Abstract
We previously reported that Sestd1 KO phenocopies Dact1 KO in mice, consistent with a model in which Sestd1 and Dact1 act together to form a crucial functional complex that regulates Vangl2 in the Wnt/Planar Cell Polarity (PCP) pathway. Here, we show that Dvl2, a binding partner of Dact1, also forms complexes with Sestd1, and does so independently of both Dact1 and Vangl2. In cell-based assays, whereas Sestd1 does not alter Dvl2 activation of the Wnt/β-catenin signaling pathway, Dvl2 enhances activation of Rho family GTPases by Dact1 and Sestd1, consistent with a role in the PCP pathway. In mice, although Dvl2 KO is recessive in an otherwise wild type background, it leads to dominant embryonic lethality in either the Sestd1 or Dact1 KO background. This genetic synergy stands in contrast to the epistasis we have previously reported between Sestd1 and Dact1 KO, and suggests independent or semi-independent functions for Dvl2 vs. Sestd1/Dact1 in the regulation of the PCP pathway during development. In conclusion, biochemical and genetic interactions between Dvl2, Sestd1, and Dact1, in addition to prior reported interactions between these same molecules and Vangl2, suggest that all these gene products can form complexes together and regulate the PCP pathway during mammalian development. However, Sestd1 and Dact1 have a closely allied function in the post-translational regulation of Vangl2 that is at least partially distinct from the functions of Dvl2 in this pathway.
Keywords: Dact1, Dvl2, Rho GTPase, Sestd1, Vangl2, planar cell polarity
Introduction
The Planar Cell Polarity (PCP) pathway is a major form of β-catenin-independent signaling that can occur in cells downstream of extracellular Wnt ligands and/or other intercellular interactions including between Wnt co-receptors and Van Gogh like (Vangl) 4-pass transmembrane proteins.1,2 The PCP pathway contributes to cell polarity and to polarized cell movements during development; one key developmental process to which it contributes is convergent-extension (CE) movement of cells in the primary germ layers and their immediate tissue derivatives that lengthen and narrow the embryo and close the neural tube. Although much remains to be elucidated mechanistically, it has been established that the PCP pathway coordinately regulates the subcellular localization of several transmembrane and associated intracellular proteins, and stimulates cytoskeletal rearrangements and presumably changes in cell adhesion that facilitate and promote this class of morphogenetic movements. Among several downstream PCP pathway effectors are the Rho superfamily of small GTPases (which include the Rho, Rac, and Cdc42 subfamilies), whose activity serves to mobilize actin and other cytoskeletal components.1,3,4 The PCP pathway contrasts with the “canonical” β-catenin-dependent Wnt pathway (Wnt/β-catenin signaling), whose primary target is the regulation of gene transcription in the nucleus to alter cell proliferation and cell fate.5
Dishevelled (Dvl), a scaffold protein with 3 conserved motifs (DIX, PDZ, and DEP), is involved in virtually all described types of Wnt signaling.6-9 There are 3 Dvl loci (Dvl1, 2, and 3) in the genomes of mice and humans, each with widespread and highly overlapping expression patterns during development and in the adult.10-12 Genetically engineered Dvl1 knockout (KO) mice develop grossly normally but display defects in communal and maternal behavior.13 In contrast, Dvl2 KO mice have complex birth defects that include cardiac, skeletal, and neural tube defects reflective of PCP pathway disruption during development.14 Genetic combination of the Dvl1 and Dvl2 KO in mice leads to more severe neural tube defects than the Dvl2 KO alone,15 suggesting partially redundant functions of these Dvl proteins in the PCP pathway. Mice and humans also have 3 Dact (Dapper Antagonist of Catenin, also known simply as “Dapper” or “Frodo”) loci (Dact1, 2, and 3), which encode Dvl-interacting regulators of Wnt signaling.16-21 Dact1 KO mice usually die within a day of birth from a spectrum of posterior malformations, including neural tube defects, reflecting abnormalities in the PCP pathway during embryonic development.22,23 Biochemical analyses support that the PCP pathway is disrupted in developing tissues of Dact1 KO animals. Moreover, the levels and localization of some components of the PCP pathway, notably the Vangl2 transmembrane protein, are misregulated in affected tissues of Dact1 KO embryos.22
We recently identified SEC14 and spectrin domains 1 (Sestd1) as a Dact1 binding partner. Remarkably, Sestd1 KO mice display a phenotypic spectrum identical to Dact1 KO mice.22 Biochemical studies show that loss of Sestd1, like loss of Dact1, leads to disruption of cellular PCP pathway readouts. Based on this and other evidence, we concluded that in developing tissues where they are required, Sestd1 and Dact1 form a complex that regulates Vangl2 post-translational stability and/or trafficking and the PCP pathway.24 Given that Dact1 can bind Dvl2 that has been implicated separately in the same pathway and in overlapping developmental events,14 we speculated that Sestd1 and Dvl2 might also be partners during embryogenesis. Here we provide biochemical and genetic evidence that supports complex formation and functional interactions among the Sestd1, Dact1, and Dvl2 proteins, while also demonstrating that there are likely to be important differences in the roles these proteins play in the PCP pathway and in post-translational Vangl2 regulation.
