Abstract
Varicose veins are elongated and dilated saphenous veins. Despite the high prevalence of this disease, its pathogenesis remains unclear.
Aims
In this study, we investigated the control of matrix metalloproteinases (MMPs) expression by prostaglandin (PG)E2 during the vascular wall remodeling of human varicose veins.
Methods and Results
Varicose (small (SDv) and large diameter (LDv)) and healthy saphenous veins (SV) were obtained after surgery. Microsomal and cytosolic PGE-synthases (mPGES and cPGES) protein and mRNA responsible for PGE2 metabolism were analyzed in all veins. cPGES protein was absent while its mRNA was weakly expressed. mPGES-2 expression was similar in the different saphenous veins. mPGES-1 mRNA and protein were detected in healthy veins and a significant decrease was found in LDv. Additionally, 15-hydroxyprostaglandin dehydrogenase (15-PGDH), responsible for PGE2 degradation, was over-expressed in varicose veins. These variations in mPGES-1 and 15-PGDH density account for the decreased PGE2 level observed in varicose veins. Furthermore, a significant decrease in PGE2 receptor (EP4) levels was also found in SDv and LDv. Active MMP-1 and total MMP-2 concentrations were significantly decreased in varicose veins while the tissue inhibitors of metalloproteinases (TIMP -1 and -2), were significantly increased, probably explaining the increased collagen content found in LDv. Finally, the MMP/TIMP ratio is restored by exogenous PGE2 in varicose veins and reduced in presence of an EP4 receptor antagonist in healthy veins.
Conclusions
In conclusion, PGE2 could be responsible for the vascular wall thickening in human varicose veins. This mechanism could be protective, strengthening the vascular wall in order to counteract venous stasis.
Introduction
Varicose saphenous veins are characterized by venous backflow and blood stagnation. [1], [2], [3], [4] This pathology is part of the chronic venous disease of the legs that is categorized into several classes from C0 to C6, where the C2 stage corresponds to varicose veins which are frequently removed by surgery. [5] Despite the high incidence of this disease, its pathogenesis is still poorly understood although some hypotheses, such as a local hypertension or a genetic predisposition, have been suggested. [2], [3].
The metabolism and the effects of bioactive lipids like prostanoids (prostaglandins (PG) and thromboxane) has been rarely investigated in the context of varicose veins. Prostanoids are produced by most blood and vascular cell types. [6] PGE2 via selective activation of EP1-4 receptor subtypes is involved in the control of vascular tone, [7], [8] inflammation, [9], [10], [11], [12] pain [13] and vascular wall remodeling. [14] PGE2 is synthesized from arachidonic acid (AA) through the enzymatic activities of two cyclooxygenases (COX-1 and/or COX-2) and three PGE synthases (PGES). [15] Furthermore, PGE2 is degraded by 15-hydroxyprostaglandin dehydrogenase (15-PGDH), the only enzyme responsible for its catabolism. Among the three PGES that specifically catalyze the final step of PGE2 biosynthesis; two are constitutive: microsomal (mPGES-2) and cytosolic (cPGES). [12], [16] The third, mPGES-1, [11], [12], [16], [17], [18], is quantitatively the most important enzymatic activity for PGE2 production. mPGES-1 and COX-2 expression are generally co-induced by inflammatory cytokines such as IL-1β. [11], [17] However, in a recent publication, [19] we have shown the absence of COX-2 in the varicose veins.
As observed during aneurysm formation or in the pathogenesis of endometriosis, [14], [20], [21], [22] PGE2 modulates vascular wall remodeling mediated by the matrix metalloproteinases (MMPs). The renewal of extracellular matrix (ECM) by MMP [23] activity is dysregulated in many vascular diseases [24] such as acute coronary artery syndrome, atherosclerosis or aneurysm. [25], [26], [27], [28], [29] Some MMPs involved in these processes are the interstitial collagenase, MMP-1, that cleaves fibrillar collagens, which are subsequently degraded by the gelatinases, MMP-2 and MMP-9. [25], [28] There are few studies in human tissues which have demonstrated the role of PGE2 on the expression/activation of MMPs. [14], [20], [21], [22] For example, PGE2 activates several MMPs via EP2/EP4-receptor stimulation in human endometriotic epithelial and stromal cells. [14] On the other hand, MMP activities are also under control of endogenous tissue inhibitor of metalloproteinase (TIMP) and changes in MMP/TIMP ratio are probably involved in vascular wall remodeling and in varicose vein formation. [30], [31], [32], [33], [34].
The aim of this study was to investigate the role of PGE2 in the mechanism involved in the formation of varicose veins. We have investigated how PGE2 and the enzymes responsible for its metabolism could participate in venous wall remodeling via MMP, TIMP and collagen deposition. Specifically, this study was designed to analyse the pathology of varicose veins. For each patient with varicose veins (C2 stage) removed by surgery, we compared a dilated segment and a non-dilated segment. These two samples were compared with healthy saphenous veins (SV). An increase in diameter between segments of the healthy SV and the lesser dilated varicose vein can be observed. Therefore, our hypothesis is that the non-dilated varicose vein segment represents an intermediate stage of the disease. In this way, our study addresses the evolution of varicose pathology.
Methods
1 Human Saphenous Veins (Healthy and Varicose)
Fifteen healthy SV from calf patients undergoing bypass surgery (10 male and 5 female, aged 71±4 years) were obtained. In most European countries, varicose veins are still frequently removed by conventional surgery. Varicose veins (n = 30, stage C2) from patients undergoing vein stripping (14 male and 16 female, aged 59±4 years) were obtained at Bichat Hospital (Paris, France) with patients’ verbal and written informed consent. The consent form, explaining all the procedure, was approved by the Bichat ethic committee. The investigation conforms to the principles outlined in the declaration of Helsinki as these tissues were anonymized. All research programs involving the use of human tissue were approved and supported by the National Institute for Health and Medical Research (INSERM) ethics committee and these tissues are considered as surgical waste in accordance with French ethical laws (L.1211-3–L.1211-9). Following harvesting, veins were rapidly placed in ice-cold saline. After surgical use, non-used healthy SV (∼3 mm) or varicose vein segments were immediately transported to the laboratory. All veins were processed within two hours after excision. They were cleaned of adipose tissue and blood. Varicose veins were separated into two different segments, of small diameter (SDv, ∼4 mm) and of large diameter (LDv, ∼8 mm). Saphenous veins obtained after bypass surgery are considered as healthy since patients were not under NSAIDs medication and veins were checked using sonographic ultrasound studies. All preparations were used with intact adventitial layers.
