Abstract
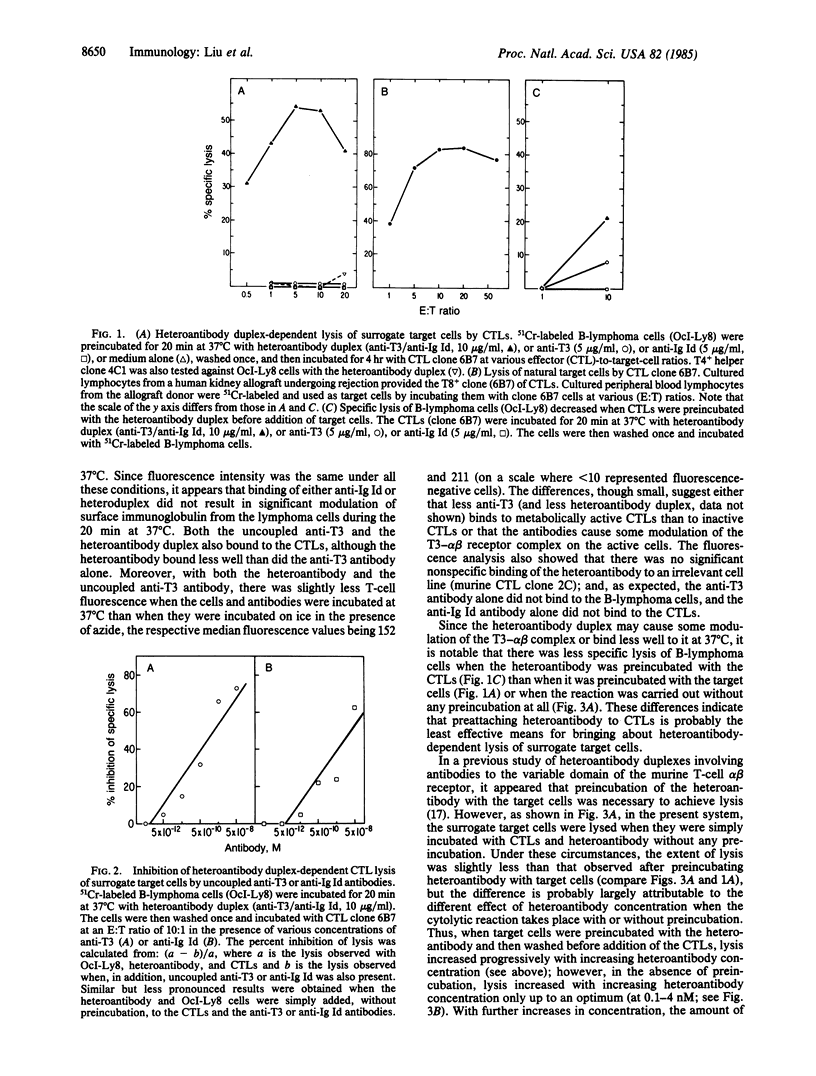
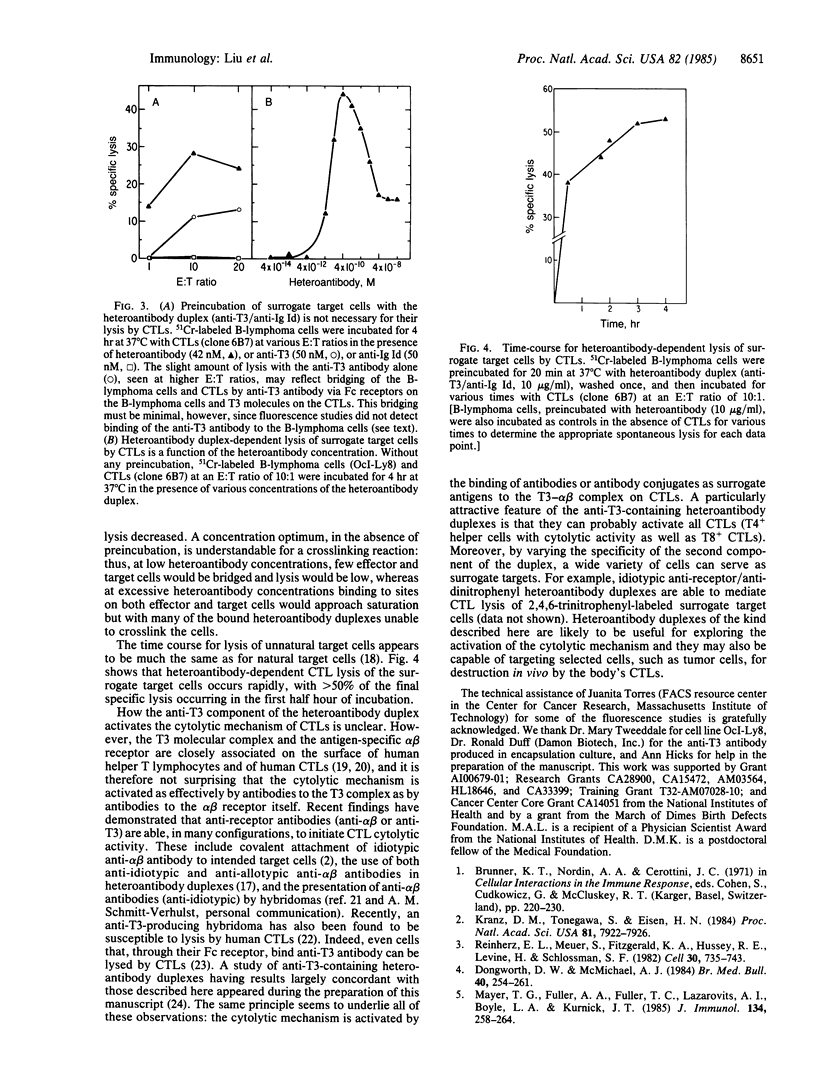
Antibodies to the clonally unique variable-region determinants (idiotype) of the antigen-specific alpha beta heterodimeric receptor of a clone of cytotoxic T cells (CTLs) were shown previously to render diverse cells, regardless of their own surface antigens, susceptible to lysis by that clone of CTLs. To extend these findings, we have sought to develop a general means for targeting cells for destruction by any CTL, without regard to its alpha beta idiotype and specificity for antigen. We explored the use of heteroantibody duplexes formed by joining covalently an antibody to the T3 complex (anti-T3), which is associated with the alpha beta receptors on all human mature T cells, and a second antibody, specific for an antigen on the intended target cell. The second antibody selected in this study was specific for the idiotype (Id) of the surface immunoglobulin of a human B-lymphoma (anti-Ig Id). In the presence of the anti-T3/anti-Ig Id heteroantibody duplex the B-lymphoma cells were lysed by a clone of human T8+ CTLs (of unrelated specificity) but not by a noncytotoxic clone of human T4+ helper T cells, and lysis by the CTLs was specifically blocked by the uncoupled anti-T3 or the uncoupled anti-Ig Id antibodies. The extent of the heteroantibody-dependent cytolysis depended both on the heteroantibody concentration and on whether the intended target cells or the CTL effectors were initially preincubated with the heteroantibody. Under optimal conditions, heteroantibody-dependent lysis of the surrogate target (B-lymphoma) cells by the CTLs compared favorably with lysis of their natural target cells by the same CTLs. Overall, our findings suggest that heteroantibody duplexes containing anti-T3 antibody may be capable of targeting selected cells, such as tumor cells, for destruction in vivo by the body's CTLs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner M. B., Trowbridge I. S., Strominger J. L. Cross-linking of human T cell receptor proteins: association between the T cell idiotype beta subunit and the T3 glycoprotein heavy subunit. Cell. 1985 Jan;40(1):183–190. doi: 10.1016/0092-8674(85)90321-6. [DOI] [PubMed] [Google Scholar]
- Brown S., Dilley J., Levy R. Immunoglobulin secretion by mouse X human hybridomas: an approach for the production of anti-idiotype reagents useful in monitoring patients with B cell lymphoma. J Immunol. 1980 Sep;125(3):1037–1043. [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongworth D. W., McMichael A. J. Inhibition of human T lymphocyte function with monoclonal antibodies. Br Med Bull. 1984 Jul;40(3):254–261. doi: 10.1093/oxfordjournals.bmb.a071986. [DOI] [PubMed] [Google Scholar]
