Abstract
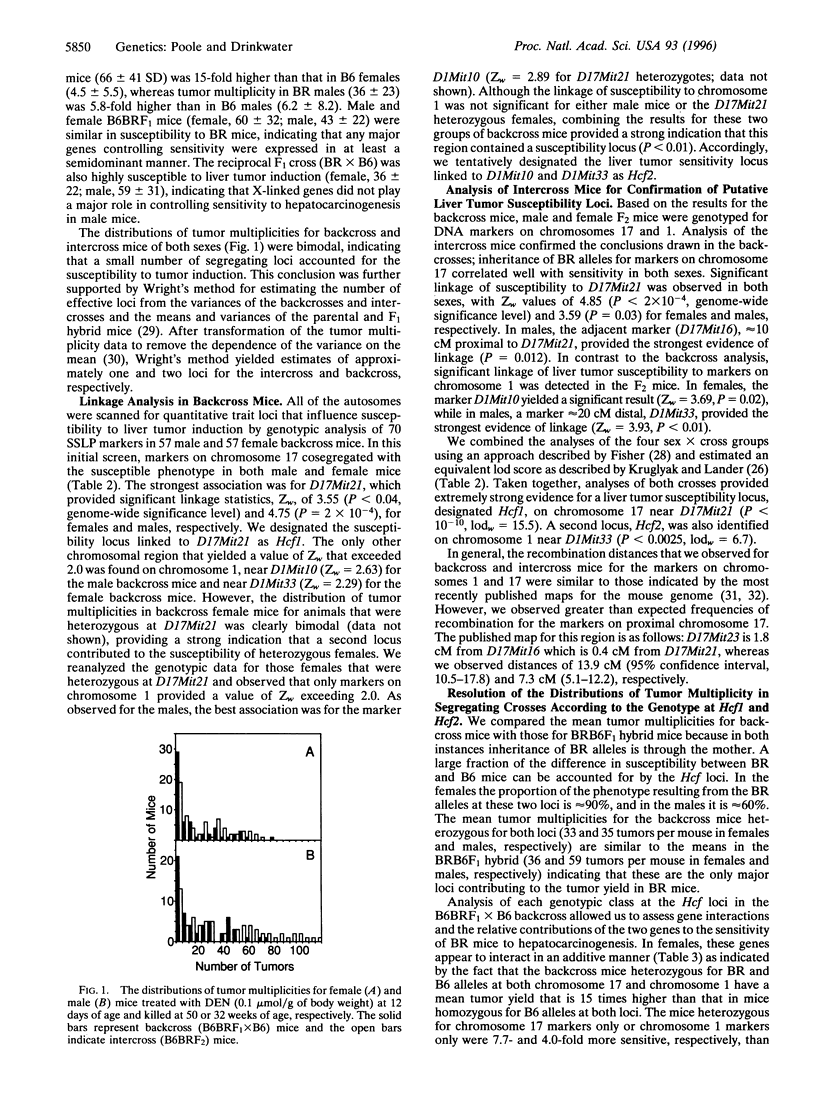
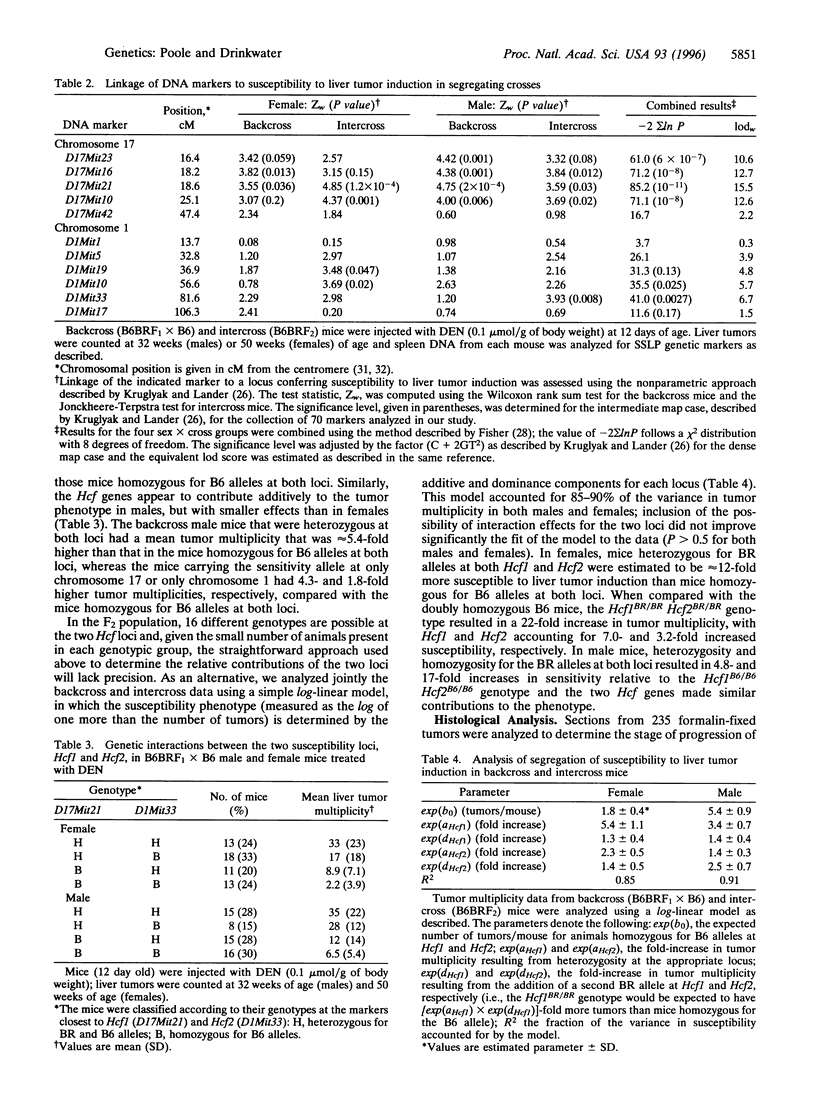
Hormonal and genetic factors strongly influence the susceptibility of inbred mice to hepatocarcinogenesis. Female C57BR/cdJ (BR) mice are extremely susceptible to liver tumor induction relative to other strains because they are genetically insensitive to the inhibition of hepatocarcinogenesis by ovarian hormones. To determine the genetic basis for the sensitivity of BR mice relative to resistant C57BL/6J (B6) mice, we treated 12-day-old B6BRF1 x B6 and B6BRF1 x B6BRF1 (F2) animals with N,N-diethylnitrosamine (0.1 micromol/g of body weight) and enumerated liver tumors at 32 weeks of age in males and at 50 weeks in females. Genomic DNA samples from backcross and F2 mice were analyzed for 70 informative simple sequence length polymorphism markers. Genetic markers on chromosome 17 (D17Mit21) and chromosome 1 (D1Mit33) cosegregated with high tumor multiplicity in both sexes. Together, these loci [designated Hcf1 and Hcf2 (Hepatocarcinogenesis in females), respectively] account for virtually all of the difference in sensitivity between BR and B6 mice. The Hcf1 locus accounts for a majority of the higher susceptibility of BR mice of both sexes. Backcross female mice heterozygous at both loci (33 +/- 23 tumors per mouse) and at Hcf1 only (17 +/- 18) were 15- and 8-fold more sensitive, respectively, than mice homozygous for the B6 alleles at Hcf1 and Hcf2 (2.2 +/- 3.9). In backcross male mice, the double heterozygotes (35 +/- 22) and Hcf1 heterozygotes (28 +/- 12) were 5.4- and 4.3-fold more sensitive than mice homozygous for B6 alleles at both loci (6.5 +/- 5.4).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERVONT H. B. Studies on the occurrence of spontaneous hepatomas in mice of strains C3H and CBA. J Natl Cancer Inst. 1950 Dec;11(3):581–592. [PubMed] [Google Scholar]
- Aitman T. J., Hearne C. M., McAleer M. A., Todd J. A. Mononucleotide repeats are an abundant source of length variants in mouse genomic DNA. Mamm Genome. 1991;1(4):206–210. doi: 10.1007/BF00352326. [DOI] [PubMed] [Google Scholar]
- Amor M., Tosi M., Duponchel C., Steinmetz M., Meo T. Liver mRNA probes disclose two cytochrome P-450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4453–4457. doi: 10.1073/pnas.82.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang D., Liberek K., Skowyra D., Zylicz M., Georgopoulos C. Biological role and regulation of the universally conserved heat shock proteins. J Biol Chem. 1991 Dec 25;266(36):24233–24236. [PubMed] [Google Scholar]