Results
Dvl2 can form a complex with Sestd1 independently of Dact1 and Vangl2
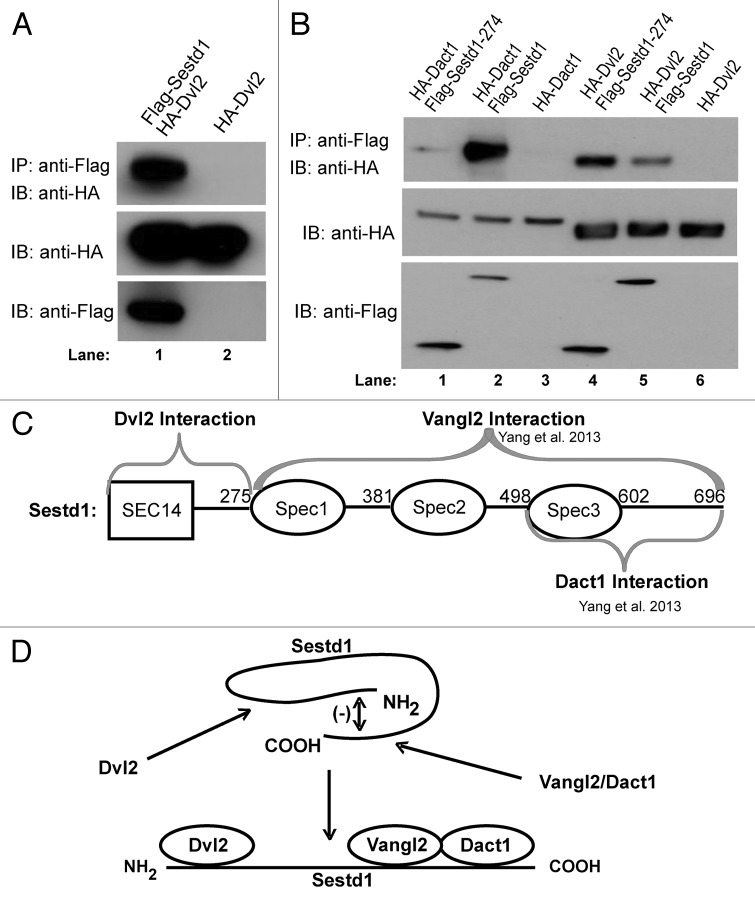
To test the hypothesis that Sestd1 can form a complex with Dvl proteins, murine Sestd1 was recombinantly coexpressed in an immortalized human cell line with one of the murine Dvl paralogs, Dvl2. Co-immunoprecipitation (co-IP) assays demonstrated that the full-length Sestd1 and Dvl2 proteins form complexes when expressed in this manner (Fig. 1A, lane 1 vs. control lane 2). However, as Dact proteins are shared binding partners of both Dvl2 and Sestd1,24,25 it remained possible that the Sestd1-Dvl2 complex formation observed in these cells might occur indirectly via mutual binding to endogenous Dact proteins. To eliminate this possibility, a murine Sestd1 deletion mutant (Sestd1 (1–274)), which does not interact with Dact proteins,24 was recombinantly coexpressed with either Dvl2 or Dact1 (as a negative control) in the same cell line and co-IP assays performed. In confirmation of our previously published finding,24 this portion of Sestd1, comprised of the SEC14-homology region and other N-terminal sequence, is stably expressed in these cells but does not form a complex above background immunoprecipitation levels with full-length Dact1 (Fig. 1B, lane 1 vs. lanes 2 and 3); nevertheless, this same N-terminal region of Sestd1 does form a complex with Dvl2 (Fig. 1B, lane 4). In fact, this region of Sestd1 associates with Dvl2 more robustly than the full-length Sestd1 protein (Fig. 1B, lane 4 vs. lane 5), suggesting that the C-terminal region of Sestd1 including the spectrin repeats, which is necessary and sufficient for association with Dact1 and Vangl2,24 inhibits association with Dvl2. This is intriguing given that the converse is true with regard to Sestd1 complex formation with Vangl2: We previously reported that the N-terminal region of Sestd1, which we show here associates with Dvl2, inhibits association with Vangl2.24 To summarize, the co-IP data suggest that Sestd1 can associate with Dvl2 independently of the Sestd1-Dact1 interaction and of the Sestd1-Vangl2 interaction (Fig. 1C), and further suggest the possibility of mutual regulatory interactions between these halves of the Sestd1 protein in complex formation (Fig. 1D).
