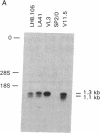
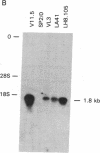
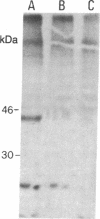
Abstract
Two different antigen-specific radiation leukemia virus (RadLV)-transformed suppressor T-cell clones, LH8.105 and LA41, exhibiting anti-lysozyme and anti-acetylcholine-receptor suppressor activity, respectively, have been examined for rearrangement and expression of genes encoding the alpha and beta chains of the T-cell receptor for antigen. LH8.105 cells express the T-cell-receptor polypeptides, as shown by specific immunoprecipitation. In both cell lines, potentially functional transcripts of alpha- and beta-chain genes are detected by RNA blot analysis. These suppressor T-cell clones exhibit alpha-chain gene rearrangements, deletion of both alleles of the constant-region (C) gene segment C beta 1, and rearrangement of the two alleles of C beta 2 when analyzed by Southern blot hybridization. Restriction analysis suggests that the DNA rearrangement is beyond the second joining-region (J) minigene of the J beta 2 cluster. These results establish that at least some mouse suppressor T-cell clones, like helper and cytotoxic T lymphocytes, rearrange and transcribe the genes coding for the alpha and beta chains of the antigen-specific T-cell receptor.
Full text
PDF

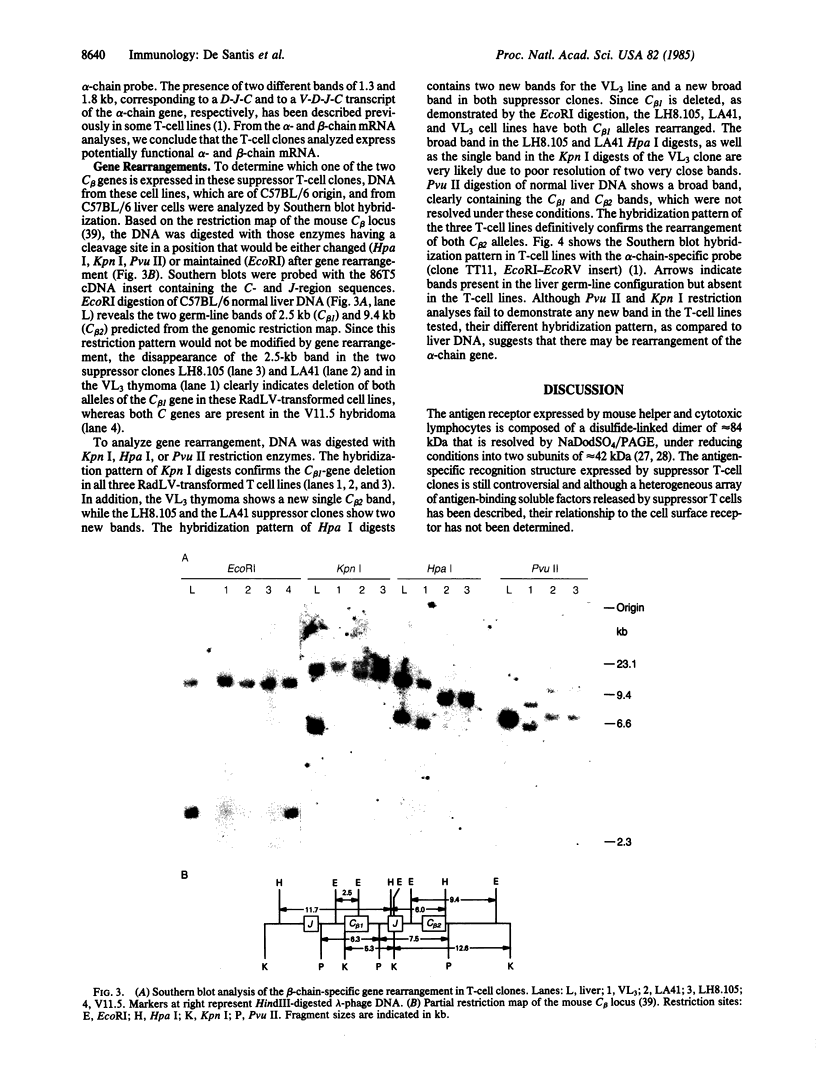


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acuto O., Fabbi M., Smart J., Poole C. B., Protentis J., Royer H. D., Schlossman S. F., Reinherz E. L. Purification and NH2-terminal amino acid sequencing of the beta subunit of a human T-cell antigen receptor. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3851–3855. doi: 10.1073/pnas.81.12.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorini L., Colizzi V., Doria G., Ricciardi-Castagnoli P. Immunoregulation of lysozyme-specific suppression. II. Hen egg-white lysozyme-specific monoclonal suppressor T cell factor suppresses the afferent phase of delayed-type hypersensitivity and induces second-order suppressor T cells. Eur J Immunol. 1984 Sep;14(9):826–830. doi: 10.1002/eji.1830140911. [DOI] [PubMed] [Google Scholar]
- Adorini L., Doria G., Ricciardi-Castagnoli P. Fine antigenic specificity and genetic restriction of lysozyme-specific suppressor T cell factor produced by radiation leukemia virus-transformed suppressor T cells. Eur J Immunol. 1982 Sep;12(9):719–724. doi: 10.1002/eji.1830120905. [DOI] [PubMed] [Google Scholar]
- Adorini L., Pini C., De Santis R., Robbiati F., Doria G., Ricciardi-Castagnoli P. Monoclonal suppressor T-cell factor displaying V H restriction and fine antigenic specificity. Nature. 1983 Jun 23;303(5919):704–706. doi: 10.1038/303704a0. [DOI] [PubMed] [Google Scholar]
