Abstract
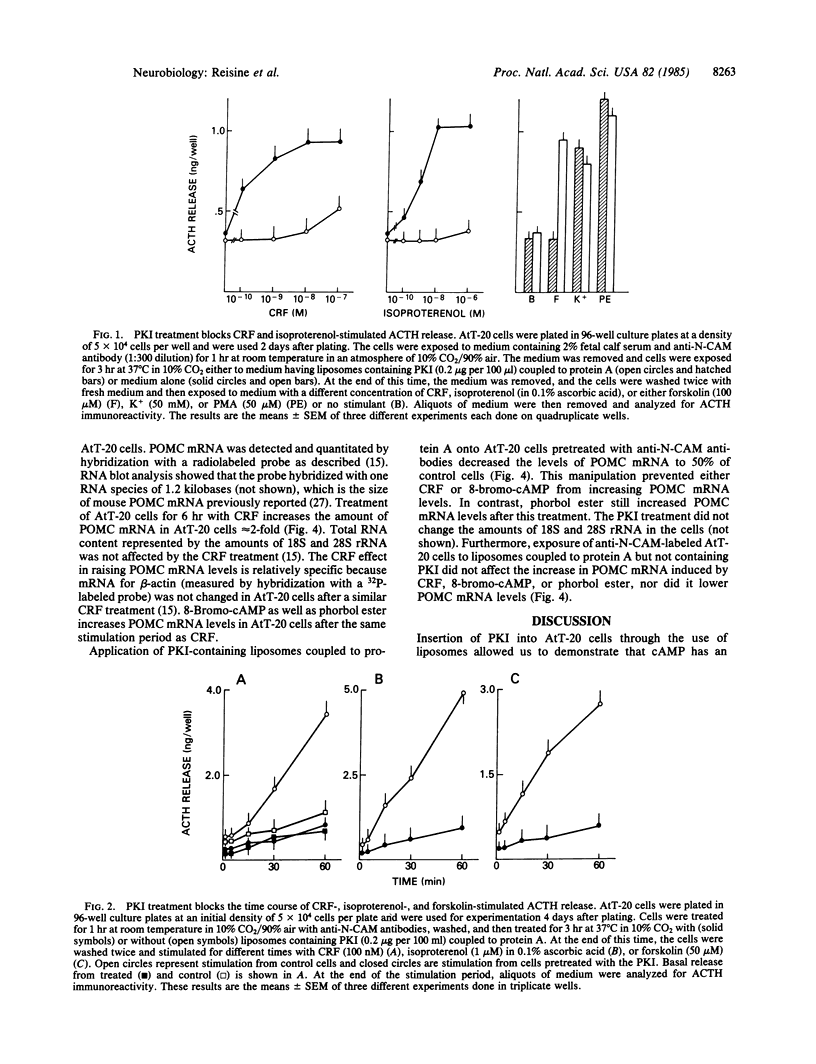
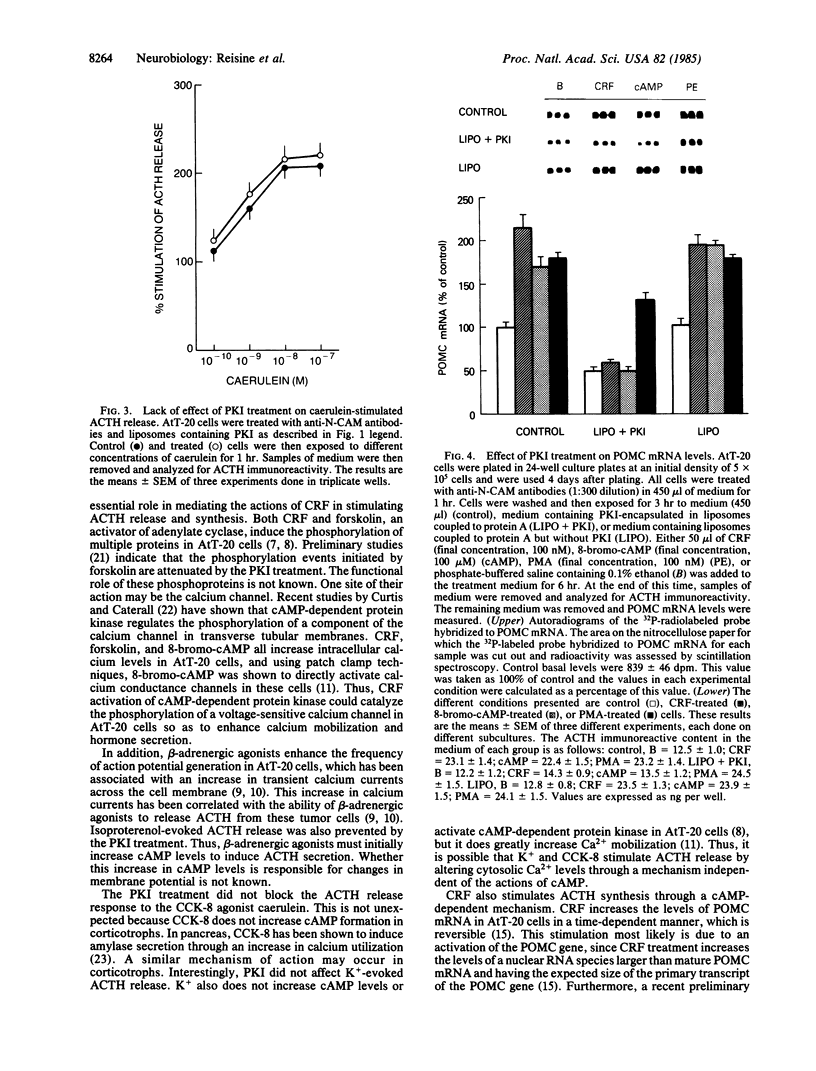
Corticotropin-releasing factor (CRF) is the most potent and effective natural stimulant of corticotropin (ACTH) secretion. In a tumor cell line of the mouse anterior pituitary (AtT-20/D16-16) consisting of a homogeneous population of corticotrophs, CRF is known to increase adenylate cyclase and cAMP-dependent protein kinase activities as well as to release ACTH. To determine whether activation of cAMP-dependent protein kinase is essential for CRF to evoke the secretion of ACTH, an inhibitor (PKI) of this kinase was inserted into AtT-20 cells. This was accomplished by first encapsulating PKI into liposomes and then covalently coupling them to protein A for binding to antibodies directed against an AtT-20 cell surface antigen, N-CAM (neural cell adhesion molecule). The binding of the liposomes to the anti-N-CAM antibodies led to the internalization of the PKI into the tumor cells. The PKI treatment greatly attenuated CRF-stimulated ACTH release as well as the secretory response to beta-adrenergic agonists. However, ACTH release in response to caerulein, an agonist of cholecystokinin 8 receptors, was not altered by the PKI treatment. CRF treatment also increased the levels of mRNA for proopiomelanocortin (POMC), the precursor for ACTH in AtT-20 cells. Application of liposomes containing PKI to AtT-20 cells blocked the ability of CRF and 8-bromo-cAMP, but not phorbol ester, to increase POMC mRNA levels. The results revealed an essential role for cAMP in mediating the effect of CRF on ACTH release and POMC gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Wong B. S., Sabol S. L., Busis N., Jackson M. B., Weight F. F. Action potentials and membrane ion channels in clonal anterior pituitary cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2086–2090. doi: 10.1073/pnas.80.7.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera G., Harwood J. P., Wilson J. X., Morell J., Brown J. H., Catt K. J. Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem. 1983 Jul 10;258(13):8039–8045. [PubMed] [Google Scholar]
