Abstract
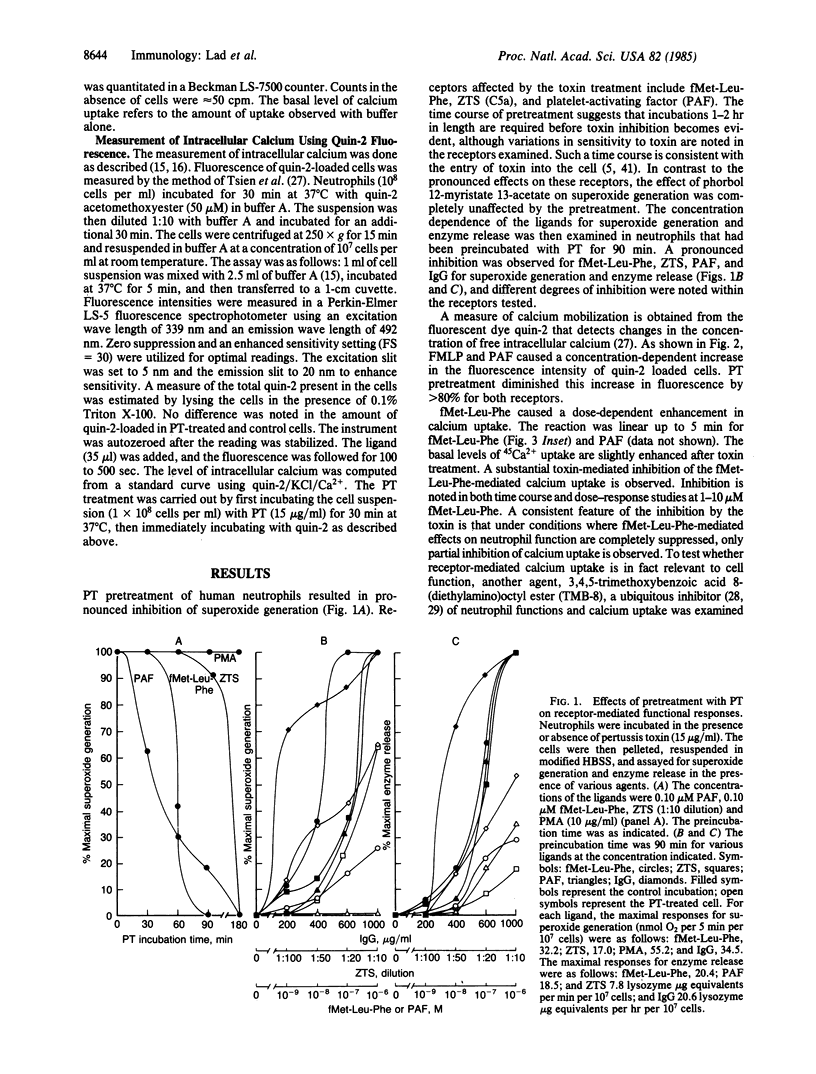
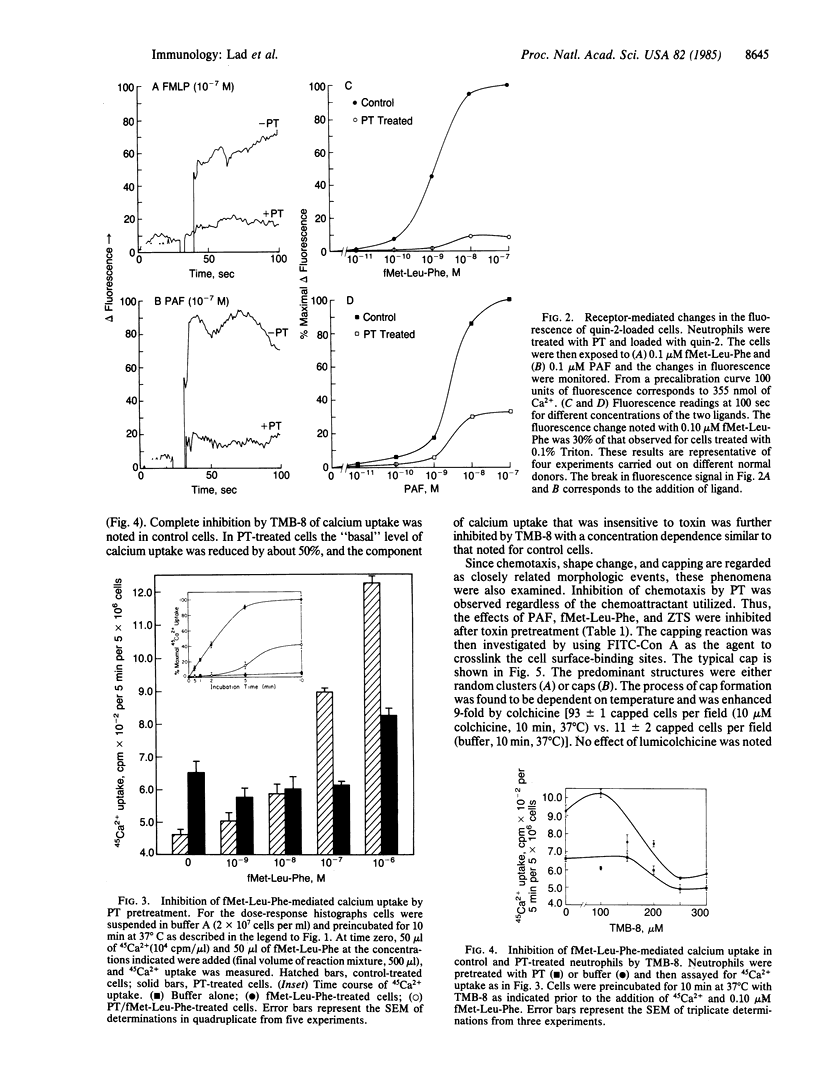
Human neutrophils treated with pertussis toxin had decreased functional responses to several agents including zymosan-treated serum, heat-aggregated immunoglobulin, platelet-activating factor, and fMet-Leu-Phe. Responses affected include superoxide generation and release of lysozyme. The degree and type of inhibition was dependent on the individual receptor and the cellular response studied. Measurement of intracellular calcium levels with quin-2 showed that both fMet-Leu-Phe- and platelet-activating factor-mediated increases in quin-2 fluorescence were diminished as a result of pertussis toxin treatment. fMet-Leu-Phe-mediated calcium uptake was also inhibited. However, under conditions where fMet-Leu-Phe-mediated effects on cell function were completely abolished, only a partial inhibition of 3,4,5-trimethoxybenzoic acid 8-(diethylamino)octyl ester (TMB-8) sensitive calcium uptake was observed. A study of the linked reactions of chemotaxis, capping, and shape change revealed that chemotaxis was inhibited regardless of the chemoattractant utilized (zymosan-treated serum, fMet-Leu-Phe, and platelet-activating factor) and the associated reactions of Con A capping and fMet-Leu-Phe- or Con A-mediated shape change were reduced in pertussis toxin-treated cells. Our results suggest that multiple mediators of inflammation act through a pertussis toxin-sensitive GTP-binding protein that regulates the mobilization of internal calcium as well as calcium uptake and is, in addition, a key control element of shape change, capping, and chemotaxis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Bourguignon G. J. Capping and the cytoskeleton. Int Rev Cytol. 1984;87:195–224. doi: 10.1016/s0074-7696(08)62443-2. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Endocytosis: relation to capping and cell locomotion. Science. 1984 May 18;224(4650):681–686. doi: 10.1126/science.6719108. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chiou C. Y., Malagodi M. H. Studies on the mechanism of action of a new Ca-2+ antagonist, 8-(N,N-diethylamino)octyl 3,4,5-trimethoxybenzoate hydrochloride in smooth and skeletal muscles. Br J Pharmacol. 1975 Feb;53(2):279–285. doi: 10.1111/j.1476-5381.1975.tb07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Johnson H. B., Spiegelberg H. L. The release of granule enzymes from human neutrophils stimulated by aggregated immunoglobulins of different classes and subclasses. J Immunol. 1972 Dec;109(6):1182–1192. [PubMed] [Google Scholar]
- Hoffstein S. T. Ultrastructural demonstration of calcium loss from local regions of the plasma membrane of surface-stimulated human granulocytes. J Immunol. 1979 Sep;123(3):1395–1402. [PubMed] [Google Scholar]
- Kammer G. M., Rudolph S. A. Regulation of human T lymphocyte surface antigen mobility by purinergic receptors. J Immunol. 1984 Dec;133(6):3298–3302. [PubMed] [Google Scholar]
