hypertension?. Clin Chem Lab Med 41: 1408–1411. [DOI] [PubMed] [Google Scholar]
- 15. van Guldener C, Nanayakkara PW, Stehouwer CD (2003) Homocysteine and blood pressure. Curr Hypertens Rep 5: 26–31. [DOI] [PubMed] [Google Scholar]
- 16. Qian X, Lu Z, Tan M, Liu H, Lu D (2007) A meta-analysis of association between C677T polymorphism in the methylenetetrahydrofolate reductase gene and hypertension. Eur J Hum Genet 15: 1239–1245. [DOI] [PubMed] [Google Scholar]
- 17. Niu WQ, You YG, Qi Y (2012) Strong association of methylenetetrahydrofolate reductase gene C677T polymorphism with hypertension and hypertension-in-pregnancy in Chinese: a meta-analysis. J Hum Hypertens 26: 259–267. [DOI] [PubMed] [Google Scholar]
- 18. Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, et al. (2005) Systematic review and meta-analysis of the association between {beta} 2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol 162: 201–211. [DOI] [PubMed] [Google Scholar]
- 19. Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24: 1291–1306. [DOI] [PubMed] [Google Scholar]
- 20. Arj-Ong S, Thakkinstian A, McEvoy M, Attia J (2010) A systematic review and meta-analysis of tumor necrosis factor alpha-308 polymorphism and Kawasaki disease. Pediatr Int 52: 527–532. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 22. Zintzaras E, Ioannidis JP (2005) Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 28: 123–137. [DOI] [PubMed] [Google Scholar]
- 23. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 24. Thompson SG, Sharp SJ (1999) Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 18: 2693–2708. [DOI] [PubMed] [Google Scholar]
- 25. Trikalinos TA, Salanti G, Zintzaras E, Ioannidis JP (2008) Meta-analysis methods. Adv Genet 60: 311–334. [DOI] [PubMed] [Google Scholar]
- 26. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sohda S, Arinami T, Hamada H, Yamada N, Hamaguchi H, et al. (1997) Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med Genet 34: 525–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakata Y, Katsuya T, Takami S, Sato N, Fu Y, et al. (1998) Methylenetetrahydrofolate reductase gene polymorphism: relation to blood pressure and cerebrovascular disease. Am J Hypertens 11: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 29. Grandone E, Margaglione M, Colaizzo D, Cappucci G, Scianname N, et al. (1999) Prothrombotic genetic risk factors and the occurrence of gestational hypertension with or without proteinuria. Thromb Haemost 81: 349–352. [PubMed] [Google Scholar]
- 30. Chikosi AB, Moodley J, Pegoraro RJ, Lanning PA, Rom L (1999) 5,10 methylenetetrahydrofolate reductase polymorphism in black South African women with pre-eclampsia. Br J Obstet Gynaecol 106: 1219–1220. [DOI] [PubMed] [Google Scholar]
- 31. O’Shaughnessy KM, Fu B, Ferraro F, Lewis I, Downing S, et al. (1999) Factor V Leiden and thermolabile methylenetetrahydrofolate reductase gene variants in an East Anglian preeclampsia cohort. Hypertension 33: 1338–1341. [DOI] [PubMed] [Google Scholar]
- 32. Powers RW, Minich LA, Lykins DL, Ness RB, Crombleholme WR, et al. (1999) Methylenetetrahydrofolate reductase polymorphism, folate, and susceptibility to preeclampsia. J Soc Gynecol Investig 6: 74–79. [DOI] [PubMed] [Google Scholar]
- 33. Kobashi G, Yamada H, Asano T, Nagano S, Hata A, et al. (2000) Absence of association between a common mutation in the methylenetetrahydrofolate reductase gene and preeclampsia in Japanese women. Am J Med Genet 93: 122–125. [DOI] [PubMed] [Google Scholar]
- 34. Kaiser T, Brennecke SP, Moses EK (2000) Methylenetetrahydrofolate reductase polymorphisms are not a risk factor for pre-eclampsia/eclampsia in Australian women. Gynecol Obstet Invest 50: 100–102. [DOI] [PubMed] [Google Scholar]
- 35. Jr Rigo J, Nagy B, Fintor L, Tanyi J, Beke A, et al. (2000) Maternal and neonatal outcome of preeclamptic pregnancies: the potential roles of factor V Leiden mutation and 5,10 methylenetetrahydrofolate reductase. Hypertens Pregnancy 19: 163–172. [DOI] [PubMed] [Google Scholar]
- 36. Rajkovic A, Mahomed K, Rozen R, Malinow MR, King IB, et al. (2000) Methylenetetrahydrofolate reductase 677 C>T polymorphism, plasma folate, vitamin B(12) concentrations, and risk of preeclampsia among black African women from Zimbabwe. Mol Genet Metab 69: 33–39. [DOI] [PubMed] [Google Scholar]
