Abstract
Bacteria of the family Rickettsiaceae have always been largely studied not only for their importance in the medical field, but also as model systems in evolutionary biology. In fact, they share a recent common ancestor with mitochondria. The most studied species, belonging to genera Rickettsia and Orientia, are hosted by terrestrial arthropods and include many human pathogens. Nevertheless, recent findings show that a large part of Rickettsiaceae biodiversity actually resides outside the group of well-known pathogenic bacteria. Collecting data on these recently described non-conventional members of the family is crucial in order to gain information on ancestral features of the whole group. Although bacteria of the family Rickettsiaceae, and of the whole order Rickettsiales, are formally described as non-flagellated prokaryotes, some recent findings renewed the debate about this feature. In this paper we report the first finding of members of the family displaying numerous flagella and active movement inside their host cells. These two new taxa are hosted in aquatic environments by protist ciliates and are described here by means of ultrastructural and molecular characterization. Data here reported suggest that the ancestor of Rickettsiales displayed flagellar movement and re-evaluate the hypothesis that motility played a key-role in the origin of mitochondria. Moreover, our study highlights that the aquatic environment represents a well exploited habitat for bacteria of the family Rickettsiaceae. Our results encourage a deep re-consideration of ecological and morphological traits of the family and of the whole order.
Introduction
The family Rickettsiaceae (Pinkerton, 1936 [1], emended by Dumler and colleagues [2]) represents one of the most studied taxonomic groups of bacteria. At present it includes members of the genera Rickettsia and Orientia, and the recently described candidate genera “Candidatus Cryptoprodotis” and “Candidatus Megaira” [3], [4]. Bacteria belonging to this family have attracted a great deal of attention and have been traditionally studied with a medical-entomological approach. The reason for such a high interest in these microorganisms resides mainly in the fact that species of the genera Rickettsia and Orientia are well-known etiological agents of many human and vertebrate diseases, having blood-feeding arthropods as vectors. Although most studies were mainly focused on pathogenic members of the family, an increasing number of non-pathogenic bacteria have been described also in non-hematophagous arthropods [5]–[9], and even associated with completely different organisms like leeches [10], cnidarians [11], [12], green algae [13] and protozoa [3], [4], [14]–[16]. Thus, it is now obvious that a large fraction of Rickettsiaceae biodiversity resides outside the group of pathogenic bacteria hosted by blood-feeding arthropods. It is then noteworthy that, among protists, ciliates harbor not only bacteria of the family Rickettsiaceae [3], [4], [15], but also several bacteria belonging to other groups of the order Rickettsiales [17]–[23]. Thus, these eukaryotic microorganisms can reasonably be considered useful biological systems for investigations on rickettsial biodiversity.
Besides their interest for the medical field, bacteria of the family Rickettsiaceae and of the whole order Rickettsiales assume additional importance. In the context of evolutionary biology, the study of these bacteria represents a key aspect in order to gain a better knowledge on one of the most fundamental steps in the history of life on earth: the evolution of mitochondria. It is now widely accepted that mitochondria originated from endosymbiotic prokaryotes [24], and that Rickettsiales bacteria and mitochondria share a common ancestor. Although the debate is still open [25], [26], this hypothesis is strongly supported by molecular data [27]–[31] and by other features of Rickettsiales such as the endosymbiotic lifestyle, the presence of similar respiratory chains and the lack of alternative energy patterns (for reviews see [32], [33]). Rickettsiales bacteria represent the closest extant relatives of the mitochondria ancestor. Therefore, the study of this group of prokaryotes can provide important indications concerning the origin of the organelle.
The majority of available data refers mainly to louse- or tick-borne rickettsial human pathogens [34]. As these species are highly specialized organisms co-evolved with their hosts, they are probably quite divergent from the rickettsial-mitochondrial common ancestor [35]. It is thus possible that some crucial evolutionary adaptations were overlooked because they are no longer retained in the deeply investigated extant species of Rickettsiales. According to some authors, protists could be considered as the ancestral hosts of members of the whole order Rickettsiales [33]. In this case, the later specialization to multicellular eukaryotic hosts coincided probably with the beginning of genome reduction in the symbionts [33]. Therefore, investigations on protist-borne Rickettsiales bacteria could provide new insights on ancestral features of the whole group.
A critical feature for understanding the evolutionary history of Rickettsiales and the steps which led to the establishment of the mitochondrial symbiosis is the presence or absence of movement in the mitochondria ancestor. Indeed, this feature could discriminate between a passive model of engulfment or a model implying a more active role of the bacterial partner [36], [37]. It has been shown that some species of Rickettsia can induce actin polymerization and move by generating actin filaments that resemble those present in filopodia [38]–[40]. The same capacity has been shown for members of the family Holosporaceae (Rickettsiales) [41]. Nevertheless, although the presence of some extracellular structures like pili or fimbriae-like elements has been demonstrated in the Rickettsiaceae [40], [42], [43], flagella have never been observed in members of the family. Bacteria of the order Rickettsiales were officially described as “bacteria with … no flagella” [44] and no evidence of active movement has been reported until now.
The recent finding of a complete set of flagellar genes in “Candidatus Midichloria mitochondrii”, a tick symbiont belonging to the order (family “Candidatus Midichloriaceae” [45]), strongly challenged the firm belief that Rickettsiales never displayed flagellar movement during their evolutionary history [46]. Indeed, genomic data obtained from “Ca. M. mitochondrii” show a pattern of vertical inheritance for the flagellar genes and strongly support the hypothesis that these genes were present in the ancestor of Rickettsiales and mitochondria as well. Although seven of these genes are expressed at the RNA level, the presence of flagella and/or of active movement has never been observed in “Ca. M. mitochondrii” [47]. Actual peritrichous flagella are present in the ciliate endosymbiont Lyticum, a close relative of “Ca. M. mitochondrii”, but this bacterium is nevertheless completely unable to move and probably maintained these structures for a different function [48].
In this paper we report the first finding of members of the family Rickettsiaceae displaying numerous flagella and active movement inside their host cells. These two new taxa of bacterial endosymbionts of protist ciliates are described by means of ultrastructural and molecular characterization.
Materials and Methods
Ciliate Host Isolation, Culturing and Identification
The study was performed on the ciliates listed in Table 1. Ciliate cultures were maintained in one of the following media: Synthetic Medium for Blepharisma (SMB), infusion of Cerophyll (Cerophyll Laboratories, Kansas City, Missouri, USA) and straw, lettuce infusion and artificial brackish water (5‰ salinity). They were periodically fed with bacteria (Raoultella planticola or Enterobacter aerogenes) or the photosynthetic flagellate Dunaliella tertiolecta.