2 Saphenous Vein Preparation and Incubation
For Western blotting, fresh vein samples were ground in liquid nitrogen and homogenized in a RIPA solution as previously described. [19] After a 15 min centrifugation at 4500 g, the supernatant was stored at −20°C. Proteins were quantified using a bicinchoninic acid (BCA) assay kit (ThermoScientific, Rockford, IL, USA). For histological studies, fresh veins were fixed for 24 h in paraformaldehyde (3.7% in PBS), embedded in paraffin and sectioned at 5 µm. Sections were deparaffinized in toluene and hydrated at the beginning of the experiments. Before enzyme immunoassay (EIA) measurement of PGE2, MMPs or TIMPs, fresh vein samples were incubated at 37°C either for 24 h (70 mg wet weight tissue/mL solution) in RPMI solution (Gibco Invitrogen, Paisley, UK) with 5% CO2 or for 30 min in Tyrode’s solution.with 5% CO2 in O2. RPMI solution always contained antibiotics (penicillin, 1000 IU/mL; streptomycin, 100 µg/mL) and antimycotic (amphotericin, 0.25 µg/mL). The fresh venous preparations were incubated for 24 h in RMPI+antibiotics solution with or without pharmacological treatments (EP receptor agonist or antagonist). The fresh venous preparations were incubated for 30 min in Tyrode’solution with or without (arachidonic acid +/− glutathione). Supernatants were harvested and frozen until use.
3 Western Blot Analysis
Protein analysis was evaluated as previously described. [19] Fifty micrograms of venous proteins or Western ready control (for all proteins, from Cayman, Ann Arbour, MI, USA) were used and specific antibodies are presented in the Methods section of Supplementary data.
4 EIA
4.1 Measurement of PGE2 levels
The concentration of PGE2 in the supernatant of healthy SV or varicose veins was determined using an enzyme immunoassay (EIA) kit (Cayman). Frozen supernatants were used and diluted with the assay buffer. The PGE2 concentration was determined according to the instructions provided with the kit. In addition, 30 min incubations in presence of arachidonic acid (1 µmol/L, Cayman) and with or without glutathione (2.5 mmol/L, Sigma) [11] were also performed (data not shown).
4.2 Measurement of MMP and TIMP levels
MMP and TIMP protein levels were measured in the supernatant of vascular preparations using commercial kits (DuoSet, R&D systems, Minneapolis, MN, USA). Tissues were incubated in absence or presence of PGE2 (10 µmol/L, Cayman) or an EP4 receptor antagonist GW627368X [35] (1 µmol/L, Cayman). Results were expressed in pg or ng per mg of wet tissue. For MMP-1, measurements were made by using two different kits. The first kit recognized only the pro MMP-1 and the second kit recognize the total MMP-1 (pro-MMP-1 and active MMP-1 together). The quantity of active MMP-1 was calculated by the subtraction of pro-MMP-1 value to total MMP-1 value for each sample. MMP-2 was measured using kit which recognizes total MMP. TIMP-1 and TIMP-2 concentrations were measured using kits (one for TIMP-1 and another one for TIMP-2), which recognize the total human TIMP-1 or -2 (free and complexed with MMPs).
5 Histological Study
Masson’s trichrome staining was used for detection of several collagen and general morphology [36] of the healthy and varicose veins.
6 Data Analysis
Due to the specificity of the experiments, several normalization procedures were performed (Supplementary data). For specific protein content measured by Western blot, the level of protein expression was normalised by the α-actin content. Optical density (OD) for the different bands was measured by Scion Image®. For EIA measurements, normalisation was done by correcting for tissue wet weight. Collagen content were measured by calculating the optical density (pixel number) with Photoshop® and normalized by the total area of the vein sections.
Statistical analyses were performed using the program SigmaStat® (Systat Software, IL, USA). All data are presented as means ± sem derived from (n) patients. A one-way-ANOVA test (followed by the Tukey post-hoc test) or a paired t-test was used and a P-value <0.05 was considered as statistically significant. Linear regression and the Pearson test were used for correlation analysis (SigmaStat®).
Results
PGES and COX Content in Varicose and Healthy Saphenous Veins
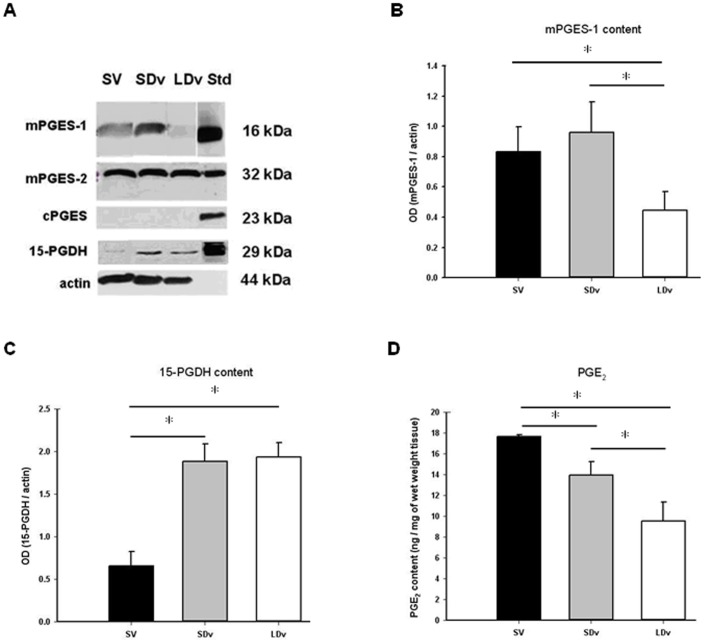
Expression of PGES isomers was measured using Western blot analysis and normalized to α-actin (Figures 1 A, B); for mPGES-1, a 16 kDa band was found in homogenates of healthy SV (n = 4) and varicose veins (n = 5) corresponding to the standard band. A significant decrease (about 50%) for mPGES-1 intensity was observed in the LDv samples as compared to the healthy SV. A 32 kDa band was found for mPGES-2 without modification of its intensity (n = 4–5) among the different venous preparations. cPGES was not detected in venous tissues, even using a greater protein load (100 µg) or another antibody for cPGES (n = 6–7). In order to confirm this result, Western blot analysis with protein extracts derived from human internal mammary artery was performed and a 23 kDa band corresponding to this isoform was detected (Figure S1). Furthermore, measurement of mPGES-1 transcript (Figure S2 and Table S1 in the File S1) confirmed the decreased expression (about 75%) of this enzyme in LDv (n = 5). In all venous preparations, mPGES-2 and cPGES mRNA were expressed (Figure S2). mRNA coding for mPGES-2 was slightly but significantly increased in LDv as compared to healthy SV. The cyclooxygenases were also analysed at the protein levels (Figure S3), COX-2 was not detectable while a significant increase was shown for COX-1 content.