- 37.Zhan S, Gao Y, Yin X, Huang Y, Hu Y, et al.. (2000) A case control study on the relationship between obnormal homocysteine metabolism and essential hypertension. Chin J Epidemiol. 21: 194–197 [In Chinese]. [PubMed]
- 38. Zusterzeel PL, Visser W, Blom HJ, Peters WH, Heil SG, et al. (2000) Methylenetetrahydrofolate reductase polymorphisms in preeclampsia and the HELLP syndrome. Hypertens Pregnancy 19: 299–307. [DOI] [PubMed] [Google Scholar]
- 39. Laivuori H, kaaja R, Ylikorkala O, Hiltunen T, Konrula K (2000) C677T polymorphism of the methylenetetrahydrofolate reductase gene and preeclampsia. Obstet Gynecol 96: 277–80. [DOI] [PubMed] [Google Scholar]
- 40. Murphy RP, Donoghue C, Nallen RJ, D’Mello M, Regan C, et al. (2000) Prospective evaluation of the risk conferred by factor V Leiden and thermolabile methylenetetrahydrofolate reductase polymorphisms in pregnancy. Arterioscler Thromb Vasc Biol 20: 266–270. [DOI] [PubMed] [Google Scholar]
- 41. Kupferminc MJ, Fait G, Many A, Gordon D, Eldor A, et al. (2000) Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol 96: 45–49. [DOI] [PubMed] [Google Scholar]
- 42.Li K, Zheng D, Xue Y, Sun Y, Chen L, et al.. (2000) The common C677T polymorphism in the methylenetetrahydrofolate reductase gene is associated with neural tube defects and preeclampsia. Chin J Med Genet 17: 76–78 [In Chinese]. [PubMed]
- 43. Kim YJ, Williamson RA, Murray JC, Andrews J, Pietscher JJ, et al. (2001) Genetic susceptibility to preeclampsia: roles of cytosineto-thymine substitution at nucleotide 677 of the gene for methylenetetrahydrofolate reductase, 68-base pair insertion at nucleotide 844 of the gene for cystathionine beta-synthase, and factor V Leiden mutation. Am J Obstet Gynecol 184: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 44. Livingston JC, Barton JR, Park V, Haddad B, Phillips O, et al. (2001) Maternal and fetal inherited thrombophilias are not related to the development of severe preeclampsia. Am J Obstet Gynecol 185: 153–157. [DOI] [PubMed] [Google Scholar]
- 45. Benes P, Kankova K, Muzik J, Groch L, Benedik J, et al. (2001) Methylenetetrahydrofolate reductase polymorphism, type II diabetes mellitus, coronary artery disease, and essential hypertension in the Czech population. Mol Genet Metab 73: 188–195. [DOI] [PubMed] [Google Scholar]
- 46. Lachmeijer AM, Arngrimsson R, Bastiaans EJ, Pals G, ten KL, et al. (2001) Mutations in the gene for methylenetetrahydrofolate reductase, homocysteine levels, and vitamin status in women with a history of preeclampsia. Am J Obstet Gynecol 184: 394–402. [DOI] [PubMed] [Google Scholar]
- 47. Raijmakers MT, Zusterzeel PL, Steegers EA, Peters WH (2001) Hyperhomocysteinaemia: a risk factor for preeclampsia?. Eur J Obstet Gynecol Reprod Biol 95: 226–228. [DOI] [PubMed] [Google Scholar]
- 48.Wei S, Zheng J, Shi D, Zou L, Bi L (2001) The relationship between MTHFR gene polymorphisms and homocysteine levels and pregnancy induced hypertension. Chin J Modern Med 11: 10–12 [In Chinese].
- 49. Alfirevic Z, Mousa HA, Martlew V, Briscoe L, Perez-Casal M, et al. (2001) Postnatal screening for thrombophilia in women with severe pregnancy complications. Obstet Gynecol 97: 753–759. [DOI] [PubMed] [Google Scholar]
- 50. Watanabe H, Hamada H, Yamakawa-Kobayashi K, Yoshikawa H, Arinami T (2001) Evidence for an association of the R485K polymorphism in the coagulation factor V gene with severe preeclampsia from screening 35 polymorphisms in 27 candidate genes. Thromb Haemost 86: 1594–1595. [PubMed] [Google Scholar]
- 51. D’ Elia AV, Driul L, Giacomello R, Colaone R, Fabbro D, et al. (2002) Frequency of factor V, prothrombin and methylenetetrahydrofolate reductase gene variants in preeclampsia. Gynecol Obstet Invest 53: 84–87. [DOI] [PubMed] [Google Scholar]
- 52. Morrison ER, Miedzybrodzka ZH, Campbell DM, Haites NE, Wilson BJ, et al. (2002) Prothrombotic genotypes are not associated with preeclampsia and gestational hypertension: results from a large population-based study and systematic review. Thromb Haemost 87: 779–785. [PubMed] [Google Scholar]
- 53. Prasmusinto D, Skrablin S, Hofstaetter C, Fimmers R, van der Ven K (2002) The methylenetetrahydrofolate reductase 677 C–>T polymorphism and preeclampsia in two populations. Obstet Gynecol 99: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Guo H, Li Y (2002) MTHFR gene C677T polymorphisms and variation of plasma homocysteine levels in Primary hypertension. Tianjin Med J 30: 579–582 [In Chinese].