Table 1. Main features of the two newly described bacterial species, as well as data of their hosts.
| Taxon | Host | Origin | Strain orpopulation | Intracellularlocalization | Flagella | Viral capsid-likestructures | Insertions |
| “ Candidatus Trichorickettsia mobilis” | Paramecium multimicronucleatum | Italy, Serchio River(freshwater pond) | PS23(polyclonal) | Macronucleus | YES | YES,cylindrical | Long |
| Paramecium multimicronucleatum | Italy, Lucca (canal) | LSA(monoclonal) | Macronucleus | YES | YES,cylindrical | Long | |
| Paramecium multimicronucleatum | Germany, Büsnau(wastewater plant) | Pm(monoclonal) | Macronucleus | YES | YES,cylindrical | Long | |
| Paramecium nephridiatum | Italy, Latina(wastewater plant) | PAR(polyclonal) | Cytoplasm | NO | YES,icosahedral | Long | |
| Euplotes aediculatus | India, New Delhi(freshwater pond) | In(polyclonal) | Cytoplasm | NO | NO | Long | |
| “ Candidatus Gigarickettsia flagellata” | Spirostomum minus | Italy, Serchio River(freshwater pond) | SS03(polyclonal) | Cytoplasm | YES | NO | Short |
Identification of the ciliate species was achieved in previous studies by Boscaro and colleagues [23], [49] or in this study by characterization of 18S rRNA gene sequence (see below) and by morphological observations according to Fokin [50]. For the morphological determination, living observations of the ciliates as well as checking of fixed material, stained by Feulgen reaction, were performed.
In vivo Observations and Transmission Electron Microscopy (TEM)
In vivo observations were performed using differential interference contrast (DIC) microscopy with a Leitz or Leica (Weitzlar, Germany) microscope at a magnification of 40–1250 X.
TEM preparations were obtained by fixing ciliate cells in glutaraldehyde and/or paraformaldehyde in cacodylate or phosphate buffer, with a postfixation in OsO4. The cells were then embedded in Epoxy embedding medium (Fluka, BioChemika) and cut using a Reichert-Jung Ultracut E or a LKB 8800 Ultrotome III microtome. Ultrathin sections were stained with uranyl acetate followed by lead citrate. The samples were visualized using a Zeiss EM 10, a Jeol JEM-1400 or a JEOL 100S.
For negative staining several LSA cells were briefly washed in distilled water and squashed; a drop of the resulting suspension was placed on a Pioloform coated grid. Bacteria were allowed to precipitate for 2–3 min, then a drop of 1% uranyl acetate in distilled water was added for no longer than 1 min. The liquid was then absorbed with filter paper and the grid was air-dried.
For Atomic Force Microscopy the ciliate cells were briefly washed in the sterile lettuce medium, squashed in a small drop of medium on the cover slip and air dried. The images were obtained with a NTEGRA Aura (NT MDT, Russia).
DNA Extraction and SSU rRNA Genes Characterization
DNA extraction was performed according to Wisotzkey and colleagues [51] for mass cultures (PAR, Pm), or using the protocol for mycelium DNA of NucleoSpin™ Plant DNA Extraction Kit (Macherey-Nagel GmbH and Co., Düren NRW, Germany), for cultures with low cell numbers (In, PS23, LSA, SS03). 18S rRNA gene sequences of host ciliates were then obtained as described elsewhere [52]. The 16S rRNA gene of the endosymbionts were amplified by a PCR reaction employing universal eubacterial primers [53], [54] or by a touchdown PCR reaction [55] employing specifically designed primers: RickFla_F69 5′-GTTAACTTAGGGCTTGCTC-3′, RickFla_F87 5′-CTCTAGGTTAATCAGTAGCAA-3′, RickBas_F166 5′-ATGCTAATGCCGTATATTCTC-3′, Rick_R1270 5′-TTTTAGGGATTTGCTCCACG-3′, Rick_R1455 5′-CCGTGGTTGGCTGCCT-3′. PCR products were then used for library construction or directly sequenced [54].
SSU rRNA gene sequences have been deposited in the European Nucleotide Archive (ENA) with accession numbers HG315605–HG315619.
Probes Design and FISH Experiments
Preliminary FISH experiments were performed using the eubacterial universal probe EUB338 [56] and the specifically designed probe Rick_697 (5′-TGTTCCTCCTAATATCTAAGAA-3′) in order to verify the presence of endosymbiotic bacteria belonging to the Rickettsiaceae family. On the basis of the obtained 16S rRNA gene sequences three probes were designed and synthesized as described elsewhere [57]: TrichoRick_142 (5′-GTTTCCAAATGTTATTCCATAC-3′), targeting all the described symbionts with the exception of that of Spirostomum minus SS03; GigaRick_436 (5′-TCATCTTCTCTGCTAAAAGA-3′), targeting only the symbiont of S. minus SS03; RickFla_430 (5′-TCTTCCCTGCTAAAAGAACTTT-3′), targeting all the described symbionts. Specificity of the new probes was tested on SILVA [58] and RDP [59] databases. FISH experiments were performed as described by Manz and colleagues [60]; probes were labeled at 5′ with Cy3 or fluoresceine. Slides were then mounted with SlowFade Gold Antifade with DAPI (Invitrogen). The newly designed probes Rick_697, TrichoRick_142, GigaRick_436 and RickFla_430 have been deposited at ProbeBase [61].
16S rRNA Gene Sequences Analysis
16S rRNA gene sequences were aligned against those present in the most recent version of the SILVA database [58] using the ARB software package [62].
Phylogenetic analysis was performed on 32 sequences. Sequence lengths were reduced to that of the shortest one, and the long inserts present only in the newly characterized taxa (see Results) were removed (obtaining a 1,338 character matrix); gaps were coded as a fifth character status. Maximum Likelihood (ML) and Bayesian Inference (BI) methods were employed, using the software PHYML [63] and MrBayes [64], respectively. The best substitution model was selected according to the AIC parameter calculated by jModelTest [65]. Bootstrapping (1,000 pseudoreplicates) was applied to the ML analysis. In order to further test the robustness of the results, the same analyses were repeated on modified matrices containing only columns with more than one non-gap character (modified matrix 1; 1,321 characters) or removing all columns containing gaps (modified matrix 2; 1,302 characters). Unless differently stated, similarity values were calculated on the unmodified matrix.
Results
Host Identification
The ciliate populations named In and PAR were previously identified as Euplotes aediculatus [23] and Paramecium nephridiatum, respectively [49]. A partial 18S rRNA gene sequence was obtained for PS23 (1,633 bp), LSA (1,710 bp) and Pm (1,710 bp). In all three cases the sequence showed more than 98% similarity with published sequences of Paramecium multimicronucleatum (e. g. strains TH105 and YM25, accession numbers AB252006 and AB252007, respectively [66]) according to NCBI Blastn. Species designation was also confirmed by morphological features like cell size and morphology of the micronuclei as well as the number of the nuclei (data not shown). A partial 18S rRNA gene sequence (831 bp) was also retrieved for population SS03. The highest similarity score (100%) was given in this case by the sequence of Spirostomum minus (AM398200; [67]), and the same species designation was suggested by morphological features like size of the cells, oral apparatus position and shape of the macronucleus (data not shown).
Symbiont Morphology, Ultrastructure and Flagellar Movement
Morphological and ultrastructural data of all the studied symbionts are summarized in Table 1. Symbionts of P. multimicronucleatum (PS23, LSA and Pm) are located almost exclusively inside the macronucleus of the host cell, not surrounded by any symbiosomal vacuole. Most of the bacteria are visible near the inner membrane of the nuclear envelope, while some have been very rarely observed in the cytoplasm of the host ciliate. The short rod-shaped bacterial symbionts possess two membranes arranged in a typical Gram-negative organization (Figure 1A, B). The cytoplasm of the symbionts is slightly electrondense and homogeneous. Highly electrondense, cylindrical corpuscles have been frequently observed inside the bacterial cells. These structures often display a somehow regular arrangement, being aligned in thick bands on the median region of the bacterial cell body. The geometrical shape of these corpuscles, their regular arrangement and their electrondensity suggest that they could be viral capsids (Figure 1A). No other complex structure associated with these capsid-like structures have ever been detected. The presence of numerous peritrichous flagella was detected on sections, on negative contrast and atomic force microscope observations (Figure 1A, C; Figure S1). The flagella are arranged all around the surface of the bacterial cell, they are quite long (more than 10 µ in most cases), often curved and even rolled when fixed (Figure 1C; Figure S1).
