Abstract
Ocean acidification (OA) has important implications for the persistence of coral reef ecosystems, due to potentially negative effects on biomineralization. Many coral reefs are dynamic with respect to carbonate chemistry, and experience fluctuations in pCO2 that exceed OA projections for the near future. To understand the influence of dynamic pCO2 on an important reef calcifier, we tested the response of the crustose coralline alga Porolithon onkodes to oscillating pCO2. Individuals were exposed to ambient (400 µatm), high (660 µatm), or variable pCO2 (oscillating between 400/660 µatm) treatments for 14 days. To explore the potential for coralline acclimatization, we collected individuals from low and high pCO2 variability sites (upstream and downstream respectively) on a back reef characterized by unidirectional water flow in Moorea, French Polynesia. We quantified the effects of treatment on algal calcification by measuring the change in buoyant weight, and on algal metabolism by conducting sealed incubations to measure rates of photosynthesis and respiration. Net photosynthesis was higher in the ambient treatment than the variable treatment, regardless of habitat origin, and there was no effect on respiration or gross photosynthesis. Exposure to high pCO2 decreased P. onkodes calcification by >70%, regardless of the original habitat. In the variable treatment, corallines from the high variability habitat calcified 42% more than corallines from the low variability habitat. The significance of the original habitat for the coralline calcification response to variable, high pCO2 indicates that individuals existing in dynamic pCO2 habitats may be acclimatized to OA within the scope of in situ variability. These results highlight the importance of accounting for natural pCO2 variability in OA manipulations, and provide insight into the potential for plasticity in habitat and species-specific responses to changing ocean chemistry.
Introduction
Anthropogenic CO2 emissions have been rising steadily since the Industrial Revolution with the increase in fossil fuel consumption, agriculture, and land development [1], resulting in greater concentrations of CO2 entering the surface ocean [2]. Ocean acidification (OA) refers to the equilibration of atmospheric CO2 with the surface ocean, and the subsequent changes to seawater carbonate chemistry. These reactions are manifest as a decrease in mean ocean pH, carbonate ion concentration (CO3 2−) and CaCO3 saturation state (Ω), an increase in pCO2 and bicarbonate ion concentration (HCO3 −), and no change in total alkalinity (AT). Saturation state is an important factor that influences rates of biomineralization, where CaCO3 precipitation is favored when Ω is >1 [3]. Changes in Ω associated with ocean acidification have important implications for biogenic calcifiers and for ecosystems that are structurally dependent on carbonate platforms, such as coral reefs [3]. The changes in carbonate chemistry due to OA have raised widespread concern, particularly because they are occurring at a rate that is at least 2 orders of magnitude faster than during previous glacial periods [4]. The accelerated rate of OA may outpace the ability of marine organisms to adapt to the changing environment [5].
Near future projections for atmospheric CO2 gas concentrations, such as in the Representative Concentration Pathways (RCPs) [6], are based on open ocean conditions where carbonate chemistry varies predictably over time and mean ocean pH is reported to be 8.02–8.07 [7]. Seawater chemistry in near shore coastal ecosystems, such as upwelling regions [8], [9], coral reefs [10]–[12], estuarine systems, kelp forests [7] and natural CO2 vents [13]–[16], is both spatially and temporally dynamic within a habitat [17]. Swings in diel pH frequently exceed the forecasted projections of ocean acidification for the end of the century [7], [12], [18]–[20]. The recent development of autonomous sensors that record high frequency in situ pH data over time [21], [22] have shed light on the extent of pH variability within habitats and across ecosystems [7], [12].
In coral reef ecosystems, particularly shallow reef flats, carbonate chemistry varies over the course of a day [17], with tidal cycles [23], [24], and even between upstream and downstream habitats [25], [26]. The shallow reef benthos often is dominated by biogenic calcifiers that can create high pCO2 and low pH conditions by depleting total alkalinity (AT) [27] and releasing CO2 during the process of calcification [28], [29]. The interplay of reef metabolism and carbonate chemistry thus creates a feedback that influences the magnitude of pH variability that resident organisms experience [12], [30]–[32]. A feedback also exists in the balance of photosynthesis and respiration, with peaks and lows in photosynthesis during the day and night respectively [10], [33], [34]. Exposure of resident marine organisms to dynamic swings in diel pH may influence the plasticity of organismal responses to changing carbonate chemistry, yet variation in pH has been incorporated in only a few ocean acidification manipulations [17], [35]–[37].
To date, much of the ocean acidification literature has focused on the effects of decreased pH/increased pCO2 on biological responses of marine organisms in static laboratory mesocosm experiments by maintaining steady CO2 treatments over time [38]. Although the magnitude of responses varies, the overall effect of ocean acidification on marine calcifiers primarily has been negative [38]–[40]. These studies have been critical to advancing the understanding of CO2 effects on marine organisms, but may underestimate the importance of natural fluctuations in pCO2 on organismal responses. For instance, coral recruits exposed to ecologically relevant oscillations between ambient and high pCO2 experienced enhanced growth and survival when compared to recruits exposed to ambient or high pCO2 conditions alone [36]. By understanding the role that natural variability plays towards influencing species responses to changing carbonate chemistry we can gain insight into the potential for organisms to acclimatize and adapt to ocean acidification. This issue is of primary concern because the accelerated rate of ocean acidification is unprecedented over geological history [41] and may preclude the adaptive potential of some marine species. Little is known about species acclimatization potential to ocean acidification, and studies are beginning to explore the concept of acclimation in the context of ocean acidification [42]. Understanding how organisms already may be acclimatized in a rapidly changing environment can improve our understanding of how coral reef community structure may change in the near future. Marine ecosystems in which organisms are exposed frequently and consistently to environmental fluctuations, such as shallow coral reef flats [7], [10], [12], provide an in situ platform in which to explore the potential for acclimatization to ocean acidification.
Although much of reef net CaCO3 deposition occurs through biogenic calcification by scleractinian corals [43], another important group of reef builders are crustose coralline algae (CCA) [44], [45]. Crustose corallines serve many important ecological functions by contributing to reef primary productivity [46]–[48], facilitating settlement of coral larvae [49]–[51], and maintaining the structural integrity of the coral reef framework by cementing reef fragments together [52], [53]. Corallines precipitate the most soluble polymorph of CaCO3, high-magnesium (Mg) calcite [54], and have been shown to be sensitive to ocean acidification. Exposure to high pCO2 conditions reduces coralline calcification and growth [40], [55]–[60], and inhibits the function of the chemical cue produced by corallines and/or their microbial assemblages that facilitates coral settlement [61]–[64]. Despite their important role in coral reef ecosystems relatively few studies have addressed the effects of OA on tropical coralline algae. Furthermore, studies have yet to explore the effects of variable pCO2 on crustose corallines, or their potential for acclimitization. Porolithon onkodes (synonymous with Hydrolithon onkodes [65]) is an important reef-building coralline alga that is abundant throughout the Indo-Pacific in shallow reef systems and has been shown to promote settlement in coral larvae [49], [51]. By exploring the response of P. onkodes from a naturally dynamic shallow reef flat to oscillating pH, we may better understand the adaptability of an important reef-building species to ocean acidification.
The aims of this study were, 1) to determine the effect of oscillating pCO2 on crustose coralline calcification, photosynthesis, and respiration, and 2) to determine the role of prior exposure to in situ pCO2 variability in influencing coralline responses to ocean acidification. To accomplish the first goal, we conducted tightly-controlled laboratory mesocosm experiments in Moorea, French Polynesia, in which we exposed P. onkodes to ambient, high, and oscillating pCO2 at levels ranging from ∼400 µatm to 660 µatm, the natural range in pCO2 on the back reef of Moorea. We hypothesized that exposure to high pCO2 alone would decrease P. onkodes calcification, as shown in previous studies, and that exposure to pCO2 oscillating within an ecologically relevant range would modulate the negative effect of high pCO2 on calcification. Further, we hypothesized that exposure to high pCO2 would enhance net photosynthesis in all corallines due to the potential promoting effects of increased dissolved CO2 on carbon fixation. To explore the potential for acclimatization to ocean acidification conditions, we used P. onkodes individuals collected from either low pH variability sites (reef crest, upstream) or high pH variability sites (200 m shoreward of the reef crest, downstream) within the same reef habitat. We hypothesized that individuals from downstream sites would calcify more in the variable pCO2 treatment than those from upstream sites because they were acclimatized in situ to variable pCO2. To our knowledge, this study is the first to quantify the response of coralline algae to variable pCO2 and to explore the potential for acclimatization of an important reef builder to high pCO2.
