Abstract
Calcium ion (Ca2+) concentration plays a key role in cell signaling in eukaryotic cells. At the cellular level, Ca2+ directly participates in such diverse cellular events as adhesion and migration, differentiation, contraction, secretion, synaptic transmission, fertilization, and cell death. As a consequence of these diverse actions, the cytosolic concentration of free Ca2+ is tightly regulated by the coordinated activity of Ca2+ channels, Ca2+ pumps, and Ca2+-binding proteins. Although many of these regulators have been studied in depth, other proteins have been described recently, and naturally far less is known about their contribution to cell physiology. Within this last group of proteins, STIM1 has emerged as a major contributor to Ca2+ signaling by means of its activity as Ca2+ channel regulator. STIM1 is a protein resident mainly, but not exclusively, in the endoplasmic reticulum (ER), and activates a set of plasma membrane Ca2+ channels termed store-operated calcium channels (SOCs) when the concentration of free Ca2+ within the ER drops transiently as a result of Ca2+ release from this compartment. Knowledge regarding the molecular architecture of STIM1 has grown considerably during the last years, and several structural domains within STIM1 have been reported to be required for the specific molecular interactions with other important players in Ca2+ signaling, such as Ca2+ channels and microtubules. Within the modulators of STIM1, phosphorylation has been shown to both activate and inactivate STIM1-dependent Ca2+ entry depending on the cell type, cell cycle phase, and the specific residue that becomes modified. Here we shall review current knowledge regarding the modulation of STIM1 by phosphorylation.
Keywords: Calcium signaling, phosphorylation, STIM1, store-operated calcium entry, ORAI1, EB1, microtubules
Background
The cytosolic free Ca2+ concentration ([Ca2+]i) in eukaryotic cells is in the low nanomolar range (~100 nM), and transient modifications of this concentration serve as an intracellular mechanism for cell signaling because of the existence of a variety of effectors that are sensitive to such transient increases. For instance, in many cases the binding of extracellular ligands to their receptors, including G protein-coupled receptors (GCPRs) located at the plasma membrane, triggers the phosphoinositide pathway by means of the activation of phospholipase C. This activation is responsible for the hydrolysis of phosphatidylinositol 4,5-bisphosphate and the subsequent generation of inositol 1,4,5-trisphosphate (Ins(1,4,5)P3) and 1,2-diacylglycerol (DAG). Soluble Ins(1,4,5)P3 binds to its receptor (Ins(1,4,5)P3R) in the endoplasmic reticulum (ER), whereas the more lipophilic DAG accumulates in the plasma membrane. Ins(1,4,5)P3R is a receptor-operated Ca2+ channel that activates by binding to its endogenous ligand Ins(1,4,5)P3, leading to transient Ca2+ release from the ER and thereby transiently increasing [Ca2+]i.1,2 Many Ca2+-sensitive effectors are found in the cytosol, which contribute to the diversity of the intracellular signaling initiated by the Ca2+ release from the ER. Alternatively, other Ca2+ channels located in the plasma membrane such as the ligand-gated plasma membrane P2X receptor and the voltage-operated Ca2+ channels families regulate the influx of extracellular Ca2+ to the cytosol upon biding to ligands or upon plasma membrane depolarization conditions, respectively. We highlight these examples to draw attention to the fact that there are two major sources of Ca2+ that may increase [Ca2+]i, - the extracellular milieu, and the intracellular compartments including the most important intracellular Ca2+ store, the ER and the specialized sarcoplasmic reticulum (SR) in muscle cells. In all cases the increase of [Ca2+]i is transient, and the high cytosolic Ca2+ levels are rapidly cut down by the activation of Ca2+ pumps in the plasma membrane and