- 55. Rodriguez-Esparragon F, Hernandez-Perera O, Rodriguez-Perez JC, Anabitarte A, Diaz-Cremades JM, et al. (2003) The effect of methylenetetrahydrofolate reductase C677T common variant on hypertensive risk is not solely explained by increased plasma homocysteine values. Clin Exp Hypertens 25: 209–220. [DOI] [PubMed] [Google Scholar]
- 56.Fu F, Liu H, Liao T, Xiong L, He X, et al.. (2003) Investigation of the relationship between polymorphism of methylenetetrahydrofolate reductase and pregnancy induced hypertension syndrome. Jiangxi Med J 38: 401–403 [In Chinese].
- 57.Zhang XY (2003) Homocysteine metabolism abnormality and pathogenesis of pregnancy induced hypertension. Master thesis, Tianjin Medical University [In Chinese].
- 58. Perez-Mutul J, Gonzalez-Herrera L, Sosa-Cabrera T, Martinez-Olivares R (2004) A mutation in the 5,10-methylenetetrahydrofolate reductase gene is not associated with preeclampsia in women of southeast Mexico. Arch Med Res 35: 231–234. [DOI] [PubMed] [Google Scholar]
- 59. Yilmaz H, Unlucerci Y, Gurdol F, Isbilen E, Isbir T (2004) Association of pre-eclampsia with hyperhomocysteinaemia and methylenetetrahydrofolate reductase gene C677T polymorphism in a Turkish population. Aust N Z J Obstet Gynaecol 44: 423–427. [DOI] [PubMed] [Google Scholar]
- 60.Wang HY, Li CM, Wang Z, Yang F (2004) Relationships between polymorphisms of angiotensin-converting enzyme and methylenetetrahydrofolate reductase genes and genetic susceptibility to pregnancy induced hypertension. Chin J Obstet Gynecol 39: 369–372 [In Chinese]. [PubMed]
- 61. Williams MA, Sanchez SE, Zhang C, Bazul V (2004) Methylenetetrahydrofolate reductase 677 C>T polymorphism and plasma folate in relation to pre-eclampsia risk among Peruvian women. J Matern Fetal Neonatal Med 15: 337–344. [DOI] [PubMed] [Google Scholar]
- 62. Pegoraro RJ, Chikosi A, Rom L, Roberts C, Moodley J (2004) Methylenetetrahydrofolate reductase gene polymorphisms in black South Africans and the association with preeclampsia. Acta Obstet Gynecol Scand 83: 449–454. [DOI] [PubMed] [Google Scholar]
- 63. De Maat MP, Jansen MW, Hille ET, Vos HL, Bloemenkamp KW, et al. (2004) Preeclampsia and its interaction with common variants in thrombophilia genes. J Thromb Haemost 2: 1588–1593. [DOI] [PubMed] [Google Scholar]
- 64. Heux S, Morin F, Lea RA, Ovcaric M, Tajouri L, et al. (2004) The methylentetrahydrofolate reductase gene variant (C677T) as a risk factor for essential hypertension in Caucasians. Hypertens Res 27: 663–667. [DOI] [PubMed] [Google Scholar]
- 65.Liu JW, Ye L, Liu J, Li XY (2004) Methylenetetrahydrofolate reductase gene polymorphism and susceptibility to peripheral arterial occlusive disease in hypertensive patients. Chin J Geriatr Brain Vessel Dis 6: 4–6 [In Chinese].