Figure 1. Dysregulation of PGE2 synthesis.
Protein measurements, representative samples of Western blot (A) of microsomal and cytosolic prostaglandin E synthases (mPGES-1, mPGES-2 or cPGES), 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in human small and large diameter varicosities (paired SDv and LDv, n = 6) and healthy saphenous veins (SV, n = 4). Standards (Std) are Western ready controls from Cayman. Histograms represent Western blot quantification of mPGES-1 (B) and 15-PGDH (C) corresponding bands. Optical density (OD, arbitrary units) was quantified by Scion Image® and the mean normalized by actin. PGE2 content (D) in paired SDv and LDv (n = 8) and healthy saphenous veins (SV, n = 4) was determined by EIA in supernatants after 24 h of incubation of the venous preparations in RPMI solution. Results are normalized by tissue wet weight. * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
Increased Content of the PGE2 Degrading Enzyme (15-PGDH) in Varicose Veins
Using Western blot analysis, a 29 kDa band corresponding to 15-PGDH was observed in the protein extracts of saphenous veins corresponding to the standard band (Ready control; Cayman). A significant increase (∼188%) was found in varicose veins (SDv and LDv; n = 5) as compared to healthy SV (n = 5; Figure 1C).
Decreased PGE2 Content in Varicose Veins
EIA measurements showed significantly decreased PGE2 concentrations in the solution incubated for 24 h (Figure 1D) and for 30 min (Figure S4) with varicose veins (SDv and LDv; n = 5) as compared to healthy SV (n = 5). Interestingly, a progressive and significant decrease of the PGE2 levels was observed between healthy SV, SDv and LDv respectively. Similar results were found after 30 min incubation with arachidonic acid (1 µmol/L), in the presence or absence of glutathione (2.5 mmol/L) (data not shown, n = 3–4). This decreased content in PGE2 in varicose veins was associated with an increased production of TxA2 and PGD2, measured as their stable metabolites TxB2 and 15d-PGJ2, respectively (Figure S5).
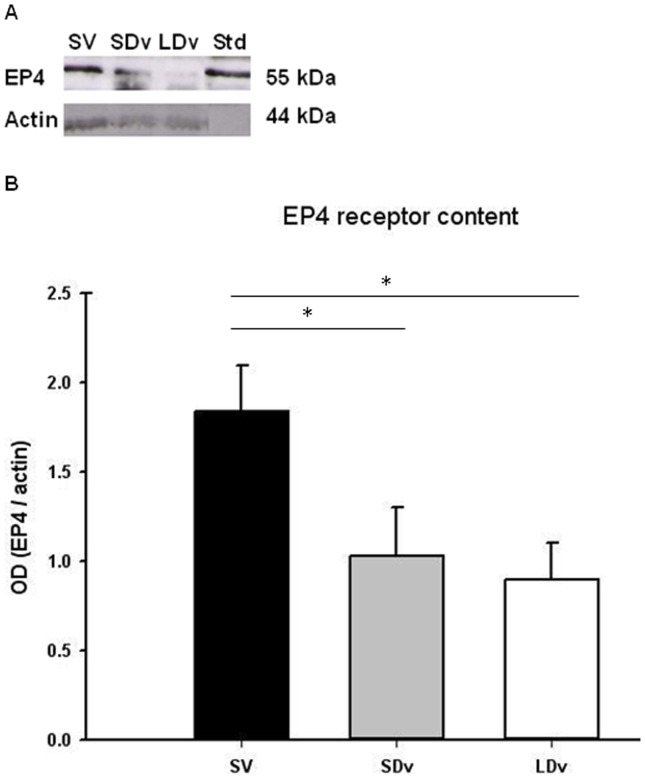
Decreased EP4 Receptor Content in Varicose Veins
Using Western blot analysis, a strong band (about 55 kDa) was observed for the EP4 receptor (Figures 2 A, B) in the homogenates of saphenous veins. Protein density in varicose veins (SDv and LDv; n = 7) was significantly lower than in healthy SV (n = 4).
Figure 2. EP4 receptor decreased in varicose veins.