Materials and Methods
Site Description
This study was conducted during May and June 2010 in Moorea, French Polynesia as part of the Moorea Coral Reef Long-Term Ecological Research site (MCR LTER) under a Protocole D'accueil (Scientific Research Permit) to RCC from the Delegation a la Recherche de la Polynesie Français (unnumbered). Moorea is located 20 km west of Tahiti and is surrounded by a barrier reef that encloses a lagoon. Water circulation is driven by oceanic and wind-driven swells that break on the reef crest and force mostly unidirectional flow of water across the back reef and into the lagoon where it then exits through one of the reef passes [66]. In the back reef on the north shore of Moorea, water residence time is dependent on offshore wave height. The transit time for water flowing over the reef crest to habitats 200 m downstream is approximately 30–45 minutes [66] and is dependent on flow speed. The carbonate chemistry of water flowing across the back reef is altered by reef metabolism, with photosynthesis and calcification decreasing pCO2 over the reef during the day, and respiration and dissolution increasing pCO2 over the reef during the night [29]. The farther a parcel of water travels from the source of oceanic inflow over the reef crest, the longer the residence time and exposure to reef metabolism and the greater potential for change in the carbonate chemistry. As a result, reef organisms inhabiting upstream locations (e.g., fore reef and reef crest) are exposed to relatively constant pCO2 of incoming oceanic seawater, while the pCO2 experienced by organisms living in downstream habitats varies on a diel basis and with the environmental conditions of light and water flow that modulate reef metabolic processes and determine water residence time over the reef [25], [26], [31], [67], [68].
Sample Collection
Samples of the common crustose coralline alga, Porolithon onkodes, were collected from upstream habitats on the shallow fore reef at a depth of 2–3 m on the north shore reef between Cook's and Opunohu Bays. Samples of the same species also were collected from the back reef approximately 200 m downstream of the reef crest at a similar depth. These two locations experience approximately the same flow [66], nutrient, and sedimentation [69] conditions. Individuals collected from the upstream location represent organisms exposed to a history of low pH variability, and individuals from the downstream location a history of higher pH variability [26], [31]. Crustose coralline samples were collected using a diamond-grit hole saw (40 mm outer diameter, 36 mm inner diameter) attached to a pneumatic drill. Coralline algal thalli were separated from the underlying dead coral skeleton to yield approximately 0.5 cm thick cores of P. onkodes. Coralline algal samples were returned to the lab and carefully cleaned of epibionts. The bottoms of the algal disks were covered with marine epoxy (Z-spar) to prevent exposure of the underlying carbonate to potential dissolution in treatment conditions. Samples were kept in a water table with a high exchange of fresh seawater, under ambient light, for three days to allow for healing. Only healthy appearing samples without obvious tissue damage were used in the experiment.
After samples were collected, the taxonomic identification of Porolithon onkodes was assigned based on Adey et al. (1982) [70] and Payri et al. (2000) [71]. We used gross morphological, reproductive, and trichocyte characteristics to identify each sample using simple laboratory procedures. Specimens were dried at 60°C for 48 hours, fragmented with diagonal cutters, and internal characteristics of the fragmented edges, including conceptacle size and shape, trichocyte arrangement, cellular organization, and margin morphology were examined under a dissecting scope. Identification of samples was verified by comparison to samples (N = 3) that had been previously confirmed by Dr. Robert Steneck (personal comm.).
CO2 Enrichment Treatments
The CO2 enrichment treatments were established in a mesocosm facility at the Richard B. Gump South Pacific Research Station consisting of 6, 150-L tanks, each with independent lighting (250 W metal halide fixtures) and temperature control. Mesocosm tanks were operated in an open configuration with each tank receiving fresh, unfiltered seawater pumped from Cook's Bay at a rate of 140 ml min−1 tank−1 resulting in approximately two complete turnovers of water each day. Each tank was fitted with a clear plexiglass lid to reduce atmospheric gas exchange and stabilize treatment pCO2 levels. Light levels (PAR) were adjusted to maintain the light level in each tank at ∼600 µmol quanta m−2 s−1 (on a 12∶12 h light:dark cycle) that resulted in a daily integrated 12-h PAR similar to that measured at the collection sites (∼26 mol quanta m−2 d−1). The temperature control for each tank was set to a target value of 28.5°C which represents the ambient temperature on the reef during the austral winter (May/June).
To simulate the effect of atmospheric CO2 on ocean surface waters, treatment tanks were bubbled with either ambient air or CO2-enriched air to establish targeted pCO2 levels. CO2-enriched air was created using a solenoid-controlled gas regulation system (Model A352, Qubit Systems) that mixed pure CO2 with ambient air to achieve the desired partial pressure (sensu Edmunds et al. (2011)) [72]. The targeted pCO2 value for the high pCO2 treatment was 650 µatm, which represents the pCO2 at downstream sites just before dawn and the projected atmospheric pCO2 in 50 years based on carbon emission scenarios [6]. The three pCO2 treatment levels used in the experiment were ambient (∼400 µatm), high pCO2 (∼660 µatm), and oscillating pCO2 (samples were exposed to a treatment that alternated between 400/660 µatm).
The physical parameters of each tank were monitored throughout the experiment including light, salinity, temperature, and carbonate chemistry. Temperature was measured daily in the morning, afternoon, and evening using a digital thermometer with an accuracy of 0.05°C (Traceable Digital Thermometer, Thermo Fisher Scientific). Light was measured daily beneath the surface of the water and at the sample locations (∼15 cm above the bottom of the tank) in each tank using a 2π LiCOR quantum sensor connected to a LiCOR 1400 meter. Salinity was measured daily on water samples from each tank using a desktop YSI 3100 conductivity meter, and pH (total scale) was measured daily using a spectrophotometric technique (described below).
Seawater Chemistry
Water samples were collected daily at 0900 hours in 20 mL glass scintillation vials for spectrophotometric analysis of pH (total scale). pH measurements were made immediately following collection using a spectrophotometer (Shimadzu UV-2450) and the indicator dye m-cresol purple according to standard operating procedure (SOP) 6 [73]. The temperature of the sample was controlled with a constant-temperature cell holder (Shimadzu) that maintained cell temperature at 25°C. The accuracy of the spectrophotometric technique was assessed by conducting pH measurements on certified reference material with a known pH (Tris Buffer in synthetic seawater, A. Dickson, Scripps Institution of Oceanography), and the mean accuracy of this technique was ±0.09% (N = 16). Water samples were collected every two days at 0900 hours for determinations of total alkalinity (AT) by siphoning treatment water into 250-ml borosilicate sample bottles [73]. Water samples for AT were analyzed within 6 hours of collection after the water sample had equilibrated with laboratory room temperature. Total alkalinity (AT) determinations were made using a Mettler T50 automated titrator to conduct modified, open cell potentiometric titrations [74] according to standard operating procedure (SOP) 3b [73]. Titrations were conducted on 100-ml samples at a temperature of 24°C (samples were equilibrated with room temperature) with a DG115-SC pH probe (Mettler Toledo) calibrated using a three-point calibration with NBS buffers (pH 4.00, 7.00, 10.00). The mean accuracy of the AT determinations was ±0.5% (N = 6) compared to Certified Reference Materials (Reference Material for Oceanic CO2 Measurements, Batch 99, A. Dickson, Scripps Institution of Oceanography).
Water samples were collected at each site over five days in order to characterize the carbonate chemistry at the upstream and downstream sites. Integrated water samples were collected during the day (from 0800 to 1800) and during the night (from 1900 to 0400) by continuously sampling water (4 mL min−1) with a submersible autonomous water sampler. Upstream and downstream water samples were collected simultaneously. Water temperature was recorded at the time of sample collection and samples were returned to the lab and analyzed as described above within 24 h of collection. The results from all samples were processed in Microsoft Excel [74], and AT, pH, salinity and temperature were used to determine the remaining carbonate parameters using CO2SYS [75] with the dissociation constants of Mehrbach et al. (1973) [76] refit by Dickson & Millero (1987) [77].