the ER. Whereas the plasma membrane Ca2+-ATPase (PMCA) extrudes Ca2+ into the extracellular milieu, the sarco(endo)plasmic Ca2+-ATPase (SERCA) pumps Ca2+ into the lumen of the ER.3 Other active Ca2+ transport systems are also involved in the restoration of cytosolic Ca2+ basal levels, such as the Na+/Ca2+ exchanger with a lower affinity for Ca2+ compared with Ca2+ pumps,4 and the mitochondrial Ca2+ uniporter (MCU) which transport Ca2+ into the mitochondria matrix.5 The combination of the activities of all Ca2+ transport systems defines specific Ca2+ transients in terms of amplitude, intensity, and duration which vary according to the initial stimulus and the cell type. Thus, the number, type and molar ratios of the molecules involved in the origin and maintenance of the Ca2+ mobilization define the spatio-temporal pattern of Ca2+ signaling. For instance, Ca2+ signaling during fertilization of mammalian oocytes is initiated by the sperm-specific phospholipase C zeta (PLCζ) that triggers the phosphoinositide pathway once PLCζ has been released into the oocyte after the fusion with the sperm.6 This pathway induces an initial increase of [Ca2+]i in the oocyte which last for 1–2 min, which is followed by repetitive and transient increases of [Ca2+]i, required for the exit from cell cycle arrest of oocytes in the metaphase of the second meiotic division.7 Another classical example of Ca2+ mobilization required to trigger downstream effects is found in skeletal muscle cells. Depolarization of plasma membrane induces a conformational change in dihydropyridine receptors (DHPRs, voltage-dependent, L-type, plasma membrane Ca2+ channels) which activate ryanodine receptors located in the SR, enabling the Ca2+ release from this intracellular store.8 These are just two classical examples of how Ca2+ fluxes regulate cell physiology, which also indicate the importance of intracellular Ca2+ stores for this signaling. To ensure the durability and maintenance of the signaling, a plasma membrane Ca2+ transport systems regulates intracellular Ca2+ stores refilling. This Ca2+ entry pathway is called store-operated Ca2+ entry (SOCE),9 a ubiquitous mechanism and one of the most important pathways for Ca2+ entry in non-excitable cells. Consistent with its descriptive name, SOCE is regulated by the Ca2+ concentration within the ER.9,10
STIM1 Regulates Ca2+ Entry
The mechanism that links luminal Ca2+ levels with plasma membrane Ca2+ entry is mediated by STIM1 (stromal interaction molecule 1), a type I transmembrane protein that senses Ca2+ within the luminal space of the ER, and activates plasma membrane Ca2+ channels (SOCs) upon Ca2+ depletion conditions in the intracellular stores.11-13 Human STIM1 protein consists of 685 aminoacids, with an SAM domain (sterile α motif) and an EF-hand domain that acts as a Ca2+ sensor within the luminal space of the ER. Upon depletion of Ca2+ within the ER, the SAM-EF domain mediates the oligomerization of STIM1,14,15 triggering this oligomerization in clusters of ~1 μm diameter. The clustering favors the relocalization of STIM1 in ER-plasma membrane (PM) junctions,15-17 required for the activation of SOCE. STIM1 directly binds to store-operated calcium channels (SOCs), activating a highly Ca2+-selective, non-voltage-gated, inwardly rectifying current known as the Ca2+ release activated Ca2+ current (ICRAC). This current is reconstituted by both STIM1 and ORAI1 (also known as CRACM1), a plasma membrane protein that constitutes a major Ca2+ channel regulated by STIM1.18-22 In addition to ORAI1, some of the transient receptor potential canonical (TRPC) channels can function in a STIM1-dependent mode. STIM1 directly binds and activates TRPC1, TRPC4 and TRPC5 channels that can therefore act as SOCs.23 However, the STIM1/TRPC molar ratio determines the STIM1-dependent or STIM1-independent mode of action of the channels.24 In addition, TRPC1 also associates with ORAI1 to produce TRPC1-ORAI1-STIM1 ternary complexes that act as SOCs.25