- 66. Tylicki L, Fodinger M, Puttinger H, Rutkowski P, Strozecki P, et al. (2005) Methylenetetrahydrofolate reductase gene polymorphisms in essential hypertension relation: with the development of hypertensive end-stage renal disease. Am J Hypertens 18: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 67. Driul L, Damante G, D’Elia A, Ianni A, Springolo F, et al. (2005) Genetic thrombophilias and uterine artery Doppler velocimetry and preeclampsia. Int J Gynaecol Obstet 88: 265–270. [DOI] [PubMed] [Google Scholar]
- 68. Davalos IP, Moran MC, Martinez-Abundis E, Gonzalez-Ortiz M, Flores-Martinez SE, et al. (2005) Methylenetetrahydrofolate reductase C677T polymorphism and Factor V Leiden variant in Mexican women with preeclampsia/eclampsia. Blood Cells Mol Dis 35: 66–69. [DOI] [PubMed] [Google Scholar]
- 69. Hernandez-Diaz S, Wu XF, Hayes C, Werler MM, Ashok TD, et al. (2005) Methylenetetrahydrofolate reductase polymorphisms and the risk of gestational hypertension. Epidemiology 16: 628–634. [DOI] [PubMed] [Google Scholar]
- 70. Also-Rallo E, Lopez-Quesada E, Urreizti R, Vilaseca MA, Lailla JM, et al. (2005) Polymorphisms of genes involved in homocysteine metabolism in preeclampsia and in uncomplicated pregnancies. Eur J Obstet Gynecol Reprod Biol 120: 45–52. [DOI] [PubMed] [Google Scholar]
- 71. Mello G, Parretti E, Marozio L, Pizzi C, Lojacono A, et al. (2005) Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension 46: 1270–1274. [DOI] [PubMed] [Google Scholar]
- 72. Ulukus M, Eroglu Z, Yeniel A, Toprak E, Kosava B, et al. (2006) Frequency of factor V leiden (G1691A), prothrombin (G20210A) and methylenetetrahydrofolate reductase (C677T) genes mutations in woman with adverse pregnancy outcomea. J Turkish-German Gynecol Assoc 7: 195–201. [Google Scholar]
- 73.Tian G, She DX, Qi QH (2005) Genetic research between gene polymorphism of homocysteine metabolism related enzymes and pre-eclampsia. Chin J Thromb Haemost 11: 197–199 [In Chinese].
- 74. Jaaskelainen E, Keski-Nisula L, Toivonen S, Romppanen EL, Helisalmi S, et al. (2006) MTHFR C677T polymorphism is not associated with placental abruption or preeclampsia in Finnish women. Hypertens Pregnancy 25: 73–80. [DOI] [PubMed] [Google Scholar]
- 75. Lwin H, Yokoyama T, Yoshiike N, Saito K, Yamamoto A, et al. (2006) Polymorphism of methylenetetrahydrofolate reductase gene (C677T MTHFR) is not a confounding factor of the relationship between serum uric acid level and the prevalence of hypertension in Japanese men. Circ J 70: 83–87. [DOI] [PubMed] [Google Scholar]
- 76. Dalmaz CA, Santos KG, Botton MR, Tedoldi CL, Roisenberg I (2006) Relationship between polymorphisms in thrombophilic genes and preeclampsia in a Brazilian population. Blood Cells Mol Dis 37: 107–110. [DOI] [PubMed] [Google Scholar]
- 77. Yalinkaya A, Erdemoglu M, Akdeniz N, Kale A, Kale E (2006) The relationship between thrombophilic mutations and preeclampsia: a prospective case-control study. Ann Saudi Med 26: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li XJ, Huang W (2009) The analysis of MTHFR gene polymorphism in patients with renal damage caused by hypertension and patients with renal parenchymal hypertension. J Capital Univ Med Sci 27: 497–500 [In Chinese].
- 79.Wang SM, Shen R, Shi XY, Jiang T, Liu XM, et al. (2006) The relationship between MTHFR gene polymorphism and gestational hypertension syndrome. Reprod Contracept 26: 378–379 [In Chinese].
- 80. Demir SC, Evruke C, Ozgunen T, Kadayifci O, Altintas U, et al. (2006) The relationship between pregnancy induced hypertension and congenital thrombophilia. Saudi Med J 27: 1161–1166. [PubMed] [Google Scholar]
- 81. Hui P, Nakayama T, Morita A, Sato N, Hishiki M, et al. (2007) Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens Res 30: 585–592. [DOI] [PubMed] [Google Scholar]
- 82. Nagy B, Hupuczi P, Papp Z (2007) High frequency of methylenetetrahydrofolate reductase 677TT genotype in Hungarian HELLP syndrome patients determined by quantitative real-time PCR. J Hum Hypertens 21: 154–158. [DOI] [PubMed] [Google Scholar]
- 83. Dusse LM, Carvalho M, Braganca WF, Paiva SG, Godoi LC, et al. (2007) Inherited thrombophilias and pre-eclampsia in Brazilian women. Eur J Obstet Gynecol Reprod Biol 134: 20–23. [DOI] [PubMed] [Google Scholar]
- 84. Stonek F, Hafner E, Philipp K, Hefler LA, Bentz EK, et al. (2007) Methylenetetrahydrofolate reductase C677T polymorphism and pregnancy complications. Obstet Gynecol 110: 363–368. [DOI] [PubMed] [Google Scholar]
- 85. Markan S, Sachdeva M, Sehrawat BS, Kumari S, Jain S, et al. (2007) MTHFR 677 CT/MTHFR 1298 CC genotypes are associated with increased risk of hypertension in Indians. Mol Cell Biochem 302: 125–131. [DOI] [PubMed] [Google Scholar]
- 86.Zhang ZH, Zhang RJ, Liu AM, Xu Q, Liu ZH (2007) Study on eNOS gene and MTHFR gene polymorphisms in preeclampsia. Chin J Birth Health Hered 15: 21–24 [In Chinese].