- Atkinson J. P., Karp D. R., Seeskin E. P., Killion C. C., Rosa P. A., Newell S. L., Shreffler D. C. H-2 S region determined polymorphic variants of the C4, Slp, C2, and B complement proteins: a compilation. Immunogenetics. 1982;16(6):617–623. doi: 10.1007/BF00372032. [DOI] [PubMed] [Google Scholar]
- Buchmann A., Bauer-Hofmann R., Mahr J., Drinkwater N. R., Luz A., Schwarz M. Mutational activation of the c-Ha-ras gene in liver tumors of different rodent strains: correlation with susceptibility to hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):911–915. doi: 10.1073/pnas.88.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D., Woods D. E., Whitehead A. S., Goldberger G., Colten H. R., Seidman J. G. Molecular map of the murine S region. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6947–6951. doi: 10.1073/pnas.80.22.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demant P., Oomen L. C., Oudshoorn-Snoek M. Genetics of tumor susceptibility in the mouse: MHC and non-MHC genes. Adv Cancer Res. 1989;53:117–179. doi: 10.1016/s0065-230x(08)60281-x. [DOI] [PubMed] [Google Scholar]
- Dietrich W. F., Miller J. C., Steen R. G., Merchant M., Damron D., Nahf R., Gross A., Joyce D. C., Wessel M., Dredge R. D. A genetic map of the mouse with 4,006 simple sequence length polymorphisms. Nat Genet. 1994 Jun;7(2 Spec No):220–245. doi: 10.1038/ng0694supp-220. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R. Genetic control of hepatocarcinogenesis in C3H mice. Drug Metab Rev. 1994;26(1-2):201–208. doi: 10.3109/03602539409029791. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R., Ginsler J. J. Genetic control of hepatocarcinogenesis in C57BL/6J and C3H/HeJ inbred mice. Carcinogenesis. 1986 Oct;7(10):1701–1707. doi: 10.1093/carcin/7.10.1701. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R., Klotz J. H. Statistical methods for the analysis of tumor multiplicity data. Cancer Res. 1981 Jan;41(1):113–119. [PubMed] [Google Scholar]
- Fitch W. M., Atchley W. R. Evolution in inbred strains of mice appears rapid. Science. 1985 Jun 7;228(4704):1169–1175. doi: 10.1126/science.4001935. [DOI] [PubMed] [Google Scholar]