Figure 1. Dvl2 forms a complex with Sestd1 independent of Dact1 or Vangl2. (A) Sestd1 interacts with Dvl2 in a human cell line. Flag-Sestd1 was expressed with or without HA-Dvl2, protein complexes immunoprecipitated (IP) with anti-FLAG agarose beads, and associated proteins detected by immunoblot (IB) with anti-HA antibody. (B) The interaction between Sestd1 and Dvl2 is independent of Dact1. Flag-Sestd1 or Flag-Sestd1 (1–274) was expressed with or without HA-Dact1 or HA-Dvl2, and co-IP assays performed as in A. (C) Schematic summary of complex formation between Sestd1 and Dvl2. Interacting regions are indicated by gray braces. (D) Schematic diagram of regulatory interactions between the N-terminal and C-terminal halves of the Sestd1 protein inferred from co-IP data with Dvl2, Dact1, and Vangl2.
Dvl2 cooperates with Dact1 and Sestd1 in activating Rho GTPase activity
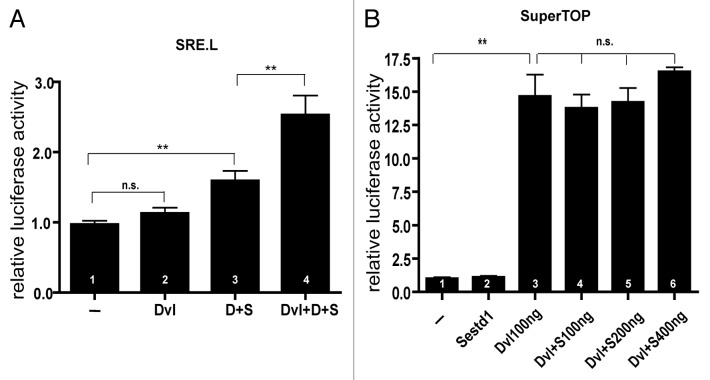
Genetic and biochemical evidence strongly suggest that Sestd1 and Dact1 act together in the PCP pathway during mouse development.22,24 Dvl2 is a key regulator of the PCP pathway and is a binding partner of both Sestd1 and Dact1.15,20,24 On this basis we reasoned that Dvl2 might affect the ability of a Sestd-Dact1 complex to activate Rho GTPases, downstream effectors of the PCP pathway.22,24 To measure this, we employed SRE.L,26 a plasmid-based luciferase reporter containing the Serum Response Element upstream of the luciferase cDNA, using conditions in an immortalized mammalian cell line that we have previously validated as a read-out of Rho GTPase activity.24 Although expression of Dvl2 alone had no effect on Rho GTPase activity based on this reporter (Fig. 2A, bar 2 vs. bar 1: Dvl2: 1.13 ± 0.078 vs. SRE: 0.97 ± 0.05, P = 0.1054), expression of Dvl2 significantly increased activity elicited in the presence of both Dact1 and Sestd1 (Fig. 2A, bar 4 vs. bar 3: Dvl2 + Dact1 + Sestd1: 2.53 ± 0.28 vs. Dact1 + Sestd1: 1.59 ± 0.14, P = 0.0086). These data suggest that Dvl2 can synergize with the Sestd1-Dact1 complex in Rho GTPase activation.
Figure 2. Dvl2 cooperates with Dact1 and Sestd1 in stimulating cellular Rho GTPase activity. (A) Dvl2, Sestd1 and Dact1 synergistically elevate Rho GTPase activity. The SRE.L reporter and Renilla plasmids were co-transfected into cells alone (–) or with Dvl2, Dact1–2A-Sestd1 (D+S) or both plasmids, and luciferase activity measured 24 h later. The graph represents relative luciferase activity, calculated as the ratio of the firefly to Renilla luciferase activity (mean ± SEM (n = 4)). (B) Dvl2 does not synergize with Sestd1 in Wnt/β-catenin-dependent luciferase reporter assay. The SuperTOP flash reporter and Renilla plasmids were co-transfected into cells alone (–) or with Sestd1, Dvl2, or both, and luciferase activity measured 24 h post-transfection. n.s., not significant (P > 0.05); **, P < 0.01.
