Abstract
Excitatory synaptic transmission takes place at actin-rich protrusions called dendritic spines. Strong synaptic input activates NMDA-type glutamate receptors and induces calcium flux into these structures, initiating a program of cytoskeletal rearrangement that results in larger spines with stronger synapses. These changes in synaptic strength are thought to be the primary cellular mechanism underlying learning and memory. We recently reported that the dual Ras/Rac1 guanine nucleotide exchange factor (GEF) RasGRF2 links calcium flux to both spine enlargement and synaptic strengthening through its Rac-GEF activity. Additionally, we demonstrated that acute Rac1 activation is sufficient to enhance synaptic transmission. Since Rac1 is a major regulator of the actin cytoskeleton, these results suggest that the cytoskeleton itself regulates synaptic strengthening. Here we discuss models for how cytoskeletal modifications may enhance synaptic AMPA-type glutamate receptor abundance during long-term potentiation.
Keywords: AMPA, Actin, Cytoskeleton, GTPase, LTP, Rac1, Spine, Synapse
Synaptic strengthening during long-term potentiation (LTP) is caused by the accumulation of AMPA-type glutamate receptors (AMPARs) at synapses.1 The mechanisms underlying this accumulation are unclear. Suggestively, the compartments that hold synapses (dendritic spines) undergo a program of cytoskeletal rearrangement during LTP that results in larger spines with enhanced filamentous (F)-actin content.2-4 Top candidates for initiating these cytoskeletal effects are the Rho-family of small GTPases, which regulate the activity of actin-binding proteins such as cofilin, profilin, and the Arp2/3 complex.5 Of these GTPases, overexpression studies have indicated that Rac1 in particular recapitulates the spine morphology effects observed during LTP6 and induces actual synaptic strengthening.7 Furthermore, pharmacological inhibition of Rac1 activation8 or actin dynamics9 inhibit LTP induction. To determine if a common Rac1 pathway could couple calcium flux to spine enlargement and synaptic strengthening, we performed a biochemical screen on the class of proteins that activates Rac1 (i.e., guanine nucleotide exchange factors (GEFs)) to determine if any gained activity during LTP.10 Consequently, we identified the dual Ras/Rac-GEF RasGRF2 and demonstrated that its Rac-GEF activity is required for both spine enlargement and synaptic strengthening.10 Furthermore, we determined that acute Rac1 activation itself (using a photoactivatable construct) rapidly affects synapse physiology.10 Since Rac1 is a major regulator of the actin cytoskeleton, these findings strongly suggest that the cytoskeletal dynamics that cause spine enlargement also cause AMPAR trafficking to the synapse.
AMPAR trafficking during LTP is regulated by at least two mechanisms: (i) exocytic pathways that increase AMPAR expression on the surface of the plasma membrane, and (ii) diffusion barriers that restrict the lateral movements of AMPARs post-insertion. Enhancement of AMPAR surface expression during LTP is well-documented,1,11 although it remains unresolved whether AMPARs are primarily exocytosed in dendrites,12,13 spines,14,15 or both. The actin cytoskeleton likely regulates several aspects of LTP-induced AMPAR exocytosis. Prior to stimulation, the membrane-associated cortical actin cytoskeleton functions as a scaffold to traffic and anchor exocytic vesicles proximal to the plasma membrane.16 Active remodeling of the cytoskeleton is required for subsequent vesicle docking and fusion events.16
Regardless of whether AMPARs are exocytosed into dendrites or spines, there is consensus that they are not exocytosed directly into synapses. Because of this, AMPARs must by necessity transverse the plasma membrane to reach the synapse boundary.13,14 This step provides an additional opportunity for regulated AMPAR trafficking. Single particle tracking experiments have demonstrated that AMPARs exhibit differential surface mobility when proximal to actin-rich structures such as spines and synapses.17-20 These AMPAR mobility effects are likely mediated by diffusion barriers, which are generated by the actin cytoskeleton or by cytoskeleton-binding proteins in the vicinity of AMPAR cytoplasmic domains.21 While the concept of the cortical actin cytoskeleton creating transmembrane protein diffusion barriers is not new, it is not clear how these barriers function to traffic AMPARs toward synapses during LTP. In particular, it is not intuitive how an enhanced cytoskeletal network (as occurs during LTP) could contribute to the synaptic accumulation of AMPARs through mass action. To assist in visualizing diffusion barrier function in our model (Fig. 1A), we will conceptualize the actin cytoskeleton as a maze; the dead ends and excessive turning intrinsic to a maze hinder net movement (i.e., diffusion) relative to an open-field (i.e., the rest of the plasma membrane). The two most plausible ways a cytoskeletal maze could be exploited to elevate AMPAR content around synapses are through the formation of a more dense cytoskeletal maze, or the formation of an enlarged cytoskeletal maze (Fig. 1B). In both of these models, which are not mutually exclusive, AMPARs will spend more time confined in these enhanced mazes due to increased maze path-length relative to the basal maze. In essence, denser or larger mazes are more difficult to exit than enter, resulting in net AMPAR accumulation into the maze, and ultimately the synapse.