It was early found that the C-terminal portion of STIM1 is sufficient for the activation of ORAI1, and that both proteins physically interact through a coiled-coil domain in the C-terminus of ORAI1.16 The minimal domain of STIM1 that activates ORAI1 is a conserved CRAC activation domain (CAD) of STIM1 of ~100 amino acids that binds directly to ORAI1. An alternative given name for this domain is STIM1-ORAI1 activation region (SOAR).26,27 The cytosolic domain (residues 234–685) contains three coiled-coil domains, CC1, CC2, and CC3. CC2 and CC3 overlap with the SOAR/CAD region required to activate Ca2+ channels (aminoacids 334–442).16,26,27 Because CC1 interacts with CC2/CC3 at resting state, keeping STIM1 in a closed conformation, the activation of STIM1 by store depletion and the binding of STIM1 with ORAI1 requires an intramolecular transition of STIM1 into an open conformation, in order to expose CC2 and CC3 so they are able to activate ORAI1 by a physical interaction of coiled-coil domains.28,29 Investigation of the mechanism of TRPC1 gating led to the proposal that it involves an electrostatic mechanism through the interaction of two negative charged aspartates in TRPC1 and the conserved polybasic domain, enriched in lysine residues, at the C-terminus of STIM1.30 An additional Ca2+ channel that is regulated by STIM1 belongs to the voltage-operated Ca2+ channels family. STIM1 binding to Cav1.2 channels suppresses the activation of these channels, an action that is mediated also by the SOAR/CAD domain of STIM1, causing long-term internalization of the channel from the membrane.31,32 Thus, multiple actions are triggered by the cytosolic domain of STIM1, including the activation and inactivation of diverse channels in response to different stimuli. It is plausible that the cytosolic domain encompasses additional domains that modulate the Ca2+ channel-regulation activity of STIM1, by controlling STIM1 subcellular localization and/or the selectivity of the Ca2+ channel to be activated or inhibited. In this regard, STIM1 has a serine/proline-rich domain (or S/P)-rich domain which could be acting as a modulator of the STIM1 activity.
Phosphorylation of STIM1 Regulates its Activity
STIM1 is a phosphoprotein33 in which large-scale mass spectrometry studies have revealed potential phosphorylation sites.34-36 Subsequent initial reports of detailed analyses of phosphoresidues from immunoprecipitated STIM1 differed in their findings, mainly because the studies had focused on different cell cycle stages.37-39 During the M-phase, SOCE becomes inactivated,40,41 and STIM1 clustering remains inactive in response to store depletion.38,39 Although Yu et al. reported that STIM1 is phosphorylated during meiosis of Xenopus oocytes, substitution mutations of target residues to mimic constitutive phosphorylation or dephosphorylation do not modulate the clustering of STIM1 in response to store depletion, an observation that supports the lack of any physiological function for STIM1 phosphorylation during meiosis in Xenopus oocytes.39 Smyth et al. found that STIM1 clustering is also inactivated during mitosis of mammalian cells,38 and they identified specific residues, such as Ser602 and Ser608, that become dephosphorylated during that process. Other sites were initially found to be constitutively phosphorylated (Ser575, Ser620, and Ser621).38 Interestingly, Ser486 and Ser668 becomes phosphorylated during mitosis, but not in interphase.38 Ser668 belongs to a consensus sequence for cyclin-dependent kinase 1 (CDK1), and is phosphorylated by CDK1 in vitro. Also, the expression of single alanine substitution mutations (S668A or S486A) does not rescue SOCE in mitotic cells. However, expression of a double mutant S486A/S668A does show SOCE responses in mitosis,38 confirming the role of STIM1 phosphorylation at Ser486 and Ser668 in SOCE inactivation during mitosis.