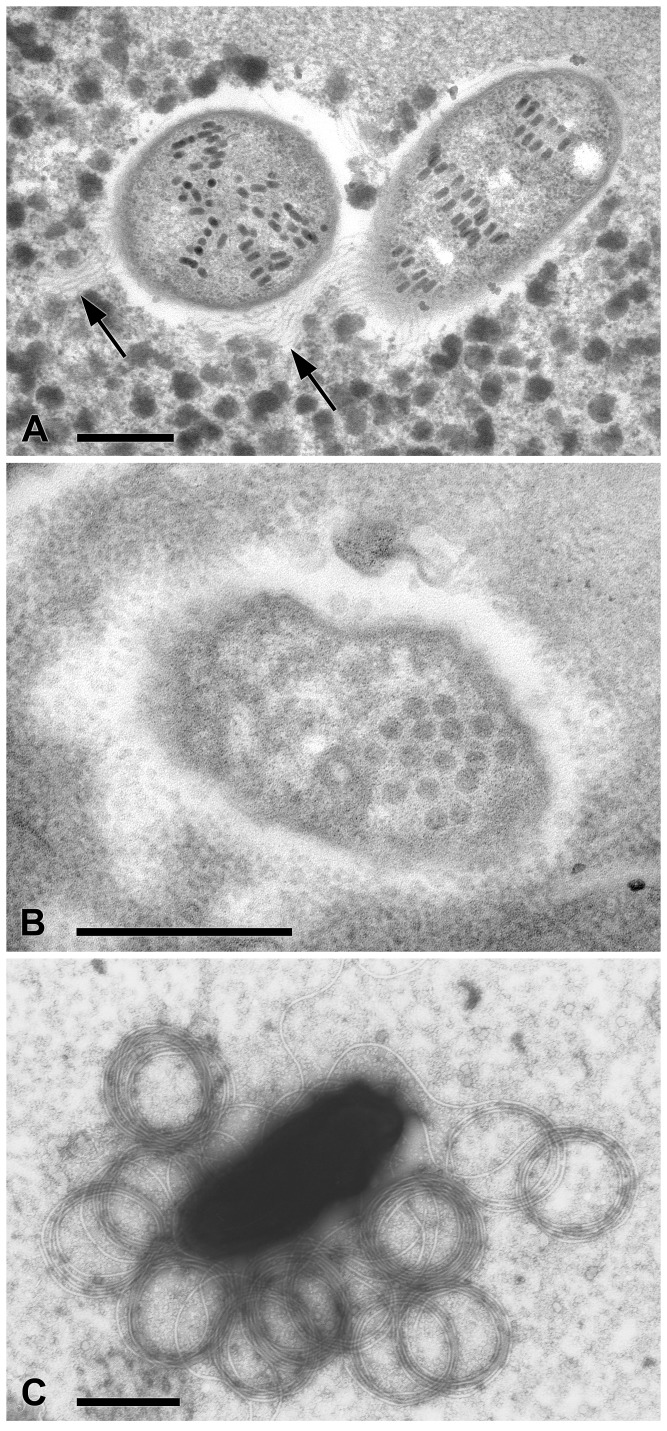
Figure 1. Transmission electron microscopy images of “Candidatus Trichorickettsia mobilis”.
(A) “Candidatus Trichorickettsia mobilis” inside the macronucleus of P. multimicronucleatum PS23; several cylindrical electrondense particles, arranged in a regular way, are visible inside the bacterium; arrows indicate the flagella of the symbionts. (B) “Candidatus Trichorickettsia mobilis” inside the cytoplasm of P. nephridiatum PAR; icosahedral electrondense particles are visible. (C) Negative staining of “Candidatus Trichorickettsia mobilis” from P. multimicronucleatum LSA: numerous and long flagella are clearly visible. Bars: 0.5 µm.
In vivo observations showed the bacterial symbionts swimming on the edge of the nucleoplasm, displaying a quite fast circular movement (Movie S1), or inside a network of “channels” where the chromatin seems to be less compact or absent (Movies S2, S3). The symbionts were rarely present in the cytoplasm of the host cell and in these cases they have never been observed to swim. On crushed ciliate preparations, the cease of movements has been observed a few minutes after the contact with the external environment. Bacteria trapped inside vesicles originating from the host nuclear envelope did not show any reduction of movement until the dissolution of the vesicle itself.
Symbiotic bacteria from P. nephridiatum PAR and of E. aediculatus In were instead always present in the ciliate hosts’ cytoplasm and never detected inside either the macro- or the micronucleus. TEM observations performed on P. nephridiatum PAR revealed that their morphology resembles that of the P. multimicronucleatum symbionts. Viral capsid-like structures were also detected, but their shape and arrangement differ from those of P. multimicronucleatum symbionts (Figure 1B). Neither flagella, nor movement have ever been observed in the symbionts of these two ciliate populations.
Bacterial symbionts of S. minus SS03 are also located in the cytoplasm of the host cell. Their most striking feature is the remarkable cell size, with some specimens attaining 20 µm in length and 1.2 µm in width (Figure 2). Their cytoplasm is generally highly electrondense and homogeneous, the membrane organization resembles that of typical Gram-negative bacteria. No viral capsid-like structures were observed inside the bacterial cells, but the presence of flagella was shown by TEM observations (Figure 2). Despite their huge size, these symbionts constantly swim in the cytoplasm of the host cell. Their movements are not erratic, but straightforward and parallel to the host cell membrane. Their cell body is flexible and bends to continue the straight movement when they reach the host cell extremities. Reversion of the direction of movement has never been observed. Rather often host organelles (e. g. nuclei) are touched or even misplaced by bacteria during their rushes. Despite this, host ciliates do not show any obvious sign of stress. Bacterial movement ceases almost immediately after contact with the external environment (e. g. after host cell disruption).
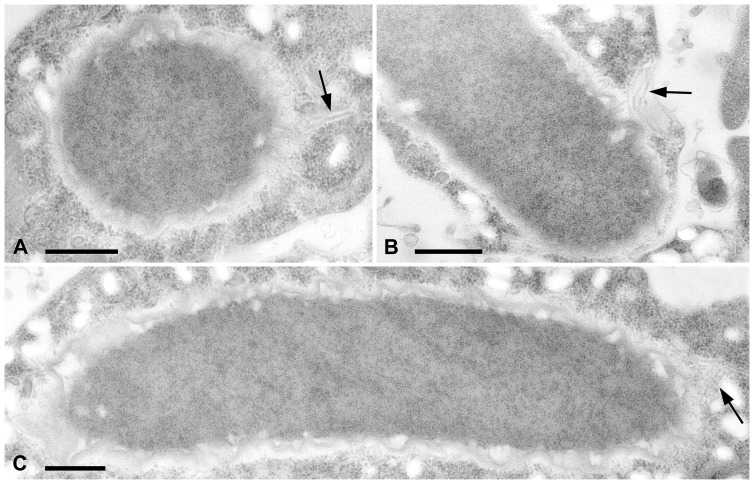
Figure 2. Transmission electron microscopy images of “Candidatus Gigarickettsia flagellata” in transverse (A) and longitudinal (B, C) sections.