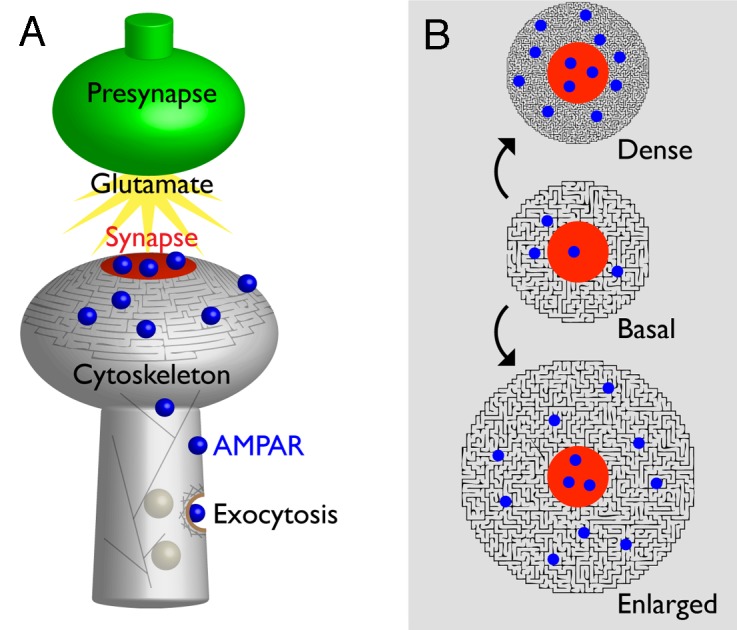
Figure 1. Models for how an enhanced cytoskeleton may induce synaptic AMPAR accumulation. (A) Illustration of AMPAR (blue) distribution on the surface of a dendritic spine in relationship to the synapse (red). AMPAR surface expression is enhanced during LTP through actin-mediated exocytosis, and movement once on the membrane surface is affected through AMPAR cytoplasmic domain interactions with the cytoskeleton. (B) Starting from a basal state, LTP-induced cytoskeleton rearrangements may result in the formation of a denser cytoskeletal maze, an enlarged cytoskeletal maze, or both. These enhanced cytoskeletal mazes would traffic AMPARs toward the synapse. Additional mechanisms may account for cytoskeletal regulation of AMPAR trafficking within the synapse proper. See text for details.
The aforementioned models provide hypothetical explanations for the cytoskeleton’s contribution to AMPAR trafficking toward the synapse during LTP. Additional complications may exist for AMPAR transit into the synapse proper. For instance, pharmacological actin depolymerization appears to actually decrease AMPAR mobility within the synapse,22 whereas the opposite is true for transmembrane proteins outside the synapse.17 This is confusing considering the molecular composition of the synapse appears to allow for AMPAR anchoring to the cytoskeleton through the following interactions: AMPAR → Stargazin → PSD-95 → GKAP/SPAR/Shank/Cortactin → F-actin.1 These interactions also appear to be dispensable for LTP since AMPARs are not specifically required to support LTP (i.e., generic glutamate receptors will suffice).23 To reconcile these findings, it has been proposed that the cytoskeleton actively exerts forces on the postsynaptic density (PSD) that promote AMPAR mobility within the synapse.22 In other words, cytoskeletal dynamics during LTP may permit AMPAR access into the PSD through an active mechanism, in contrast to the passive diffusion barriers we have proposed here to traffic AMPARs toward the PSD. Finally, actin-dependent spine morphogenesis may itself contribute to synaptic strengthening. In addition to creating more room to accommodate larger synapses, it is possible that cytoskeleton remodeling changes the topography of the synaptic zone (i.e., a shape change from convex to concave relative to the presynapse), resulting in enhanced glutamate sensitivity without AMPAR trafficking. In summary, we propose that the actin cytoskeleton regulates a sequence of postsynaptic events that contribute to synaptic potentiation: AMPAR insertion at extrasynaptic sites, lateral movement of AMPAR toward synapses, positioning or anchoring of receptors at the synapse, and morphology alterations that result in greater AMPAR capacity or glutamate sensitivity. The ability of the actin cytoskeleton to regulate multiple steps in synaptic strengthening may explain why the majority of neuronal F-actin resides in dendritic spines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/27343
References
- 1.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–97. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–6. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto K-I, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nat Neurosci. 2004;7:1104–12. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- 4.Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–29. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govek E-E, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–40. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Wiens KM, Lin H, Liao D. Rac1 induces the clustering of AMPA receptors during spinogenesis. J Neurosci. 2005;25:10627–36. doi: 10.1523/JNEUROSCI.1947-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez LA, Tejada-Simon MV. Pharmacological inactivation of the small GTPase Rac1 impairs long-term plasticity in the mouse hippocampus. Neuropharmacology. 2011;61:305–12. doi: 10.1016/j.neuropharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CH, Lisman JE. A role of actin filament in synaptic transmission and long-term potentiation. J Neurosci. 1999;19:4314–24. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwechter B, Rosenmund C, Tolias KF. RasGRF2 Rac-GEF activity couples NMDA receptor calcium flux to enhanced synaptic transmission. Proc Natl Acad Sci U S A. 2013;110:14462–7. doi: 10.1073/pnas.1304340110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 12.Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–21. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron. 2009;64:381–90. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci. 2006;26:2000–9. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–5. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 16.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell Mol Life Sci. 2013;70:2099–121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards DA, De Paola V, Caroni P, Gähwiler BH, McKinney RA. AMPA-receptor activation regulates the diffusion of a membrane marker in parallel with dendritic spine motility in the mouse hippocampus. J Physiol. 2004;558:503–12. doi: 10.1113/jphysiol.2004.062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehlers MD, Heine M, Groc L, Lee M-C, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–60. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heine M, Groc L, Frischknecht R, Béïque J-C, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–5. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Triller A, Choquet D. New concepts in synaptic biology derived from single-molecule imaging. Neuron. 2008;59:359–74. doi: 10.1016/j.neuron.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct. 2005;34:351–78. doi: 10.1146/annurev.biophys.34.040204.144637. [DOI] [PubMed] [Google Scholar]
- 22.Kerr JM, Blanpied TA. Subsynaptic AMPA receptor distribution is acutely regulated by actin-driven reorganization of the postsynaptic density. J Neurosci. 2012;32:658–73. doi: 10.1523/JNEUROSCI.2927-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]