In addition to its activity in the PCP pathway, Dvl2 can also regulate the Wnt/β-catenin signaling pathway. Unlike Dact1, which when recombinantly expressed can antagonize Wnt/β-catenin signaling by inducing degradation of co-expressed Dvl2,25,27 Sestd1 does not induce degradation of co-expressed Dvl2 in cells (Fig. 1B, lane 5 vs. 6 anti-HA blot). It has also been demonstrated that a Dact1 paralog can synergize with a co-expressed Dvl protein to activate the Wnt/β-catenin pathway under some conditions.21 To test whether Sestd1 can similarly potentiate or otherwise affect Dvl2-mediated Wnt/β-catenin signal pathway activation, we employed an assay in an immortalized mammalian cell line based on a plasmid-based reporter (SuperTOP) containing multiple T-cell factor binding sites upstream of the luciferase cDNA.28,29 As expected from previous studies,27 recombinant expression of Dvl2 significantly activated the Wnt/β-catenin signaling pathway as measured by this assay (Fig. 2B, bar 3 vs. bar 1: Dvl2: 14.64 ± 1.63 vs. SuperTOP only (–): 1.0 ± 0.10, P < 0.0001). However, recombinant expression of Sestd1 did not activate this reporter by itself (Fig. 2B, bar 2 vs. bar 1: Sestd1: 1.1 ± 0.09 vs. SuperTOP only (–): 1.0 ± 0.10, P = 0.47), nor did it affect activation of this reporter by Dvl2 (100ng) regardless of Sestd1 dose (Fig. 2B, bars 4, 5, 6 each compared vs. bar 3: Dvl2+Sestd1 100ng, 13.76 ± 1.01, P = 0.66; Dvl2+Sestd1 200ng, 14.20 ± 1.07, P = 0.83; Dvl2+Sestd1 400ng, 16.49 ± 0.34, P = 0.31; each compared vs. Dvl2 only, 14.64 ± 1.63). Together, the signaling reporter data suggest that Sestd1 functionally interacts with Dvl2 in the PCP, but not the Wnt/β-catenin, pathway.
Sestd1 and Dact1 KO display similar synergistic genetic interactions with Dvl2 KO in mice
Mutation in components of the PCP pathway, including Dact1, Sestd1, and Dvl2, lead to a CE deficit during embryonic axis elongation and to neural tube defects.14,22,24 Biochemical interactions between the Dvl2 and Sestd1 proteins suggest that mutations at the corresponding loci may genetically interact in functionally revealing ways. To test this hypothesis, an intercross was made between Sestd1–/+; Dvl2–/+ mice, and neonatal offspring genotyped and phenotyped (Table 1). Although Dvl2 is recessive (i.e., Dvl2 heterozygotes are viable and phenotypically wild type), and although mice that are null for either Dvl2 or Sestd1 can survive to birth, Sestd1–/–; Dvl2–/+ (Sestd1 null in a Dvl2 heterozygous background) and Sestd1–/–; Dvl2–/– (Sestd1 null in a Dvl2 null background) neonates are never observed. This indicates that loss of Sestd1 combined with loss of either one or both copies of Dvl2 leads to embryonic lethality. This is similar to data we obtained by intercrossing Dact1–/+; Dvl2–/+ mice. Here again, although mice that are null for either Dvl2 or Dact1 can survive to birth, and although Dvl2 is recessive, Dact1–/–; Dvl2–/+ (Dact1 null in a Dvl2 heterozygous background) neonates are reduced in number by half, and Dact1–/–; Dvl2–/– (Dact1 null in a Dvl2 null background) neonates are never observed, indicating that simultaneous loss of Dact1 and Dvl2 leads to embryonic lethality (Table 2).
Table 1. Loss of the Dvl2 gene leads to dominant embryonic lethality in a Sestd1 null background.
| Genotype |
Sestd1–/+ Dvl2–/+ |
Sestd1–/+ | Dvl2–/+ |
Sestd1–/+ Dvl2–/– |
Sestd1–/– Dvl2–/+ |
Sestd1–/– | Dvl2–/– | WT |
Sestd1–/– Dvl2–/– |
|---|---|---|---|---|---|---|---|---|---|
|
Neonatal Phenotype |
Normal | Normal | Normal | Normal | embryonic lethality |
Short tail No genital tubercle |
Normal | Normal | embryonic lethality |
|
Neonates Observed |
13 | 6 | 7 | 4 | 0 | 4 | 2 | 2 | 0 |
|
Neonates Expected |
9.5 | 4.75 | 4.75 | 4.75 | 4.75 | 2.375 | 2.375 | 2.375 | 2.375 |
|
Predicted Mendelian Ratio |
1/4 | 1/8 | 1/8 | 1/8 | 1/8 | 1/16 | 1/16 | 1/16 | 1/16 |
Sestd1–/+; Dvl2–/+ X Sestd1–/+; Dvl2–/+ (total = 38)
Table 2. Loss of the Dvl2 gene leads to dominant embryonic lethality in a Dact1 null background.
