Abstract
Oxidative damage and clustering of band 3 in the membrane have been implicated in the removal of senescent human erythrocytes from the circulation at the end of their 120-day life span. However, the biochemical and mechanistic events leading to band 3 cluster formation have yet to be fully defined. Here we show that while neither membrane peroxidation nor MetHb formation on their own can induce band 3 clustering in the human erythrocytes, they can do so when acting in combination. We further show that MetHb binding to the cytoplasmic domain of band 3 in peroxidized, but not in untreated erythrocyte membranes, induces cluster formation. Age-fractionated populations of erythrocytes from normal human blood, obtained by a density-gradient procedure, have enabled us to examine a subpopulation, highly enriched in senescent cells. We have found that band 3 clustering is a feature of only this small fraction, amounting to ~ 0.1% of total circulating erythrocytes. These senescent cells are characterized by an increased proportion of MetHb as a result of reduced NADH-dependent reductase activity, and accumulated oxidative membrane damage. These findings have enabled us to establish that the combined effects of membrane peroxidation and MetHb formation are necessary for band 3 clustering, and this is a very late event in erythrocyte life. A plausible mechanism for the combined effects of membrane peroxidation and MetHb is proposed, involving high-affinity cooperative binding of MetHb to the cytoplasmic domain of oxidized band 3, probably due to its carbonylation, rather than other forms of oxidative damage. This modification leads to dissociation of ankyrin from the band 3, enabling the tetrameric MetHb to cross-link the resulting freely diffusible band 3 dimers, with formation of clusters.
During its 120-day circulatory life span the human erythrocyte is continuously exposed to oxidative stress (1, 2) that can result in oxidation of hemoglobin (Hb) to methemoglobin (MetHb) (3) and may also inflict oxidative damage on membrane lipids and proteins (4–6). Reductases, such as NADH-dependent MetHb reductase, protect Hb against oxidation (7), whereas oxidative damage to membrane components, as by carbonylation and reaction with malondialdehyde (MDA)- and 4-hydroxy-2-nonenal (HNE) is in general irreversible (4, 5, 8, 9). A number of perturbations of the cell and its membrane changes have been documented during aging, including loss of membrane surface, diminution of cell volume (10–12), clustering of band 3 (13–15), and surface exposure of phosphatidylserine (16). These changes may all contribute in varying degree to the removal of senescent erythrocytes from the circulation at the end of their normal life span (16–19). However, the relation of oxidative damage to these senescence markers has yet to be fully delineated.
Clustering of band 3, generation of MetHb and its denaturation, leading to Heinz-body formation as well as lipid peroxidation (MDA accumulation) in the membrane has been documented in a number of erythrocyte disorders, including sickle cell anemia, thalassemias and glucose-6-phosphate dehydrogenase (G6PD) deficiency (20–25). It has been shown that naturally occurring anti-band 3 antibodies in plasma bind to clustered band 3, resulting in removal of the cells by splenic macrophages (18, 19). Thus it is very likely that band 3 clustering plays a role in the elimination of senescent normal, as well as various pathological, erythrocytes.
While oxidized Hb has been implicated in band 3 clustering, the mechanism by which such clusters develop in senescent normal erythrocytes is obscure. In the present study, we show that both membrane peroxidation and MetHb formation are inseparable from cluster formation, and that the phenomenon is restricted to senescent circulating erythrocytes. We also demonstrate reduced-NADH-dependent reductase activity in senescent erythrocytes, and an ensuing increase in MetHb formation. We further show that MetHb binding to the cytoplasmic domain of band 3 in peroxidized, but not in unoxidized erythrocyte membranes, leads to band 3 clustering. These findings establish that the combined effects of membrane peroxidation and MetHb formation are preconditions for band 3 clustering and that this is a terminal event in the erythrocyte life span.
MATERIALS AND METHODS
Materials
Percoll, Q Sepharose Fast Flow, and Sephadex-G25 fine were from GE Healthcare, UK. PVDF membrane was from Merck Millipore, Billerica, MA, U.S.A. FITC- and HRP-conjugated anti-rabbit IgGs and HRP-conjugated anti-mouse IgG were from Dako, Glostrup, Denmark. Bis(sulfosuccinimidyl)suberate (BS3) was from Pierce, Rockford, IL, U.S.A. Anti-Hbβmonoclonal antibody was from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, U.S.A. Cytochrome b5, 5,5’-Dithiobis-(2–nitrobenzonic acid) (DTNB), Flavin mononucleotide, Trichloroacetic acid (TCA), Thiobarbituric acid (TBA), 1,1-3,3-Tetraethoxypropane, Potassium iodide, and Tween 20 were from Wako, Tokyo, Japan. PMSF and tert-butyl hydroperoxide (t-BHP) were from Sigma-Aldrich, St. Louis, MO, U.S.A. NADH and NADPH was from Orient Yeast Co., LTD., Tokyo, Japan. Potassium ferricyanide was from Kanto Chemical Co., Inc., Tokyo, Japan. Diamide was from MP Biochemicals, Solon, OH, U.S.A.
Density separation of human erythrocytes using Percoll gradients
After obtaining informed consent, venous blood was drawn from healthy human volunteers and used in all the studies outlined. Erythrocytes were collected and washed three times with phosphate buffered saline (PBS; 137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4). A discontinuous gradient of Percoll was made by overlaying 2 ml of 90%, 81.8%, 80.2% and 75.8% Percoll in 0.25 M sucrose, 120 mM KCl and 10 mM Tris/Cl (pH 7.4). Two ml of washed erythrocytes was layered on top of the Percoll gradient, followed by centrifugation using a swing rotor at 1,000 × g for 30 min at 4°C. Erythrocytes on top of the 75.8% Percoll layer were removed and cells between 75.8% and 80.2%, and 80.2% and 81.8% were collected and designated as Fractions 1 (F1) and 2 (F2), respectively. For separation of the most dense erythrocytes, six ml of washed erythrocytes were overlaid on top of 20 ml of 89% Percoll (density of 1.153 g/ml) in 0.25 M sucrose, 120 mM KCl, and 10 mM Tris/Cl (pH 7.4) and centrifuged using an angle rotor at 33,500 × g for 15 min at 4°C to generate a continuous density gradient. Erythrocytes in the very bottom dense layer were collected and designated as F3.
Mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC) were measured using an automated hematology analyzer. Protein 4.1a/b ratio was calculated by densitometric analysis of immunoblots performed using polyclonal antibodies against protein 4.1a and protein 4.1b prepared in our laboratory. MDA concentrations were measured by MDA-TBA method. Briefly, membrane ghosts (300 µg protein), obtained from density-separated erythrocytes were suspended in 500 µl of 5 mM sodium phosphate and mixed with 500 µl of 10% TCA for 10 min and then centrifuged at 1,000 × g for 10 min. 900 µl of 1% TBA was added to 900 µl of the supernatants, and the samples were boiled for 10 min followed by measuring the absorbance at 530 nm. MDA standard curve was generated as previously described (26).
Preparation of resealed ghosts and MetHb
Washed erythrocytes were lysed and washed three times in 5 mM phosphate buffer at 4°C. In some experiments, the membranes were peroxidized by incubation with 0.6 mM t-BHP for 30 min at 37°C (27). The membranes were resealed in PBS at 37°C for 40 min in the presence of either Hb or MetHb. Hb was prepared from the lysate obtained by freeze thawing of erythrocytes followed by gel filtration through Sephadex-G25 column in PBS. MetHb was prepared according to the method of Hensley et al. with minor modifications (28). Briefly, Hb was incubated with 5% potassium ferricyanide in PBS for 30 min at 37°C to convert Hb to MetHb, followed by rapid removal of potassium ferricyanide by gel filtration through Sephadex-G25 column. MetHb was used within one hour following its preparation. Wave length scan of MetHb incubated additionally for 30 min at 37°C (same condition with below binding assays) showed a typical pattern of MetHb and not of hemichrome (data not shown).