The environmental conditions in the established treatments were stable over the 14-d experiment. Temperature, salinity, light, and total alkalinity were similar across all treatments and also similar to ambient levels in the field (Table 1). The pCO2 treatments created by bubbling ambient and CO2-enriched air resulted in distinct, non-overlapping pH and pCO2 levels (Figure 1A) and saturation states of calcite (Ωcalc) (Table 1). The pCO2 levels in the elevated treatments (680±15, mean ± SE) were 65% higher than in the ambient treatments (411±14) (Figure 1A) and Ωcalc was reduced by 28% from 5.67±0.04 to 4.09±0.06 as a result of a reduction in the concentration of carbonate. There was no temporal variation in pCO2 within the treatment tanks over the 2, 24-h periods that were sampled (Figure 1B).
Table 1. Carbonate chemistry measurements.
| Treatment | Temp (°C) | Salinity (psu) | Light† | pH‡ | TA (µmol/kg) | pCO2 (µatm) | ΩCa | ΩAr |
| Ambient | 28.7±0.02 | 35.5±0.02 | 607±15 | 8.041±0.007 | 2311±8 | 417±16 | 5.58±0.13 | 3.72±0.09 |
| Ambient | 28.5±0.02 | 35.5±0.03 | 631±15 | 8.035±0.005 | 2312±7 | 404±21 | 5.66±0.15 | 3.78±0.10 |
| Elevated | 28.5±0.02 | 35.5±0.04 | 651±17 | 7.863±0.014 | 2315±8 | 667±30 | 4.12±0.15 | 2.75±0.10 |
| Elevated | 28.7±0.02 | 35.5±0.03 | 654±15 | 7.863±0.013 | 2314±3 | 663±42 | 4.17±0.19 | 2.78±0.12 |
| Var/Amb | 28.7±0.02 | 35.5±0.02 | 668±16 | 8.027±0.005 | 2329±6 | 432±6 | 5.52±0.06 | 3.68±0.04 |
| Var/Elev | 28.4±0.02 | 35.5±0.02 | 634±12 | 7.865±0.014 | 2322±3 | 666±67 | 4.17±0.32 | 2.78±0.21 |
Values (means ± SE) for physical variables in each tank over the course of the 14-d experiment. N = 14 sampling days for temperature, salinity, light, and pH, and N = 5 sampling days for TA, pCO2, ΩCa, and ΩAr.
Light = photosynthetically active radiation (PAR, µmol quanta),
pH = total scale pH units, ΩCa = the saturation state of calcite, ΩAr = the saturation state of aragonite
Figure 1. Mean daily and hourly pCO2 of treatment conditions.
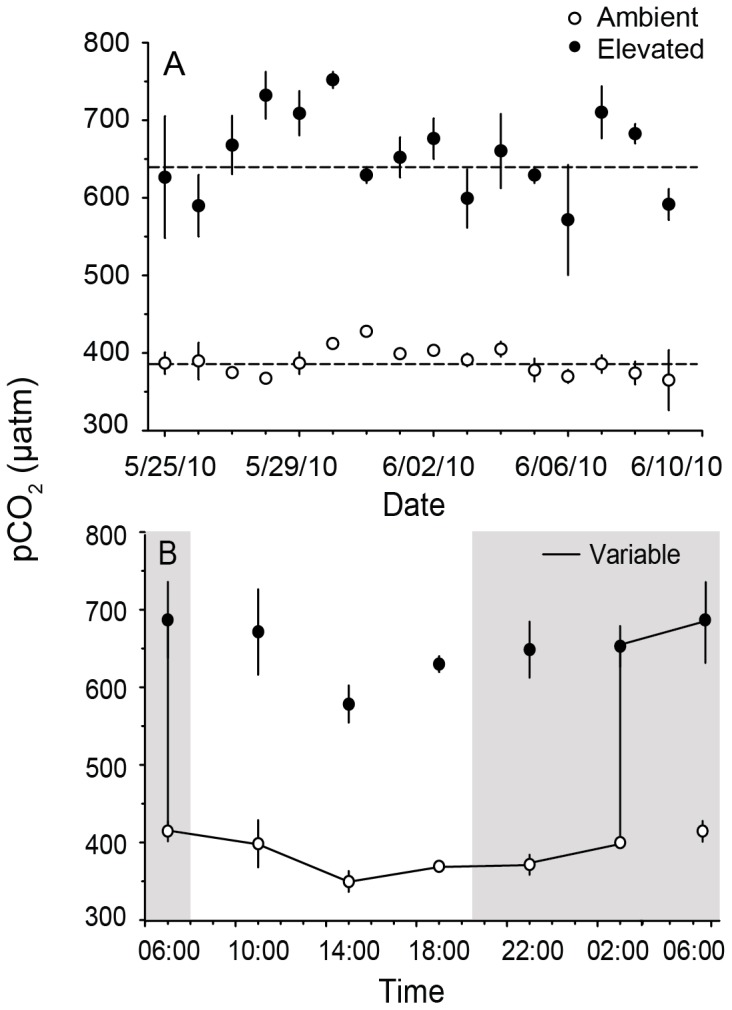
Mean (± SE) daily pCO2 of ambient (open circles) treatment tanks (N = 3), elevated (closed circles) treatment tanks (N = 3), and the overall treatment means for the duration of the experiment (dashed line) (A). Mean pCO2 of ambient and elevated treatments measured over one diel cycle (B). The solid line shows the transition of the variable treatment samples from ambient pCO2 into the elevated pCO2 treatment 0000 and then back into the ambient treatment at 0600. pCO2 was calculated using CO2SYS and measurements of total alkalinity and pH (total scale).
Experimental Incubations and Response Variables
Algal samples were allocated randomly to one of three pCO2 treatments: 1) ambient pCO2 (∼400 µatm), 2) elevated pCO2 (∼660 µatm), and 3) a variable pCO2 treatment that cycled between 400 and 660 µatm (Figure 1). The elevated pCO2 was chosen based on pre-dawn measurements of pCO2 at the downstream collection site (Table 2). Prior to placing coralline algal samples into the treatments, each sample was buoyant weighed with the epoxy base (to the nearest mg) [78]. Six samples from each habitat origin (upstream, downstream) were placed on elevated PVC racks in each tank (15 cm above the bottom). The ambient controls and pCO2 treatments were fully replicated twice (N = 2 tanks). The variable pCO2 treatment was accomplished by placing samples in an ambient pCO2 tank for 18 h (from 0600 to 0000) and then transferring the samples to a dedicated elevated pCO2 tank (i.e., samples in different treatments were not mingled) for 6 h (from 0000 to 0600) each day to simulate the timing of elevated pCO2 in downstream habitats in the field (Figure 1B). At each time of transfer, samples from the ambient and elevated treatments also were removed from their tanks and returned to the same tank to serve as a sample handling control.
Table 2. Daily integrated pCO2 for upstream and downstream backreef habitats.
| Habitat | Diel Period | pCO2 (µatm) |
| Upstream | Day | 424.8±9.4 |
| Night | 421.0±9.9 | |
| Downstream | Day | 385.8±5.9 |
| Night | 700.4±55.5 |
Values (means ± SE) of in situ pCO2 from daily integrated water samples at upstream and downstream sites of collection (N = 5 days). Upstream sites were located at the reef crest and downstream sites were located 200 m shoreward of the reef crest. Continuous water samples were collected simultaneously and autonomously from each site during the day (from 0800 to 1800) and during the night (from 1900 to 0400).