Protein measurements, representative samples of Western blot (A) of EP4 receptor in human small and large diameter varicosities (paired SDv and LDv, n = 7) and healthy saphenous veins (SV, n = 4). Western blot quantification of EP4 (B). Optical density (OD, arbitrary units) was quantified by Scion Image® and normalized by α-actin; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
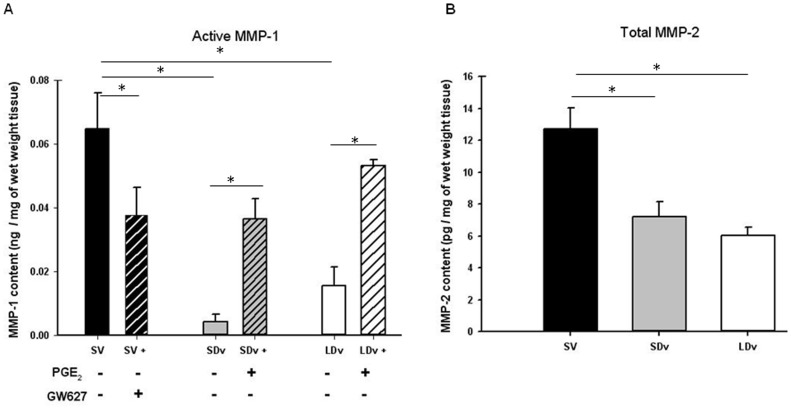
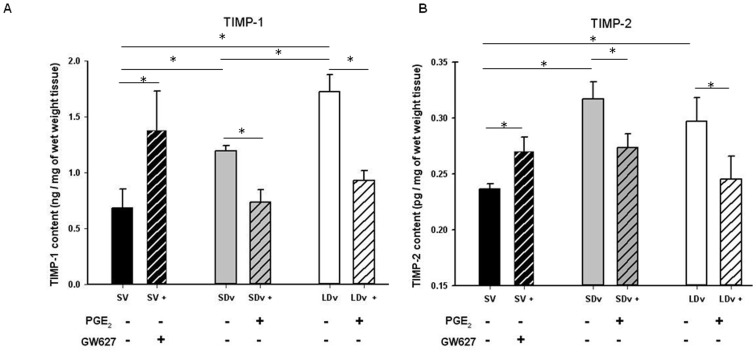
Expression of MMPs and TIMPs
EIA measurements for MMP-1 and MMP-2 (Figures 3 A, B), TIMP-1 and TIMP-2 (Figures 4 A, B) were performed in the same samples to allow valid comparison and show significant changes. For MMP-1, measurements of pro-MMP and total MMP were made and the quantity of active MMP-1 was calculated. The subtraction showed a decrease in active MMP-1 in varicose veins (SDv and LDv; n = 6) as compared with healthy SV (n = 4). Incubation with PGE2 led to the activation of MMP-1 in varicose veins (SDv and LDv) to the same level as in healthy SV. The saphenous veins incubated with an EP4 antagonist showed a significant decrease in MMP-1 activation (Figure 3A). Total MMP-2 concentration was also measured in varicose veins (n = 5) and compared with healthy SV (n = 5) after a 30 min incubation. A significant decrease in MMP-2 expression was found in varicose veins (SDv and LDv) compared to healthy SV (Figure 3B). TIMP-1 measurement showed a significant increase in the varicose veins (SDv and LDv) compared to healthy SV (Figure 4A). Addition of PGE2 induced a decreased TIMP expression in varicose veins (SDv and LDv) whereas the healthy veins incubated with the EP4 antagonist showed a significant increase in TIMP. The EIA measurement for TIMP-2 (Figure 4B) showed similar results as for TIMP-1, a significant increase in its content in varicose veins, both small and large diameter, as compared to the healthy SV. For TIMP-1, we also observed a significant increase in its content in LDv as compared with SDv. Incubation with PGE2 significantly decreased TIMP-2 content in SDv and in LDv. In the case of healthy SV, incubation with the EP4 antagonist resulted in increased TIMP-2 content.
Figure 3. PGE2 is responsible for decreased MMP-1 activity.
(A) Active MMP-1 content in human small and large diameter varicosities (paired SDv and LDv, n = 6) and healthy saphenous veins (SV, n = 4) after 24 h of incubation. Treatments with either PGE2 10 µmol/L or GW62768X 1 µmol/L are represented by hatched bars. (B) Total MMP-2 content in varicose veins (n = 5) and SV (n = 4) after 30 min of incubation. Values were determined by EIA in supernatants and normalized by tissue wet weight; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
Figure 4. PGE2 is responsible for increased TIMP production.
TIMP-1 (A) and TIMP-2 (B) contents in human small and large diameter varicosities (paired SDv and LDv, n = 6) and healthy saphenous veins (SV, n = 4). Treatments with either PGE2 10 µmol/L or GW62768X 1 µmol/L are represented by hatched bars. Values were determined by EIA in supernatants after 24 h of incubation. Values are normalized by tissue wet weight; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
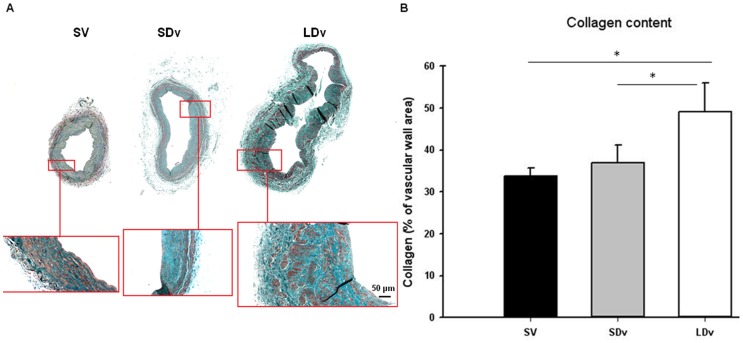
Increased Collagen Content in Varicose Veins
Using histomorphometry, collagen content was measured on cross sections of healthy (n = 3) and varicose (n = 5) SV (Figures 5 A, B). A significantly higher content of collagen was found in LDv as compared to healthy SV and SDv.
Figure 5. Increase of collagen content in varicose veins.
Histomorphometric measurement of collagen content after Masson’s Trichrome staining (A) in human small and large diameter varicosities (paired SDv and LDv, n = 5) and healthy saphenous veins (magnificence 4X and 10X) (SV, n = 3). (B) Values obtained after Photoshop measurement of colour density. Values are normalized by total surface area; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
Discussion
Our results demonstrate that in the varicose veins, PGE2 plays a major role in vascular wall remodeling and in collagen over-expression. The reduced synthesis (via decreased mPGES-1) and/or increased degradation (via increased 15-PGDH) of PGE2 are responsible for its lower concentration in human varicose saphenous veins when compared to healthy ones. This reduced PGE2 content and the lower density of its receptor (EP4) are responsible for the down-regulation of the MMP/TIMP ratio in varicose veins. The consequence of this biological cascade is a reduction of active collagenase content and an accumulation of collagen in the vascular wall of varicose veins and could explain the intima hyperplasia and the thickening observed in the varicose wall [4], [37]. This phenomenon is in complete accordance with a recent publication where selective deletion of mPGES-1 in both endothelial and vascular smooth muscle cells resulted in hyperplasia by enhancing the neointimal proliferative response to vascular injury in mice [38].