Further evidence was that extracellular signal-regulated kinases 1/2 (ERK1/2) phosphorylate STIM1 in vitro at Ser575, Ser608, and Ser62137 and that STIM1 phosphorylation at ERK1/2 target sites regulates SOCE in HEK293 cells.37,42 The phosphorylation of STIM1 at Ser575, Ser608, and Ser621 was revealed by mass spectrometry using immunoprecipitated STIM1 from asynchronous HEK293 cells,37 and later with phospho-specific antibodies against phosphorylated residues.42 This latter strategy demonstrated that STIM1 phosphorylation at ERK1/2 target sites increases during SOCE activation, and consequently the alanine substitution mutation of these sites nullifies SOCE, whereas Ser-to-Glu mutation enhances Ca2+ entry.37,42 In contrast to the results reported in [38], phospho-specific antibodies against phosphoSer575, phosphoSer608, and phosphoSer621 revealed a dynamic phosphorylation of STIM1 that was strongly dependent on the Ca2+ store filling state.42 Thus, Ca2+ store depletion is accompanied by an increase of STIM1 phosphorylation at ERK1/2 target sites, whereas Ca2+ store refilling triggers STIM1 dephosphorylation at these sites.42 Many aspects of the molecular mechanism by which the phosphorylation of STIM1 regulates SOCE remain unclear, but the inhibition of STIM1 phosphorylation decreases STIM1 clustering in response to store depletion42 and impairs STIM1-ORAI1 binding, as monitored by fluorescence resonance energy transfer (FRET) and by co-immunoprecipitation.37 In an attempt to resolve the open question of the requirement of STIM1 phosphorylation at Ser575, Ser608, and Ser621 to activate SOCE in HEK293 cells during interphase, we recently found that phosphorylation of STIM1 at ERK1/2 target sites regulates the association of STIM1 with EB1 (end-binding protein 1), a regulator of growing microtubule ends.43-45 The role of the cytoskeleton in SOCE regulation has been studied in depth,46-50 and it was early established that STIM1 colocalizes with tubulin and that the treatment of HEK293 cells with nocodazole, which triggers microtubule depolymerization, severly reduces SOCE.46 Later, Sampieri et al. reported that STIM1 travels through the ER in association with EB1 under resting conditions, confirming the connection between STIM1 and microtubules, and that the association ceases upon depletion of the ER, facilitating the aggregation of STIM1 into large clusters.51 Those authors also observed that STIM1 re-associates with EB1 when intracellular calcium stores are replenished, i.e., STIM1-EB1 dissociation is fully reversible. Because STIM1 binds to EB1 in the resting state, STIM1 can be considered a microtubule plus-end-tracking protein (+TIP).52 There are two modes of direct interaction between EB1 and +TIPs - either through a cytoskeleton-associated protein glycine-rich (CAP-Gly) domain or by binding to a Ser/Thr-x-Ile-Pro (S/TxIP) consensus sequence.53 As a +TIP, STIM1 localization is dependent on microtubule formation, and a STIM1 sequence encompassing residues 642–645 (Thr-Arg-Ile-Pro) has been found to be essential for the binding to EB1.54 For those +TIPs with an S/TxIP motif, examples of regulation by phosphorylation in the vicinity of this domain, but not within this sequence, have been reported. These examples include APC,55,56 MCAK,57 and CLASP2,58,59 all of which are phosphorylated in the vicinity of the S/TxIP sequence, regulating their interaction with microtubules. In all cases, as with STIM1, sequences flanking the S/TxIP motif contain a high number of proline, serine, and basic residues, leading to a net positive charge in the surroundings of this EB1 binding domain. This observation might explain why phosphorylation in the vicinity of this sequence blocks the localization of +TIPs to microtubule ends. In this regard, the phosphorylation of STIM1 at residues Ser575, Ser608, and Ser621 triggers the dissociation of STIM1 from EB1.42 Because refilling of Ca2+ stores is accompanied by dephosphorylation of these residues, this reversible phosphorylation constitutes a mechanism that fully explains the reversible interaction of STIM1 and EB1 (see Figure 1). Indeed, Ser-to-Ala substitution mutants of STIM1 do not dissociate from EB1 under store depletion conditons, and Ser-to-Glu mutants remain dissociated from EB1 even under resting conditions.42 In accordance with the proposed model described in Figure 1, those stimuli that induce store depletion and ERK1/2 activation lead to phosphorylation of STIM1 at Ser575, Ser608, and Ser621. This specific phosphorylation triggers the dissociation of STIM1 from EB1, which in turn facilitates STIM1 clustering and the binding to SOCs to activate Ca2+ entry. Replenisment of Ca2+ stores induces STIM1 dephosphorylation by as yet to be described phosphatases, leading to the reassociation of STIM1 with EB1, and the microtubule localization pattern of STIM1 (Fig. 1).