| Genotype |
Dact1–/+ Dvl2–/+ |
Dact1–/+ | Dvl2–/+ |
Dact1–/+ Dvl2–/– |
Dact1–/– Dvl2–/+ |
Dact1–/– | Dvl2–/– | WT |
Dact1–/– Dvl2–/– |
|---|---|---|---|---|---|---|---|---|---|
|
Neonatal Phenotype |
Normal | Normal | Normal | Normal | Short tail No genital tubercle |
Short tail No genital tubercle |
Normal | Normal | embryonic lethality |
|
Neonates Observed |
14 | 8 | 13 | 9 | 4 | 3 | 7 | 2 | 0 |
|
Neonates Expected |
15 | 7.5 | 7.5 | 7.5 | 7.5 | 3.75 | 3.75 | 3.75 | 3.75 |
|
Predicted Mendelian Ratio |
1/4 | 1/8 | 1/8 | 1/8 | 1/8 | 1/16 | 1/16 | 1/16 | 1/16 |
Dact1–/+; Dvl2–/+ X Dact1–/+; Dvl2–/+ (total = 60)
Discussion
We have shown here that Dvl2 forms complexes with Sestd1 independently of interactions with Dact1 and Vangl2. We have also shown in cells that recombinantly expressed Dvl2 can stimulate Rho GTPase activity cooperatively with Dact1 and Sestd1, consistent with these proteins all contributing to the PCP pathway. Our genetic data in mice, demonstrating synergistic (phenotypically super-additive) developmental interactions between KO mutations at the Dvl2 and Sestd1 loci as well as between KO mutations at the Dvl2 and Dact1 loci, similarly suggest that the Dvl2, Sestd1, and Dact1 proteins functionally converge on a key developmental process (i.e., PCP) in the embryo—but indicate that Dvl2 also contributes in some fashion that is not entirely dependent on either Dact1 or Sestd1. This contrasts with the completely epistatic genetic relationship between mutations in Dact1 and Sestd1: KO of both of these loci together leads to precisely the same phenotype as KO of either locus by itself,24 indicating that the Dact1 and Sestd1 proteins act in a single (linear) biochemical pathway such that a loss of either protein alone has the same functional consequence as loss of both proteins together. Further supporting such a model, the Dvl2, Dact1, and Sestd1 loci substantially differ in their genetic interactions with the Vangl2 locus in mice: Dvl2 mutations synergize with,15 whereas Dact1 and Sestd1 mutations rescue, Vangl2 mutations.22,24
Several lines of evidence suggest that all 3 of these scaffold proteins—Dact1, Sestd1, and Dvl2—participate in the trafficking of Vangl2. Sestd1 has a SEC14 domain that by homology implicates this protein in lipid signaling and membrane trafficking.30,31 In previous work, we have linked the unique Dact1 and Sestd1 KO phenotypic spectrum and genetic interactions to increased Vangl2 protein levels at the plasma membrane in the affected tissue of mutant embryos.22 Conversely, Vangl2 missense mutations that reduce Vangl2 protein at the plasma membrane also interfere with the ability of the corresponding Vangl2 mutant proteins to form complexes with Dvl proteins.32,33 Supporting a link between Dact, Dvl, and protein trafficking or turnover, loss of individual Dact family members has been reported to increase Dvl protein levels in some models,23,27 whereas recombinantly overexpressed Dact family members reproducibly reduce Dvl protein levels in many cell lines.25,27 Finally, both Dvl and Dact family members display a punctate intracellular distribution,20,27,34,35 and although controversial,25,36 in both cases these puncta have been equated with a membrane-bound compartment in some studies.37,38
In sum, genetic, developmental, cell biological, and biochemical data suggest a model in which Dvl2, Dact1, and Sestd1 all contribute to the PCP pathway during embryonic development—but that Dact1 and Sestd1 have a relatively unique functional alliance in Vangl2 trafficking that is not wholly shared by Dvl2, whereas Dvl2 participates in another aspect of PCP pathway signal transduction that is not entirely dependent on either Dact1 or Sestd1 (Fig. 3). This model, clearly incomplete in its details, is nonetheless useful in that it highlights gaps in our understanding and questions that remain: Does a Dact1/Sestd1 complex participate primarily in the biochemical regulation of Vangl2 stability, in Vangl2 endocytosis and associated degradation or recycling, or in Vangl2 trafficking from the Golgi to the plasma membrane? Does Dvl2 participate in the same Dact1/Sestd1 complex or in some separate aspect of Vangl2 cell biology? How might complex formation between these proteins relate mechanistically to these and other steps in the PCP pathway, including activation of downstream effectors such as Rho GTPases? These and other questions can be addressed through further experiments making use of the existing KO mouse lines and novel transgenic models, combined with state-of-the-art cell biological, developmental, signaling, and molecular imaging techniques.