After 14 days in treatment conditions, all coralline algal samples were buoyant weighed again and 3 samples from each origin and pCO2 treatment replicate were chosen randomly for measurements of photosynthesis and respiration. Photosynthesis measurements were estimated during the day from changes in dissolved oxygen during 30-min incubations of each sample in a 102-ml acrylic chamber fitted with a PreSens dipping oxygen optode connected to a Fibox 3 transmitter (Precision Sensing GmbH, Germany). The oxygen probe was calibrated at the start of incubations using a 2-point calibration with temperature correction in water-saturated air (100%) and seawater with no oxygen (supersaturated sodium dithionite, Na2S2O4). Oxygen concentrations automatically were corrected for temperature using a PreSens probe that simultaneously measured temperature in the incubation chamber (Precision Sensing GmbH, Germany). A water jacket surrounding the acrylic chamber was connected to a circulating water bath (Lauda) that provided temperature control (28°C). A stir bar at the base of the chamber created vigorous water motion. Light was provided by two fiber optic halogen lights that created PAR levels in the chamber similar to those in the mesocosms (∼600 µmol quanta m−2 s−1). Following photosynthesis measurements, samples were returned to their respective treatments and respiration estimates were made on the same samples at night between 1900 and 2300. During respiration measurements the chamber was kept dark using an opaque shroud. For each incubation (photosynthesis and respiration), the chamber was filled completely with seawater from the appropriate treatment and new seawater was used for each incubation. Seawater from the ambient treatment was used for both ambient and variable treatment incubations (because samples in the variable treatment were not placed in the elevated treatment until 0000), and seawater from the elevated treatment was used for the elevated incubations. Oxygen concentrations were recorded each minute and photosynthesis and respiration rates were calculated from the linear slopes over time. Metabolic rates were normalized to surface area of the coralline algal samples, estimated by image analysis (ImageJ, NIH) of digital photographs of each sample. Net calcification rates for each sample were calculated by converting the buoyant weight gained during the experiment to the dry weight gained using the density of calcite (2.71 g cm−3) [78] and normalizing the rates to surface area.
Statistical Analyses
Normalized rates of net calcification, gross photosynthesis, respiration, and net photosynthesis were analyzed using a mixed model ANOVA, with habitat origin (upstream/downstream) and pCO2 treatment (ambient, elevated, variable pCO2) as fixed factors and tank as a random nested factor. If the tank factor was not significant in the initial analysis (P>0.25), it was eliminated from the subsequent analysis and a two-way fixed factor ANOVA was used [79]. Additionally, an explicit a priori contrast tested specifically for the effect of the variable pCO2 treatment on coralline algae from upstream and downstream habitats. The assumptions of normality and homoscedasticity were examined prior to all analyses through the examination of residuals and Cochran's test, respectively.
Results
Response Variables
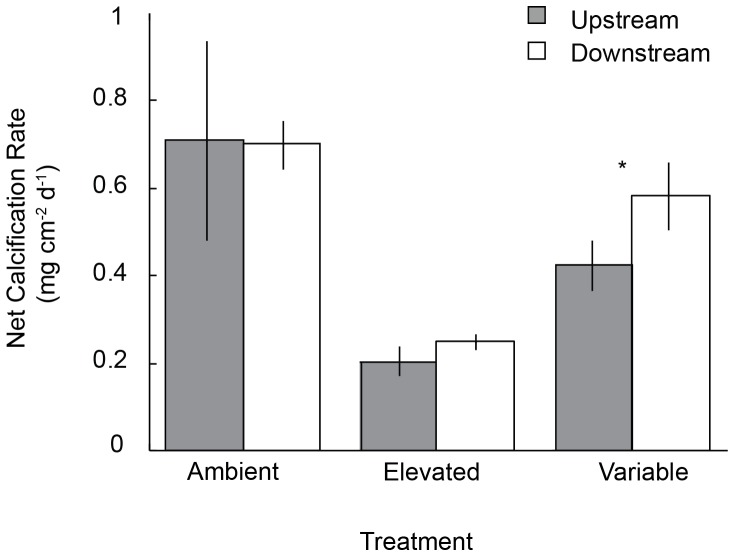
The nested tank effect in the mesocosm experiment was not significant (P>0.25) in the initial analysis of net calcification so it was removed from the subsequent analysis [79]. Net calcification of P. onkodes was significantly affected by an interaction between the pCO2 treatment and habitat origin (F2,42 = 4.02, P = 0.025), with significant effects of both pCO2 treatment (F2,42 = 18.42, P<0.0001), and habitat origin (F1,42 = 5.18, P = 0.028) (Figure 2, Table S1). As demonstrated in previous studies, net calcification was reduced by over 70% in the elevated pCO2 treatment compared to ambient conditions and this trend was exhibited by individuals from both upstream and downstream habitats. The overall response of P. onkodes net calcification to the variable pCO2 treatment was an intermediate decrease that might be explained most parsimoniously by the proportion of time spent in the ambient (75%) and elevated (25%) pCO2 treatments, although the overall decrease in calcification was closer to 35%. Notably, the a priori comparison between P. onkodes collected from upstream and downstream habitats revealed significantly different calcification responses to the variable pCO2 treatment (F1,14 = 22.43, P<0.001). Following exposure to the variable pCO2 treatment, corallines from the downstream habitats experienced a 17% decrease in net calcification as compared to the ambient controls, while corallines from the upstream habitats experienced a 41% decrease in calcification compared to ambient controls (Figure 2).
Figure 2. Calcification response of Porolithon onkodes to ocean acidification treatments.
Mean (±SE) rates of net calcification for P. onkodes collected from upstream and downstream habitats after 14 days of exposure to three pCO2 treatments. The asterisk denotes a significant difference of an a priori comparison between upstream and downstream origins under the variable pCO2 treatment.
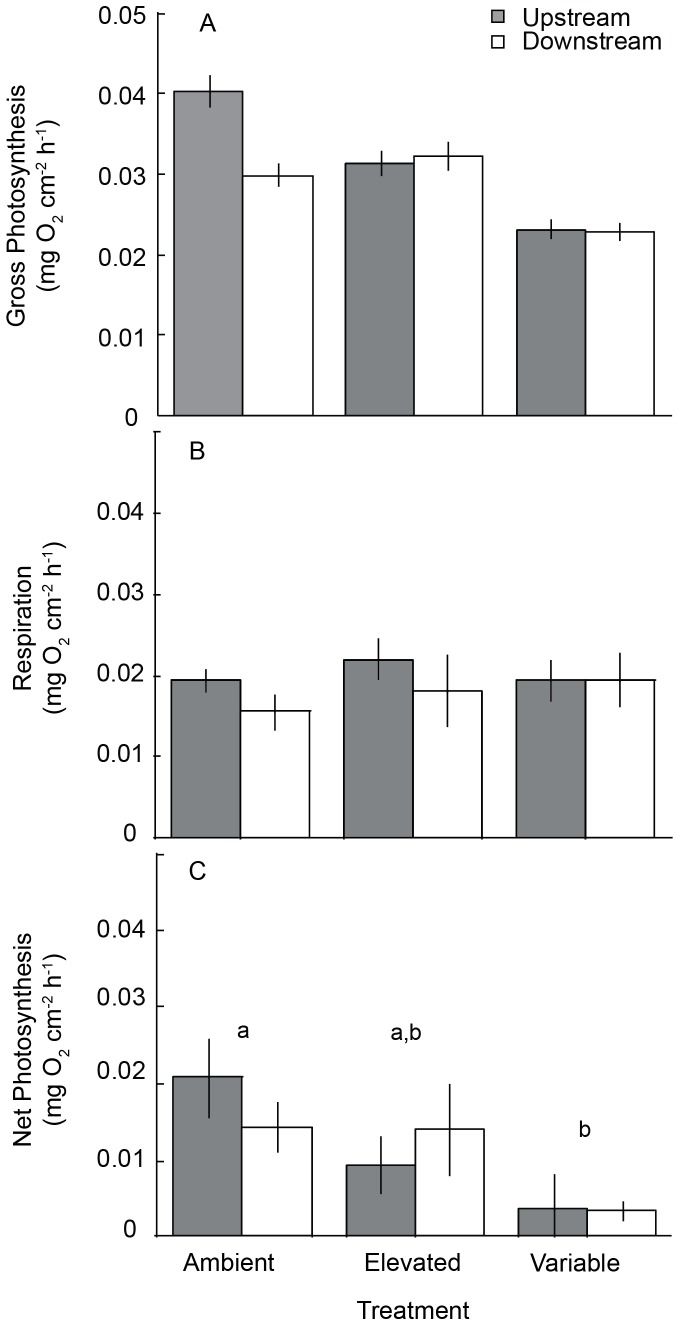
There was no significant effect of nesting tank within treatment in the initial analyses of the metabolic response variables (P>0.25), therefore the tank effect was pooled in subsequent analyses [79]. Rates of gross photosynthesis were not affected significantly by pCO2 treatment (F2,18 = 3.03, P = 0.086), habitat origin (F1,18 = 0.630, P = 0.443), or the interaction between factors (F2,18 = 0.738, P = 0.499) (Fig. 3A, Table S1). Similarly, there was no effect of pCO2 treatment (F2,18 = 0.414, P = 0.667), habitat origin (F1,18 = 1.26, P = 0.276), or the interaction between factors (F2,18 = 0.020, P = 0.980) on P. onkodes rates of respiration (Fig. 3B, Table S1). However, the net photosynthetic rate of P. onkodes was significantly lower in the variable pCO2 treatment compared to the ambient treatment (F2,18 = 5.59, P = 0.013). There was no effect of habitat origin (F1,18 = 0.041, P = 0.843) or the interaction between factors (F2,18 = 0.882, P = 0.431) on net photosynthesis (Fig. 3C, Table S1). Data are archived at the Moorea Coral Reef LTER data repository (http://mcr.lternet.edu/data/).