In human saphenous vein preparations, PGES are coupled only with the constitutive COX-1 since COX-2 was not detectable [19] and this kind of coupling was also previously observed in non-inflammatory conditions. [15] In our study, protein (Figure 1) and mRNA (Figure S2) levels of the different PGES isoforms were analysed. cPGES protein was not found in all sample and was only detectable at the mRNA level. In contrast, mPGES-2 protein was found at similar levels in all venous preparations. Our results concerning mPGES-1 were unexpected since we found this enzyme in the SV while mPGES-1 expression is classically induced by inflammatory conditions via NF-κB. [9] However, this isoform has also been found as a constitutive enzyme in some human cell types, [18], [39] such as fibroblasts, [40] the astroglioma cell line [41] or vascular smooth muscle cells. [12] In these studies, positive correlations between the presence of mPGES-1 and a higher level of PGE2 are shown. We also showed a significant decrease (50–75%) in mPGES-1, at both protein and mRNA levels, in LDv compared to the other segments (SV and SDv). This decreased content could be due to several mechanisms such as an increased of PGD2 metabolite, 15d-PGJ2 (as observed in Figure S5) which is known to down regulate mPGES-1 [42] or by other endogenous regulator. In addition, we found that 15-PGDH content was increased in varicose veins (SDv and LDv). Taken together, our measurements of these enzymes involved in the synthesis and degradation of PGE2 are in favour of a reduced concentration of this prostaglandin in varicose veins. This effect is not due to a decreased expression of COX-1 since the protein content was increased (Supplementary data). This dysregulation of PGE2 production led to a reorganisation of PG synthesis such as an increased thromboxane A2 and PGD2 production (Figure S5).
In our human saphenous vein preparations, the accumulation of released PGE2 is in the “pg/mg of tissue” range after 30 min incubation as previously described [43] and greater (1000 fold) after 24 h incubation as previously shown. [44] However, in both cases, in comparison to SV, similar and significant reductions in PGE2 concentrations were found, i.e. 22–23% and 44–59% in the supernatant of SDv and LDv, respectively. These decreases in PGE2 concentration in SDv, and even more so in LDv, correlate with the severity of the pathology and are in complete accordance with our results concerning the expression of the enzymes responsible for PGE2 metabolism. More precisely, in SDv, only increased 15-PGDH expression could explain the decrease in PGE2, while in LDv, both the increase in15-PGDH and the decrease in mPGES-1 may participate in the more pronounced decrease in PGE2 concentration. An inverse regulation has been observed in human pancreatic tumors where enhanced PGE2 production proceeds via the over-expressions of COX-2 and microsomal PGES-1 and the down-regulation of 15-PGDH by SNAI2. [45] In addition, the lower concentrations of PGE2 measured in varicose veins will have lower EP4-mediated effects since a significant decrease in this receptor density was found in varicose (SDv and LDv) as compared with SV. For example, the EP4-mediated vasodilation previously reported in human healthy saphenous veins [43] should be reduced in varicose veins. Similarly, MMP expression could be modified by decreases in both PGE2 and EP4 receptor expression.
MMP -1, -2, -9, some enzymes responsible for ECM degradation, and/or their inhibitors TIMP-1, -2, have been measured in human varicose veins and the results of many studies are controversial (see reviews [2], [34]). MMP quantity or activity was shown to be increased or decreased in varicose veins as compared to healthy SV. In these publications, the varicose vein preparations originated from different locations and from patients with various clinical stages of chronic venous disease ranging from C1 to C6, facts which could explain the variable results described. In many of the studies, only total MMP was quantified, there was no distinction between the pro- and active- MMP and the calculations of the ratio active-MMP versus TIMP were even rarer. Our results describe a decrease in active MMP-1, total MMP-2 (while total MMP-1 is not modified in varicose veins) and an increase in TIMP-1 and TIMP-2 concentrations in varicose veins. They are in accordance with previously reported measurements in the same tissues [33], [46], [47] and several immunohistochemistry experiments. [48], [49], [50].
The relation between PGE2 and MMPs has been demonstrated in some vessel types but there is no report in human saphenous veins. Our experiments are in favour of a clear relation between PGE2 and MMP activation, a process that is decreased in human varicose veins. In healthy SV, the addition of the EP4 antagonist, GW627368X, produced both a decrease in active MMP-1 and an increase in TIMP-1/-2 concentrations; a reverse effect was observed in varicose veins with exogenous PGE2 stimulation. These MMP/TIMP expressions regulated by PGE2 are mostly dependent upon EP4 receptor activation, as suggested by the GW627368X antagonism. However, PGE2 or EP4 antagonist did not totally restore or abolish the activation of MMP-1, respectively. It is unlikely that the EP2 receptor could be involved since, EP2 mRNA, was not found in human saphenous vein. [43] Other pathways, such as Reactive Oxygen Species, could be implicated. [51].
Our results on the histological quantification of collagen, a major vascular wall component, support our results on MMP/TIMP regulation between human healthy and varicose veins. Masson’s trichrome staining showed significant increases in collagen content in the LDv where MMP-1, the enzyme responsible for its degradation, and to a lesser extent, MMP-2, are significantly reduced as compared to healthy SV. Furthermore, this result is in accordance with previous studies where investigators found an increase in type I collagen content in segments of varicose veins compared to normal veins. Hydroxyproline, the major amino acid of collagen was also augmented. [30], [52], [53].
In contrast to varicose veins, in many other human vascular pathologies the reverse is observed: vascular wall remodeling is associated with an increased PGE2 synthesis. Gomez-Hernandez et al. [26] demonstrated that in the plasma of patients with acute coronary syndrome, higher plasma PGE2 concentrations were found and these correlated with MMP-9 activity. In human atherosclerotic plaques, mPGES-1 and EP4 receptors are over-expressed. [27], [54], [55] Similarly, studies on the vascular wall of human aneurysms showed an increase in mPGES-1 expression, [56] in PGE2 production [57] and in EP4-receptor presence. [22] A selective EP4-receptor agonist (ONO-329-AE1) increased the activation of MMP-2 in human aortic aneurysm explants. [22] Finally, changes in the vascular wall composition and formation of aortic aneurysms could be prevented in mice knocked-out for mPGES-1 or EP4-receptors. [22], [58], [59] This discrepancy between these other vascular pathologies and human varicose vein pathology could be explained by the inflammatory environment which is absent only in the latter case. [19] Although in the above-mentioned vascular pathologies and in varicose veins an opposite process is displayed, overall, these studies showed a control of vascular wall remodeling via PGE2 metabolism and EP4 receptor stimulation.
In conclusion, the reduction of PGE2 concentrations in human varicose veins is due to a decrease in mPGES-1 and an increase in 15-PGDH. These effects lead to the imbalance of vascular wall remodeling by decreasing the MMP/TIMP ratio (Table 1) and could result in the accumulation of collagen in varicose veins. This endogenous mechanism could be a protective effect of the saphenous vein in order to restrain the blood stasis by reinforcing the vascular wall, avoiding ectasic segment formation and venous wall rupture.
