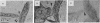Abstract
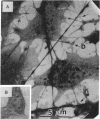
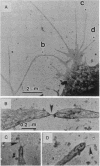
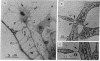
Adhesive contacts made by filopodia of developing neurons are important in neurite growth and in the formation of synaptic junctions. In the present work, filopodial interactions of cultured chicken retina neurons were studied by using video-enhanced contrast, differential interference contrast (VEC-DIC) microscopy and the high-voltage electron microscope (HVEM). Use of the HVEM to examine whole mounts of fixed cells showed that filopodia in older cultures developed an appearance that might be expected of nascent synapses, becoming enlarged at their endings and accumulating organelles resembling synaptic vesicles. VEC-DIC microscopy, used to observe the motility and adhesive properties of filopodia in living cells, showed there was a particularly high affinity between filopodia tips. Contacting filopodia typically repositioned themselves so they could attach at a tip-to-tip position, occasionally bending as much as 90 degrees to achieve this preferred orientation. Interacting filopodia frequently remained together as they pushed or pulled on each other, moved laterally together, or stretched tightly and underwent intense vibratory movements. Such linked motility occurred even when apparent gaps existed between the filopodia. Examination of these gaps with the HVEM revealed filamentous structures linking the apposed membranes. The filamentous links were 10-13 nm in diameter and 30-100 nm long. Although it has not yet been established that the filaments reflect the native configuration of the interconnecting materials, the structures seem likely to be associated with the strongly adhesive behavior of the filopodial tips. The possible significance of these structural and functional properties of filopodia tips to axon growth and synapse formation is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Allen N. S., Travis J. L. Video-enhanced contrast, differential interference contrast (AVEC-DIC) microscopy: a new method capable of analyzing microtubule-related motility in the reticulopodial network of Allogromia laticollaris. Cell Motil. 1981;1(3):291–302. doi: 10.1002/cm.970010303. [DOI] [PubMed] [Google Scholar]
- Allen R. D., Metuzals J., Tasaki I., Brady S. T., Gilbert S. P. Fast axonal transport in squid giant axon. Science. 1982 Dec 10;218(4577):1127–1129. doi: 10.1126/science.6183744. [DOI] [PubMed] [Google Scholar]
- Barbera A. J. Adhesive recognition between developing retinal cells and the optic tecta of the chick embryo. Dev Biol. 1975 Sep;46(1):167–191. doi: 10.1016/0012-1606(75)90095-0. [DOI] [PubMed] [Google Scholar]
- Bentley D., Keshishian H. Pathfinding by peripheral pioneer neurons in grasshoppers. Science. 1982 Dec 10;218(4577):1082–1088. doi: 10.1126/science.218.4577.1082. [DOI] [PubMed] [Google Scholar]
- Berry M., McConnell P., Sievers J. Dendritic growth and the control of neuronal form. Curr Top Dev Biol. 1980;15(Pt 1):67–101. doi: 10.1016/s0070-2153(08)60117-9. [DOI] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Electron microscopy of critical point dried whole cultured cells. J Microsc. 1975 Jul;104(2):107–120. doi: 10.1111/j.1365-2818.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Combes P. C., Privat A., Pessac B., Calothy G. Differentiation of chick embryo neuroretina cells in monolayer cultures. An ultrastructural study. I. Seven-day retina. Cell Tissue Res. 1977 Dec 13;185(2):159–173. doi: 10.1007/BF00220661. [DOI] [PubMed] [Google Scholar]
- Gottlieb D. I., Glaser L. Cellular recognition during neural development. Annu Rev Neurosci. 1980;3:303–318. doi: 10.1146/annurev.ne.03.030180.001511. [DOI] [PubMed] [Google Scholar]
- Ho R. K., Goodman C. S. Peripheral pathways are pioneered by an array of central and peripheral neurones in grasshopper embryos. Nature. 1982 Jun 3;297(5865):404–406. doi: 10.1038/297404a0. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Cell-substratum adhesion of neurite growth cones, and its role in neurite elongation. Exp Cell Res. 1979 Nov;124(1):127–138. doi: 10.1016/0014-4827(79)90263-5. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Immunocytochemical evidence for colocalization in neurite growth cones of actin and myosin and their relationship to cell--substratum adhesions. Dev Biol. 1981 Jul 15;85(1):113–122. doi: 10.1016/0012-1606(81)90240-2. [DOI] [PubMed] [Google Scholar]
- Letourneau P. C. Possible roles for cell-to-substratum adhesion in neuronal morphogenesis. Dev Biol. 1975 May;44(1):77–91. doi: 10.1016/0012-1606(75)90378-4. [DOI] [PubMed] [Google Scholar]
- Lilien J. E. Specific enhancement of cell aggregation in vitro. Dev Biol. 1968 Jun;17(6):657–678. doi: 10.1016/0012-1606(68)90012-2. [DOI] [PubMed] [Google Scholar]
- Marsh L., Letourneau P. C. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J Cell Biol. 1984 Dec;99(6):2041–2047. doi: 10.1083/jcb.99.6.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAI J. Dissociated dorsal root ganglia in tissue culture. Am J Anat. 1956 Jul;99(1):81–129. doi: 10.1002/aja.1000990105. [DOI] [PubMed] [Google Scholar]
- NAKAI J. Studies on the mechanism determining the course of nerve fibers in tissue culture. II. The mechanism of fasciculation. Z Zellforsch Mikrosk Anat. 1960;52:427–449. doi: 10.1007/BF00339758. [DOI] [PubMed] [Google Scholar]
- Pfenninger K. H., Maylié-Pfenninger M. F. Lectin labeling of sprouting neurons. I. Regional distribution of surface glycoconjugates. J Cell Biol. 1981 Jun;89(3):536–546. doi: 10.1083/jcb.89.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees R. P., Bunge M. B., Bunge R. P. Morphological changes in the neuritic growth cone and target neuron during synaptic junction development in culture. J Cell Biol. 1976 Feb;68(2):240–263. doi: 10.1083/jcb.68.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Klier F. G., Birdwell C. A role for adherons in neural retina cell adhesion. J Cell Biol. 1983 Apr;96(4):990–998. doi: 10.1083/jcb.96.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H. C., Ris H., Klein W. L. Ultrastructural networks in growth cones and neurites of cultured central nervous system neurons. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5779–5783. doi: 10.1073/pnas.80.18.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui H. T., Lankford K. L., Ris H., Klein W. L. Novel organization of microtubules in cultured central nervous system neurons: formation of hairpin loops at ends of maturing neurites. J Neurosci. 1984 Dec;4(12):3002–3013. doi: 10.1523/JNEUROSCI.04-12-03002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D., Schnapp B. J., Reese T. S., Sheetz M. P. Movement of organelles along filaments dissociated from the axoplasm of the squid giant axon. Cell. 1985 Feb;40(2):449–454. doi: 10.1016/0092-8674(85)90159-x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Daniels M. P., Nirenberg M. Synapse and acetylcholine receptor synthesis by neurons dissociated from retina. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2370–2374. doi: 10.1073/pnas.73.7.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Microtrabecular lattice of the cytoplasmic ground substance. Artifact or reality. J Cell Biol. 1979 Jul;82(1):114–139. doi: 10.1083/jcb.82.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]