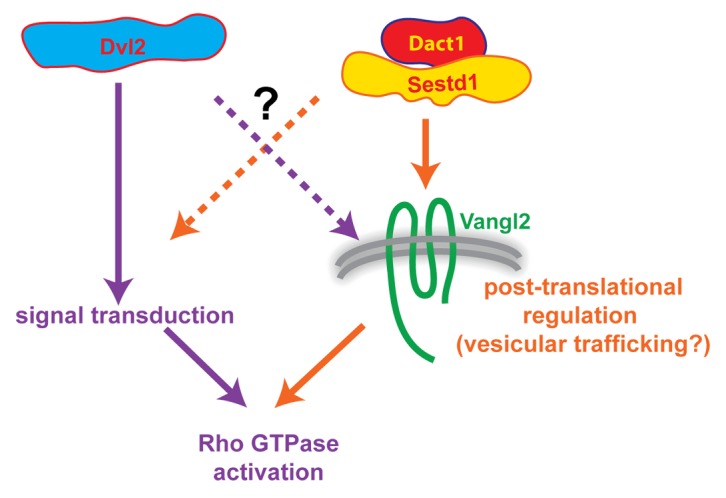
Figure 3. Working model of inferred PCP pathway relationships and cell biological functions to be explored through further experimentation. Although Dvl2 can form a complex with Sestd1 and Dact1 as well as with Vangl2, genetic and other evidence suggest that Dact1 and Sestd1 work closely together in Vangl2 trafficking (right side, orange arrows), whereas Dvl2 makes at least partially independent contributions to signal transduction (left side, purple arrows). Both sets of proteins may nevertheless contribute to both processes (dashed arrows), which may indeed be functionally intertwined.
Methods
Immunoprecipitation
A human embryonic kidney (HEK293T) cell line was transfected with the HA-Dvl2 or HA-Dact1 plasmid either with or without the Flag-Sestd1 or Flag-Sestd1 (1–274) plasmid using Lipofectamine 2000 (Life Technologies). After 48 h, transfected cells were lysed, pre-cleared, and incubated with anti-FLAG M2 beads for 3 h. Beads were collected and washed as described previously.25 Protein complexes were separated by SDS-PAGE followed by detection using anti-HA antibody.
Luciferase reporter activity assays
To measure Rho family GTPase activity, an immortalized mouse fibroblast (NIH3T3) cell line was transfected in triplicate with the SRE.Luciferase reporter plasmid, a Renilla plasmid, and either empty vector, HA-Dvl2, FlagDact1–2A-HASestd1,24 or the HA-Dvl2 and FlagDact1–2A-HASestd1 plasmids together, using Lipofectamine 2000 (Life Technologies). In previous work, we showed that stimulation of this reporter by Dact1 + Sestd1 is blocked by co-expression of the C3 ADP ribosyltransferase, indicating that it reflects activition of the Rho subfamily of small GTPases under these conditions.24,39 To measure Wnt/β-catenin signaling activity, HEK293T cells were transfected in triplicate with the SuperTOPflash reporter plasmid, a Renilla plasmid, and either empty vector, Flag-Sestd1, HA-Dvl2 or the Flag-Sestd1 and HA-Dvl2 plasmids together, using Lipofectamine 2000 (Life Technologies). One day after transfection, luciferase activity was measured by a luminometer (Veritas) as previously described.24,40
Statistical analyses
Prism software (GraphPad) was used for all data analysis; P values were calculated by unpaired, parametric, 2-tailed t test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank our colleagues Anthony Wynshaw-Boris, Randall T Moon, and Kozo Kaibuchi for providing the Dvl2 KO mouse line, pSuperTOP, and pSRE.L respectively.
Funding
Supported by National Institutes of Health Grants R01HD055300 (Cheyette BNR) and T32 MH089920 (Yang XY), and also by the Department of Psychiatry and the Center for Neurobiology and Psychiatry at the University of California San Francisco.