FACS analysis of clustered band 3
The rabbit anti-band 3 polyclonal antibody that we generated using the multiple antigenic peptide (MAP) corresponding to amino acid residues 538 to 554 (asymmetric 4 branches) as an immuogen was used for FACS analysis. Resealed ghosts were incubated with the antibody at 1:10 dilution in PBS containing 2% BSA. After washing three times with the same buffer, the resealed cells were incubated with FITC-anti-rabbit IgG for 60 min, washed three times with PBS followed by FACS analysis (Beckman Coulter, Inc., Brea, CA, U.S.A.). For the assessment of specificity of this antibody, resealed ghosts prepared from erythrocyte were treated with both 1 mM ZnCl2 and 1 mM BS3, a chemical cross-linker, to induce irreversible band 3 clustering (29), or with 2 mM diamide, an oxidant of SH groups, to induce reversible band 3 clustering (25), and were analyzed by FACS. Furthermore, diamide-treated ghosts treated with reducing agents, 20 mM DTT or 10 mM β-mercaptoethanol (β-ME) were also analyzed by FACS.
Measurement of MetHb and MetHb reductase activity
Density fractionated erythrocytes were lysed with double distilled water and centrifuged at 1,000 × g for 10 min. MetHb content as % of total cell Hb was determined by dividing A630 of the resultant supernatants by A630 of 2% potassium ferricyanide-treated supernatants. The activities of NADH- and NADPH-dependent MetHb reductases in fractionated erythrocytes were measured according to methods described by Kuma et al. (30) and Yubisui et al. (31), respectively. The activities of F2 and F3 fractions were expressed as percentage of the activity of F1 fraction.
MetHb binding to peroxidized and non-oxidized inside out vesicles (IOVs)
IOVs were prepared from ghosts as described previously (32). Both ankyrin and protein 4.1 remained associated with the IOVs. In some experiments, IOVs were peroxidized with 0.6 mM t-BHP for 30 min at 37°C. The peroxidized IOVs were treated with 5 µg/ml trypsin for 30 min on ice to digest and remove cytoplasmic domain of band 3 (33). Ten µg of IOVs were incubated with MetHb (0 to 31.3 µM) for 30 min at 37°C and IOVs with bound MetHb were collected by centrifugation at 100,000 × g for 20 min at 4°C through 8% sucrose cushion. Bound MetHb was separated by SDS-PAGE and the extent of binding was determined by densitometric analysis of the MetHb band in immunoblots performed with anti-Hbβ monoclonal antibody. The amount of ankyrin associated with IOVs was quantitated by densitometric analysis of the ankyrin band in immunoblots performed using anti-ankyrin antibody made in our laboratory. The experiments were performed using three different IOV preparations.
Ankyrin binding to peroxidized and non-oxidized potassium iodide-treated IOVs (KI-IOVs)
Ankyrin was purified according to the method described by Tyler et al. (34) with minor modifications. KI-IOVs were prepared as described by Bennett et al. (35) and KI-IOVs were peroxidized with 0.6 mM t-BHP. The various concentrations of purified ankyrin (0 to 2.0 µM) was added to 10 µg of KI-IOVs or peroxidized KI-IOVs and the samples were incubated for 30 min at 37°C and subsequently centrifuged at 100,000 × g for 20 min through 8% sucrose cushion to separate unbound ankyrin from KI-IOVs. The pellets were collected and bound ankyrin was separated by SDS-PAGE. Amount of ankyrin was measured by densitometric analysis of CBB-stained band of ankyrin as described above. These experiments were performed using 3 to 5 different membrane preparations.
Detection of carbonylated band 3 on peroxidized membrane
Ghosts and IOVs were incubated with 0.6 mM t-BHP for up to 120 min at 37°C. IOVs were then treated with 5 µg/ml α-chymotrypsin for 45 min at 0°C to cleave cytoplasmic domain of band 3 (43 kDa) from the transmembrane domain of band 3. Proteins of these ghosts and IOVs were separated by SDS-PAGE (8% or 10% gels, respectively) and transferred to PVDF membranes. Carbonylated proteins were detected by derivatization of carbonyl groups using dinitrophenylhydrazine (DNPH) and the following immunoreaction with anti-DNP antibody. These experiments were performed using OxiSelect Protein Carbonyl Immunoblot Kit according to the manual (CELL BIOLABS, Inc., San Diego, CA, U.S.A.). The carbonylated signal was detected by exposing PVDF membranes to a film for either 5 or 90 seconds.
Statistical analysis
All statistical analyses were performed by Student’s t-test.
RESULTS
MetHb induced clustering of band 3
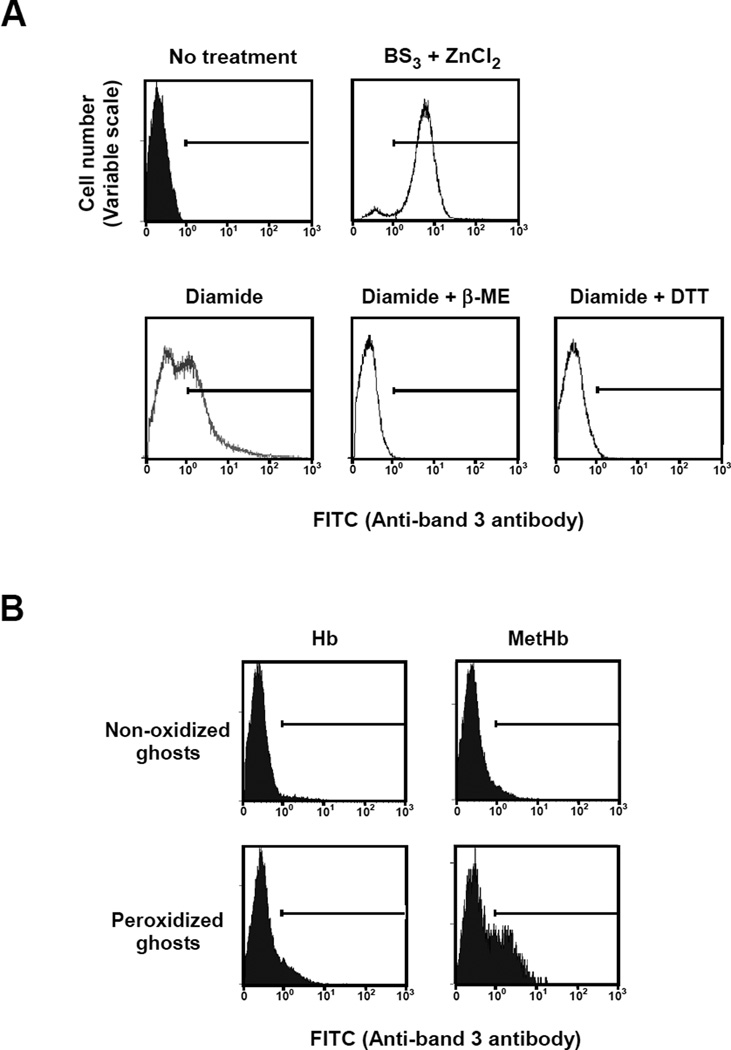
To validate the specificity of the anti-band 3 antibody, which we generated, we measured by FACS its capacity to bind normal erythrocyte membranes and membranes in which band 3 clustering had been induced by treatment with BS3 and ZnCl2. The antibody specifically recognized clustered band 3 in treated cells but not normal band 3 in untreated cells (Fig. 1A). The antibody similarly recognized clustered band 3 in cells treated with diamide which induces clustering by disulfide bond formation between cytoplasmic domain of band 3 molecules (25). Binding of the antibody was lost after reduction of the diamide-treated cells by DTT or β-ME (Fig. 1A). To confirm that the antibody preferentially recognizes clustered band 3, we performed inhibition analyses using either band 3 peptide 538-554 or band 3 MAP 538-554. The latter efficiently inhibited antibody binding to clustered band 3 induced by both BS3/ZnCl2 and diamide. These results imply that the antibody we used preferentially binds to clustered band 3 but not to native unclustered band 3 (Fig. S1).
Figure 1. FACS analysis of band 3 clustering in normal and variously treated ghosts.
(A) To assess the specificity of the peptide antibody recognizing clustered band 3, ghosts prepared from normal erythrocytes were incubated in the absence (upper left panel) or presence (upper right panel) of 1 mM BS3 and ZnCl2 to chemically cross-link band 3 or in the presence of 2 mM diamide (lower left panel) to reversibly cross-link band 3 by disulfide bonds. Moreover, diamide-treated ghosts reduced by 20 mM DTT (lower middle panel) or 10 mM β-ME (lower right panel) were also prepared to assess the reversible effect. FACS analysis of the cells was performed following incubation with rabbit anti-band 3 antibody and FITC-conjugated anti-rabbit IgG antibody. (B) Ghosts prepared from normal erythrocytes were incubated with (peroxidized ghosts) or without (non-oxidized ghosts) 0.6 mM t-BHP, a lipid peroxidation reagent, and then resealed with either Hb or MetHb. These resealed ghosts were subjected to FACS analysis as described above.