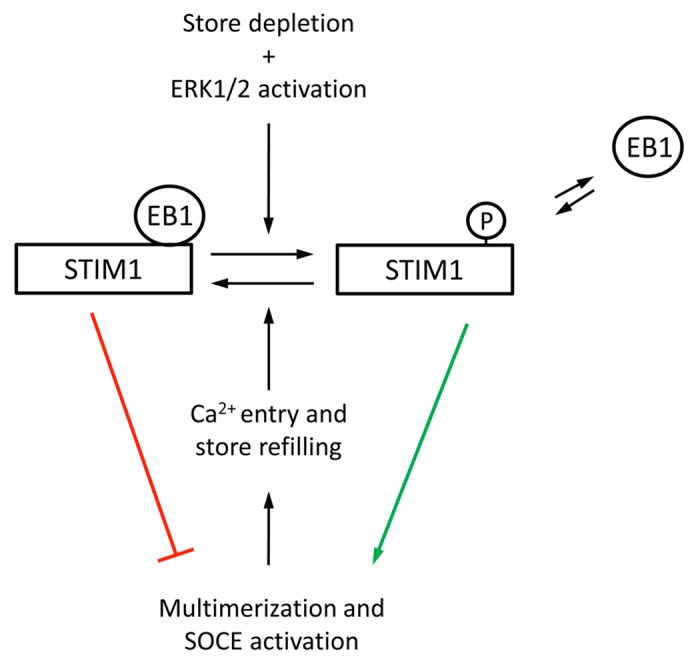
Figure 1. Mechanism for the phosphor-regulation of STIM1-EB1 interaction, proposed in [42].
As has been reported for other +TIPs, the phosphor-regulation of STIM1-EB1 interaction could be more complex, and other phosphoresidues might trigger a diversity of actions. For instance, the Ser-to-Ala substitution mutation of 10 residues in STIM1 leads to ER mislocalization by pulling ER tubules into the mitotic spindle.60 Because in wild-type STIM1-expressing cells ER tubules are excluded from this mitotic spindle, those results suggest that hyperphosphorylation of STIM1 is required to maintain ER normal structure in mitosis. However, it would be necessary to monitor the phosphorylation state of individual residues to discover which, if not all, of the residues are involved in this specific control of localization.
Finally, other phosphorylation sites at Tyr residues could be important for the initial steps of SOCE activation, because an increase of total phosphorylated Tyr has been detected in STIM1 from platelets under store depletion conditions, and the inhibition of this phosphorylation decreases the binding of STIM1 to ORAI1 and therefore SOCE.61 However, the identification of the specific modified residues and the molecular mechanism underlying this regulation remains to be clarified.
With the recent studies of the role of STIM1 phosphorylation on cell physiology, one has just started to understand the physiological relevance of this modulation. In addition to HEK293 cells, STIM1 phosphorylation, enhanced by thapsigargin, has been reported in neonatal rat ventricular myocytes.62 Also, it is known that phosphorylation of STIM1 at Ser575, one of the target sites of the ERK1/2 activity, promotes the differentiation of cultured mouse C2C12 myoblasts to myocytes.63 In parallel, it has been shown that STIM1 is necessary and sufficient for cardiomyocyte hypertrophy in vitro and in the adult heart in vivo.64,65 Taken togeteher, these recent results indicate that the activity of STIM1 as a Ca2+ channels regulator is important for skeletal and cardiac muscle cells development, and that STIM1 phosphorylation constitutes a potential pharmacological target for the treatment of muscle hypertrophy.
The MEK-ERK pathway was early suggested as being involved in the activation of SOCE in human platelets, probably as a downstream effector of Ras proteins,66 and it has recently been demonstrated that constitutive dephosphorylation of STIM1 at ERK1/2 target sites impairs platelet adhesion to fibrinogen,67 supporting a physiological role for STIM1 phosphorylation by this signaling pathway.