- 87.Xing XR, Hua Q (2007) Relationships between the polymorphism of methylenetetrahydrofolate reductase gene C677T and hypertension, cardiac structure and function. Med J Chin PLA 32: 741–744 [In Chinese].
- 88.Hu RL, Zhao SQ, Niu GM, Zhang CY, Hu RL, et al. (2007) The association between gene polymorphisms of N5,10 methylenetetrahydrofolate reductase (MTHFR) and Mongol nation patients with primary hypertension disease and hypertension complicating cerebrovascular disease. Stroke Nerv Dis 14: 13–15 [In Chinese].
- 89.Fan LP (2007) Association between methylenetetrahydrofolate reductase gene polymorphism and hypertension in pregnancy. Master thesis, Nanchang University [In Chinese].
- 90. Lin PT, Cheng CH, Wei JC, Huang YC (2008) Low plasma pyridoxal 5′phosphate concentration and MTHFR677 C-T genotypes are associated with increased riskof hypertension. Int J Vitam Nutr Res 78: 33–40. [DOI] [PubMed] [Google Scholar]
- 91. Canto P, Canto-Cetina T, Juarez-Velazquez R, Rosas-Vargas H, Rangel-Villalobos H, et al. (2008) Methylenetetrahydrofolate reductase C677T and glutathione S-transferase P1 A313G are associated with a reduced risk of preeclampsia in Maya-Mestizo women. Hypertens Res 31: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 92. Ilhan N, Kucuksu M, Kaman D, Ilhan N, Ozbay Y (2008) The 677 C/T MTHFR polymorphism is associated with essential hypertension, coronary artery disease, and higher homocysteine levels. Arch Med Res 39: 125–130. [DOI] [PubMed] [Google Scholar]
- 93. Muetze S, Leeners B, Ortlepp JR, Kuse S, Tag CG, et al. (2008) Maternal factor V Leiden mutation is associated with HELLP syndrome in Caucasian women. Acta Obstet Gynecol Scand 87: 635–642. [DOI] [PubMed] [Google Scholar]
- 94.Ding YS, Guan LX, Wang YH, Zhao L, Chen W (2008) Study on MTHFR and eNOS gene polymorphisms in pregnancy-induced hypertension in the Hans of Weifang area. Chin J Birth Health Hered 16: 12–14 [In Chinese].
- 95.Luo JW, Tang Y, Chen H, Wu XY, Wu YA, et al. (2008) Study on MTHFR C677T polymorphism in hypertensive subjects with blood stasis syndrome. J Beijing Uni Tradit Chin Med 31: 351–353 [In Chinese].
- 96.Wang SM, Wang LG, Liu XJ, Wu AH, Yu JC, et al. (2008) Investigation on the association between MTHFR gene C677T polymorphism and preeclampsia. Chin J Woman Child Health 23: 552–554 [In Chinese].
- 97.Zhang XY, Sun D, Sun J (2008) Relationship between homocysteine metabolism abnormality and preeclampsia. Chin J Perinat Med 11: 245–248 [In Chinese].
- 98. Ng X, Boyd L, Dufficy L, Naumovski N, Blades B, et al. (2009) Folate nutritional genetics and risk for hypertension in an elderly population sample. J Nutrigenet Nutrigenomics 2: 1–8. [DOI] [PubMed] [Google Scholar]
- 99. Fakhrzadeh H, Mirarefin M, Sharifi F, Ghotbi S, Rezaei HM, et al. (2009) Association of methylenetetrahydrofolate reductase gene polymorphism (C677T) with metabolism syndrome in an Iranian population: Tehran Homocysteine study. Iran J Diabetes Lipid Disord 9: 37–46. [Google Scholar]
- 100. Stiefel P, Miranda ML, Bellido LM, Luna J, Jimenez L, et al. (2009) Genotype of the CYBA promoter -930A/G, polymorphism C677T of the MTHFR and APOE genotype in patients with hypertensive disorders of pregnancy: an observational study. Med Clin (Barc) 133: 657–661. [DOI] [PubMed] [Google Scholar]
- 101. Kahn SR, Platt R, McNamara H, Rozen R, Chen MF, et al. (2009) Inherited thrombophilia and preeclampsia within a multicenter cohort: the Montreal Preeclampsia Study. Am J Obstet Gynecol 200: 151.e1–e9. [DOI] [PubMed] [Google Scholar]
- 102.Cai YM, Gong WX (2009) Linkage study on methylenetetrahydrofolate reductase single nucleotide polymorphisms and hypertension in the elderly with rheumatoid arthritis. Chin J Birth Health Heredity 17: 14–17 [In Chinese].