To investigate the ability of MetHb to induce band 3 clustering in erythrocyte membranes, we resealed either MetHb- or Hb-loaded ghosts prepared from normal erythrocytes. We ensured that reductases present in the cytosol had been removed by extensive washing prior to resealing. While resealing with Hb failed to induce antibody binding, incorporation of MetHb resulted in antibody binding to a small fraction (5.9%) of resealed cells (Fig. 1B). In marked contrast, following incorporation of MetHb into ghosts prepared from erythrocytes previously treated with t-BHP (a lipid-peroxidizing agent), a substantial increase in the proportion of cells displaying band 3 clusters (29.6%) was observed (Fig. 1B). Hb incorporation into peroxidized membranes resulted in a much smaller increase in antibody-labelled cells (9.0%). Peroxidation after treatment with t-BHP was confirmed by the resulting increased membrane-associated MDA relative to that in untreated cells (2.20 and 1.82 nmol/mg membrane protein, respectively). We conclude that MetHb can promote band 3 clustering in peroxidized erythrocyte membranes. We note that tyrosine phosphorylation of cytoplasmic domain of band 3 was not observed in these peroxidized membranes (data not sown).
MetHb binding to IOVs
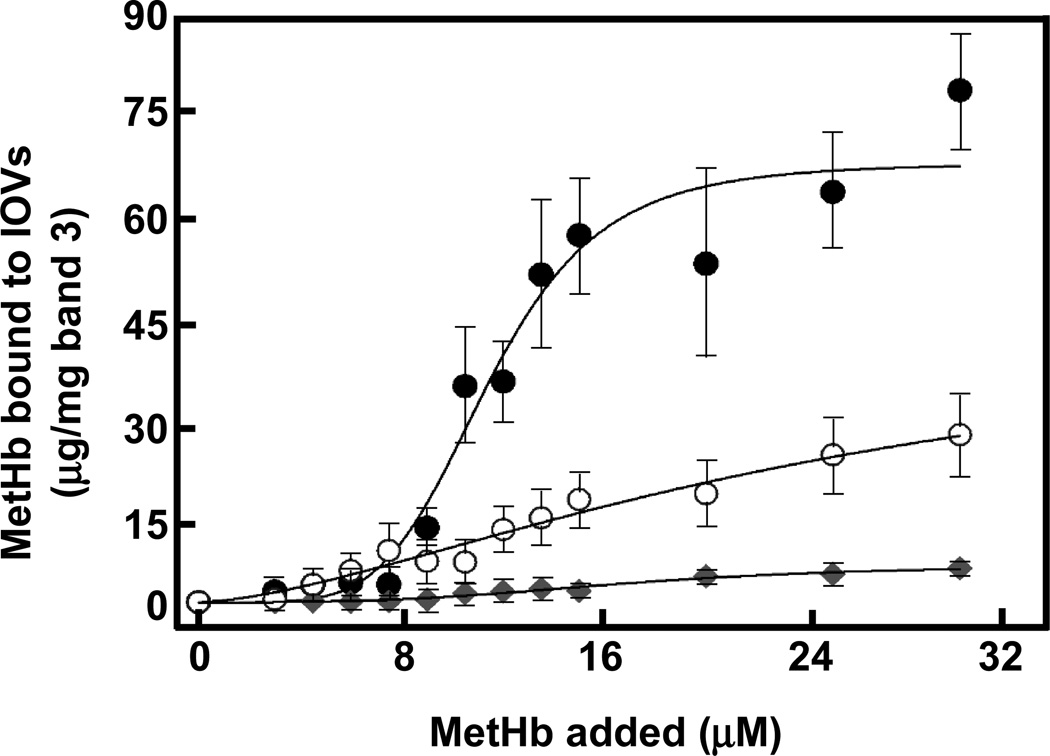
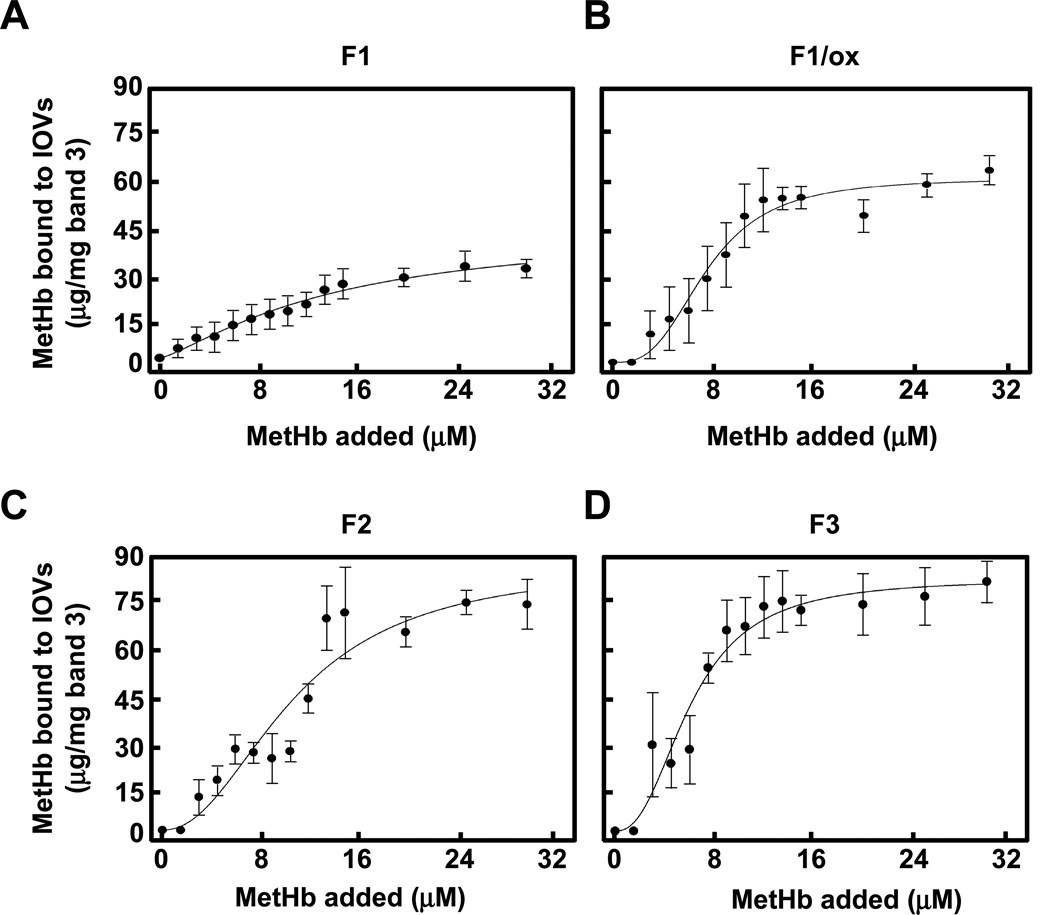
We measured the binding of MetHb and Hb to the cytoplasmic domain of band 3 in IOVs prepared from untreated and peroxidized erythrocyte membranes. Whereas concentration-dependent binding of MetHb to IOVs was observed (Fig. 2), Hb showed no perceptible binding. MetHb uptake by normal ghosts increased in a concentration-dependent manner, saturating at about 31.3 µM. Analysis of the binding profile revealed the presence of a single class of sites with Kd of 26 µM and estimated maximal binding of 45.3 µg/mg band 3 (Table 1). In marked contrast, the binding of MetHb to IOVs prepared from t-BHP-treated membranes, displayed a sigmoidal curve typical of positively cooperative binding with a Hill coefficient of 3.2 (Table 1). The Kd of binding to these IOVs was derived to be 11.9 µM with an estimated a maximal binding capacity of 67.6 µg/mg band 3. The binding of MetHb was almost entirely eliminated by treatment of the membranes with trypsin. This shows that binding to the lipid bilayer is minimal. Specificity of MetHb binding to band 3 (not other proteins) was shown by inhibition assay using cytoplasmic domain of band 3 that had been purified from peroxidized membranes (Fig. S2). With increasing concentrations of the cytoplasmic domain of band 3 the amount of MetHb associated with IOV decreased. Thus, while MetHb binds to the cytoplasmic domain of band 3 in a simple saturable manner in normal membranes, peroxidation of membranes leads to a somewhat higher affinity and cooperativity of binding most likely as a consequence of conformational coupling between the binding sites on the two subunits of the band 3 dimer.