In conclusion, phosphorylation regulates the activation of STIM1. However, the phosphorylation of different target residues has diverse effects. Because a differential phosphorylation profile can trigger opposing actions, the phosphorylation state of target residues needs to be defined under different stimuli so that one can establish molecular models capable of predicting STIM1 activities, particularly under pathophysiological conditions. In this scenario, phosphorylation target residues could be investigated as potential pharmacological targets for the treatment of diseases, since this would not alter upstream signaling cascades, and would therefore limit the intensity of side effects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by grant BFU2011–22798 of the Spanish Ministerio de Economia y Competitividad and European Social Fund.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26283
References
- 1.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–25. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 3.Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- 4.Carafoli E, Santella L, Branca D, Brini M. Generation, control, and processing of cellular calcium signals. Crit Rev Biochem Mol Biol. 2001;36:107–260. doi: 10.1080/20014091074183. [DOI] [PubMed] [Google Scholar]
- 5.Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, Wright L, Pallafacchina G, Terrin A, Mammucari C, et al. The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem. 2013;288:10750–8. doi: 10.1074/jbc.R112.420752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129:3533–44. doi: 10.1242/dev.129.15.3533. [DOI] [PubMed] [Google Scholar]
- 7.Cuthbertson KS, Cobbold PH. Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca(2+) Nature. 1985;316:541–2. doi: 10.1038/316541a0. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe T, Beam KG, Adams BA, Niidome T, Numa S. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 1990;346:567–9. doi: 10.1038/346567a0. [DOI] [PubMed] [Google Scholar]
- 9.Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Putney JW., Jr. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–10. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Jr., Meyer T. STIM is a Ca(2+) sensor essential for Ca(2+)-store-depletion-triggered Ca(2+) influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, et al. STIM1, an essential and conserved component of store-operated Ca(2+) channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, Ellisman MH, Stauderman KA, Cahalan MD. STIM1 is a Ca(2+) sensor that activates CRAC channels and migrates from the Ca(2+) store to the plasma membrane. Nature. 2005;437:902–5. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J Biol Chem. 2006;281:35855–62. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 15.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. Proc Natl Acad Sci USA. 2007;104:9301–6. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muik M, Frischauf I, Derler I, Fahrner M, Bergsmann J, Eder P, Schindl R, Hesch C, Polzinger B, Fritsch R, et al. Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J Biol Chem. 2008;283:8014–22. doi: 10.1074/jbc.M708898200. [DOI] [PubMed] [Google Scholar]
- 17.Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr. Ca2+-store-dependent and -independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–72. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soboloff J, Spassova MA, Tang XD, Hewavitharana T, Xu W, Gill DL. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–5. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- 19.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–9. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 21.Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca(2+) entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 23.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–45. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KP, Yuan JP, So I, Worley PF, Muallem S. STIM1-dependent and STIM1-independent function of transient receptor potential canonical (TRPC) channels tunes their store-operated mode. J Biol Chem. 2010;285:38666–73. doi: 10.1074/jbc.M110.155036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong HL, Cheng KT, Liu X, Bandyopadhyay BC, Paria BC, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh BB, et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–16. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136:876–90. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan JP, Zeng W, Dorwart MR, Choi YJ, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol. 2009;11:337–43. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korzeniowski MK, Manjarres IM, Varnai P, Balla T. Activation of STIM1-Orai1 involves an intramolecular switching mechanism. Sci Signal. 