- 103.Shen XN, Huang YP, Tang SH, Zhang CL, Chen WS (2009) The relationship between the polymorphism of MTHFR gene and preeclampsia. J Pract Obstet Gynecol 25: 236–238 [In Chinese].
- 104. Rojas JC, Luna M, Rangel-Nava H, Nanos D, Collados MT (2010) Genetic thrombophilia and endothelial activation markers in patients with preeclampsia. Ginecol Obstet Mex 78: 401–409. [PubMed] [Google Scholar]
- 105.Yu W (2010) Relationship between polymorphism of methylenetetrahydrofolate reductase gene and pregnancy induced hypertension syndrome. J Henan Med Col Staff Workers 22: 648–651 [In Chinese].
- 106.Wang H, Su AJ, Wu GZ, Zhang Y, Chen YL, et al. (2010) Relationship of homocysteine, methylenetetrahydrofolate reductase (MTHFR) A1298C polymorphism and essential hypertension in Xinjiang Kazakhs. Chin J Misdiagn 10: 3787–3790 [In Chinese].
- 107.Zhong L, Feng ZF, Zhu JJ, Wang RX, Jiang JC (2010) Research on polymorphism of homocysteic acid (Hcy) metabolic enzymes genes among patients with pregnancy hypertension. Chin J Health Lab Tech 20: 2128–2130 [In Chinese].
- 108. Procopciuc LM, Caracostea G, Zaharie G, Puscas M, Iordache G, et al. (2010) Mutant maternal and fetal thrombophilic genotypes as a risk factor for preeclampsia. Gineco Ro 6: 74–81. [Google Scholar]
- 109. Klai S, Fekih-Mrissa N, El HS, Kaabechi N, Nsiri B, et al. (2011) Association of MTHFR A1298C polymorphism (but not of MTHFR C677T) with elevated homocysteine levels and placental vasculopathies. Blood Coagul Fibrinolysis 22: 374–378. [DOI] [PubMed] [Google Scholar]
- 110. Demirel Y, Dogan S, Uludag A, Silan C, Atik S, et al. (2011) Combined effect of Factor V Leiden, MTHFR, and angiotensin-converting enzyme (insertion/deletion) gene mutations in hypertensive adult individuals: a population-based study from Sivas and Canakkale, Turkey. Genet Test Mol Biomarkers 15: 785–791. [DOI] [PubMed] [Google Scholar]
- 111. Aggarwal S, Dimri N, Tandon I, Agarwal S (2011) Preeclampsia in North Indian women: the contribution of genetic polymorphisms. J Obstet Gynaecol Res 37: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 112. Dogan OO, Simsek Y, Celen S, Danisman N (2011) Frequency of hereditary thrombophilia, anticoagulant activity, and homocysteine levels in patients with hemolysis, elevated liver functions and low thrombocyte count (HELLP) syndrome. J Obstet Gynaecol Res 37: 527–533. [DOI] [PubMed] [Google Scholar]
- 113. Mislanova C, Martsenyuk O, Huppertz B, Obolenskaya M (2011) Placental markers of folate-related metabolism in preeclampsia. Reproduction 142: 467–476. [DOI] [PubMed] [Google Scholar]
- 114. Mendilcioglu I, Bilgen T, Arikan Y, Keser I, Simsek M, et al. (2011) The association between inherited thrombophilias and pregnancy-related hypertension recurrence. Arch Gynecol Obstet 284: 837–841. [DOI] [PubMed] [Google Scholar]
- 115.Jin Y, Zhao LY, Hou YT, Wang ZF (2011) Association between MTHFR gene polymorphism and primary hypertension complicated with CHD. Chin J Geriatr Heart Brain Vessel Dis 13: 1081–1083 [In Chinese].
- 116.Ma J, Yang P (2011) The correlation between the gene polymorphisms of MTHFR and eNOS and essential hypertension of the Han nationality in Yunnan province, Chin J Cardio Res 9: 909–912 [In Chinese].
- 117.Liu HY, Chen SP, Ma P, Xu QB (2011) The association between gene polymorphisms of N5,10-methylenetetrahydrofolate reductase and essential hypertension inpatients of Ningxia Hui nationality. Tianjin Med J 39: 1095–1098 [In Chinese].
- 118.Su NJ, Li B, Fen JH, Yu B (2011) Study on the relationship between N 5,10-methtlenetetrahydrofolate reductase gene and endothelial nitric oxide synthase gene polymorphisms and preeclampsia and eclampsia in the Han nationality women of Guangdong. Prog Obstet Gynecol 20: 199–202 [In Chinese].