- Ballinari D., Castelli C., Traversari C., Pierotti M. A., Parmiani G., Palmieri G., Ricciardi-Castagnoli P., Adorini L. Disulfide-linked surface molecules of monoclonal antigen-specific suppressor T cells: evidence for T cell receptor structures. Eur J Immunol. 1985 Aug;15(8):855–860. doi: 10.1002/eji.1830150822. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Chien Y., Becker D. M., Lindsten T., Okamura M., Cohen D. I., Davis M. M. A third type of murine T-cell receptor gene. Nature. 1984 Nov 1;312(5989):31–35. doi: 10.1038/312031a0. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Yoshikai Y., Taylor S., Siu G., Hood L., Mak T. W. Identification of a diversity segment of human T-cell receptor beta-chain, and comparison with the analogous murine element. 1984 Sep 27-Oct 3Nature. 311(5984):387–389. doi: 10.1038/311387a0. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Goodfellow P. N., Spurr N. K., Solomon E., Tanigawa G., Tonegawa S., Owen M. J. The human T-cell receptor alpha-chain gene maps to chromosome 14. Nature. 1985 Mar 21;314(6008):273–274. doi: 10.1038/314273a0. [DOI] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Rosenthal P. N., Lung M. L., Kaplan H. S. Physicochemical, biological and serological properties of a leukemogenic virus isolated from cultured RadLV-induced lymphomas of C57BL/Ka mice. Virology. 1978 Oct 1;90(1):23–35. doi: 10.1016/0042-6822(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Dembić Z., Bannwarth W., Taylor B. A., Steinmetz M. The gene encoding the T-cell receptor alpha-chain maps close to the Np-2 locus on mouse chromosome 14. Nature. 1985 Mar 21;314(6008):271–273. doi: 10.1038/314271a0. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Chien Y., Becker D. M., Kavaler J., Davis M. M. Genomic organization and sequence of T-cell receptor beta-chain constant- and joining-region genes. Nature. 1984 Aug 2;310(5976):387–391. doi: 10.1038/310387a0. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum C. H., Kappler J. W., Trowbridge I. S., Marrack P., Freed J. H. Immunoglobulin-like nature of the alpha-chain of a human T-cell antigen/MHC receptor. Nature. 1984 Nov 1;312(5989):65–67. doi: 10.1038/312065a0. [DOI] [PubMed] [Google Scholar]
- Hayday A. C., Saito H., Gillies S. D., Kranz D. M., Tanigawa G., Eisen H. N., Tonegawa S. Structure, organization, and somatic rearrangement of T cell gamma genes. Cell. 1985 Feb;40(2):259–269. doi: 10.1016/0092-8674(85)90140-0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Cohen D. I., Nielsen E. A., Davis M. M. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984 Mar 8;308(5955):149–153. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S. M., Germain R. N., Bevan M. J., Dorf M., Engel I., Fink P., Gascoigne N., Heber-Katz E., Kapp J., Kaufmann Y. Rearrangement and transcription of a T-cell receptor beta-chain gene in different T-cell subsets. Proc Natl Acad Sci U S A. 1985 Jan;82(2):531–535. doi: 10.1073/pnas.82.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. M., Nielsen E. A., Kavaler J., Cohen D. I., Davis M. M. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature. 1984 Mar 8;308(5955):153–158. doi: 10.1038/308153a0. [DOI] [PubMed] [Google Scholar]
- Kronenberg M., Goverman J., Haars R., Malissen M., Kraig E., Phillips L., Delovitch T., Suciu-Foca N., Hood L. Rearrangement and transcription of the beta-chain genes of the T-cell antigen receptor in different types of murine lymphocytes. Nature. 1985 Feb 21;313(6004):647–653. doi: 10.1038/313647a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malissen M., Minard K., Mjolsness S., Kronenberg M., Goverman J., Hunkapiller T., Prystowsky M. B., Yoshikai Y., Fitch F., Mak T. W. Mouse T cell antigen receptor: structure and organization of constant and joining gene segments encoding the beta polypeptide. Cell. 1984 Jul;37(3):1101–1110. doi: 10.1016/0092-8674(84)90444-6. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. Biosynthesis and processing of murine T-cell antigen receptor. Cell. 1984 Oct;38(3):659–665. doi: 10.1016/0092-8674(84)90260-5. [DOI] [PubMed] [Google Scholar]