Figure 3. Metabolic responses of Porolithon onkodes to ocean acidification treatments.
Mean (±SE) rates of gross photosynthesis (A), respiration (B), and net photosynthesis (C) of P. onkodes collected from upstream and downstream habitats after 14 days of exposure to three pCO2 treatments. There were no significant differences among treatments or origins for gross photosynthesis. Small case letters represent results of post hoc multiple comparisons for net photosynthesis where treatments sharing letters are not significantly different.
Discussion
Our results demonstrate that the response of a coralline alga to variability in pCO2 depends upon the conditions in the original habitat. Porolithon onkodes collected from a downstream habitat, with prior exposure to high pCO2 variability, calcified significantly more in the variable pCO2 treatment than individuals from the upstream habitat, with history of exposure to lower and diurnally stable pCO2. These findings support our hypothesis that exposure to oscillating, high pCO2 within the natural range of variability, had a modulating effect on coralline calcification compared to the static high pCO2 treatment. We suggest that because the original habitat of P. onkodes had a significant impact on the coralline calcification response to variable pCO2, a history of exposure to dynamic carbonate conditions may facilitate plasticity in organismal responses to ocean acidification. Our findings emphasize the importance of incorporating oscillating pCO2 levels, where appropriate, into laboratory experiments because prior conditioning to high pCO2 may confound calcification responses to ocean acidification.
Corallines exposed to the static treatment of high CO2 calcified >70% less than those in the ambient treatment, regardless of the origin of collection. This finding corroborates several recent studies that have shown static, high pCO2 conditions to have severe consequences for crustose coralline calcification and growth [40], [55]–[60], [80]. The reduction in calcified biomass following exposure to high pCO2 may be a result of decreased calcification and/or increased dissolution associated with a lower Ω. Although the high pCO2 treatments did not reach undersaturation with respect to aragonite or calcite saturation state (Ω <1), calcification becomes increasingly difficult and potentially energetically more costly as it approaches the saturation horizon (Ω = 1) [3]. The difference in the reduction of net calcification for upstream and downstream organisms between the variable treatment and ambient pCO2 treatments (41% and 17%, respectively) indicates that P. onkodes from downstream habitats were able to mitigate some of the negative effects of elevated pCO2 on calcification, and/or the promoting effects of higher pCO2 on calcium carbonate dissolution. The response of a marine calcifier to oscillating pCO2 in a laboratory ocean acidification experiment has been demonstrated in few other studies, thus far. Dufault et al. (2012) showed that coral recruits exposed to oscillating pCO2 (between ∼450 and ∼850 µatm) experienced enhanced growth and survival compared to recruits exposed to both a static ambient and high pCO2 treatment [36]. Together these findings indicate that variability in carbonate chemistry should be incorporated into future OA experiments in order to enhance the ecological relevance of laboratory manipulations.
The increase in dissolved CO2 associated with ocean acidification and CO2 enrichment has the potential to fertilize photosynthesis by increasing the amount of substrate available to the photosynthetic enzyme Ribulose-1,5-bisphosphate carboxylase oxygenase (RuBISCO) [81], [82]. High pCO2 has been shown to stimulate photosynthesis in a variety of marine primary producers including seagrasses, phytoplankton, and some macroalgae [39], [81], [82]. However, species-specific responses may be influenced by the presence and activity of carbon concentrating mechanisms (CCMs) and whether a primary producer is carbon limited. We hypothesized that CO2 enrichment would stimulate photosynthetic rates in P. onkodes by providing more substrate for carbon fixation. We found no effect of pCO2 treatment on rates of P. onkodes gross photosynthesis or respiration, but a significant effect of the pCO2 treatment on net photosynthesis, with highest rates in the ambient pCO2 treatment, and lowest rates in the variable pCO2 treatment. Because net photosynthesis was determined based on gross photosynthesis and respiration rates, our finding may be a statistical result that has little biological basis. The role of CCMs in coralline algae is not well understood, and differences in CCM activity may have contributed to the mixed results found here and in the recent literature. For example, Anthony et al. (2008) found that exposure to extremely high pCO2 conditions (pH 7.6 and 7.8) decreased P. onkodes net productivity by 50% to 100%, respectively [55]. Furthermore, Martin et al. (2013) found that high pCO2 (700 µatm) decreased net photosynthetic efficiency of the Mediterranean coralline alga Lithophyllum cabiochae, and similar to our study found high pCO2 to have no effect on coralline respiration [83]. Respiration measurements were conducted between 1900 and 2300 using water from the appropriate treatments. For the variable pCO2 treatments, samples were incubated with ambient water because samples were not placed into high pCO2 conditions until midnight (0000). This methodology did not account for the response of the variable treatment samples to high pCO2 and, therefore, may underestimate the impact of the variable pCO2 treatment on rates of coralline respiration.
We suggest that the effect of habitat origin on the P. onkodes calcification response to variable pCO2 provides evidence of in situ acclimatization to naturally occurring variable pCO2. Although the terms ‘acclimation’ and ‘acclimatization’ often are used interchangeably, here we follow the terminology of Edmunds and Gates (2008), wherein acclimatization refers to the physiological compensation of an organism to a regime of natural co-occurring environmental stimuli [84]. The relatively short distance between sites (200 m) and lack of physical barriers makes the possibility of genetic differentiation, thus adaptation, between upstream and downstream coralline populations unlikely. If local adaptation were the underlying mechanism to the significance of the origin by pCO2 treatment interaction, we would expect both upstream and downstream individuals to exhibit the same response to variable pCO2. Form & Reibesell (2012) were among the first to document an example of acclimation of a marine calcifier to acidified conditions [42]. The cold-water coral, Lophelia pertuse, acclimated to acidified conditions after six months of exposure in a controlled laboratory mesocosm [42]. This provides an example of temporal acclimation to conditions similar to ocean acidification. In the current study, we suggest that corallines from downstream habitats are acclimatized to high pCO2, but only in the context of oscillating conditions that simulated the natural environment.
It is difficult to discern the exact mechanisms driving lower coralline calcification rates in the static and variable pCO2 treatments, in part because calcification in coralline algae is not well understood. Corallines calcify intracellularly, where high-Mg calcite is deposited directly within the organic matrix of the cell wall [47], [85]. In order for intracellular CaCO3 precipitation to occur, the internal microenvironment must be supersaturated with respect to CaCO3 (high pH). Calcification in corallines may be either biologically controlled or biologically induced [85] and influenced by both photosynthesis and calcification because they are inextricably linked. Biological control over calcification may be driven by photosynthesis, where photosynthesis directly provisions the energy necessary for calcification. Calcification may also be biologically induced as photosynthesis indirectly maintains the chemical conditions needed for CaCO3 precipitation by producing OH− ions [86]. For example, precipitation of magnesite and dolomite in P. onkodes cells is thought to be biologically induced by changes in pH due to metabolic activity [85]. Calcification is also influenced by external seawater chemistry, because calcified algae must uptake calcium ions (Ca2+) and carbonate ions (CO3 2−) directly from the seawater or through the intracellular conversion of HCO3 − to CO3 2− [87], [88]. Biomineralization becomes more difficult when the CaCO3 saturation state (Ω) approaches undersaturation (<1) [3], as is predicted to occur in the near future with ocean acidification.