Arrows indicate the flagella. Bars: 0.5 µm.
16S rRNA Gene Characterization of Symbionts and FISH Experiments
Almost full-length 16S rRNA gene sequences of the symbionts were obtained from all the examined samples, with a length ranging from 1,314 bp to 1,624 bp (accession numbers HG315609–HG315619). The most peculiar feature of the obtained sequences is the presence of two quite long inserted elements in positions 207 and 212 (Escherichia coli), respectively. They are separated only by a 4 bp long region (consensus sequence: 5′-TTTA-3′) present in all other sequences included in the analysis. These insertions are not present in any other 16S rRNA gene sequence obtained from bacteria belonging to the order Rickettsiales.
Sequences obtained from Paramecium (PAR, PS23, Pm, LSA) and Euplotes (In) samples show an insertion of 48 bp in position 207 and another one of 152 bp in position 212. The concatenated sequences of the two insertions share a similarity value range of 90.7–100% among different strains. This range is quite larger than that calculated among the same strains using the entire sequence (98.4–100%), or the sequence without the insertions (99.7–100%). A Blastn search using the concatenated sequence of the insertions provided only low-score hits (72–88% similarity, and 9e−8-2e−10 e-values for the best 10 hits) from genomic sequences of Rickettsia species. The most represented homolog is the gene for the beta subunit of RNA polymerase. All these data suggest that the insertions were transferred as mobile elements from other genomic regions of a Rickettsia-like organism (likely the symbiont itself). Although further investigations are required in order to verify this point, they could be non-functional and subjected to weak purifying selection.
The consensus sequence of clones obtained from the symbiont of S. minus SS03 possesses two shorter insertions of 23 bp each.
Once the insertions are removed, the similarity between the sequence of SS03 symbiont and the sequences of other symbionts here described is 95.0–95.1%. Similarities shared with other members of Rickettsiaceae are below 94.0% (SS03 symbiont) and below 97.32% (other symbionts).
The probe Rick_697 matched 249 16S rRNA gene sequences on SILVA database. 90% of these sequences belongs to the Rickettsiaceae family, while the remaining 10% includes Wolbachia pipientis, the bacterial symbiont of Sitophilus zeamais and some uncultured bacteria. The same probe matched 626 sequences on RDP database, 95% of which belonging to members of the Rickettsiaceae family and the rest to uncultured bacteria. Probes specifically designed for the newly characterized symbionts were shown to be highly specific. Indeed, probe RickFla_430 matched no sequence on SILVA database and only 8 sequences on RDP (all belonging to unclassified organisms), probe TrichoRick_142 matched no sequence on SILVA and only 3 sequences on RDP (all from unclassified organisms) and probe GigaRick_436 matched no sequences on SILVA nor on RDP.
In order to confirm 16S rRNA gene characterization of the newly described symbionts, FISH experiments were performed using the specifically designed probes (Figure 3, Figure S2). While probe RickFla_430 gave a bright and sharp signal inside ciliate cells of all the studied samples, probe TrichoRick_142 gave a positive signal only on samples of P. multimicronucleatum PS23, Pm and LSA, P. nephridiatum PAR and E. aediculatus In. On the contrary, probe GigaRick_436 gave a positive signal only inside the cytoplasm of S. minus SS03.
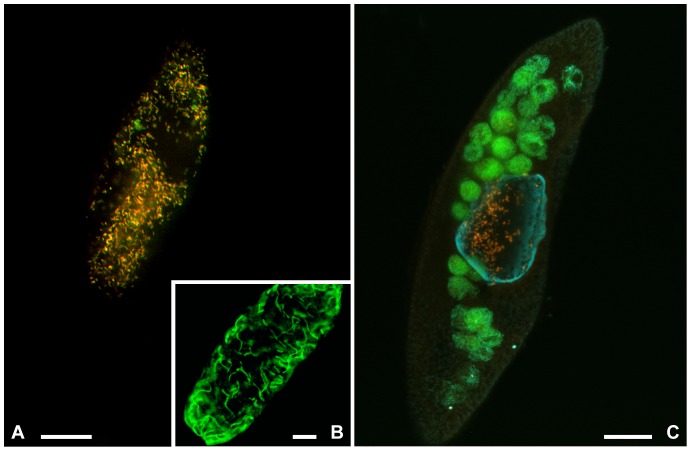
Figure 3. FISH experiments performed on “Candidatus Trichorickettsia mobilis” and “Candidatus Gigarickettsia flagellata”.
Symbionts inside P. nephridiatum PAR (A) and inside P. multimicronucleatum LSA (C) with probe RickFla_430 (Cy3, red signal) together with eubacterial probe EUB338 (fluoresceine, green signal). Food bacteria, labeled only in green, are visible inside food vacuoles in LSA (C). FISH experiment performed on “Candidatus Gigarickettsia flagellata” with probe GigaRick_436 (green signal) is shown in B. Bars: 20 µm.
Phylogeny of Symbionts
All the 16S rRNA gene sequences obtained in this work are tightly associated in a highly supported cluster, with the exception of the sequence from the S. minus SS03 symbiont. This bacterium does form a monophyletic group with the others here characterized, but stands at the top of a much longer branch. Moreover, statistical support to the group including all new sequences is relatively low (70/0.98 in the analysis performed on the non-modified matrix; up to 75/0.99 with the modified matrix 2).
The topology of the ingroup does not differ between ML and BI analyses, and shows only minor differences in unsupported nodes when calculated on modified matrices. The clade formed by the ciliate endosymbionts characterized herein is the sister group of validly described Rickettsia species, exclusively found in arthropods, and related organisms that were not formally described (e. g. “Rickettsia limoniae”) and that possess a wider host spectrum. Other bacterial symbionts of ciliates belonging to the Rickettsiaceae family are more distantly related, and scattered in different clades together with symbionts of other aquatic organisms (Figure 4).
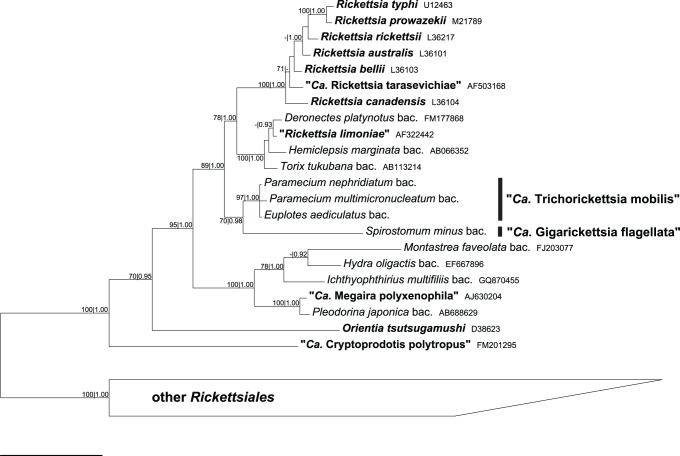
Figure 4. Bayesian Inference tree of the family Rickettsiaceae.
The tree was built on the unmodified character matrix (see text) employing the GTR+I+G (8 gamma categories) model. Numbers associated to nodes represent Maximum Likelihood bootstraps and Posterior Probabilities, respectively (values below 70|0.85 are omitted). Taxa that received a formal or provisional binomial name are in bold. The bar stands for an inferred sequence divergence of 5%. “Ca.”, “Candidatus”; “bac.”, “bacterium associated to”.