- Albert K. A., Helmer-Matyjek E., Nairn A. C., Müller T. H., Haycock J. W., Greene L. A., Goldstein M., Greengard P. Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7713–7717. doi: 10.1073/pnas.81.24.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M., Bilezikjian L. M., Vale W. W., Rosenfeld M. G., Evans R. M. Independent effects of growth hormone releasing factor on growth hormone release and gene transcription. Nature. 1985 Mar 21;314(6008):279–281. doi: 10.1038/314279a0. [DOI] [PubMed] [Google Scholar]
- Birnberg N. C., Lissitzky J. C., Hinman M., Herbert E. Glucocorticoids regulate proopiomelanocortin gene expression in vivo at the levels of transcription and secretion. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6982–6986. doi: 10.1073/pnas.80.22.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn T. O., Sutton R. E., Rivier C. L., Vale W. W. Corticotropin-releasing factor regulates proopiomelanocortin messenger ribonucleic acid levels in vivo. Neuroendocrinology. 1984 Aug;39(2):170–175. doi: 10.1159/000123974. [DOI] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Comb M., Douglass J., Lissitzky J. C., Uhler M., Herbert E. Regulation of opioid gene expression. Peptides. 1983 Sep-Oct;4(5):651–656. doi: 10.1016/0196-9781(83)90013-x. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2528–2532. doi: 10.1073/pnas.82.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Litvin Y., PasMantier R., Fleischer N., Erlichman J. Hormonal activation of the cAMP-dependent protein kinases in AtT20 cells. Preferential activation of protein kinase I by corticotropin releasing factor, isoproterenol, and forskolin. J Biol Chem. 1984 Aug 25;259(16):10296–10302. [PubMed] [Google Scholar]
- Miyazaki K., Reisine T., Kebabian J. W. Adenosine 3',5'-monophosphate (cAMP)-dependent protein kinase activity in rodent pituitary tissue: possible role in cAMP-dependent hormone secretion. Endocrinology. 1984 Nov;115(5):1933–1945. doi: 10.1210/endo-115-5-1933. [DOI] [PubMed] [Google Scholar]