Glossary
Abbreviations:
- CE
convergent-extension cell movements
- co-IP
co-immunoprecipitation
- Dact1
Dapper Antagonist of Catenin 1
- Dvl2
Dishevelled 2
- KO
genetically engineered knock-out
- PCP
planar cell polarity
- Sestd1
SEC14 and Spectrin Domains 1
- Vangl2
Van Gogh-like 2
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/26834
References
- 1.Tada M, Kai M. Planar cell polarity in coordinated and directed movements. Curr Top Dev Biol. 2012;101:77–110. doi: 10.1016/B978-0-12-394592-1.00004-1. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–9. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–5. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 4.Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–77. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- 5.Huang H, He X. Wnt/beta-catenin signaling: new (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–25. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klingensmith J, Nusse R, Perrimon N. The Drosophila segment polarity gene dishevelled encodes a novel protein required for response to the wingless signal. Genes Dev. 1994;8:118–30. doi: 10.1101/gad.8.1.118. [DOI] [PubMed] [Google Scholar]
- 7.Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–25. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- 8.Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22:717–27. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Wynshaw-Boris A. Dishevelled: in vivo roles of a multifunctional gene family during development. Curr Top Dev Biol. 2012;101:213–35. doi: 10.1016/B978-0-12-394592-1.00007-7. [DOI] [PubMed] [Google Scholar]
- 10.Sussman DJ, Klingensmith J, Salinas P, Adams PS, Nusse R, Perrimon N. Isolation and characterization of a mouse homolog of the Drosophila segment polarity gene dishevelled. Dev Biol. 1994;166:73–86. doi: 10.1006/dbio.1994.1297. [DOI] [PubMed] [Google Scholar]
- 11.Klingensmith J, Yang Y, Axelrod JD, Beier DR, Perrimon N, Sussman DJ. Conservation of dishevelled structure and function between flies and mice: isolation and characterization of Dvl2. Mech Dev. 1996;58:15–26. doi: 10.1016/S0925-4773(96)00549-7. [DOI] [PubMed] [Google Scholar]
- 12.Tsang M, Lijam N, Yang Y, Beier DR, Wynshaw-Boris A, Sussman DJ. Isolation and characterization of mouse dishevelled-3. Dev Dyn. 1996;207:253–62. doi: 10.1002/(SICI)1097-0177(199611)207:3<253::AID-AJA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Lijam N, Paylor R, McDonald MP, Crawley JN, Deng CX, Herrup K, Stevens KE, Maccaferri G, McBain CJ, Sussman DJ, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/S0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- 14.Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–38. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–78. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waxman JS, Hocking AM, Stoick CL, Moon RT. Zebrafish Dapper1 and Dapper2 play distinct roles in Wnt-mediated developmental processes. Development. 2004;131:5909–21. doi: 10.1242/dev.01520. [DOI] [PubMed] [Google Scholar]
- 17.Fisher DA, Kivimäe S, Hoshino J, Suriben R, Martin PM, Baxter N, Cheyette BN. Three Dact gene family members are expressed during embryonic development and in the adult brains of mice. Dev Dyn. 2006;235:2620–30. doi: 10.1002/dvdy.20917. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X, Tan J, Li J, Kivimäe S, Yang X, Zhuang L, Lee PL, Chan MT, Stanton LW, Liu ET, et al. DACT3 is an epigenetic regulator of Wnt/beta-catenin signaling in colorectal cancer and is a therapeutic target of histone modifications. Cancer Cell. 2008;13:529–41. doi: 10.1016/j.ccr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okerlund ND, Kivimäe S, Tong CK, Peng IF, Ullian EM, Cheyette BN. Dact1 is a postsynaptic protein required for dendrite, spine, and excitatory synapse development in the mouse forebrain. J Neurosci. 2010;30:4362–8. doi: 10.1523/JNEUROSCI.0354-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheyette BN, Waxman JS, Miller JR, Takemaru K, Sheldahl LC, Khlebtsova N, Fox EP, Earnest T, Moon RT. Dapper, a Dishevelled-associated antagonist of beta-catenin and JNK signaling, is required for notochord formation. Dev Cell. 2002;2:449–61. doi: 10.1016/S1534-5807(02)00140-5. [DOI] [PubMed] [Google Scholar]
- 21.Gloy J, Hikasa H, Sokol SY. Frodo interacts with Dishevelled to transduce Wnt signals. Nat Cell Biol. 2002;4:351–7. doi: 10.1038/ncb784. [DOI] [PubMed] [Google Scholar]