Discussion
Features and Characterization of the Novel Symbionts
According to the obtained data, the characterized bacterial symbionts unambiguously belong to the order Rickettsiales and to the family Rickettsiaceae. The same data, together with similarity analysis, indicate that we are dealing with two distinct bacterial species [68]. The first one, represented by the symbionts detected in samples of Paramecium (PS23, Pm, LSA, PAR) and Euplotes (In), will be thereafter referred to as “Candidatus Trichorickettsia mobilis”. The second one is represented by the symbiont of S. minus (SS03) and will be thereafter referred to as “Candidatus Gigarickettsia flagellata”, distinctive for its much larger size, different ultrastructural features and shorter insertion elements in the 16S rRNA genes.
Obtained results show that “Candidatus Trichorickettsia mobilis” inhabits the macronucleus and possesses flagella and motility inside P. multimicronucleatum. It is worthy of mention that a flagellated endosymbiont showing the same morphological features and moving ability inside the host macronucleus was briefly described in the past without any molecular characterization in P. multimicronucleatum populations isolated in Boston, USA, and Moldova [69]. On the other hand, “Candidatus Trichorickettsia mobilis” is localized in the cytoplasm and does not show either flagella or motility inside P. nephridiatum and E. aediculatus. Viral capsid-like structures were found in several samples, but according to morphology they belong to at least two different types. The characterization of this bacterium from several different samples pointed out the possible expression of remarkably different features within the same bacterial species. Differences in intracellular localization and moving ability indicate a certain degree of plasticity, apparently driven by the host species.
Nuclear localization has already been reported for bacteria belonging to the family Rickettsiaceae. Indeed, members of the Spotted Fever Group (SFG) in the genus Rickettsia were already known to reside also in the nucleus of the eukaryotic host cell [70]. Moreover, Ogata and colleagues [40] reported for the species Rickettsia bellii the capability of colonizing the host cell nucleus and dividing inside it. It has been speculated that the nucleus can represent both a protected, nutrient-rich environment to be exploited for these endosymbiotic bacteria and an optimal location for possible interference with host gene expression [71]. Other members of the orders Rickettsiales that typically invade the host nuclei are the ciliate symbionts belonging to the family Holosporaceae [22], [72] and the genus Caedibacter [19], [73].
Flagellar Motility and its Implications
The most surprising feature of the two newly described bacterial species is the unambiguous presence of active flagellar movement. This is an absolute novelty not only in the family Rickettsiaceae, but also in the entire order Rickettsiales. Available data indicate that flagellar movement could represent an ancestral character for all the Alphaproteobacteria, which was secondarily lost in Rickettsiales [74]. The finding of flagella and of vertically inherited flagellar genes in “Candidatus Midichloriaceae” family [46], [48] and, now, the finding of flagellar movement in two bacteria belonging to the family Rickettsiaceae suggest that not only the genes, but also their full expression and functioning, have been retained with a spotted pattern in the different evolutionary lineages of the order. According to this scenario, the possibility that the ancestor of Rickettsiales displayed flagellar movement re-evaluates the hypothesis that motility played a key-role in the origin of mitochondria.
Alternatively, it could be also hypothesized that flagellar movement has been re-acquired by the symbionts here described. In this case, a horizontal gene transfer could have occurred, for example, with some other bacterium associated to protist ciliates. Indeed, horizontal gene transfer between bacteria associated to protists has already been documented [40]. The presence of electrondense particles, resembling viral capsids, which has been shown inside “Candidatus Trichorickettsia mobilis”, suggests also the possibility of gene exchange involving a viral partner, although the presence of flagella and capsid-like structures do not always correlate (Table 1). The acquisition of additional information on these particles and on their possible activity will help address this issue. To summarize, an independent re-acquisition of flagella by different members of the order cannot be excluded, but its explanation requires at present several speculative assumptions. Therefore, all these hypotheses need to be tested by genome characterization and analysis, an approach that has only recently been applied to bacterial symbionts of ciliates [75]. Moreover, further screenings on non-conventional (i. e. non-blood-feeding arthropods) potential hosts from different environments could enlarge the record of motile Rickettsia-related bacteria. This would possibly shed light on the evolutionary path of this peculiar feature and its adaptive meaning. In any case, data here reported definitively invalidate the hypothesis of a complete absence of such an important character in the entire order.
Diversity of Rickettsiales Bacteria in Ciliates
Symbiotic systems involving protists as hosts are turning out to be a precious source of information on the diversity and evolution of bacteria belonging to the order Rickettsiales. Our results strongly suggest the hypothesis that several ancestral features of Rickettsiales could have been retained mainly in bacteria colonizing unicellular hosts [33]. In the light of the new findings here reported, the hypothesis that protists could represent the ancestral hosts of the Rickettsiales should be perhaps re-evaluated and better investigated.
Beside evolutionary aspects, some ecological considerations merit attention. The two new bacterial species here described have been detected in samples derived from aquatic habitats. Considering that all the best-known Rickettsiaceae bacteria were isolated from terrestrial habitats, this means that the new species inhabit a completely different environment. This finding has to be added to some recent reports of other bacteria belonging to the family both from marine [12], [15] and freshwater habitats (see for example [3], [4], [11], [13]). Taking all these data into account, it seems evident that the aquatic environment represents a well-exploited and important habitat for bacteria of the family Rickettsiaceae. Moreover, it is noteworthy that two of the studied population (PAR and Pm) were sampled in waste water treatment plants. Perhaps, protists colonizing such highly impacted environments should deserve more attention as possible reservoirs of bacteria related to pathogenic groups.
Taxonomy and Diagnosis
According to the guidelines of Fournier and Raoult [76] a bacterial species can be included in the genus Rickettsia only if its 16S rRNA gene sequence shows a similarity value of 98.1% or higher with respect to other members of the genus. Following this rule, some new bacteria of the family Rickettsiaceae, like the yet-undescribed “Rickettsia limoniae” and others which have been recently characterized (see for example [10], [11]), should not be considered representatives of the genus Rickettsia. For this reason, we describe the two newly characterized bacteria as two novel bacterial genera within the family Rickettsiaceae. Their classification in two different genera is a precaution due to the conspicuous sequence differences between them, and the relatively low statistical support underlying their association in phylogenetic analysis. The putative clade formed by these taxa can be identified through the FISH oligonucleotide probe RickFla_430 (5′-TCTTCCCTGCTAAAAGAACTTT-3′) and the presence of insertion elements in the 16S rRNA gene, although its monophyly should be further tested.
Description of “Candidatus Trichorickettsia mobilis”. Trichorickettsia mobilis (Tric.ho.ric.ket’tsi.a mo’bi.lis; Gr. masc. n. thrix, hair, N.L. fem. n. Rickettsia, from the name of a related genus, N.L. fem. n. Trichorickettsia, hairy Rickettsia; L. adj. mobilis, motile). Rod-shaped bacterium, up to 2.6 µm in length and 1.3 µm in width. Macronuclear or cytoplasmic symbiont of protist ciliates of the genera Paramecium and Euplotes. Displaying flagella and swimming behavior inside the host cell of P. multimicronucleatum. Electrondense cytoplasm, frequently hosting particles resembling viral capsids with various shapes arranged in regular fashions. Belonging to the family Rickettsiaceae in the order Rickettsiales. Basis of assignment: 16S rRNA gene sequence (accession number: HG315612) and positive match with the specific FISH oligonucleotide probe TrichoRick_142 (5′ – GTTTCCAAATGTTATTCCATAC -3′). Uncultured thus far.