Figure 2. Binding of MetHb to IOVs prepared from non-oxidized and peroxidized normal erythrocyte membranes.
Ten µg of non-oxidized IOVs (○), IOVs peroxidized by 0.6 mM t-BHP (●), and peroxidized IOVs digested by 5 µg/ml trypsin (♦) were incubated with MetHb (0 to 31.3 µM) and then IOVs were collected in the pellets by centrifugation through 8% sucrose cushion followed by SDS-PAGE and immunoblotting with anti-Hbβ monoclonal antibody. The amount of MetHb bound to IOVs was calculated by densitometric analysis. The data are the mean values ± S.D. of three individual experiments and the lines represent the best fit of the data using the Hill equation.
Table 1.
Kinetics analysis of MetHb to IOVs prepared from washed erythrocytes.
| IOV | Kd (µM) | Binding max | Hill coefficient | R2 |
|---|---|---|---|---|
| (µg/mg band 3) | ||||
| Non-oxidized | 26.4 ± 1.5 | 45.3 ± 19.6 | 1.6 ± 0.4 | 0.97 |
| Peroxidized | 11.9 ± 0.6 | 67.6 ± 4.5 | 3.2 ± 1.2 | 0.96 |
Characterization of density-fractionated human erythrocytes
To determine whether the clustering of band 3 in the cell in vitro, engendered by the combined effects of membrane oxidative damage and MetHb binding, is also a feature of erythrocyte aging in vivo, we examined cellular and membrane alterations in three distinct population of human erythrocytes. These were obtained by density-gradient fractionation, on the grounds that cell density increases with cell age. In our Percoll density fractionation protocol, 59.5% of all cells could be recovered in the intermediate-density fraction (F1), while only 1.0% and 0.1% were found in the higher-density F2 and F3 fractions, respectively. Measured values for MCV, MCHC, protein 4.1a/b ratio and MDA levels for each of the three fractions isolated from whole blood are shown in Table 2. As previously noted, with increasing cell density (F3>F2>F1), MCV decreases and MCHC increases reflecting erythrocyte aging in vivo. It should be noted all the monitored parameters showed little difference between the original whole blood and F1. As protein 4.1b is deamidated to 4.1a in non-enzymatic, irreversible and time-dependent manner, the ratio 4.1a/b is an excellent indicator of the age of erythrocytes in circulation (36, 37). The 0.1% dehydrated, dense red cells making up F3, had the highest ratio 4.1a/b, confirming that this represents the most senescent erythrocytes still circulating, while the 1% of cells in F2 are less senescent, but still more so than those in the bulk F1. It is also interesting to note that the MDA level of F3 was significantly higher than that of F2, but below that of F1 and of the unfractionated cells. These findings imply that erythrocyte membranes accumulate oxidant damage during cell aging in vivo.
Table 2.
Characterization of fractionated erythrocytes.
| Fraction | Ratio (% of total cells in each fraction) |
MCV (fl) |
MCHC (g/dl) |
Protein 4.1a/b | MDA (nmol/mg membrane protein) |
|---|---|---|---|---|---|
| F1 | 59.5 | 97.7 | 35.8 | 0.84 | 1.80 |
| F2 | 1.0 | 95.5 | 37.1 | 2.07 | 2.14 |
| F3 | 0.1 | 86.1 | 41.5 | 2.10 | 2.96 |
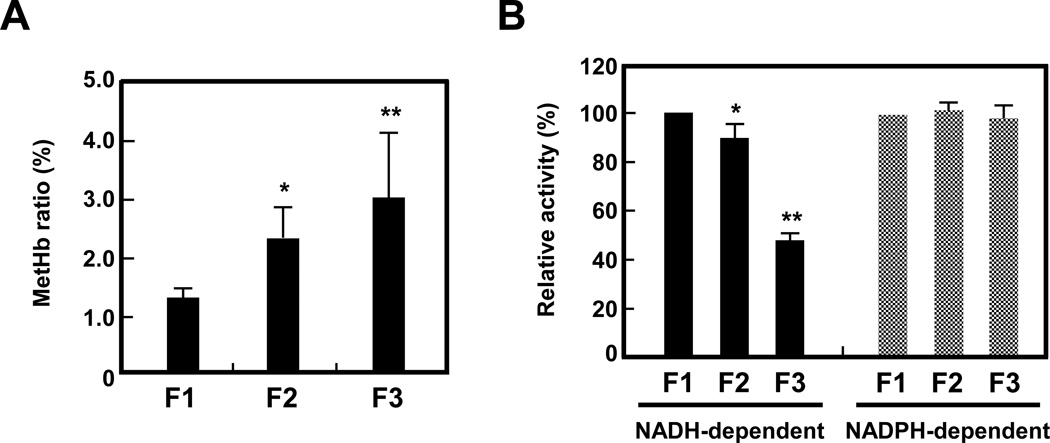
MetHb content of erythrocytes in the most dense cell fraction (F3) was significantly higher (3.0% of total Hb) than that in the least dense F1 fraction (1.3% of total Hb), while erythrocytes in F2 exhibit an intermediate level of MetHb (Fig. 3A). Correspondingly, NADH-dependent MetHb reductase activity was lower by some 10% and 50% respectively in the F3 and F2 cells relative to those in F1. NADPH-dependent reductase activity remained essentially unchanged in all fractions (Fig. 3B). Since NADH-dependent reductase is the enzyme primarily responsible for MetHb reduction (7, 38), the fall in its activity during red cell aging is responsible for MetHb accumulation in senescent erythrocytes.
Figure 3. MetHb content and MetHb reductase activity of fractionated erythrocytes.
(A) MetHb content of erythrocytes in F1, F2, and F3 fractions from 4 individuals was measured by the cyan methemoglobin method described in Methods. Data indicate the means ± S.D. *P<0.05, **P<0.01 vs. F1. (B) NADH- and NADPH-dependent MetHb reductase activities were measured in F1, F2, and F3 erythrocytes as described in Methods. Enzyme activities (%) in F2 and F3 erythrocytes were expressed as relative to the values derived for F1 erythrocytes. Data indicate the means ± S.D. (n = 3). *P<0.05, **P<0.01 vs. F1.
Characterization of band 3 clustering in density-fractionated human erythrocytes
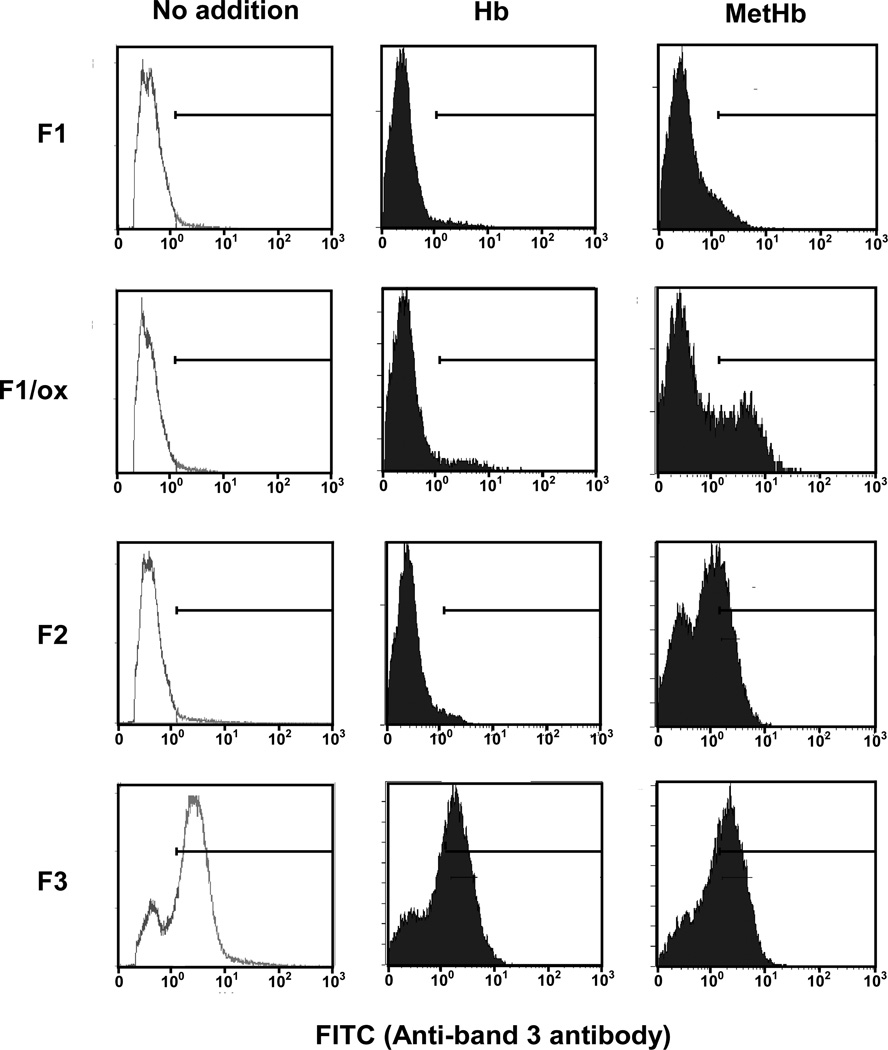
Using our band 3 antibody that specifically recognizes clustered band 3, we explored the extent of band 3 clustering in native density-fractionated human erythrocytes, and in resealed cells, loaded with either Hb or MetHb (Fig. 4). No clustered band 3 could be detected in native erythrocyte membranes prepared from either the F1 or F2 fractions. On the other hand, clustered band 3 could be readily detected in membranes of erythrocytes from F3 fraction, indicating that band 3 clustering is a feature of senescent cells. Peroxidization of F1 membranes (F1/ox) to the degree occurring naturally in F2 cells (MDA 2.23 nmol/mg membrane protein) did not induce band 3 clustering.