- 22.Suriben R, Kivimäe S, Fisher DA, Moon RT, Cheyette BN. Posterior malformations in Dact1 mutant mice arise through misregulated Vangl2 at the primitive streak. Nat Genet. 2009;41:977–85. doi: 10.1038/ng.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen J, Chiang YJ, Gao C, Xue H, Xu J, Ning Y, Hodes RJ, Gao X, Chen YG. Loss of Dact1 disrupts planar cell polarity signaling by altering dishevelled activity and leads to posterior malformation in mice. J Biol Chem. 2010;285:11023–30. doi: 10.1074/jbc.M109.085381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Cheyette BN. SEC14 and spectrin domains 1 (Sestd1) and Dapper antagonist of catenin 1 (Dact1) scaffold proteins cooperatively regulate the Van Gogh-like 2 (Vangl2) four-pass transmembrane protein and planar cell polarity (PCP) pathway during embryonic development in mice. J Biol Chem. 2013;288:20111–20. doi: 10.1074/jbc.M113.465427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivimäe S, Yang XY, Cheyette BNR. All Dact (Dapper/Frodo) scaffold proteins dimerize and exhibit conserved interactions with Vangl, Dvl, and serine/threonine kinases. BMC Biochem. 2011;12:33. doi: 10.1186/1471-2091-12-33. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sah VP, Seasholtz TM, Sagi SA, Brown JH. The role of Rho in G protein-coupled receptor signal transduction. Annu Rev Pharmacol Toxicol. 2000;40:459–89. doi: 10.1146/annurev.pharmtox.40.1.459. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Gao X, Wen J, Ning Y, Chen YG. Dapper 1 antagonizes Wnt signaling by promoting dishevelled degradation. J Biol Chem. 2006;281:8607–12. doi: 10.1074/jbc.M600274200. [DOI] [PubMed] [Google Scholar]
- 28.Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–7. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 29.DasGupta R, Kaykas A, Moon RT, Perrimon N. Functional genomic analysis of the Wnt-wingless signaling pathway. Science. 2005;308:826–33. doi: 10.1126/science.1109374. [DOI] [PubMed] [Google Scholar]
- 30.Miehe S, Bieberstein A, Arnould I, Ihdene O, Rütten H, Strübing C. The phospholipid-binding protein SESTD1 is a novel regulator of the transient receptor potential channels TRPC4 and TRPC5. J Biol Chem. 2010;285:12426–34. doi: 10.1074/jbc.M109.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mousley CJ, Tyeryar KR, Vincent-Pope P, Bankaitis VA. The Sec14-superfamily and the regulatory interface between phospholipid metabolism and membrane trafficking. Biochim Biophys Acta. 2007;1771:727–36. doi: 10.1016/j.bbalip.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torban E, Wang HJ, Groulx N, Gros P. Independent mutations in mouse Vangl2 that cause neural tube defects in looptail mice impair interaction with members of the Dishevelled family. J Biol Chem. 2004;279:52703–13. doi: 10.1074/jbc.M408675200. [DOI] [PubMed] [Google Scholar]
- 33.Iliescu A, Gravel M, Horth C, Kibar Z, Gros P. Loss of membrane targeting of Vangl proteins causes neural tube defects. Biochemistry. 2011;50:795–804. doi: 10.1021/bi101286d. [DOI] [PubMed] [Google Scholar]
- 34.Itoh K, Antipova A, Ratcliffe MJ, Sokol S. Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol Cell Biol. 2000;20:2228–38. doi: 10.1128/MCB.20.6.2228-2238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller JR, Rowning BA, Larabell CA, Yang-Snyder JA, Bates RL, Moon RT. Establishment of the dorsal-ventral axis in Xenopus embryos coincides with the dorsal enrichment of dishevelled that is dependent on cortical rotation. J Cell Biol. 1999;146:427–37. doi: 10.1083/jcb.146.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwarz-Romond T, Merrifield C, Nichols BJ, Bienz M. The Wnt signalling effector Dishevelled forms dynamic protein assemblies rather than stable associations with cytoplasmic vesicles. J Cell Sci. 2005;118:5269–77. doi: 10.1242/jcs.02646. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Zhou H, Su Y, Sun Z, Zhang H, Zhang L, Zhang Y, Ning Y, Chen YG, Meng A. Zebrafish Dpr2 inhibits mesoderm induction by promoting degradation of nodal receptors. Science. 2004;306:114–7. doi: 10.1126/science.1100569. [DOI] [PubMed] [Google Scholar]
- 38.Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature. 2002;419:726–9. doi: 10.1038/nature01056. [DOI] [PubMed] [Google Scholar]
- 39.Wilde C, Genth H, Aktories K, Just I. Recognition of RhoA by Clostridium botulinum C3 exoenzyme. J Biol Chem. 2000;275:16478–83. doi: 10.1074/jbc.M910362199. [DOI] [PubMed] [Google Scholar]
- 40.Louie SH, Yang XY, Conrad WH, Muster J, Angers S, Moon RT, Cheyette BN. Modulation of the beta-catenin signaling pathway by the dishevelled-associated protein Hipk1. PLoS One. 2009;4:e4310. doi: 10.1371/journal.pone.0004310. [DOI] [PMC free article] [PubMed] [Google Scholar]