- Korchak H. M., Rutherford L. E., Weissmann G. Stimulus response coupling in the human neutrophil. I. Kinetic analysis of changes in calcium permeability. J Biol Chem. 1984 Apr 10;259(7):4070–4075. [PubMed] [Google Scholar]
- Korchak H. M., Vienne K., Rutherford L. E., Wilkenfeld C., Finkelstein M. C., Weissmann G. Stimulus response coupling in the human neutrophil. II. Temporal analysis of changes in cytosolic calcium and calcium efflux. J Biol Chem. 1984 Apr 10;259(7):4076–4082. [PubMed] [Google Scholar]
- Lad P. M., Glovsky M. M., Richards J. H., Learn D. B., Reisinger D. M., Smiley P. A. Identification of receptor regulatory proteins, membrane glycoproteins, and functional characteristics of adenylate cyclase in vesicles derived from the human neutrophil. Mol Immunol. 1984 Jul;21(7):627–639. doi: 10.1016/0161-5890(84)90048-8. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Glovsky M. M., Smiley P. A., Klempner M., Reisinger D. M., Richards J. H. The beta-adrenergic receptor in the human neutrophil plasma membrane: receptor-cyclase uncoupling is associated with amplified GTP activation. J Immunol. 1984 Mar;132(3):1466–1471. [PubMed] [Google Scholar]
- Lad P. M., Goldberg B. J., Smiley P. A., Olson C. V. Receptor-specific threshold effects of cyclic AMP are involved in the regulation of enzyme release and superoxide production from human neutrophils. Biochim Biophys Acta. 1985 Aug 30;846(2):286–295. doi: 10.1016/0167-4889(85)90076-x. [DOI] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Smiley P. A. Association of the N-formyl-Met-Leu-Phe receptor in human neutrophils with a GTP-binding protein sensitive to pertussis toxin. Proc Natl Acad Sci U S A. 1985 Feb;82(3):869–873. doi: 10.1073/pnas.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Reisinger D. M., Smiley P. A. Ligand requirements for the relaxation of adenylate cyclase from activated and inhibited states. Biochemistry. 1983 Jun 21;22(13):3278–3284. doi: 10.1021/bi00282a037. [DOI] [PubMed] [Google Scholar]
- Lucisano Y. M., Mantovani B. Lysosomal enzyme release from polymorphonuclear leukocytes induced by immune complexes of IgM and of IgG. J Immunol. 1984 Apr;132(4):2015–2020. [PubMed] [Google Scholar]
- Matsumoto K., Osakabe K., Katayama H., Yoshizawa N., Seki M., Miyaji H., Nagura Y., Ohi H., Hatano M. Concanavalin-A-induced suppressor cell activity in idiopathic membranous nephropathy. Int Arch Allergy Appl Immunol. 1982;69(1):26–29. doi: 10.1159/000233141. [DOI] [PubMed] [Google Scholar]