Figure 4. FACS analysis of Hb- or MetHb-incorporated ghosts prepared from density fractionated erythrocytes.
Hb or MetHb were resealed into the ghosts made from erythrocytes isolated in F1, F2, and F3 fractions, and peroxidized F1 ghosts (F1/ox). Band 3 clustering in these resealed ghosts was analyzed by FACS as described in the Methods.
We next examined whether incorporation of either MetHb or Hb can induce band 3 clustering in the different populations of density-fractionated erythrocytes (Fig. 4). In no case did incorporation of Hb generate additional band 3 clusters, although clusters did appear in the membranes of a small proportion (ca. 12%) Hb-loaded peroxidized ghosts prepared from the low-density F1 fraction. MetHb, by contrast, caused varying degrees of cluster formation in all three cell populations. Thus, while MetHb had little effect on native low-density cells, it engendered clusters in 35% of the same cell fraction after peroxidation. In the native intermediate density population of cells (F2), MetHb induced band 3 clusters in about 60% of the cells. MetHb did not further increase the already large fraction of cells in the high-density fraction (F3) containing prominent clusters (Fig. 4).
MetHb binding to IOVs prepared from density-fractionated erythrocytes
The binding of MetHb and Hb to IOVs prepared from membranes of defined subpopulations of density-fractionated red cells (F1, peroxidized F1, F2 and F3) was measured (Fig. 5). At Hb concentrations of up to 31 µM, no detectable binding to IOVs could be discerned. In marked contrast, MetHb bound to IOV preparations to varying degrees. Detectable binding of MetHb to F1 IOVs was observed at 4 µM, rising with increasing MetHb concentration to a plateau, corresponding to saturation at 30 µg/mg band 3, conforming to a single Kd of 10−5 M (Table 3). MetHb bound to F2 and F3 IOVs with higher affinity (Kd = 10−6 M) and with about twice as many binding sites at saturation than on F1 IOVs (76 and 74 µg/mg, respectively, as against 30 µg/mg). The binding profiles for F2 and F3 IOVs were sigmoidal, reflecting positive cooperative binding with Hill coefficients of 2.2 and 2.4, respectively (Table 3). Following peroxidation of F1 membranes, both the binding affinity and the maximal binding capacity for MetHb to F1/ox IOVs increased significantly (Kd = 10−6 M and 48 µg/mg, respectively). Perplexingly, this binding profile showed positive cooperativity with a Hill coefficient of 3.0. These findings imply that the peroxidation of membrane induces positive cooperative binding of MetHb to band 3.
Figure 5. Binding of MetHb to IOVs prepared from density fractionated erythrocytes.
The amount of MetHb bound to IOVs prepared from F1 (A), F1/ox (B), F2 (C), and F3 (D) ghosts was measured and showed as described in the Methods and the legend of Fig. 2.
Table 3.
Kinetic analysis of MetHb to IOVs prepared from fractionated erythrocytes.
| IOV | Kd (µM) | Binding max | Hill coefficient | R2 |
|---|---|---|---|---|
| (µg/mg band 3) | ||||
| F1 | 21.1 ± 6.3 | 30.4 ± 4.3 | 1.1 ± 0.4 | 0.93 |
| F1/ox | 7.8 ± 0.3 | 48.2 ± 18.0 | 3.0 ± 0.5 | 0.97 |
| F2 | 8.5 ± 1.4 | 75.8 ± 9.6 | 2.2 ± 0.6 | 0.96 |
| F3 | 6.4 ± 0.6 | 73.5 ± 5.1 | 2.4 ± 0.6 | 0.94 |
Effect of MetHb binding to band 3 on its function
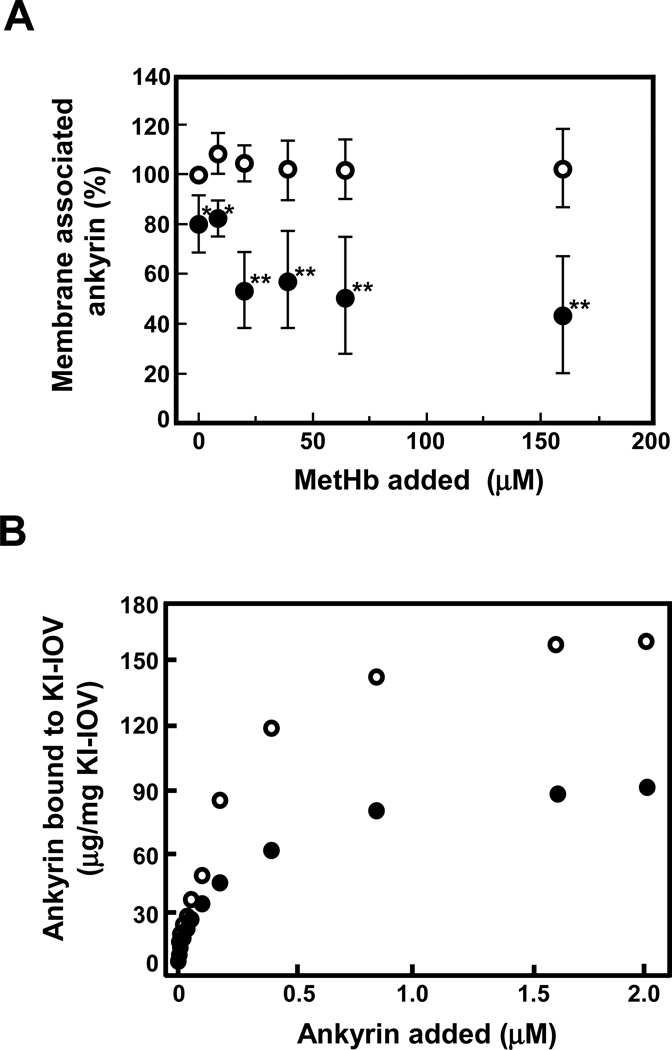
We next examined whether MetHb binding to the cytoplasmic domain of band 3 affects its interactions with its binding partners, ankyrin and protein 4.1 (39). Following bindings of increasing amounts of MetHb to IOVs prepared from normal membranes, both ankyrin and protein 4.1 remained associated with IOVs (data not shown). By contrast, MetHb binding to IOVs prepared from t-BHP-treated membranes resulted in dose-dependent dissociation of ankyrin from the membranes (Fig. 6A), implying decreased affinity of band 3 binding to ankyrin following oxidative damage. No concomitant change in membrane content of protein 4.1 was detected (data not shown). It should be noted that peroxidation by itself appeared to decrease the ankyrin content of IOVs by about 20%. To further support our conclusions, we measured the binding of ankyrin to normal and peroxidized and ankyrin-stripped IOVs (KI-IOVs). While both IOV preparations bound ankyrin in a concentration-dependent manner, maximal binding to t-BHP-treated KI-IOVs was only half of that to normal KI-IOVs (Fig. 6B). We infer that MetHb binding to the cytoplasmic domain of band 3 in peroxidized membrane induces a steric or conformational effect that reduces its ability to bind ankyrin.