Plasticity in the coralline response to high pCO2 variability therefore may have been driven by mechanisms associated with both photosynthesis and calcification. Identifying the mechanisms that promote coralline acclimatization to variable pCO2 was beyond the scope of this study. Here we propose potential biological and mineralogical mechanisms that may have contributed to the differences in coralline calcification by habitat. Two mechanisms (assuming biological control over calcification) by which corallines may maintain intracellular pH at the site of calcification, thus facilitating acclimatization to variable pCO2, are: 1) enhancement of photosynthesis from increased dissolved CO2 concentrations, and 2) opportunistic use of HCO3− to mitigate the decrease in CO3 2−. Photosynthesis may be fertilized by high CO2 and thus facilitate supersaturation of the intracellular microenvironment. However, since photosynthesis was not higher in the variable pCO2 treatment, this mechanism likely does not explain our calcification results. The second proposed mechanism, bicarbonate usage, applies to algae such as crustose corallines that can utilize HCO3 − [88] through the conversion of HCO3 − to CO2 with the enzyme carbonic anhydrase. The enzyme-catalyzed conversion releases a hydroxyl ion (OH−) and increases the local pH environment in favor of CaCO3 precipitation [54]. CCMs, including carbonic anhydrases (CA) and HCO3 − transporters, are important for supplying carbon to photosynthesis and calcification [89], [90]. Production and expression of CCMs is energetically costly and are influenced by environmental conditions [90], [91]. The temperate coralline alga, Corallina officinalis, exhibited a trend of increasing internal CA activity following exposure to high pCO2 [92], and an inverse trend between external CA activity and CO2 concentration [93]. Corallines frequently exposed to high pCO2 therefore may regulate CCMs, such as CA activity, in order to mitigate decreased CO3 2− concentrations associated with OA by switching to HCO3 − utilization [88]. A third mechanism (assuming abiotic control over calcification) may have been differences in calcification due to mineralogy. Coralline crusts from different habitats may exhibit differences in magnesium content, and thus corallines from upstream and downstream habitats may have differed in mineralogy from the start of the experiment. Mineralogy, rather than acclimatization, may have driven the calcification response to variable pCO2 if abiotic calcification (cell infilling) by magnesite and dolomite [85] was affected differentially by pCO2 conditions. In order to conclusively differentiate between mineralogical and physiological mechanisms, future studies should quantify coralline mineralogy before and after exposure to treatment conditions.
Based on our findings, biogenic calcifiers may be found in greater abundances at microhabitats with lower daily pCO2 variability, a finding that corroborates field-based studies that showed the distribution and abundance of benthic calcifiers to be correlated with pCO2/pH variability [12]–[14], [16]. As seen in other studies of shallow reef flats [7], [12], our data show that shallow reef environments may be shaped by diel pH variability. Our findings are corroborated by seawater chemistry measurements from a shallow reef system in Okinawa, Japan, where the alkalinity and DIC are consistently lower on the back reef during the day than on the reef crest [10]. Furthermore, the Tiahura reef transect, a similar section of back reef 6.5 km west of our sites on the north shore of Moorea, has been revisited since the early 1980's and calcification and carbonate chemistry have been thoroughly characterized, supporting our characterization of the upstream and downstream sites as having high and low pCO2 variability respectively [31], [67], [94]–[97]. In addition to differences in the magnitude of carbonate chemistry variability, other factors that may influence coralline growth rates within a habitat include grazing intensity, nutrient availability, water flow, and light levels. Our coralline samples were collected from the same depth at upstream and downstream sites and samples were acclimated to the same light conditions prior to the start of the experiment. Previous light history in the original habitat (at the same depths) likely did not impact the observed growth rates among treatments. Although upstream and downstream habitats may be characterized by different flow regimes, this experiment was conducted during the Austral winter (May-June), when differences in flow between the upstream and downstream site are minimal [66]. Furthermore, flow-through water within mesocosm tanks, aeration, and water pumped from the chilling system created water flow within the tanks (∼10 cm s−1). Although many factors influence coralline growth, the significant results from our laboratory experiment and the previous literature suggest that changes in the patterns of calcification rate among treatments were driven most likely by the carbonate chemistry conditions in the original habitats.
This study documents a modulated response of P. onkodes calcification to high pCO2 in the oscillating OA treatment which was based upon the natural range of pCO2 experienced in situ. The significant impact of the original habitat on the coralline calcification response to pCO2 treatments indicates that variability in carbonate chemistry likely is an important factor influencing biogenic calcification rates. These results are important because they emphasize that the original environmental conditions of individuals used in OA experiments can have a significant effect on experimental outcomes. Variability in carbonate chemistry in the original habitat may be a potential confounding factor in past OA studies, and may explain some of the ambiguity in calcification results in the literature. Further, we suggest that organisms in highly variable pCO2 environments may be acclimatized to near-future changes associated with ocean acidification. Studies should continue to explore the adaptive potential of marine organisms to the changing environment by exploring both in situ acclimatization and adaptation. Our findings have important implications applicable to other ecosystems that experience similar diel fluctuations in pH such as upwelling regions and estuarine systems. Given the potential dire consequences of ocean acidification for coastal ecosystems it is important to consider how acclimatization may facilitate the survival of marine organisms in the near future.
Supporting Information
ANOVA tables. Results from two-way ANOVA, with pCO2 treatment and habitat origin as fixed and interacting factors. Each response variable was analyzed separately. During initial analyses tank was treated as a random factor nested within treatment, but was not significant for all response variables (P>0.25) and was removed from subsequent analyses. Significant values (P<0.05) are highlighted in bold.
(DOCX)
Acknowledgments
We thank A. Brown, J. Gowan, and S. Swanson for lab and field assistance; and J. O'Leary and two anonymous reviewers for a constructive review of the manuscript. This is a contribution of the Moorea Coral Reef (MCR) Long Term Ecological Research site, and is contribution no. 206 of the California State University, Northridge, Marine Biology Program.