Description of “Candidatus Gigarickettsia flagellata”. Gigarickettsia flagellata (Gi.ga.ric.ket’tsi.a fla.gel.la’ta; Gr. masc. n. gigas, giant, N.L. fem. n. Rickettsia, from the name of a related genus, N.L. fem. n. Gigarickettsia, giant Rickettsia; L. adj. flagellata, with flagella). Long rod shaped bacterium, up to 20 µm in length and 1.2 µm in width. Cytoplasmic bacterial symbiont of Spirostomum minus (Ciliophora, Heterotrichea). Possessing flagella and swimming behavior inside the host cell. Belonging to the family Rickettsiaceae in the order Rickettsiales. Basis of assignment: 16S rRNA gene sequence (accession number: HG315613) and positive match with the specific FISH oligonucleotide probe GigaRick_436 (5′-TCATCTTCTCTGCTAAAAGA-3′). Uncultured thus far.
Supporting Information
Atomic force microscope image of “ Candidatus Trichorickettsia mobilis” from P. multimicronucleatum LSA. Flagella are clearly visible.
(JPG)
FISH performed on “Candidatus Trichorickettsia mobilis” inside the macronuclei of P. multimicronucleatum Pm (A) and P. multimicronucleatum PS23 (C), and the cytoplasm of E. aediculatus In (B) with the probe RickFla_430 (red signal) and, only in (C), with the eubacterial probe EUB338 (green signal). Bars: 10 micrometers.
(JPG)
“ Candidatus Trichorickettsia mobilis” swimming on the edge of the nucleoplasm of P. multimicronucleatum PS23.
(MP4)
“ Candidatus Trichorickettsia mobilis” swimming inside the macronucleus of a crushing ciliate cell of P. multimicronucleatum PS23.
(MP4)
“ Candidatus Trichorickettsia mobilis” swimming inside the macronucleus of P. multimicronucleatum LSA. The beating of somatic and oral ciliature of the protist host is also visible.
(MP4)
Acknowledgments
The authors wish to thank A. Ristori and B. Moretti for their help in characterizing the symbiont of P. nephridiatum and of E. aediculatus, respectively. S. Gabrielli is gratefully acknowledged for his help with graphic artworks. Studies on LSA strain were partly performed using the equipment of the Core Facility Centre for Microscopy and Microanalysis and the Core Facility Centre “Development of Molecular and Cell Technologies” of St.-Petersburg State University.
Supplementary information is available at PLoS ONE website.
Funding Statement
The study was supported by European Commission FP7-PEOPLE-2009-IRSES project CINAR-PATHOBACTER (247658), by Italian Research Ministry (MIUR) PRIN fellowship (protocol 2008R9WRTB), and by the COST action BM1102. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pinkerton H (1936) Criteria for the accurate classification of the rickettsial diseases (rickettsioses) and of their etiological agents. Parasitology 28: 172–189. [Google Scholar]
- 2. Dumler JS, Barbet AF, Bekker CPJ, Dasch GA, Palmer GH, et al. (2001) Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia, and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila . Int J Syst Evol Microbiol 51: 2145–2165. [DOI] [PubMed] [Google Scholar]
- 3. Ferrantini F, Fokin SI, Modeo L, Andreoli I, Dini F, et al. (2009) “Candidatus Cryptoprodotis polytropus,” a novel Rickettsia-like organism in the ciliated protist Pseudomicrothorax dubius (Ciliophora, Nassophorea). J Eukaryot Microbiol 56: 119–129. [DOI] [PubMed] [Google Scholar]
- 4. Schrallhammer M, Ferrantini F, Vannini C, Galati S, Schweikert M, et al. (2013) “Candidatus Megaira polyxenophila” gen. nov., sp. nov.: considerations on evolutionary history, host range and shift of early divergent rickettsiae. PLoS One 8: e72581 10.1371/journal.pone.0072581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen DQ, Campbell BC, Purcell AH (1996) A new Rickettsia from a herbivorous insect, the pea aphid Acyrthosiphon pisum (Harris). Curr Microbiol 33: 123–128. [DOI] [PubMed] [Google Scholar]
- 6. Davis MJ, Ying ZT, Brunner BR, Pantoja A, Ferwerda FH (1998) Rickettsial relative associated with papaya bunchy top disease. Curr Microbiol 36: 80–84. [DOI] [PubMed] [Google Scholar]
- 7. Fukatsu T, Shimada M (1999) Molecular characterization of Rickettsia sp in a bruchid beetle, Kytorhinus sharpianus (Coleoptera : Bruchidae). Appl Entomol Zool 34: 391–397. [Google Scholar]
- 8. Campbell CL, Mummey DL, Schmidtmann ET, Wilson WC (2004) Culture-independent analysis of midgut microbiota in the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae). J Med Entomol 41: 340–348. [DOI] [PubMed] [Google Scholar]
- 9. Czarnetzki AB, Tebbe CC (2004) Diversity of bacteria associated with Collembola - a cultivation-independent survey based on PCR-amplified 16S rRNA genes. FEMS Microbiol Ecol 49: 217–227. [DOI] [PubMed] [Google Scholar]
- 10. Kikuchi Y, Sameshima S, Kitade O, Kojima J, Fukatsu T (2002) Novel clade of Rickettsia spp. from leeches. Appl Environ Microbiol 68: 999–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fraune S, Bosch TCG (2007) Long-term maintenance of species-specific bacterial microbiota in the basal metazoan Hydra . Proc Natl Acad Sci U S A 104: 13146–13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sunagawa S, DeSantis TZ, Piceno YM, Brodie EL, De Salvo MK, et al. (2009) Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata . ISME J 3: 512–521. [DOI] [PubMed] [Google Scholar]
- 13. Kawafune K, Hongoh Y, Hamaji T, Nozaki H (2012) Molecular identification of rickettsial endosymbionts in the non-phagotrophic volvocalean green algae. PLoS One 7: e31749 10.1371/journal.pone.0031749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dyková I, Veverková M, Fiala I, Machácková B, Pecková H (2003) Nuclearia pattersoni sp. n. (Filosea), a new species of amphizoic amoeba isolated from gills of roach (Rutilus rutilus), and its rickettsial endosymbiont. Folia Parasitol 50: 161–170. [PubMed] [Google Scholar]
- 15. Vannini C, Petroni G, Verni F, Rosati G (2005) A bacterium belonging to the Rickettsiaceae family inhabits the cytoplasm of the marine ciliate Diophrys appendiculata (Ciliophora, Hypotrichia). Microb Ecol 49: 434–442. [DOI] [PubMed] [Google Scholar]
- 16. Sun HY, Noe J, Barber J, Coyne RS, Cassidy-Hanley D, et al. (2009) Endosymbiotic bacteria in the parasitic ciliate Ichthyophthirius multifiliis . Appl Environ Microbiol 75: 7445–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Amann R, Springer N, Ludwig W, Görtz HD, Schleifer KH (1991) Identification in situ and phylogeny of uncultured bacterial endosymbionts. Nature 351: 161–164. [DOI] [PubMed] [Google Scholar]
- 18. Springer N, Ludwig W, Amann R, Schmidt HJ, Görtz HD, et al. (1993) Occurrence of fragmented 16S rRNA in an obligate bacterial endosymbiont of Paramecium caudatum . Proc Natl Acad Sci U S A 90: 9892–9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schrallhammer M, Fokin SI, Schleifer KH, Petroni G (2006) Molecular characterization of the obligate endosymbiont “Caedibacter macronucleorum” Fokin and Görtz, 1993 and of its host Paramecium duboscqui strain Ku4–8. J Eukaryot Microbiol 53: 499–506. [DOI] [PubMed] [Google Scholar]
- 20. Eschbach E, Pfannkuchen M, Schweikert M, Drutschmann D, Brümmer F, et al. (2009) “Candidatus Paraholospora nucleivisitans”. an intracellular bacterium in Paramecium sexaurelia shuttles between the cytoplasm and the nucleus of its host. Syst Appl Microbiol 32: 490–500. [DOI] [PubMed] [Google Scholar]
- 21. Vannini C, Ferrantini F, Schleifer KH, Ludwig W, Verni F, et al. (2010) “Candidatus Anadelfobacter veles” and “Candidatus Cyrtobacter comes,” two new Rickettsiales species hosted by the protist ciliate Euplotes harpa (Ciliophora, Spirotrichea). Appl Environ Microb 76: 4047–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boscaro V, Fokin SI, Schrallhammer M, Schweikert M, Petroni G (2013) Revised systematics of Holospora-like bacteria and characterization of “Candidatus Gortzia infectiva”. a novel macronuclear symbiont of Paramecium jenningsi Microb Ecol 65: 255–267. [DOI] [PubMed] [Google Scholar]
- 23. Boscaro V, Petroni G, Ristori A, Verni F, Vannini C (2013) “Candidatus Defluviella procrastinata” and “Candidatus Cyrtobacter zanobii”, two novel ciliate endosymbionts belonging to the Midichloria clade. Microb Ecol 65: 302–310. [DOI] [PubMed] [Google Scholar]
- 24.Margulis L (1970) Origin of eukaryotic cells. New Haven (CT): Yale University Press.