- Mazurek N., Schindler H., Schürholz T., Pecht I. The cromolyn binding protein constitutes the Ca2+ channel of basophils opening upon immunological stimulus. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6841–6845. doi: 10.1073/pnas.81.21.6841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco C., Rink T. J., Pozzan T., Metcalfe J. C. Triggering of lymphocyte capping appears not to require changes in potential or ion fluxes across the plasma membrane. Biochim Biophys Acta. 1980;595(1):65–70. doi: 10.1016/0005-2736(80)90248-5. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Mechanisms that regulate the structural and functional architecture of cell surfaces. Int Rev Cytol. 1982;74:55–94. doi: 10.1016/s0074-7696(08)61169-9. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Lalchandani R., Becker E. L. Actin redistribution during Concanavalin A cap formation in rabbit neutrophils. J Reticuloendothel Soc. 1977 May;21(5):359–364. [PubMed] [Google Scholar]
- Oliver J. M., Zurier R. B., Berlin R. D. Concanavalin a cap formation on polymorphonuclear leukocytes of normal and beige (chediak-higashi) mice. Nature. 1975 Feb 6;253(5491):471–473. doi: 10.1038/253471a0. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. L., Mounessa N. L., Gallin J. I. Increasing extracellular potassium causes calcium-dependent shape change and facilitates concanavalin A capping in human neutrophils. J Immunol. 1984 Apr;132(4):2000–2006. [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. Membrane and cytoplasmic changes in B lymphocytes induced by ligand-surface immunoglobulin interaction. Adv Immunol. 1976;24:37–165. doi: 10.1016/s0065-2776(08)60329-6. [DOI] [PubMed] [Google Scholar]
- Sekura R. D., Fish F., Manclark C. R., Meade B., Zhang Y. L. Pertussis toxin. Affinity purification of a new ADP-ribosyltransferase. J Biol Chem. 1983 Dec 10;258(23):14647–14651. [PubMed] [Google Scholar]
- Shefcyk J., Yassin R., Volpi M., Molski T. F., Naccache P. H., Munoz J. J., Becker E. L., Feinstein M. B., Sha'afi R. I. Pertussis but not cholera toxin inhibits the stimulated increase in actin association with the cytoskeleton in rabbit neutrophils: role of the "G proteins" in stimulus-response coupling. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1174–1181. doi: 10.1016/0006-291x(85)90309-2. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Chemotactic factor-induced generation of superoxide radicals by human neutrophils: evidence for the role of sodium. J Immunol. 1979 Nov;123(5):2428–2435. [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Properties of concanavalin A-elicited granule exocytosis from human polymorphonuclear neutrophils. Inflammation. 1980 Dec;4(4):343–358. doi: 10.1007/BF00916046. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Chemoattractant receptors on phagocytic cells. Annu Rev Immunol. 1984;2:257–281. doi: 10.1146/annurev.iy.02.040184.001353. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese M. W., Smith C. D., Snyderman R. Potential role for a guanine nucleotide regulatory protein in chemoattractant receptor mediated polyphosphoinositide metabolism, Ca++ mobilization and cellular responses by leukocytes. Biochem Biophys Res Commun. 1985 Mar 15;127(2):450–457. doi: 10.1016/s0006-291x(85)80181-9. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]