Funding Statement
Funding was provided by grants from the National Science Foundation (OCE-0417412, OCE-10-26852, OCE-1041270) and gifts from the Gordon and Betty Moore Foundation. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Houghton RA (1995) Land-use change and the carbon-cycle. Glob Change Biol 1: 275–287. [Google Scholar]
- 2. Sabine CL, Feely RA, Gruber N, Key RM, Lee K, et al. (2004) The oceanic sink for anthropogenic CO2 . Science 305: 367–371. [DOI] [PubMed] [Google Scholar]
- 3. Kleypas JA, Buddemeier RW, Archer D, Gattuso JP, Langdon C, et al. (1999) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284: 118–120. [DOI] [PubMed] [Google Scholar]
- 4. Friedrich T, Timmerman A, Abe-Ouchi A, Bates NR, Chikamoto MO, et al. (2012) Detecting regional anthropogenic trends in ocean acidification against natural variability. Nat Clim Change 2: 167–171. [Google Scholar]
- 5. Guinotte JM, Fabry VJ (2008) Ocean acidification and its potential effects on marine ecosystems. Ann NY Acad Sci 1134: 320–342. [DOI] [PubMed] [Google Scholar]
- 6. Meinshausen M, Smith SJ, Calvin K, Daniel JS, Kainuma MLT, et al. (2011) The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Climatic Change 109: 213–241. [Google Scholar]
- 7. Hofmann GE, Smith JE, Johnson KS, Send U, Levin LA, et al. (2011) High-Frequency dynamics of ocean pH: a multi-ecosystem comparison. PloS One 6: e28983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fabry VJ, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65: 414–432. [Google Scholar]
- 9. Thomsen J, Gutowska MA, Saphörster J, Heinemann A, Trübenbach K, et al. (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7: 3879–3891. [Google Scholar]
- 10. Ohde S, van Woesik R (1999) Carbon dioxide flux and metabolic processes of a coral reef, Okinawa. Bull Mar Sci 65: 559–576. [Google Scholar]
- 11. Gagliano M, Mccormick MI, Moore JA, Depczynski M (2010) The basics of acidification: baseline variability of pH on Australian coral reefs. Mar Biol 157: 1849–1856. [Google Scholar]
- 12. Price NN, Martz TR, Brainard RE, Smith JE (2012) Diel variability in seawater pH relates to calcification and benthic community structure on coral reefs. PloS One 7: e43843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, et al. (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454: 96–99. [DOI] [PubMed] [Google Scholar]
- 14. Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, et al. (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Change 1: 165–169. [Google Scholar]
- 15. Kerrison P, Hall-Spencer JM, Suggett DJ, Hepburn LJ, Steinke M (2011) Assessment of pH variability at a coastal CO2 vent for ocean acidification studies. Estuar Coast Shelf Sci 94: 129–137. [Google Scholar]
- 16. Crook ED, Potts D, Rebolledo-Vieyra M, Hernandez L, Paytan A (2012) Calcifying coral abundance near low-pH springs: implications for future ocean acidification. Coral Reefs 31: 239–245. [Google Scholar]
- 17. Andersson AJ, Mackenzie FT (2012) Revisiting four scientific debates in ocean acidification research. Biogeosciences 9: 893–905. [Google Scholar]
- 18. Suzuki A, Nakamori T, Kayanne H (1995) The mechanism of production enhancement in coral reef carbonate systems: model and empirical results. Sediment Geol 99: 259–280. [Google Scholar]
- 19. Yates KK, Halley RB (2006) CO3 2- concentration and pCO2 thresholds for calcification and dissolution on the Molokai reef flat, Hawaii. Biogeosciences 3: 357–369. [Google Scholar]
- 20. Bates NR, Amat A, Andersson AJ (2010) Feedbacks and responses of coral calcification on the Bermuda reef system to seasonal changes in biological processes and ocean acidification. Biogeosciences 7: 2509–2530. [Google Scholar]
- 21. Seidel MP, Degrandpre MD, Dickson AG (2008) A sensor for in situ indicator-based measurements of seawater pH. Mar Chem 109: 18–28. [Google Scholar]
- 22. Martz TR, Connery JG, Johnson KS (2010) Testing the Honeywell Durafet (R) for seawater pH applications. Limnol Oceanogr Meth 8: 172–184. [Google Scholar]
- 23. Smith SV (1973) Carbon dioxide dynamics: record of organic carbon production, respiration and calcification in Eniwetok reef flat community. Limnol Oceanogr 18: 106–120. [Google Scholar]
- 24. Bates NR, Samuels L, Merlivat L (2001) Biogeochemical and physical factors influencing seawater fCO2, and air-sea CO2 exchange on the Bermuda coral reef. Limnol Oceanogr 46: 833–846. [Google Scholar]
- 25. Barnes DJ (1983) Profiling coral-reef productivity and calcification using pH and oxygen electrodes. J Exp Mar Biol Ecol 66: 149–161. [Google Scholar]
- 26. Gattuso JP, Pichon M, Delesalle B, Canon C, Frankignoulle M (1996) Carbon fluxes in coral reefs. 1. Lagrangian measurement of community metabolism and resulting air-sea CO2 disequilibrium. Mar Ecol Prog Ser 145: 109–121. [Google Scholar]
- 27. Kinsey DW (1978) Alkalinity changes and coral-reef calcification. Limnol Oeanogr 23: 989–991. [Google Scholar]
- 28. Ware JR, Smith SV, Reakakudla ML (1992) Coral reefs: sources or sinks of atmospheric CO2 . Coral Reefs 11: 127–130. [Google Scholar]
- 29. Frankignoulle M, Canon C (1994) Marine calcification as a source of carbon dioxide- positive feedback of increasing atmospheric CO2 . Limnol Oceanogr 39: 458–462. [Google Scholar]
- 30. Watanabe A, Kayanne H, Hata H, Kudo S, Nozaki K, et al. (2006) Analysis of the seawater CO2 system in the barrier reef-lagoon system of Palau using total alkalinity-dissolved inorganic carbon diagrams. Limnol Oceanogr 51: 1614–1628. [Google Scholar]
- 31. Kleypas JA, Anthony KRN, Gattuso JP (2011) Coral reefs modify their seawater carbon chemistry - case study from a barrier reef (Moorea, French Polynesia). Glob Change Biol 17: 3667–3678. [Google Scholar]
- 32. Anthony KRN, Kleypas JA, Gattuso JP (2011) Coral reefs modify their seawater carbon chemistry - implications for impacts of ocean acidification. Glob Change Biol 17: 3655–3666. [Google Scholar]
- 33. Schmalz RF, Swanson FJ (1969) Diurnal variations in carbonate saturation of seawater. J Sediment Petrol 39: 255–267. [Google Scholar]
- 34. Bensoussan N, Gattuso JP (2007) Community primary production and calcification in a NW Mediterranean ecosystem dominated by calcareous macroalgae. Mar Ecol Prog Ser 334: 37–45. [Google Scholar]
- 35. Andersson AJ, Kuffner IB, Mackenzie FT, Jokiel PL, Rodgers KS, et al. (2009) Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: mesocosm-scale experimental evidence. Biogeosciences 6: 1811–1823. [Google Scholar]
- 36. Dufault AM, Cumbo VR, Fan TY, Edmunds PJ (2012) Effects of diurnally oscillating pCO2 on the calcification and survival of coral recruits. Proc Biol Sci 279: 2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comeau S, Edmunds PJ, Spindel NB, Carpenter RC (in press) Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification.
- 38. Kleypas JA, Langdon C (2006) Coral reefs and changing seawater carbonate chemistry. Coast Est Stud 61: 73–110. [Google Scholar]
- 39. Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13: 1419–1434. [DOI] [PubMed] [Google Scholar]
- 40. Comeau S, Carpenter RC, Edmunds PJ (2013) The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol Oeanogr 58: 388–398. [Google Scholar]
- 41. Lüthi D, Le Floch M, Bereiter B, Blunier T, Barnola JM, et al. (2008) High-resolution carbon dioxide concentration record 650,000-800,000 years before present. Nature 453: 379–382. [DOI] [PubMed] [Google Scholar]
- 42. Form AU, Riebesell U (2012) Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa . Glob Change Biol 18: 843–853. [Google Scholar]
- 43. Kleypas JA, Buddemeier RW, Gattuso JP (2001) The future of coral reefs in an age of global change. Int J Earth Sci 90: 426–437. [Google Scholar]
- 44. Littler MM (1971) Standing stock measurements of crustose coralline algae Rhodophyta and other saxicolous organisms. J Exp Mar Biol Ecol 6: 91–99. [Google Scholar]
- 45. Tribollet A, Payri C (2001) Bioerosion of the coralline alga Hydrolithon onkodes by microborers in the coral reefs of Moorea, French Polynesia. Oceanol Acta 24: 329–342. [Google Scholar]
- 46. Adey WH, Macintyre IG (1973) Crustose coralline algae: a re-evaluation in geological sciences. Geol Soc Am Bull 84: 883–904. [Google Scholar]
- 47. Borowitzka MA (1981) Photosynthesis and calcification in the articulated coralline red algae Amphiroa anceps and Amphiroa foliacea . Mar Biol 62: 17–23. [Google Scholar]
- 48. Chisholm JRM (2003) Primary productivity of reef-building crustose coralline algae. Limnol Oceanogr 48: 1376–1387. [Google Scholar]
- 49. Harrington L, Fabricius K, De'ath G, Negri A (2004) Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85: 3428–3437. [Google Scholar]