Table 1. Ratio (Active MMP-1)/TIMP-1 and TIMP-2.
| Tissue | (active MMP-1)/TIMP-1 | (active MMP-1)/TIMP-2 |
| SV n = 4 | 0.10±0.024 | 254.13±75.92 |
| SDv n = 6 | 0.008±0.003* | 40.19±14.48* |
| LDv n = 6 | 0.011±0.002* | 59.43±10.55* |
Measurements were obtained by ELISA for all human small and large diameter varicosities (paired SDv and LDv) and healthy saphenous veins (SV). Values are obtained after division of active MMP-1 values by TIMP-1 and TIMP-2 values normalized per mg of weight tissue. Statistical analysis was performed using one-way-ANOVA followed by the Tukey post-hoc test or by paired t test.
*indicates a significant difference (P<0.05) with SV.
Supporting Information
cPGES expression in human internal mammary artery (IMA). (A) Histogram represent western blot analysis for internal mammary arteries (IMA, n = 3) for cPGES. (B) Representative samples. Optical density (OD, arbitrary units) was measured by Scion Image® and the mean normalized by actin.
(TIF)
Dysregulation in PGES mRNA. mRNA expression in human small and large diameter varicosities (paired SDv and LDv, n = 5) and healthy saphenous veins (SV, n = 5). mRNA levels of prostaglandin E synthases (mPGES-1, mPGES-2 or cPGES) were determined by Real-Time PCR and normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Increased COX-1 protein in varicose veins. Protein measurements (A), representative samples of Western blot of cyclooxygenases (COX-1 or COX-2) in human small and large diameter varicosities (paired SDv and LDv, n = 6) and healthy saphenous veins (SV, n = 4). Standards (Std) are Western ready controls from Cayman. Histogram (B) represents Western blot quantification of COX-1 corresponding band. Optical density (OD, arbitrary units) was measured by Scion Image® and the mean normalized by actin; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Decreased PGE2 content in 30 min. PGE2 content in human small and large diameter varicosities (paired SDv and LDv, n = 8) and healthy saphenous veins (SV, n = 4). Values were determined by EIA in supernatants after 30 min of incubation of the venous preparations in Tyrode solution. Results are normalized by tissue wet weight; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Prostanoids expression. Thromboxane (Tx) B2 and 15d-PGJ2 content in human small and large diameter varicosities (paired SDv and LDv, n = 5) and healthy saphenous veins (SV, n = 5). Values were determined by EIA in supernatants after 24 hours of incubation of the venous preparations in RPMI solution. Results are normalized by tissue wet weight; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Supporting information about experimentals protocols. Table S1. Primers used for Real-Time PCR, s: sense, as: anti sense.
(DOC)
Acknowledgments
We would like to thanks Dr Nadir Cheurfa (Inserm U698; Paris, France) and Tariya Zaoui (CHU X. Bichat; Paris, France) for their help in obtaining the vascular preparations and Dr Mary Osborne-Pellegrin for help in editing the manuscript.
Funding Statement
This work was supported by INSERM (Institut National de la Santé et de la Recherche Médicale). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Raffetto JD, Khalil RA (2008) Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology 23: 85–98. [DOI] [PubMed] [Google Scholar]
- 2. Lim CS, Davies AH (2009) Pathogenesis of primary varicose veins. Br J Surg 96: 1231–1242. [DOI] [PubMed] [Google Scholar]
- 3. Golledge J, Quigley FG (2003) Pathogenesis of varicose veins. Eur J Vasc Endovasc Surg 25: 319–324. [DOI] [PubMed] [Google Scholar]
- 4. Oklu R, Habito R, Mayr M, Deipolyi AR, Albadawi H, et al. (2012) Pathogenesis of varicose veins. J Vasc Interv Radiol 23: 33–39. [DOI] [PubMed] [Google Scholar]
- 5. Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, et al. (2006) Chronic venous disease. N Engl J Med 355: 488–498. [DOI] [PubMed] [Google Scholar]
- 6. Foudi N, Gomez I, Benyahia C, Longrois D, Norel X (2012) Prostaglandin E(2) receptor subtypes in human blood and vascular cells. Eur J Pharmacol 695: 1–6. [DOI] [PubMed] [Google Scholar]
- 7. Norel X (2007) Prostanoid receptors in the human vascular wall. ScientificWorldJournal 7: 1359–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foudi N, Louedec L, Cachina T, Brink C, Norel X (2009) Selective cyclooxygenase-2 inhibition directly increases human vascular reactivity to norepinephrine during acute inflammation. Cardiovasc Res 81: 269–277. [DOI] [PubMed] [Google Scholar]
- 9.Gomez I, Foudi N, Longrois D, Norel X (2013) The role of prostaglandin E in human vascular inflammation. Prostaglandins Leukot Essent Fatty Acids. [DOI] [PubMed]
- 10. Gomez PF, Pillinger MH, Attur M, Marjanovic N, Dave M, et al. (2005) Resolution of inflammation: prostaglandin E2 dissociates nuclear trafficking of individual NF-kappaB subunits (p65, p50) in stimulated rheumatoid synovial fibroblasts. J Immunol 175: 6924–6930. [DOI] [PubMed] [Google Scholar]
- 11. Jakobsson PJ, Thoren S, Morgenstern R, Samuelsson B (1999) Identification of human prostaglandin E synthase: a microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc Natl Acad Sci U S A 96: 7220–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Camacho M, Gerboles E, Escudero JR, Anton R, Garcia-Moll X, et al. (2007) Microsomal prostaglandin E synthase-1, which is not coupled to a particular cyclooxygenase isoenzyme, is essential for prostaglandin E(2) biosynthesis in vascular smooth muscle cells. J Thromb Haemost 5: 1411–1419. [DOI] [PubMed] [Google Scholar]
- 13. St-Jacques B, Ma W (2013) Prostaglandin E2/EP4 signalling facilitates EP4 receptor externalization in primary sensory neurons in vitro and in vivo. Pain 154: 313–323. [DOI] [PubMed] [Google Scholar]
- 14. Lee J, Banu SK, Subbarao T, Starzinski-Powitz A, Arosh JA (2011) Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol 332: 306–313. [DOI] [PubMed] [Google Scholar]
- 15. Park JY, Pillinger MH, Abramson SB (2006) Prostaglandin E2 synthesis and secretion: the role of PGE2 synthases. Clin Immunol 119: 229–240. [DOI] [PubMed] [Google Scholar]