2011;3:ra82. doi: 10.1126/scisignal.2001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muik M, Fahrner M, Schindl R, Stathopulos P, Frischauf I, Derler I, Plenk P, Lackner B, Groschner K, Ikura M, et al. STIM1 couples to ORAI1 via an intramolecular transition into an extended conformation. EMBO J. 2011;30:1678–89. doi: 10.1038/emboj.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeng W, Yuan JP, Kim MS, Choi YJ, Huang GN, Worley PF, Muallem S. STIM1 gates TRPC channels, but not Orai1, by electrostatic interaction. Mol Cell. 2008;32:439–48. doi: 10.1016/j.molcel.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CY, Shcheglovitov A, Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–5. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, Soboloff J, Tang XD, Gill DL. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–9. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manji SS, Parker NJ, Williams RT, van Stekelenburg L, Pearson RB, Dziadek M, Smith PJ. STIM1: a novel phosphoprotein located at the cell surface. Biochim Biophys Acta. 2000;1481:147–55. doi: 10.1016/S0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 34.Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–48. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 35.Villen J, Beausoleil SA, Gerber SA, Gygi SP. Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci USA. 2007;104:1488–93. doi: 10.1073/pnas.0609836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan C, Gnad F, Olsen JV, Mann M. Quantitative phosphoproteome analysis of a mouse liver cell line reveals specificity of phosphatase inhibitors. Proteomics. 2008;8:4534–46. doi: 10.1002/pmic.200800105. [DOI] [PubMed] [Google Scholar]
- 37.Pozo-Guisado E, Campbell DG, Deak M, Alvarez-Barrientos A, Morrice NA, Alvarez IS, Alessi DR, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites modulates store-operated calcium entry. J Cell Sci. 2010;123:3084–93. doi: 10.1242/jcs.067215. [DOI] [PubMed] [Google Scholar]
- 38.Smyth JT, Petranka JG, Boyles RR, DeHaven WI, Fukushima M, Johnson KL, Williams JG, Putney JW., Jr. Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat Cell Biol. 2009;11:1465–72. doi: 10.1038/ncb1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu F, Sun L, Machaca K. Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci USA. 2009;106:17401–6. doi: 10.1073/pnas.0904651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machaca K, Haun S. Store-operated calcium entry inactivates at the germinal vesicle breakdown stage of Xenopus meiosis. J Biol Chem. 2000;275:38710–5. doi: 10.1074/jbc.M007887200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston SF, Sha'afi RI, Berlin RD. Regulation of Ca2+ influx during mitosis: Ca2+ influx and depletion of intracellular Ca2+ stores are coupled in interphase but not mitosis. Cell Regul. 1991;2:915–25. doi: 10.1091/mbc.2.11.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pozo-Guisado E, Casas-Rua V, Tomas-Martin P, Lopez-Guerrero AM, Alvarez-Barrientos A, Martin-Romero FJ. Phosphorylation of STIM1 at ERK1/2 target sites regulates interaction with the microtubule plus-end binding protein EB1. J Cell Sci. 2013;126:3170–80. doi: 10.1242/jcs.125054. [DOI] [PubMed] [Google Scholar]
- 43.Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–6. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berrueta L, Kraeft SK, Tirnauer JS, Schuyler SC, Chen LB, Hill DE, Pellman D, Bierer BE. The adenomatous polyposis coli-binding protein EB1 is associated with cytoplasmic and spindle microtubules. Proc Natl Acad Sci USA. 1998;95:10596–601. doi: 10.1073/pnas.95.18.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison EE, Wardleworth BN, Askham JM, Markham AF, Meredith DM. EB1, a protein which interacts with the APC tumour suppressor, is associated with the microtubule cytoskeleton throughout the cell cycle. Oncogene. 1998;17:3471–7. doi: 10.1038/sj.onc.1202247. [DOI] [PubMed] [Google Scholar]
- 46.Smyth JT, DeHaven WI, Bird GS, Putney JW., Jr. Role of the microtubule cytoskeleton in the function of the store-operated Ca2+ channel activator STIM1. J Cell Sci. 2007;120:3762–71. doi: 10.1242/jcs.015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monastyrskaya K, Babiychuk EB, Hostettler A, Wood P, Grewal T, Draeger A. Plasma membrane-associated annexin A6 reduces Ca2+ entry by stabilizing the cortical actin cytoskeleton. J Biol Chem. 2009;284:17227–42. doi: 10.1074/jbc.M109.004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russa AD, Ishikita N, Masu K, Akutsu H, Saino T, Satoh Y. Microtubule remodeling mediates the inhibition of store-operated calcium entry (SOCE) during mitosis in COS-7 cells. Arch Histol Cytol. 2008;71:249–63. doi: 10.1679/aohc.71.249. [DOI] [PubMed] [Google Scholar]