Figure 6. Interaction of ankyrin with band 3 in non-oxidized and peroxidized IOVs.
(A) The amount of ankyrin associated with non-oxidized (○) or peroxidized (●) IOVs was measured in the presence or absence of MetHb by immunoblotting using anti-ankyrin antibody as described in Methods. The data represent the mean ratio (%) ± S.D. normalized by the amount of ankyrin bound to non-oxidized IOVs in the absence of MetHb (n = 3). *P<0.05, **P<0.01. (B) KI-IOVs pretreated with (●) or without (○) t-BHP were incubated with increasing concentrations of purified ankyrin. The amount of bound ankyrin to KI-IOVs was measured as described in Method and plotted against the concentration of ankyrin added.
Carbonylation of band 3 in peroxidized membranes
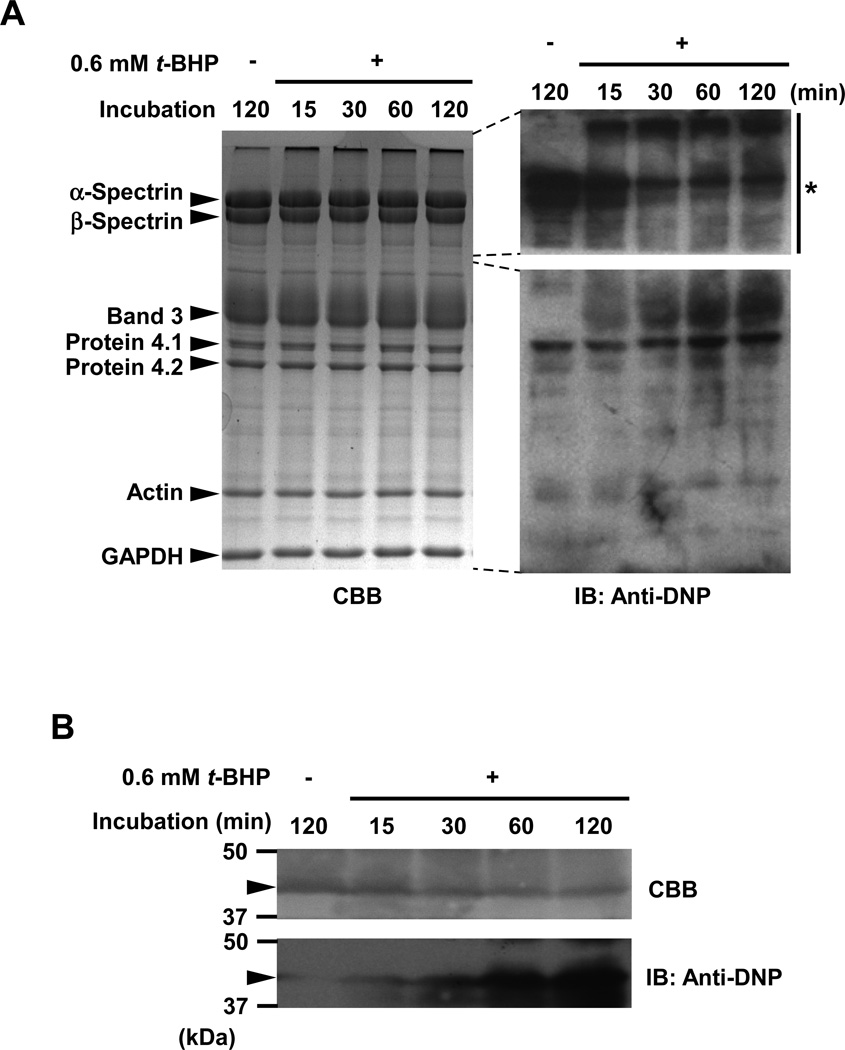
Carbonylated proteins of the ghosts incubated with or without 0.6 mM t-BHP for up to 120 min were analyzed by derivatization of carbonyl groups using DNPH and western blotting using anti-DNP antibody. Carbonylation of band 3 increased with increasing time of incubation with the reagent (Fig. 7A). Progressive carbonylation of the cytoplasmic domain of band 3 was also observed (Fig. 7B). Spectrin and protein 4.1 were both carbonylated in native ghosts, but the extent of carbonylation of both proteins was unchanged by treatment with t-BHP (Fig. 7A). We suggest that the observed carbonylation of spectrin is a consequence of endogenous modification with 4-hydroxy-2-nonenal (HNE), a carbonylation reaction in a broad sense, because DNPH also reacts with HNE-modified proteins generated by Michael reaction and Schiff-base formation (8, 9). We showed previously that only α- and β-spectrin were endogenously modified with HNE in erythrocyte membrane proteins and HNE-modified spectrin readily formed aggregates (40). The signals of carbonylated spectrin corresponding to the original position of spectrin were shifted to upper edge of the gel (high molecular weight region) by t-BHP-treatment. Immunoblot analyses to assess HNE- and MDA-dependent modification of band 3 in t-BHP-treated membranes were performed using anti-HNE or MDA antibodies (Academy Bio-medical, Houston, TX, U.S.A.), respectively. Neither HNE- nor MDA-induced modification of band 3 could be detected in t-BHP-treated ghosts (data not shown). Thiol groups in the cytoplasmic domain of band 3 purified from ghosts that had been treated with t-BHP were assayed with Ellman’s reagent (DTNB). Oxidation of SH groups in peroxidized cytoplasmic domain of band 3 was not found to have occurred (data not shown).
Figure 7. Carbonylation of band 3 induced by membrane peroxidation.
Carbonylated proteins of the ghosts which had been treated with or without 0.6 mM t-BHP for up to 120 min were analyzed as described in Methods. (A) Signal intensity of carbonylated proteins corresponding to the position of band 3 in the gel increased in time-dependent manner. The asterisk indicates a part of the gel that was exposed for a very short period (5 sec), showing that spectrin was endogenously carbonylated under the experimental conditions used to monitor carbonylation but did not show time-dependent changes. (B) Carbonylation of cytoplasmic domain of band 3 (43 kDa; arrow head) in t-BHP-treated IOVs. Carbonylation of cytoplasmic domain of band 3 increased with increasing incubation time.