- 119. Alghasham A, Settin AA, Ali A, Dowaidar M, Ismail H (2012) Association of MTHFR C677T and A1298C gene polymorphisms with hypertension. Int J Health Sci (Qassim) 6: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Fowdar JY, Lason MV, Szvetko AL, Lea RA, Griffiths LR (2012) Investigation of homocysteine-pathway-related variants in essential hypertension. Int J Hypertens 2012: 190923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lykke JA, Bare LA, Olsen J, Lagier R, Arellano AR, et al. (2012) Thrombophilias and adverse pregnancy outcomes: results from the Danish National Birth Cohort. J Thromb Haemost 10: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 122. Yin RX, Wu JZ, Liu WY, Wu DF, Cao XL, et al. (2012) Association of several lipid-related gene polymorphisms and blood pressure variation in the Bai Ku Yao population. Am J Hypertens 25: 927–936. [DOI] [PubMed] [Google Scholar]
- 123. Dissanayake VH, Sirisena ND, Weerasekera LY, Gammulla CG, Seneviratne HR, et al. (2012) Candidate gene study of genetic thrombophilic polymorphisms in pre-eclampsia and recurrent pregnancy loss in Sinhalese women. J Obstet Gynaecol Res 38: 1168–1176. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Y, Wang H, Zhang XY, Wang L, Wu GZ (2012) Relationship between homocysteine, methylenetetrahydrofolate reductase C677T polymorphism and essential hypertension in Kazak nationality in Xinjiang. J Clin Cardiol 28: 570–573 [In Chinese].
- 125. Ibrahim ZM, Metawie MAE, El-Baz AM, El-Bahie MA (2012) Methylenetetrahydrofolate C677T polymorphism and pre-eclamptic Egyptian women. Middle East Fertility Society Journal 17: 105–110. [Google Scholar]
- 126. Said JM, Higgins JR, Moses EK, Walker SP, Monagle PT, et al. (2012) Inherited thrombophilias and adverse pregnancy outcomes: a case-control study in an Australian population. Acta Obstet Gynecol Scand 91: 250–255. [DOI] [PubMed] [Google Scholar]
- 127. Coral-Vazquez RM, Romero AJ, Canizales-Quinteros S, Coronel A, Valencia VE, et al. (2013) Analysis of polymorphisms and haplotypes in genes associated with vascular tone, hypertension and oxidative stress in Mexican-Mestizo women with severe preeclampsia. Clin Biochem 46: 627–632. [DOI] [PubMed] [Google Scholar]
- 128. Fridman O, Porcile R, Morales AV, Gariglio LO, Potenzoni MA, et al. (2013) Association of methylenetetrahydrofolate reductase gene 677C>T polymorphism with hypertension in older women in a population of Buenos Aires City. Clin Exp Hypertens 35: 159–166. [DOI] [PubMed] [Google Scholar]
- 129. Kaur L, Puri M, Kaushik S, Sachdeva MP, Trivedi SS, et al. (2013) Genetic thromobophilia in pregnancy: a case-control study among North Indian women. J Thromb Thrombolysis 35: 250–256. [DOI] [PubMed] [Google Scholar]
- 130. Rahimi Z, Malek-Khosravi S, Rahimi Z, Jalilvand F, Parsian A (2013) MTHFR C677T and eNOS G894T variants in preeclamptic women: Contribution to lipid peroxidation and oxidative stress. Clin Biochem 46: 143–147. [DOI] [PubMed] [Google Scholar]
- 131.Cao ZY (2012) The relationship between MTHFR C677T polymorphism and H type hypertension with acute myocardial infarction in elderly population. Chin J Gerontol 32: 5118–5120 [In Chinese].
- 132.Yao R, Zhang HM, Zhang JL, Li DB, Fan YY (2013) Association of MTHFR C677T polymorphism with essential hypertension among Han nationality in Henan province. Chin J Gerontol 33: 1001–1003 [In Chinese].
- 133. Deveer R, Engin-Ustun Y, Akbaba E, Halisdemir B, Cakar E, et al. (2013) Association between pre-eclampsia and inherited thrombophilias. Fetal Pediatr Pathol 32: 213–217. [DOI] [PubMed] [Google Scholar]
- 134.Deng FM (2007) Polymorphism of eNOS and MTHFR genes, environmental factors and their interaction involved in the pathogenesis of essential hypertension in Kazakh ethnic of Xinjiang. Doctrol thesis, Sichuan University [In Chinese].
- 135.Saravani M, Salimi S, Yaghmaei M, Mokhtari M, Jafari M. (2011)Association between methylenetetrahydrofolate reductase gene C677T polymorphism with preeclampsis in South east of Iran. Zahedan J Res Med Sci (ZJRMS) 7: 39–43 [In Persian].