- McIntyre B. W., Allison J. P. The mouse T cell receptor: structural heterogeneity of molecules of normal T cells defined by xenoantiserum. Cell. 1983 Oct;34(3):739–746. doi: 10.1016/0092-8674(83)90530-5. [DOI] [PubMed] [Google Scholar]
- Pierotti M., DeLeo A. B., Pinter A., O'Donnell P. V., Hämmerling U., Fleissner E. The GIX antigen of murine leukemia virus: an analysis with monoclonal antibodies. Virology. 1981 Jul 30;112(2):450–460. doi: 10.1016/0042-6822(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi-Castagnoli P., Robbiati F., Barbanti E., Colizzi V., Pini C., De Santis R., Doria G., Adorini L. Immunosuppression by cell-free translation products from monoclonal antigen-specific suppressor T cell mRNA. Eur J Immunol. 1985 Apr;15(4):351–355. doi: 10.1002/eji.1830150409. [DOI] [PubMed] [Google Scholar]
- Robertson M. Receptor gene rearrangement and ontogeny of T lymphocytes. 1984 Sep 27-Oct 3Nature. 311(5984):305–306. doi: 10.1038/311305a0. [DOI] [PubMed] [Google Scholar]
- Royer H. D., Bensussan A., Acuto O., Reinherz E. L. Functional isotypes are not encoded by the constant region genes of the beta subunit of the T cell receptor for antigen/major histocompatibility complex. J Exp Med. 1984 Sep 1;160(3):947–952. doi: 10.1084/jem.160.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984 Nov 1;312(5989):36–40. doi: 10.1038/312036a0. [DOI] [PubMed] [Google Scholar]
- Saito H., Kranz D. M., Takagaki Y., Hayday A. C., Eisen H. N., Tonegawa S. Complete primary structure of a heterodimeric T-cell receptor deduced from cDNA sequences. 1984 Jun 28-Jul 4Nature. 309(5971):757–762. doi: 10.1038/309757a0. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Yagüe J., Nelson J., Marrack P., Palmer E., Augustin A., Kappler J. Primary structure of human T-cell receptor alpha-chain. Nature. 1984 Dec 20;312(5996):771–775. doi: 10.1038/312771a0. [DOI] [PubMed] [Google Scholar]
- Sinigaglia F., Gotti C., Castagnoli R., Clementi F. Acetylcholine receptor-specific suppressive T-cell factor from a retrovirally transformed T-cell line. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7569–7573. doi: 10.1073/pnas.81.23.7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu G., Clark S. P., Yoshikai Y., Malissen M., Yanagi Y., Strauss E., Mak T. W., Hood L. The human T cell antigen receptor is encoded by variable, diversity, and joining gene segments that rearrange to generate a complete V gene. Cell. 1984 Jun;37(2):393–401. doi: 10.1016/0092-8674(84)90369-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Yanagi Y., Suciu-Foca N., Mak T. W. Presence of T-cell receptor mRNA in functionally distinct T cells and elevation during intrathymic differentiation. Nature. 1984 Aug 9;310(5977):506–508. doi: 10.1038/310506a0. [DOI] [PubMed] [Google Scholar]