- 16. Gudis K, Tatsuguchi A, Wada K, Futagami S, Nagata K, et al. (2005) Microsomal prostaglandin E synthase (mPGES)-1, mPGES-2 and cytosolic PGES expression in human gastritis and gastric ulcer tissue. Lab Invest 85: 225–236. [DOI] [PubMed] [Google Scholar]
- 17. Thoren S, Weinander R, Saha S, Jegerschold C, Pettersson PL, et al. (2003) Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J Biol Chem 278: 22199–22209. [DOI] [PubMed] [Google Scholar]
- 18. Samuelsson B, Morgenstern R, Jakobsson PJ (2007) Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol Rev 59: 207–224. [DOI] [PubMed] [Google Scholar]
- 19. Gomez I, Benyahia C, Le Dall J, Payre C, Louedec L, et al. (2013) Absence of inflammatory conditions in human varicose saphenous veins. Inflamm Res 62: 299–308. [DOI] [PubMed] [Google Scholar]
- 20. Yen JH, Kocieda VP, Jing H, Ganea D (2011) Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem 286: 38913–38923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanchez C, Gabay O, Salvat C, Henrotin YE, Berenbaum F (2009) Mechanical loading highly increases IL-6 production and decreases OPG expression by osteoblasts. Osteoarthritis Cartilage 17: 473–481. [DOI] [PubMed] [Google Scholar]
- 22. Yokoyama U, Ishiwata R, Jin MH, Kato Y, Suzuki O, et al. (2012) Inhibition of EP4 signaling attenuates aortic aneurysm formation. PLoS One 7: e36724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vargova V, Pytliak M, Mechirova V (2012) Matrix metalloproteinases. Exs 103: 1–33. [DOI] [PubMed] [Google Scholar]
- 24. Benjamin MM, Khalil RA (2012) Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exs 103: 209–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jacob MP (2003) Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomed Pharmacother 57: 195–202. [DOI] [PubMed] [Google Scholar]
- 26. Gomez-Hernandez A, Sanchez-Galan E, Ortego M, Martin-Ventura JL, Blanco-Colio LM, et al. (2008) Effect of intensive atorvastatin therapy on prostaglandin E2 levels and metalloproteinase-9 activity in the plasma of patients with non-ST-elevation acute coronary syndrome. Am J Cardiol 102: 12–18. [DOI] [PubMed] [Google Scholar]
- 27. Cipollone F, Fazia M, Iezzi A, Ciabattoni G, Pini B, et al. (2004) Balance between PGD synthase and PGE synthase is a major determinant of atherosclerotic plaque instability in humans. Arterioscler Thromb Vasc Biol 24: 1259–1265. [DOI] [PubMed] [Google Scholar]
- 28. Newby AC (2012) Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol 56: 232–244. [DOI] [PubMed] [Google Scholar]
- 29. Kurihara T, Shimizu-Hirota R, Shimoda M, Adachi T, Shimizu H, et al. (2012) Neutrophil-derived matrix metalloproteinase 9 triggers acute aortic dissection. Circulation 126: 3070–3080. [DOI] [PubMed] [Google Scholar]
- 30. Sansilvestri-Morel P, Fioretti F, Rupin A, Senni K, Fabiani JN, et al. (2007) Comparison of extracellular matrix in skin and saphenous veins from patients with varicose veins: does the skin reflect venous matrix changes? Clin Sci (Lond) 112: 229–239. [DOI] [PubMed] [Google Scholar]
- 31. Kowalewski R, Sobolewski K, Wolanska M, Gacko M (2004) Matrix metalloproteinases in the vein wall. Int Angiol 23: 164–169. [PubMed] [Google Scholar]
- 32. Gillespie DL, Patel A, Fileta B, Chang A, Barnes S, et al. (2002) Varicose veins possess greater quantities of MMP-1 than normal veins and demonstrate regional variation in MMP-1 and MMP-13. J Surg Res 106: 233–238. [DOI] [PubMed] [Google Scholar]
- 33. Badier-Commander C, Verbeuren T, Lebard C, Michel JB, Jacob MP (2000) Increased TIMP/MMP ratio in varicose veins: a possible explanation for extracellular matrix accumulation. J Pathol 192: 105–112. [DOI] [PubMed] [Google Scholar]
- 34. Kucukguven A, Khalil RA (2013) Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr Drug Targets 14: 287–324. [PMC free article] [PubMed] [Google Scholar]
- 35. Wilson RJ, Giblin GM, Roomans S, Rhodes SA, Cartwright KA, et al. (2006) GW627368X ((N-{2-[4-(4,9-diethoxy-1-oxo-1,3-dihydro-2H-benzo[f]isoindol-2-yl)phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol 148: 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho-Tin-Noe B, Le Dall J, Gomez D, Louedec L, Vranckx R, et al. (2011) Early atheroma-derived agonists of peroxisome proliferator-activated receptor-gamma trigger intramedial angiogenesis in a smooth muscle cell-dependent manner. Circ Res 109: 1003–1014. [DOI] [PubMed] [Google Scholar]
- 37. Badier-Commander C, Couvelard A, Henin D, Verbeuren T, Michel JB, et al. (2001) Smooth muscle cell modulation and cytokine overproduction in varicose veins. An in situ study. J Pathol 193: 398–407. [DOI] [PubMed] [Google Scholar]
- 38. Chen L, Yang G, Xu X, Grant G, Lawson JA, et al. (2013) Cell selective cardiovascular biology of microsomal prostaglandin E synthase-1. Circulation 127: 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murakami M, Nakashima K, Kamei D, Masuda S, Ishikawa Y, et al. (2003) Cellular prostaglandin E2 production by membrane-bound prostaglandin E synthase-2 via both cyclooxygenases-1 and -2. J Biol Chem 278: 37937–37947. [DOI] [PubMed] [Google Scholar]
- 40. Bage T, Kats A, Lopez BS, Morgan G, Nilsson G, et al. (2011) Expression of prostaglandin E synthases in periodontitis immunolocalization and cellular regulation. Am J Pathol 178: 1676–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Payner T, Leaver HA, Knapp B, Whittle IR, Trifan OC, et al. (2006) Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E(2)-dependent activation of type II protein kinase A. Mol Cancer Ther. 5: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 42. Mendez M, LaPointe MC (2003) PPARgamma inhibition of cyclooxygenase-2, PGE2 synthase, and inducible nitric oxide synthase in cardiac myocytes. Hypertension 42: 844–850. [DOI] [PubMed] [Google Scholar]