- 50. Price NN (2010) Habitat selection, facilitation, and biotic settlement cues affect distribution and performance of coral recruits in French Polynesia. Oecologia 163: 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Leary JK, Potts DC, Braga JC, McClanahan TR (2012) Indirect consequences of fishing: reduction of coralline algae suppresses juvenile coral abundance. Coral Reefs 31: 547–559. [Google Scholar]
- 52. Camoin GF, Montaggioni LF (1994) High-energy coralalgal-stromatolite frameworks from Holocene reefs (Tahiti, French Polynesia). Sedimentology 41: 655–676. [Google Scholar]
- 53. Adey WH (1998) Coral reefs: algal structured and mediated ecosystems in shallow, turbulent, alkaline waters. J Phycol 34: 393–406. [Google Scholar]
- 54. Borowitzka MA (1977) Algal calcification. Oceanogr Mar Biol 15: 189–223. [Google Scholar]
- 55. Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA 105: 17442–17446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, et al. (2008) Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27: 473–483. [Google Scholar]
- 57. Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1: 114–117. [Google Scholar]
- 58. Semesi IS, Kangwe J, Bjork M (2009) Alterations in seawater pH and CO2 affect calcification and photosynthesis in the tropical coralline alga, Hydrolithon sp (Rhodophyta). Estuar Coast Shelf Sci 84: 337–341. [Google Scholar]
- 59. Diaz-Pulido G, Anthony KRN, Kline DI, Dove S, Hoegh-Guldberg O (2012) Interactions between ocean acidification and warming on the mortality and dissolution of coralline algae. J Phycol 48: 32–39. [DOI] [PubMed] [Google Scholar]
- 60. Johnson MD, Carpenter RC (2012) Ocean acidification and warming decrease calcification in the crustose coralline alga Hydrolithon onkodes and increase susceptibility to grazing. J Exp Mar Biol Ecol 434: 94–101. [Google Scholar]
- 61. Albright R, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata . Proc Natl Acad Sci USA 107: 20400–20404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby PJ (2012) Ocean acidification reduces coral recruitment by disrupting intimate larval-algal settlement interactions. Ecol Lett 15: 338–346. [DOI] [PubMed] [Google Scholar]
- 63. Doropoulous C, Diaz-Pulido G (2013) High CO2 reduces the settlement of a spawning coral on three common species of crustose coralline algae. Mar Ecol Prog Ser 475: 93–99. [Google Scholar]
- 64. Webster NS, Uthicke S, Botté ES, Flores F, Negri AP (2013) Ocean acidification reduces induction of coral settlement by crustose coralline algae. Glob Change Biol 19: 303–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kato A, Baba M, Suda S (2011) Revision of the Mastophoroideae (Corallinales, Rhodophyta) and polyphyly in nongeniculate species widely distributed on pacific coral reefs. J Phycol 47: 662–672. [DOI] [PubMed] [Google Scholar]
- 66. Hench JL, Leichter JJ, Monismith SG (2008) Episodic circulation and exchange in a wave-driven coral reef and lagoon system. Limnol Oceanogr 53: 2681–2694. [Google Scholar]
- 67. Le Campion-Alsumard T, Romano JC, Peyrot-Clausade M, Le Campion J, Paul R (1993) Influence of some coral reef communities on the calcium carbonate budget of Tiahura reef (Moorea, French Polynesia). Mar Biol 115: 685–693. [Google Scholar]
- 68.Shaw EC, McNeil BI, Tilbrook B (2012) Impacts of ocean acidification in naturally variable coral reef flat ecosystems. J Geophys Res 117.. [Google Scholar]
- 69.Gowan J (2011) The effects of water flow and sedimentation on interactions between massive Porites and algal turf. MS Thesis, California State Univ, Northridge 99 pp. [Google Scholar]
- 70.Adey WH, Townsend RA, Boykins WT (1982) The crustose coralline algae (Rhodophyta: Corallinaceae) of the Hawaiian Islands. Smithsonian Contributions to the Marine Sciences.
- 71.Payri C, N'Yeurt AD, Orempuller J (2000) Algues de Polynésie francaise: Algae of French Polynesia. Au Vent de Iles, Editions Tahiti.
- 72. Edmunds PJ (2011) Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol Oceanogr 56: 2402–2410. [Google Scholar]
- 73.Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. PISCES Special Publication.
- 74. Fangue NA, O'donnell MJ, Sewell MA, Matson PG, Macpherson AC, et al. (2010) A laboratory-based, experimental system for the study of ocean acidification effects on marine invertebrate larvae. Limnol Oceanogr Meth 8: 441–452. [Google Scholar]
- 75.Pierrot D, Lewis E, Wallace DWR (2006) MS Excel program developed by CO2 system calculations. Carbon dioxide Information Center, Oak Ridge National Laboratory, US Department of Energy.
- 76. Mehrbach C, Culberso CH, Hawley JE, Pytkowic RM (1973) Measurement of apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol Oceanogr 18: 897–907. [Google Scholar]
- 77. Dickson AG, Millero FJ (1987) A comparison of the equilibrium-constants for the dissociation of carbonic-acid in seawater media. Deep Sea Res 34: 1733–1743. [Google Scholar]
- 78. Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biol 101: 389–395. [Google Scholar]
- 79.Quinn GP, Keough MJ (2002) Experimental Design and Data Analysis for Biologists, New York, Cambridge University Press.
- 80. Martin S, Gattuso JP (2009) Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob Change Biol 15: 2089–2100. [Google Scholar]
- 81. Koch M, Bowes G, Ross C, Zhang XH (2013) Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob Change Biol 19: 103–132. [DOI] [PubMed] [Google Scholar]
- 82. Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, et al. (2012) Effects of climate change on global seaweed communities. J Phycol 48: 1064–1078. [DOI] [PubMed] [Google Scholar]
- 83. Martin S, Cohu S, Vignot C, Zimmerman G, Gattuso JP (2013) One-year experiment on the physiological response of the Mediterranean crustose coralline alga, Lithophyllum cabiochae, to elevated pCO2 and temperature. Ecol Evol 3: 676–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Edmunds PJ, Gates RD (2008) Acclimatization in tropical reef corals. Mar Ecol Prog Ser 361: 307–310. [Google Scholar]
- 85. Nash MC, Troitzsch U, Opdyke BN, Trafford JM, Russell BD, et al. (2011) First discovery of dolomite and magnesite in living coralline algae and its geobiological implications. Biogeosciences 8: 3331–3340. [Google Scholar]
- 86. Ries JB (2011) A physicochemical framework for interpreting the biological calcification response to CO2 induced ocean acidification. Geochim Cosmochim Ac 75: 4053–4064. [Google Scholar]
- 87. Ries JB (2011) Skeletal mineralogy in a high-CO2 world. J Exp Mar Biol Ecol 403: 54–64. [Google Scholar]
- 88. Comeau S, Carpenter RC, Edmunds PJ (2012) Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc Biol Sci 280: 20122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Raven JA (1970) Exogenous inorganic carbon sources in plant photosynthesis. Biol Rev 45: 167–220. [Google Scholar]
- 90. Giordano M, Beardall J, Raven JA (2005) CO2 concentrating mechanisms in algae: Mechanisms, environmental modulation, and evolution. Ann Rev Plant Biol 56: 99–131. [DOI] [PubMed] [Google Scholar]
- 91. Raven JA, Giordano M, Beardall J, Maberly SC (2011) Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynth Res 109: 281–296. [DOI] [PubMed] [Google Scholar]
- 92. Hofmann LC, Yildiz G, Hanelt D, Bischof K (2012) Physiological responses of the calcifying rhodophyte, Corallina officinalis (L.), to future CO2 levels. Mar Biol 159: 783–792. [Google Scholar]
- 93. Hofmann LC, Straub S, Bischof K (2013) Elevated CO2 levels affect the activity of nitrate reductase and carbonic anhydrase in the calcifying rhodophyte Corallina officinalis . J Exp Bot 64: 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Payri CE (1987) Zonation and seasonal variation of the commonest algae on Tiahura reef (Moorea Island, French Polynesia). Bot Mar 30: 141–149. [Google Scholar]
- 95. Gattuso JP, Pichon M, Delesalle B, Frankignoulle M (1993) Community metabolism and air-sea CO2 fluxes in a coral-reef ecosystem (Moorea, French Polynesia). Mar Ecol Prog Ser 96: 259–267. [Google Scholar]
- 96. Boucher G, Clavier J, Hily C, Gattuso JP (1998) Contribution of soft-bottoms to the community metabolism (primary production and calcification) of a barrier reef flat (Moorea, French Polynesia). J Exp Mar Biol Ecol 225: 269–283. [Google Scholar]
- 97. Gattuso JP, Payri CE, Pichon M, Delesalle B, Frankignoulle M (1997) Primary production, calcification, and air-sea CO2 fluxes of a macroalgal-dominated coral reef community (Moorea, French Polynesia). J Phycol 33: 729–738. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ANOVA tables. Results from two-way ANOVA, with pCO2 treatment and habitat origin as fixed and interacting factors. Each response variable was analyzed separately. During initial analyses tank was treated as a random factor nested within treatment, but was not significant for all response variables (P>0.25) and was removed from subsequent analyses. Significant values (P<0.05) are highlighted in bold.
(DOCX)