- 136.Yang F, Cai WJ, Hu N, Song X, Wang SL, et al. (2013) Association of the plasma homocysteine levels and methylenetetrahydrofolate reductase C677T polymorphism with essential hypertension in Han nationality in Xinjiang. J Xi’an Jiaotong Univ (Med Sci) [In Chinese]. Available online: http://www.cnki.net/kcms/detail/61.1399.R.20131029.1634.002.html. Accessed December 10,2013.
- 137.Bayramoglu A, Kucuk MU, Guler HI, Abaci O, kucukkaya Y, et al. (2013) Is there any genetic predisposition of MMP-9 gene C1562T and MTHFR gene C677T polymorphisms with essential hypertension?. Cytotechmology DOI: 10.1007/s10616–013–9665–0. [DOI] [PMC free article] [PubMed]
- 138.Alaniz FV, Marquez ML, Carrillo S, Duran MA, Hernandez EM, et al. (2013) Association of COMT G675A and MTHFR C677T polymorphisms with hypertensive disorders of pregnancy in Mexican mestizo population. Preg Hyper: An Int J Women’s Card Health. Available online: http://www.sciencedirect.com/science/article/pii/S2210778913002134. Accessed December 10,2013.
- 139. Kosmas IP, Tatsioni A, Ioannidis JP (2004) Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene with hypertension in pregnancy and pre-eclampsia: a meta-analysis. J Hypertens 22: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 140. Xia XP, Chang WW, Cao YX (2012) Meta-analysis of the methylenetetrahydrofolate reductase C677T polymorphism and susceptibility to pre-eclampsia. Hypertens Res 35: 1129–1134. [DOI] [PubMed] [Google Scholar]
- 141. Wang XM, Wu HY, Qiu XJ (2013) Methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism and risk of preeclampsia: an updated meta-analysis based on 51 studies. Arch Med Res 44: 159–168. [DOI] [PubMed] [Google Scholar]
- 142. Ruano-Ravina A, Perez-Rios M, Barros-Dios JM (2008) Population-based versus hospital-based controls: are they comparable?. Gac Sanit 22: 609–613. [DOI] [PubMed] [Google Scholar]
- 143. Zhao Y, Chen Z, Ma Y, Xia Q, Zhang F, et al. (2013) Lack of association between methionine synthase A2756G polymorphism and digestive system cancer risk: evidence from 3,9327 subjects. PLoS One 8: e61511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhong S, Xu J, Li W, Chen Z, Ma T, et al. (2013) Methionine synthase A2756G polymorphism and breast cancer risk: An up-to-date meta-analysis. Gene 527: 510–515. [DOI] [PubMed] [Google Scholar]
- 145. Heller T, Kirchheiner J, Armstrong VW, Luthe H, Tzvetkov M, et al. (2006) AmpliChip CYP450 GeneChip: a new gene chip that allows rapid and accurate CYP2D6 genotyping. Ther Drug Monit 28: 673–677. [DOI] [PubMed] [Google Scholar]
- 146. Li L, Li CJ, Zhang YJ, Zheng L, Jiang HX, et al. (2011) Simultaneous detection of CYP3A5 and MDR1 polymorphisms based on the SNaPshot assay. Clin Biochem 44: 418–422. [DOI] [PubMed] [Google Scholar]
- 147. Zhou C, Ni J, Zhao Y, Su B (2006) Rapid detection of epidermal growth factor receptor mutations in non-small cell lung cancer using real-time polymerase chain reaction with TaqMan-MGB probes. Cancer J 12: 33–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in homozygous codominant model (TT vs. CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in heterozygous codominant model (CT vs. CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in dominant model (TT+CT vs. CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in recessive model (TT vs. CT+CC).
(TIF)
Forest plot of the association between MTHFR C677T polymorphism and H & HIP in allele contrast model (T vs. C).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphism and H & HIP in homozygous codominant model (CC vs. AA).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphism and H & HIP in heterozygous codominant model (AC vs. AA).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphis and H & HIP in dominant model (CC+AC vs. AA).
(TIF)
Forest plot of the association between MTHFR A1298C polymorphism and H & HIP in recessive model (CC vs AC+AA).
(TIF)
Forest plot of the association between MTHFR A1298 polymorphism and H & HIP in allele contrast model (C vs A).
(TIF)
The cumulative forest plot of OR with 95% CI for MTHFR C677T polymorphism and H &HIP in dominant model.
(TIF)
The cumulative forest plot of OR with 95% CI for MTHFR A1298C polymorphism and H & HIP in dominant model.
(TIF)
Baseline characteristics of qualified studies in this meta-analysis.
(DOC)
Distribution of genotype and allele frequencies of the MTHFR C677T polymorphism.
(DOC)
Distribution of genotype and allele frequencies of the MTHFR A1298C polymorphism.
(DOC)
PRISMA checklist.
(DOC)