- 43. Foudi N, Kotelevets L, Gomez I, Louedec L, Longrois D, et al. (2011) Differential reactivity of human mammary artery and saphenous vein to prostaglandin E(2) : implication for cardiovascular grafts. Br J Pharmacol 163: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bishop-Bailey D, Pepper JR, Haddad EB, Newton R, Larkin SW, et al. (1997) Induction of cyclooxygenase-2 in human saphenous vein and internal mammary artery. Arterioscler Thromb Vasc Biol 17: 1644–1648. [DOI] [PubMed] [Google Scholar]
- 45. Pham H, Chen M, Li A, King J, Angst E, et al. (2010) Loss of 15-hydroxyprostaglandin dehydrogenase increases prostaglandin E2 in pancreatic tumors. Pancreas 39: 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parra JR, Cambria RA, Hower CD, Dassow MS, Freischlag JA, et al. (1998) Tissue inhibitor of metalloproteinase-1 is increased in the saphenofemoral junction of patients with varices in the leg. J Vasc Surg 28: 669–675. [DOI] [PubMed] [Google Scholar]
- 47. Woodside KJ, Hu M, Burke A, Murakami M, Pounds LL, et al. (2003) Morphologic characteristics of varicose veins: possible role of metalloproteinases. J Vasc Surg 38: 162–169. [DOI] [PubMed] [Google Scholar]
- 48. Aravind B, Saunders B, Navin T, Sandison A, Monaco C, et al. (2010) Inhibitory effect of TIMP influences the morphology of varicose veins. Eur J Vasc Endovasc Surg 40: 754–765. [DOI] [PubMed] [Google Scholar]
- 49. Chang JW, Maeng YH, Kim SW (2011) Expression of matrix metalloproteinase-2 and -13 and tissue inhibitor of metalloproteinase-4 in varicose veins. Korean J Thorac Cardiovasc Surg 44: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ishikawa Y, Asuwa N, Ishii T, Ito K, Akasaka Y, et al. (2000) Collagen alteration in vascular remodeling by hemodynamic factors. Virchows Arch 437: 138–148. [DOI] [PubMed] [Google Scholar]
- 51. Kar S, Subbaram S, Carrico PM, Melendez JA (2010) Redox-control of matrix metalloproteinase-1: a critical link between free radicals, matrix remodeling and degenerative disease. Respir Physiol Neurobiol 174: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gandhi RH, Irizarry E, Nackman GB, Halpern VJ, Mulcare RJ, et al. (1993) Analysis of the connective tissue matrix and proteolytic activity of primary varicose veins. J Vasc Surg 18: 814–820. [PubMed] [Google Scholar]
- 53. Sansilvestri-Morel P, Rupin A, Badier-Commander C, Kern P, Fabiani JN, et al. (2001) Imbalance in the synthesis of collagen type I and collagen type III in smooth muscle cells derived from human varicose veins. J Vasc Res 38: 560–568. [DOI] [PubMed] [Google Scholar]
- 54. Cipollone F, Fazia ML, Iezzi A, Cuccurullo C, De Cesare D, et al. (2005) Association between prostaglandin E receptor subtype EP4 overexpression and unstable phenotype in atherosclerotic plaques in human. Arterioscler Thromb Vasc Biol 25: 1925–1931. [DOI] [PubMed] [Google Scholar]
- 55. Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, et al. (2002) Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem 277: 44147–44154. [DOI] [PubMed] [Google Scholar]
- 56. Hasan D, Hashimoto T, Kung D, Macdonald RL, Winn HR, et al. (2012) Upregulation of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: preliminary results. Stroke 43: 1964–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reilly JM, Miralles M, Wester WN, Sicard GA (1999) Differential expression of prostaglandin E2 and interleukin-6 in occlusive and aneurysmal aortic disease. Surgery 126: 624–627 discussion 627–628. [PubMed] [Google Scholar]
- 58. Cao RY, St Amand T, Li X, Yoon SH, Wang CP, et al. (2012) Prostaglandin receptor EP4 in abdominal aortic aneurysms. Am J Pathol 181: 313–321. [DOI] [PubMed] [Google Scholar]
- 59. Wang M, Lee E, Song W, Ricciotti E, Rader DJ, et al. (2008) Microsomal prostaglandin E synthase-1 deletion suppresses oxidative stress and angiotensin II-induced abdominal aortic aneurysm formation. Circulation 117: 1302–1309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
cPGES expression in human internal mammary artery (IMA). (A) Histogram represent western blot analysis for internal mammary arteries (IMA, n = 3) for cPGES. (B) Representative samples. Optical density (OD, arbitrary units) was measured by Scion Image® and the mean normalized by actin.
(TIF)
Dysregulation in PGES mRNA. mRNA expression in human small and large diameter varicosities (paired SDv and LDv, n = 5) and healthy saphenous veins (SV, n = 5). mRNA levels of prostaglandin E synthases (mPGES-1, mPGES-2 or cPGES) were determined by Real-Time PCR and normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA level; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Increased COX-1 protein in varicose veins. Protein measurements (A), representative samples of Western blot of cyclooxygenases (COX-1 or COX-2) in human small and large diameter varicosities (paired SDv and LDv, n = 6) and healthy saphenous veins (SV, n = 4). Standards (Std) are Western ready controls from Cayman. Histogram (B) represents Western blot quantification of COX-1 corresponding band. Optical density (OD, arbitrary units) was measured by Scion Image® and the mean normalized by actin; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Decreased PGE2 content in 30 min. PGE2 content in human small and large diameter varicosities (paired SDv and LDv, n = 8) and healthy saphenous veins (SV, n = 4). Values were determined by EIA in supernatants after 30 min of incubation of the venous preparations in Tyrode solution. Results are normalized by tissue wet weight; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Prostanoids expression. Thromboxane (Tx) B2 and 15d-PGJ2 content in human small and large diameter varicosities (paired SDv and LDv, n = 5) and healthy saphenous veins (SV, n = 5). Values were determined by EIA in supernatants after 24 hours of incubation of the venous preparations in RPMI solution. Results are normalized by tissue wet weight; * P<0.05 as determined by one-way-ANOVA followed by the Tukey post-hoc test and by a paired t-test for varicose veins.
(TIF)
Supporting information about experimentals protocols. Table S1. Primers used for Real-Time PCR, s: sense, as: anti sense.
(DOC)