- 49.Redondo PC, Harper AG, Sage SO, Rosado JA. Dual role of tubulin-cytoskeleton in store-operated calcium entry in human platelets. Cell Signal. 2007;19:2147–54. doi: 10.1016/j.cellsig.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 50.Itagaki K, Kannan KB, Singh BB, Hauser CJ. Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol. 2004;172:601–7. doi: 10.4049/jimmunol.172.1.601. [DOI] [PubMed] [Google Scholar]
- 51.Sampieri A, Zepeda A, Asanov A, Vaca L. Visualizing the store-operated channel complex assembly in real time: identification of SERCA2 as a new member. Cell Calcium. 2009;45:439–46. doi: 10.1016/j.ceca.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Grigoriev I, Gouveia SM, van der Vaart B, Demmers J, Smyth JT, Honnappa S, Splinter D, Steinmetz MO, Putney JW, Jr., Hoogenraad CC, et al. STIM1 is a MT-plus-end-tracking protein involved in remodeling of the ER. Curr Biol. 2008;18:177–82. doi: 10.1016/j.cub.2007.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura N, Draviam VM. Microtubule plus-ends within a mitotic cell are 'moving platforms' with anchoring, signalling and force-coupling roles. Open Biol. 2012;2:120132. doi: 10.1098/rsob.120132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Honnappa S, Gouveia SM, Weisbrich A, Damberger FF, Bhavesh NS, Jawhari H, Grigoriev I, van Rijssel FJ, Buey RM, Lawera A, et al. An EB1-binding motif acts as a microtubule tip localization signal. Cell. 2009;138:366–76. doi: 10.1016/j.cell.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 55.Zumbrunn J, Kinoshita K, Hyman AA, Nathke IS. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3 beta phosphorylation. Curr Biol. 2001;11:44–9. doi: 10.1016/S0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 56.Nathke IS. The adenomatous polyposis coli protein: the Achilles heel of the gut epithelium. Annu Rev Cell Dev Biol. 2004;20:337–66. doi: 10.1146/annurev.cellbio.20.012103.094541. [DOI] [PubMed] [Google Scholar]
- 57.Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev Cell. 2004;6:253–68. doi: 10.1016/S1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe T, Noritake J, Kakeno M, Matsui T, Harada T, Wang S, Itoh N, Sato K, Matsuzawa K, Iwamatsu A, et al. Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J Cell Sci. 2009;122:2969–79. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- 59.Kumar P, Lyle KS, Gierke S, Matov A, Danuser G, Wittmann T. GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment. J Cell Biol. 2009;184:895–908. doi: 10.1083/jcb.200901042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smyth JT, Beg AM, Wu S, Putney JW, Jr., Rusan NM. Phosphoregulation of STIM1 leads to exclusion of the endoplasmic reticulum from the mitotic spindle. Curr Biol. 2012;22:1487–93. doi: 10.1016/j.cub.2012.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lopez E, Jardin I, Berna-Erro A, Bermejo N, Salido GM, Sage SO, Rosado JA, Redondo PC. STIM1 tyrosine-phosphorylation is required for STIM1-Orai1 association in human platelets. Cell Signal. 2012;24:1315–22. doi: 10.1016/j.cellsig.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 62.Zhu-Mauldin X, Marsh SA, Zou L, Marchase RB, Chatham JC. Modification of STIM1 by O-linked N-Acetylglucosamine (O-GlcNAc) Attenuates Store-operated Calcium Entry in Neonatal Cardiomyocytes. J Biol Chem. 2012;287:39094–106. doi: 10.1074/jbc.M112.383778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee HJ, Bae GU, Leem YE, Choi HK, Kang TM, Cho H, Kim ST, Kang JS. Phosphorylation of Stim1 at serine575 via netrin-2/Cdo-activated ERK1/2 is critical for the promyogenic function of Stim1. Mol Biol Cell. 2012;23:1376–87. doi: 10.1091/mbc.E11-07-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hulot JS, Fauconnier J, Ramanujam D, Chaanine A, Aubart F, Sassi Y, Merkle S, Cazorla O, Ouille A, Dupuis M, et al. Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation. 2011;124:796–805. doi: 10.1161/CIRCULATIONAHA.111.031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luo X, Hojayev B, Jiang N, Wang ZV, Tandan S, Rakalin A, Rothermel BA, Gillette TG, Hill JA. STIM1-dependent store-operated Ca(2)(+) entry is required for pathological cardiac hypertrophy. J Mol Cell Cardiol. 2012;52:136–47. doi: 10.1016/j.yjmcc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosado JA, Sage SO. Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. J Biol Chem. 2001;276:15659–65. doi: 10.1074/jbc.M009218200. [DOI] [PubMed] [Google Scholar]
- 67.Elvers M, Herrmann A, Seizer P, Munzer P, Beck S, Schonberger T, Borst O, Martin-Romero FJ, Lang F, May AE, et al. Intracellular cyclophilin A is an important Ca2+ regulator in platelets and critically involved in arterial thrombus formation. Blood. 2012;120:1317–26. doi: 10.1182/blood-2011-12-398438. [DOI] [PubMed] [Google Scholar]