DISCUSSION
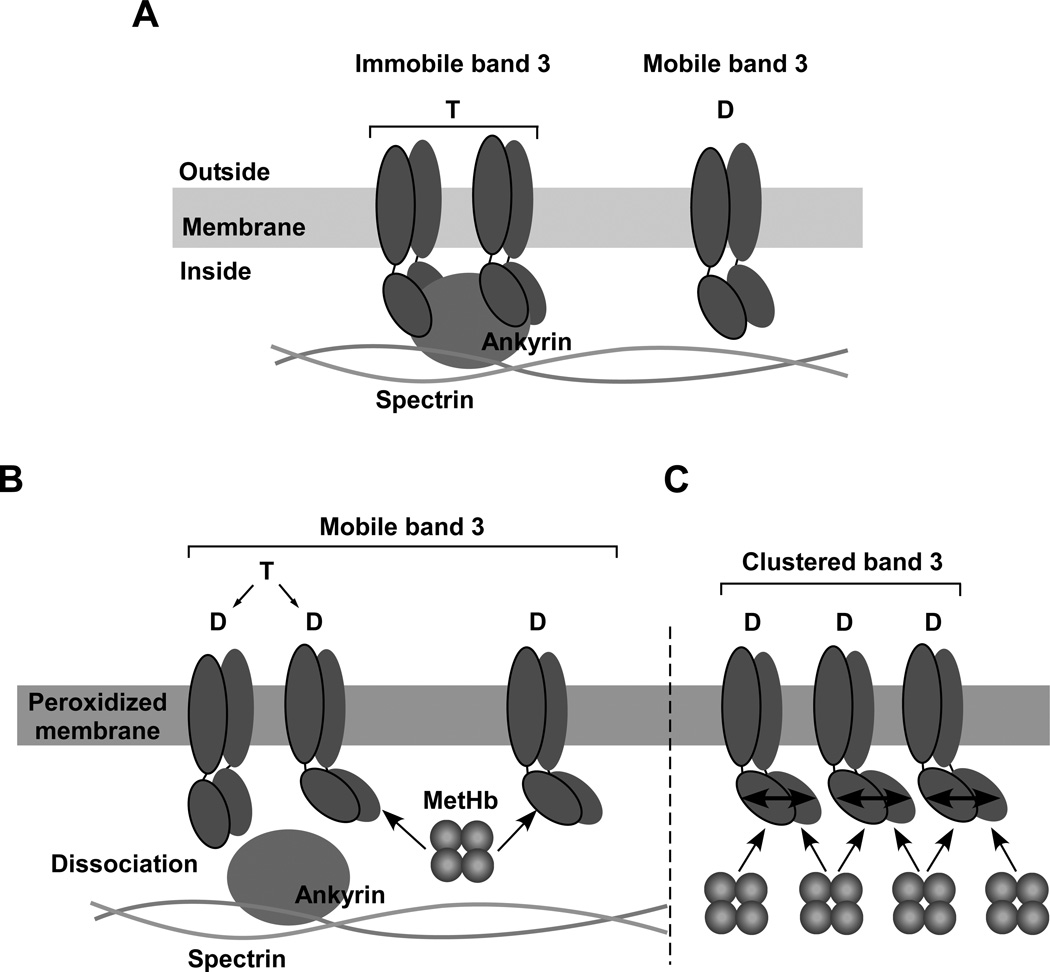
The results set out above reveal that membrane peroxidation is a prerequisite for MetHb-induced clustering of band 3 in erythrocytes. A possible mechanism for the combined effects of membrane peroxidation and MetHb in inducing band 3 clustering is depicted in Fig.8. Peroxidation of membranes results in a moderate degree of dissociation of ankyrin from its site of attachment on the cytoplasmic domain of band 3, and also a high-affinity cooperative interaction between band 3 and MetHb. Binding of MetHb promoted the loss of ankyrin. It is known (39) that dissociation of ankyrin releases the constraint on band 3 from lateral diffusion in the membrane. Binding of MetHb to one subunit of cytoplasmic domain of band 3 induces a conformational change in the cytoplasmic domains of the other subunits of the band 3 dimer, thereby increasing the affinity of their MetHb binding sites. This would account for the observed cooperativity of binding. As MetHb is a tetramer, it can cross-link the freely diffusing band 3 dimers to induce clustering.
Figure 8. A potential mechanism for the combined effect of membrane peroxidation and MetHb in inducing band 3 clustering.
(A) Band 3 tetramer formed by two dimers and linked to the skeletal network through interaction with ankyrin is immobile and is thus uniformly distributed in the membrane. The mobile band 3 dimer is not connected to the skeleton but its area of diffusion is restricted to within the lattice (fence) of the network. (B) Peroxidation of erythrocyte membranes results in a modest degree of dissociation of ankyrin from cytoplasmic domain of band 3 and high affinity cooperative binding of MetHb to cytoplasmic domain of band 3 accelerates this dissociation process probably due to carbonylation of cytoplasmic domain of band 3. Following dissociation from ankyrin, band 3 is released from its constraints for lateral diffusion in the membrane. (C) Binding of MetHb to one subunit of cytoplasmic domain of band 3 induces a conformational change in the cytoplasmic domains of other associated band 3 subunits leading to high affinity cooperative binding of MetHb. As MetHb is a tetramer, it can form a bridge between a number of diffusing band 3 dimers to induce clustering.
Our finding reveal that band 3 clustering is a feature of a very small fraction (0.1% of total) of circulating normal erythrocytes, indicating that band 3 clustering is very late event in normal erythrocyte aging and is a feature of senescent cells, which are then rapidly removed from the circulation. The finding that this fraction of cells exhibits elevated levels of MDA and increased MetHb binding in association with band 3 clustering supports our hypothesis that both membrane peroxidation and MetHb are necessary for band 3 clustering. This conclusion is further supported by our finding that neither membrane peroxidation nor MetHb alone induce band 3 clustering. The lack of band 3 clusters in the vast majority of circulating erythrocytes is a probable consequence of the two protective mechanisms: normal reductase activity, sufficient to prevent accumulation of MetHb, and effective functioning of scavengers (superoxide dismutase, glutathione, or vitamins C and E) to protect against lipid peroxidation (1, 41). It is interesting that we also found no band 3 clustering in a small subpopulation of cells (F2) with high MDA but little MetHb, but in which clustering could be induced following incorporation of MetHb (Fig. 4). Taken together these findings lend strong support to our thesis that both membrane peroxidation and MetHb are necessary for band 3 clustering in senescent erythrocytes.
Erythrocytes are continuously exposed to oxidative stress from such sources as reactive oxygen species (ROS) from both the internal and external environments (1, 2). Many forms of oxidative modification of proteins can be induced directly by ROS, possibly through modification of amino acid residues such as Pro, Arg, Lys, and Thr, resulting in protein carbonylation (9, 42). We indeed could observe carbonylation of both band 3 and its cytoplasmic domain as a consequence of membrane peroxidation, rather than other oxidative processes, such as oxidation of thiol groups (Fig. 7). The finding that ankyrin dissociates from the cytoplasmic domain of band 3 in peroxidized membranes suggests a possible conformational perturbation at the ankyrin binding site in this domain (43–45), probably due carbonylation, either direct or mediated by carbonylation of transmembrane domain of band 3. Ankyrin dissociation is necessary for band 3 clustering as discussed above (Fig. 8). The high-affinity cooperative bindings of MetHb to band 3 in peroxidized membranes could also be the result of a conformational change in an as yet unidentified MetHb binding site in band 3. The crystal structure of the cytoplasmic domain of band 3 indicates a tight symmetric dimer formed by interlocking dimerization arms of the two monomers (46). We suggest that MetHb binding to one domain may induce conformational change in the partner domain. Analysis of the co-crystal structure of a complex of MetHb and cytoplasmic domain of band 3 obtained from peroxidized membranes should provide insights into an induced conformational change.
We note finally that ROS also causes membrane lipid peroxidation leading to generation of high levels of reactive aldehydes such as MDA and HNE (8). These products readily bind covalently to proteins with consequent changes in conformation and function (8, 47, 48). In erythrocytes, HNE has been shown to modify spectrin (40), but our failure to detect modifications of band 3 by either MDA or HNE following peroxidation suggests that such modifications may not play a direct role.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Grant-in-aid for Scientific Research 22591112 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and NIH grant DK26263.