- Phillips M., Tashjian A. H., Jr Characterization of an early inhibitory effect of glucocorticoids on stimulated adrenocorticotropin and endorphin release from a clonal strain of mouse pituitary cells. Endocrinology. 1982 Mar;110(3):892–900. doi: 10.1210/endo-110-3-892. [DOI] [PubMed] [Google Scholar]
- Reisine T. Somatostatin desensitization: loss of the ability of somatostatin to inhibit cyclic AMP accumulation and adrenocorticotropin hormone release. J Pharmacol Exp Ther. 1984 Apr;229(1):14–20. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Tissue- and developmental stage-specific forms of a neural cell surface antigen linked to differences in glycosylation of a common polypeptide. EMBO J. 1982;1(10):1239–1244. doi: 10.1002/j.1460-2075.1982.tb00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Rivier C., Vale W. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6517–6521. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A. Correlation between electrical activity and ACTH/beta-endorphin secretion in mouse pituitary tumor cells. J Cell Biol. 1982 Nov;95(2 Pt 1):559–566. doi: 10.1083/jcb.95.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilders F. J., Berkenbosch F., Smelik P. G. Adrenergic mechanisms involved in the control of pituitary-adrenal activity in the rat: a beta-adrenergic stimulatory mechanism. Endocrinology. 1982 Jan;110(1):114–120. doi: 10.1210/endo-110-1-114. [DOI] [PubMed] [Google Scholar]
- Vale W., Spiess J., Rivier C., Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981 Sep 18;213(4514):1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Yates F. E., Russell S. M., Dallman M. F., Hodge G. A., McCann S. M., Dhariwal A. P. Potentiation by vasopressin of corticotropin release induced by corticotropin-releasing factor. Endocrinology. 1971 Jan;88(1):3–15. doi: 10.1210/endo-88-1-3. [DOI] [PubMed] [Google Scholar]
- Zatz M., Reisine T. D. Lithium induces corticotropin secretion and desensitization in cultured anterior pituitary cells. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1286–1290. doi: 10.1073/pnas.82.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]