- 25. Lang BF, Brinkmann H, Koski LB, Fujishima M, Görtz HD, et al. (2005) On the origin of mitochondria and Rickettsia-related eukaryotic endosymbionts. Jpn J Protozool 38: 171–183. [Google Scholar]
- 26. Burger G, Gray MW, Forget L, Lang BF (2013) Strikingly bacteria-like and gene-rich mitochondrial genomes throughout jacobid protists. Genome Biol Evol 5: 418–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsen GJ, Woese CR, Overbeek R (1994) The winds of (evolutionary) change – breathing new life into microbiology. J Bacteriol 176: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Viale AM, Arakaki AK (1994) The chaperone connection to the origins of the eukaryotic organelles. FEBS Lett 341: 146–151. [DOI] [PubMed] [Google Scholar]
- 29. Sicheritz-Pontén T, Kurland CG, Andersson SGE (1998) A phylogenetic analysis of the cytochrome b and cytochrome c oxidase I genes supports an origin of mitochondria from within the Rickettsiaceae . Biochim Biophys Acta 1365: 545–551. [DOI] [PubMed] [Google Scholar]
- 30. Fitzpatrick DA, Creevey CJ, McInerney JO (2006) Genome phylogenies indicate a meaningful alpha-proteobacterial phylogeny and support a grouping of the mitochondria with the Rickettsiales . Mol Biol Evol 23: 74–85. [DOI] [PubMed] [Google Scholar]
- 31. Williams KP, Sobral BW, Dickerman AW (2007) A robust species tree for the Alphaproteobacteria . J Bacteriol 189: 4578–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Emelyanov VV (2001) Evolutionary relationship of Rickettsiae and mitochondria. FEBS Lett 501: 11–18. [DOI] [PubMed] [Google Scholar]
- 33. Blanc G, Ogata H, Robert C, Audic S, Suhre K, et al. (2007) Reductive genome evolution from the mother of Rickettsia . PLoS Genet 3: e14 10.1371/journal.pgen.0030014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perlman SJ, Hunter MS, Zchori-Fein E (2006) The emerging diversity of Rickettsia . Proc R Soc Lond B Biol Sci 273: 2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fournier PE, El Karkouri K, Leroy Q, Robert C, Giumelli B, et al. (2009) Analysis of the Rickettsia africae genome reveals that virulence acquisition in Rickettsia species may be explained by genome reduction. BMC Genomics 10: 166 10.1186/1471-2164-10-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guerrero R, Pedros-Alio C, Esteve I, Mas J, Chase D, et al. (1986) Predatory prokaryotes: predation and primary consumption evolved in bacteria. Proc Natl Acad Sci U S A 83: 2138–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davidov Y, Jurkevitch E (2009) Predation between prokaryotes and the origin of eukaryotes. Bioessays 31: 748–757. [DOI] [PubMed] [Google Scholar]
- 38. Heinzen RA (2003) Rickettsial actin-based motility - Behavior and involvement of cytoskeletal regulators. In: Hechemy, KE; AvsicZupanc, T; Childs, JE; Raoult, DA (eds). Rickettsiology: present and future directions. Annals of the New York Academy of Sciences 990: 535–547. [DOI] [PubMed] [Google Scholar]
- 39. Gouin E, Egile C, Dehoux P, Villiers V, Adams J, et al. (2004) The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 427: 457–461. [DOI] [PubMed] [Google Scholar]
- 40. Ogata H, La Scola B, Audic S, Renesto P, Blanc G, et al. (2006) Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet 2: e76 10.1371/journal.pgen.0020076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sabaneyeva EV, Derkacheva ME, Benken KA, Fokin SI, Vainio S, et al. (2009) Actin-based mechanism of Holospora obtusa trafficking in Paramecium caudatum . Protist 160: 205–219. [DOI] [PubMed] [Google Scholar]
- 42. Ogata H, Renesto P, Audic S, Robert C, Blanc G, et al. (2005) The genome sequence of Rickettsia felis identifies the first putative conjugative plasmid in an obligate intracellular parasite. PLoS Biol 3: e248 10.1371/journal.pbio.0030248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Walker DH, Yu XJ (2005) Progress in rickettsial genome analysis from pioneering of Rickettsia prowazekii to the recent Rickettsia typhi . In: Annals of the New York Academy of Sciences Hechemy KE, Oteo JA, Raoult DA, Silverman DJ, Blanco JR, editors. Rickettsioses: from Genome to Proteome, Pathobiology, and Rickettsiae as an International Threat. 1063: 13–25. [DOI] [PubMed] [Google Scholar]
- 44.Dumler JS, Walker DH (2005) Order II. Rickettsiales Gieszczykiewicz 1939, 25AL emend. Dumler, Barbet, Bekker, Dasch, Palmer, Ray, Rikihisa and Rurangirwa 2001, 2156. In: Garrity GM, Boone DR, Castenholz RW, editors. Bergey’s manual of systematic bacteriology. Volume 2. 2nd edition. East Lansing (MI): Springer.