- Hatzubai A., Maloney D. G., Levy R. The use of a monoclonal anti-idiotype antibody to study the biology of a human B cell lymphoma. J Immunol. 1981 Jun;126(6):2397–2402. [PubMed] [Google Scholar]
- Hoffman R. W., Bluestone J. A., Leo O., Shaw S. Lysis of anti-T3-bearing murine hybridoma cells by human allospecific cytotoxic T cell clones and inhibition of that lysis by anti-T3 and anti-LFA-1 antibodies. J Immunol. 1985 Jul;135(1):5–8. [PubMed] [Google Scholar]
- Karpovsky B., Titus J. A., Stephany D. A., Segal D. M. Production of target-specific effector cells using hetero-cross-linked aggregates containing anti-target cell and anti-Fc gamma receptor antibodies. J Exp Med. 1984 Dec 1;160(6):1686–1701. doi: 10.1084/jem.160.6.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. P., Li Y., Kochoumian L. Preparation of protein conjugates via intermolecular disulfide bond formation. Biochemistry. 1978 Apr 18;17(8):1499–1506. doi: 10.1021/bi00601a022. [DOI] [PubMed] [Google Scholar]
- Kranz D. M., Tonegawa S., Eisen H. N. Attachment of an anti-receptor antibody to non-target cells renders them susceptible to lysis by a clone of cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7922–7926. doi: 10.1073/pnas.81.24.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurnick J. T., Grönvik K. O., Kimura A. K., Lindblom J. B., Skoog V. T., Sjöberg O., Wigzell H. Long term growth in vitro of human T cell blasts with maintenance of specificity and function. J Immunol. 1979 Apr;122(4):1255–1260. [PubMed] [Google Scholar]
- Mayer T. G., Fuller A. A., Fuller T. C., Lazarovits A. I., Boyle L. A., Kurnick J. T. Characterization of in vivo-activated allospecific T lymphocytes propagated from human renal allograft biopsies undergoing rejection. J Immunol. 1985 Jan;134(1):258–264. [PubMed] [Google Scholar]
- Meeker T. C., Lowder J., Maloney D. G., Miller R. A., Thielemans K., Warnke R., Levy R. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985 Jun;65(6):1349–1363. [PubMed] [Google Scholar]
- Mentzer S. J., Barbosa J. A., Burakoff S. J. T3 monoclonal antibody activation of nonspecific cytolysis: a mechanism of CTL inhibition. J Immunol. 1985 Jul;135(1):34–38. [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P., Hoffman R. W., Shaw S., Bluestone J. A., Segal D. M. Specific targeting of cytotoxic T cells by anti-T3 linked to anti-target cell antibody. Nature. 1985 Jul 25;316(6026):354–356. doi: 10.1038/316354a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Russell J. H. Internal disintegration model of cytotoxic lymphocyte-induced target damage. Immunol Rev. 1983;72:97–118. doi: 10.1111/j.1600-065x.1983.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Kanagawa O., Bevan M. J. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Apr 18;314(6012):628–631. doi: 10.1038/314628a0. [DOI] [PubMed] [Google Scholar]