ABBREVIATIONS
- BS3
bis(sulfosuccinimidyl)suberate
- IOV
inside-out vesicle
- Kd
dissociation constant
- KI-IOV
potassium iodide-treated inside-out vesicle
- HNE
4-hydroxy-2-nonenal
- MDA
malondialdehyde
- MetHb
methemoglobin
- ROS
reactive oxygen species
- SDS
sodium dodecyl sulfate
- t-BHP
tert-butyl hydroperoxide
Footnotes
Supporting Information
Two supporting figures (Figures S1 and S2) are included to confirm the specific recognition of clustered band 3 by the anti-band 3 antibody and the specific binding between MetHb and band 3 on IOVs. These materials are available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Burak Cimen MY. Free radical metabolism in human erythrocytes. Clinica. Chimica. Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 2.Pandey KB, Rizvi SI. Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxid. Med. Cell. Longev. 2010;3:2–12. doi: 10.4161/oxim.3.1.10476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Signorini C, Ferrali M, Ciccoli L, Sugherini L, Magnani A, Comporti M. Iron release, membrane protein oxidation and erythrocyte ageing. FEBS Lett. 1995;362:165–170. doi: 10.1016/0014-5793(95)00235-2. [DOI] [PubMed] [Google Scholar]
- 4.Hochstein P, Jain SK. Association of lipid peroxidation and polymerization of membrane proteins with erythrocyte aging. Fed. Proc. 1981;40:183–188. [PubMed] [Google Scholar]
- 5.Jain SK. Evidence for membrane lipid peroxidation during the in vivo aging of human erythrocytes. Biochim. Biophys. Acta. 1988;937:205–210. doi: 10.1016/0005-2736(88)90242-8. [DOI] [PubMed] [Google Scholar]
- 6.Piccinini G, Minetti G, Balduini C, Brovelli A. Oxidation state of glutathione and membrane proteins in human red cells of different age. Mech. Ageing Dev. 1995;78:15–26. doi: 10.1016/0047-6374(94)01511-j. [DOI] [PubMed] [Google Scholar]
- 7.Scott EM, Duncan IW, Ekstrand V. The reduced pyridine nucleotide dehydrogenases of human erythrocytes. J. Biol. Chem. 1965;240:481–485. [PubMed] [Google Scholar]
- 8.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 9.Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A. Protein carbonylation in human diseases. Trends Mol. Med. 2003;9:169–176. doi: 10.1016/s1471-4914(03)00031-5. [DOI] [PubMed] [Google Scholar]
- 10.Nash GB, Wyard SJ. Changes in surface area and volume measured by micropipette aspiration for erythrocytes ageing in vivo. Biorheology. 1980;17:479–484. [PubMed] [Google Scholar]
- 11.Linderkamp O, Meiselman HJ. Geometric, osmotic, and membrane mechanical properties of density-separated human red cells. Blood. 1982;59:1121–1127. [PubMed] [Google Scholar]
- 12.Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 13.Low PS, Waugh SM, Zinke K, Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- 14.Schluter A, Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6137–6141. doi: 10.1073/pnas.83.16.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kannan R, Yuan J, Low PS. Isolation and partial characterization of antibody- and globin-enriched complexes from membranes of dense human erythrocytes. Biochem. J. 1991;278:57–62. doi: 10.1042/bj2780057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor J, Pak CC, Schroit AJ. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J. Biol. Chem. 1994;269:2399–2404. [PubMed] [Google Scholar]
- 17.Singer JA, Jennings LK, Jackson CW, Docker ME, Morrison M, Walker WS. Erythrocyte homeostasis: antibody-mediated recognition of the senescent state by macrophages. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5498–5501. doi: 10.1073/pnas.83.15.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz HU, Bussolino F, Flepp R, Fasler S, Stammler P, Kazatchkine MD, Arese P. Naturally occurring anti-band-3 antibodies and complement together mediate phagocytosis of oxidatively stressed human erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 1987;84:7368–7372. doi: 10.1073/pnas.84.21.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turrini F, Arese P, Yuan J, Low PS. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J. Biol. Chem. 1991;266:23611–23617. [PubMed] [Google Scholar]
- 20.Waugh SM, Willardson BM, Kannan R, Labotka RJ, Low PS. Heinz bodies induce clustering of band 3, glycophorin, and ankyrin in sickle cell erythrocytes. J. Clin. Invest. 1986;78:1155–1160. doi: 10.1172/JCI112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood KC, Granger DN. Sickle cell disease: role of reactive oxygen and nitrogen metabolites. Clin. Exp. Pharmacol. Physiol. 2007;34:926–932. doi: 10.1111/j.1440-1681.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- 22.Rachmilewitz EA, Lubin BH, Shohet SB. Lipid membrane peroxidation in beta-thalassemia major. Blood. 1976;47:495–505. [PubMed] [Google Scholar]
- 23.Mannu F, Arese P, Cappellini MD, Fiorelli G, Cappadoro M, Giribaldi G, Turrini F. Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in beta-thalassemia intermedia erythrocytes. Blood. 1995;86:2014–2020. [PubMed] [Google Scholar]
- 24.Pantaleo A, Giribaldi G, Mannu F, Arese P, Turrini F. Naturally occurring anti-band 3 antibodies and red blood cell removal under physiological and pathological conditions. Autoimmun. Rev. 2008;7:457–462. doi: 10.1016/j.autrev.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo A, Ferru E, Giribaldi G, Mannu F, Carta F, Matte A, Franceschi LD, Turrini F. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem. J. 2009;418:359–367. doi: 10.1042/BJ20081557. [DOI] [PubMed] [Google Scholar]
- 26.Schmedes A, Holmer G. A new thiobarbituric (TBA) method for determining free malondialdehyde (MDA) and hydroperoxides selectively as a measure of lipid peroxidation. J. Am. Oil Chem. Soc. 1989;66:813–817. [Google Scholar]
- 27.Trotta RJ, Sullivan SG, Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem. J. 1983;212:759–772. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hensley P, Moffat K, Edelstein SJ. Influence of inositol hexaphosphate binding on subunit dissociation in methemoglobin. J. Biol. Chem. 1975;250:9391–9396. [PubMed] [Google Scholar]
- 29.Chiarantini L, Rossi L, Fraternale A, Magnani M. Modulated red blood cell survival by membrane protein clustering. Mol. Cell. Biochem. 1995;144:53–59. doi: 10.1007/BF00926740. [DOI] [PubMed] [Google Scholar]
- 30.Kuma F. Properties of methemoglobin reductase and kinetic study of methemoglobin reduction. J. Biol. Chem. 1981;256:5518–5523. [PubMed] [Google Scholar]
- 31.Yubisui T, Takeshita M, Yoneyama Y. Reduction of methemoglobin through flavin at the physiological concentration by NADPH-flavin reductase of human erythrocytes. J. Biochem. 1980;87:1715–1720. doi: 10.1093/oxfordjournals.jbchem.a132915. [DOI] [PubMed] [Google Scholar]
- 32.Manno S, Takakuwa Y, Mohandas N. Identification of a functional role for lipid asymmetry in biological membranes: Phosphatidylserine-skeletal protein interactions modulate membrane stability. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1943–1948. doi: 10.1073/pnas.042688399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.An X, Takakuwa Y, Nunomura W, Manno S, Mohandas N. Modulation of band 3-ankyrin interaction by protein 4.1. Functional implications in regulation of erythrocyte membrane mechanical properties. J. Biol. Chem. 1996;271:33187–33191. doi: 10.1074/jbc.271.52.33187. [DOI] [PubMed] [Google Scholar]
- 34.Tyler JM, Reinhardt BN, Branton D. Associations of erythrocyte membrane proteins. Binding of purified bands 2.1 and 4.1 to spectrin. J. Biol. Chem. 1980;255:7034–7039. [PubMed] [Google Scholar]
- 35.Bennett V, Stenbuck PJ. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J. Biol. Chem. 1980;255:6424–6432. [PubMed] [Google Scholar]
- 36.Inaba M, Maede Y. Correlation between protein 4.1a/4.1b ratio and erythrocyte life span. Biochim. Biophys. Acta. 1988;944:256–264. doi: 10.1016/0005-2736(88)90439-7. [DOI] [PubMed] [Google Scholar]
- 37.Inaba M, Gupta KC, Kuwabara M, Takahashi T, Benz EJ, Jr, Maede Y. Deamidation of human erythrocyte protein 4.1: possible role in aging. Blood. 1992;79:3355–3361. [PubMed] [Google Scholar]
- 38.Haymond S, Cariappa R, Eby CS, Scott MG. Laboratory assessment of oxygenation in methemoglobinemia. Clin. Chem. 2005;51:434–444. doi: 10.1373/clinchem.2004.035154. [DOI] [PubMed] [Google Scholar]
- 39.Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112:3939–3948. doi: 10.1182/blood-2008-07-161166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arashiki N, Otsuka Y, Ito D, Yang M, Komatsu T, Sato K, Inaba M. The covalent modification of spectrin in red cell membranes by the lipid peroxidation product 4-hydroxy-2-nonenal. Biochem. Biophys. Res. Commun. 2010;391:1543–1547. doi: 10.1016/j.bbrc.2009.12.121. [DOI] [PubMed] [Google Scholar]
- 41.Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011;51:1000–1013. doi: 10.1016/j.freeradbiomed.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis L, Lux SE, Bennett V. Mapping the ankyrin-binding site of the human erythrocyte anion exchanger. J. Biol. Chem. 1989;264:9665–9672. [PubMed] [Google Scholar]
- 44.Ding Y, Casey JR, Kopito RR. The major kidney AE1 isoform does not bind ankyrin (Ank1) in vitro. An essential role for the 79 NH2-terminal amino acid residues of band 3. J. Biol. Chem. 1994;269:32201–32208. [PubMed] [Google Scholar]
- 45.Ding Y, Kobayashi S, Kopito R. Mapping of ankyrin binding determinants on the erythroid anion exchanger, AE1. J. Biol. Chem. 1996;271:22494–22498. doi: 10.1074/jbc.271.37.22494. [DOI] [PubMed] [Google Scholar]
- 46.Zhang D, Kiyatkin A, Bolin JT, Low PS. Crystallographic structure and functional interpretation of the cytoplasmic domain of erythrocyte membrane band 3. Blood. 2000;96:2925–2933. [PubMed] [Google Scholar]
- 47.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.