- 45. Montagna M, Sassera D, Epis S, Bazzocchi C, Vannini C, et al. (2013) “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alpha-proteobacteria. Appl Environ Microbiol 79: 3241–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sassera D, Lo N, Epis S, D’Auria G, Montagna M, et al. (2011) Phylogenomic evidence for the presence of a flagellum and cbb 3 oxidase in the free-living mitochondrial ancestor. Mol Biol Evol 28: 3285–3296. [DOI] [PubMed] [Google Scholar]
- 47. Mariconti M, Epis S, Sacchi L, Biggiogera M, Sassera D, et al. (2012) A study on the presence of flagella in the order Rickettsiales: the case of ‘Candidatus Midichloria mitochondrii’. Microbiology-SGM 158: 1677–1683. [DOI] [PubMed] [Google Scholar]
- 48. BoscaroV, Schrallhammer M, Benken KA, Krenek S, Szokoli F, et al. (2013) Rediscovering the genus Lyticum, multiflagellated symbionts of the order Rickettsiales . Sci Rep 3: 3305 10.1038/srep03305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Boscaro V, Vannini C, Fokin SI, Verni F, Petroni G (2012) Characterization of “Candidatus Nebulobacter yamunensis” from the cytoplasm of Euplotes aediculatus (Ciliophora, Spirotrichea) and emended description of the family Francisellaceae . Syst Appl Microbiol 35: 432–440. [DOI] [PubMed] [Google Scholar]
- 50. Fokin SI (2010/11) Paramecium genus: biodiversity, some morphological features and the key to the main morphospecies discrimination. Protistology 6: 227–235. [Google Scholar]
- 51. Wisotzkey JD, Jurtshuk P, Fox GE (1990) PCR amplification of 16S rDNA from lyophilized cell cultures facilitates studies in molecular systematics. Curr Microbiol 21: 325–327. [DOI] [PubMed] [Google Scholar]
- 52. Rosati G, Modeo L, Melai M, Petroni G, Verni F (2004) A multidisciplinary approach to describe protists: a morphological, ultrastructural, and molecular study on Peritromus kahli Villeneuve-Brachon, 1940 (Ciliophora, Heterotrichea). J Eukaryot Microbiol 51: 49–59. [DOI] [PubMed] [Google Scholar]
- 53.Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acids techniques in bacterial systematics. Wiley, New York. 115–147.
- 54. Vannini C, Rosati G, Verni F, Petroni G (2004) Identification of the bacterial endosymbionts of the marine ciliate Euplotes magnicirratus (Ciliophora, Hypotrichia) and proposal of “Candidatus Devosia euplotis”. Int J Syst Evol Microbiol 54: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 55. Don RH, Cox PT, Wainwright BJ, Baker K, Mattick JS (1991) ‘Touchdown’ PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res 19: 4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, et al. (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56: 1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Petroni G, Rosati G, Vannini C, Modeo L, Dini F, et al. (2003) In situ identification by fluorescently labeled oligonucleotide probes of morphologically similar, closely related ciliate species. Microb Ecol 45: 156–162. [DOI] [PubMed] [Google Scholar]
- 58. Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, et al. (2007) SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 35: 7188–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37: D141–D145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Manz W, Amann RI, Wagner M, Schleifer KH (1992) Phylogenetic oligodeoxynucleotide probes for the major subclasses of Proteobacteria: problems and solutions. Syst Appl Microbiol 15: 593–600. [Google Scholar]
- 61. Loy A, Maixner F, Wagner M, Horn M (2007) probeBase – an online resource for rRNA-targeted oligonucleotide probes: new features 2007. Nucleic Acids Res 35: D800–D804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ludwig W, Strunk O, Westram R, Richter L, Meier H, et al. (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32: 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 64. Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- 65. Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 66. Hoshina R, Hayashi S, Imamura N (2006) Intraspecific genetic divergence of Paramecium bursaria and reconstruction of the parameciam phylogenetic tree. Acta Protozool 45: 377–386. [Google Scholar]
- 67. Schmidt SL, Treuner T, Schlegel M, Bernhard D (2007) Multiplex PCR approach for species detection and differentiation within the genus Spirostomum (Ciliophora, Heterotrichea). Protist 158: 139–145. [DOI] [PubMed] [Google Scholar]
- 68. Stackebrandt E, Ebers E (2006) Taxonomic parameters revisited: tarnished gold standards. Microbiology Today 35: 153–154. [Google Scholar]
- 69.Vishnyakov A, Rodionova G (1999) Motile intranuclear symbionts of ciliate Paramecium multimicronucleatum. In: Wagner, M et al. (eds) From symbiosis to eukaryotism. Endocytobiology VII. University of Geneva, Geneva, 169–177.
- 70.Yu XJ, Walker DH (2005) Family I. Rickettsiaceae Pinkerton 1936, 186AL emend. Dumler, Barbet, Bekker, Dasch, Palmer, Ray, Rikihisa and Rurangirwa 2001, 2156. In: Garrity, GM, Boone, DR, Castenholz, RW (eds). Bergey’s manual of systematic bacteriology: 2nd edition. Springer: East Lansing (MI), p 96.
- 71. Bierne H, Cossart P (2012) When bacteria target the nucleus: the emerging family of nucleomodulins. Cell Microbiol 14: 622–633. [DOI] [PubMed] [Google Scholar]
- 72.Görtz HD (2006) Symbiotic associations between ciliates and prokaryotes. In: Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E. (Eds.), The Prokaryotes. Third Edition, vol. 1, Springer-Verlag, New York, NY, 364–402.
- 73. Schmidt HJ, Görtz HD, Quackenbush RL (1987) Caedibacter caryophila sp. nov., a killer symbiont inhabiting the macronucleus of Paramecium caudatum. . Int J Syst Bacteriol 37: 459–462. [Google Scholar]
- 74. Greene SE, Brilli M, Biondi EG, Komeili A (2012) Analysis of the CtrA pathway in Magnetospirillum reveals an ancestral role in motility in Alphaproteobacteria. J Bacteriol 194: 2973–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Boscaro V, Felletti M, Vannini C, Ackerman MS, Chain PSG, et al. (2013) Polynucleobacter necessarius, a model for genome reduction in both free-living and symbiotic bacteria. Proc Natl Acad Sci U S A 110: 18590–18595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fournier PE, Raoult D (2009) Current knowledge on phylogeny and taxonomy of Rickettsia spp. Rickettsiology and Rickettsial Diseases-Fifth International Conference: Ann. N.Y. Acad. Sci. 1166: 1–11 (2009). doi: 10.1111/j.1749-6632.2009.04528.x c 2009 New York Academy of Sciences. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Atomic force microscope image of “ Candidatus Trichorickettsia mobilis” from P. multimicronucleatum LSA. Flagella are clearly visible.
(JPG)
FISH performed on “Candidatus Trichorickettsia mobilis” inside the macronuclei of P. multimicronucleatum Pm (A) and P. multimicronucleatum PS23 (C), and the cytoplasm of E. aediculatus In (B) with the probe RickFla_430 (red signal) and, only in (C), with the eubacterial probe EUB338 (green signal). Bars: 10 micrometers.
(JPG)
“ Candidatus Trichorickettsia mobilis” swimming on the edge of the nucleoplasm of P. multimicronucleatum PS23.
(MP4)
“ Candidatus Trichorickettsia mobilis” swimming inside the macronucleus of a crushing ciliate cell of P. multimicronucleatum PS23.
(MP4)
“ Candidatus Trichorickettsia mobilis” swimming inside the macronucleus of P. multimicronucleatum LSA. The beating of somatic and oral ciliature of the protist host is also visible.
(MP4)