- Forejt J., Artzt K., Barlow D. P., Hamvas R. M., Lindahl K. F., Lyon M. F., Klein J., Silver L. M. Mouse chromosome 17. Mamm Genome. 1994;5(Spec No):S238–S258. [PubMed] [Google Scholar]
- Gardner S. M., Mock B. A., Hilgers J., Huppi K. E., Roeder W. D. Mouse lymphotoxin and tumor necrosis factor: structural analysis of the cloned genes, physical linkage, and chromosomal position. J Immunol. 1987 Jul 15;139(2):476–483. [PubMed] [Google Scholar]
- Gariboldi M., Manenti G., Canzian F., Falvella F. S., Pierotti M. A., Della Porta G., Binelli G., Dragani T. A. Chromosome mapping of murine susceptibility loci to liver carcinogenesis. Cancer Res. 1993 Jan 15;53(2):209–211. [PubMed] [Google Scholar]
- Hanigan M. H., Kemp C. J., Ginsler J. J., Drinkwater N. R. Rapid growth of preneoplastic lesions in hepatocarcinogen-sensitive C3H/HeJ male mice relative to C57BL/6J male mice. Carcinogenesis. 1988 Jun;9(6):885–890. doi: 10.1093/carcin/9.6.885. [DOI] [PubMed] [Google Scholar]
- Hoopes C. W., Taketo M., Ozato K., Liu Q., Howard T. A., Linney E., Seldin M. F. Mapping of the mouse Rxr loci encoding nuclear retinoid X receptors RXR alpha, RXR beta, and RXR gamma. Genomics. 1992 Nov;14(3):611–617. doi: 10.1016/s0888-7543(05)80159-4. [DOI] [PubMed] [Google Scholar]
- Kemp C. J., Drinkwater N. R. Genetic variation in liver tumor susceptibility, plasma testosterone levels, and androgen receptor binding in six inbred strains of mice. Cancer Res. 1989 Sep 15;49(18):5044–5047. [PubMed] [Google Scholar]
- Kruglyak L., Lander E. S. A nonparametric approach for mapping quantitative trait loci. Genetics. 1995 Mar;139(3):1421–1428. doi: 10.1093/genetics/139.3.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G. H., Bennett L. M., Carabeo R. A., Drinkwater N. R. Identification of hepatocarcinogen-resistance genes in DBA/2 mice. Genetics. 1995 Jan;139(1):387–395. doi: 10.1093/genetics/139.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London W. T. Primary hepatocellular carcinoma--etiology, pathogenesis, and prevention. Hum Pathol. 1981 Dec;12(12):1085–1097. doi: 10.1016/s0046-8177(81)80329-2. [DOI] [PubMed] [Google Scholar]
- Manenti G., Binelli G., Gariboldi M., Canzian F., De Gregorio L., Falvella F. S., Dragani T. A., Pierotti M. A. Multiple loci affect genetic predisposition to hepatocarcinogenesis in mice. Genomics. 1994 Sep 1;23(1):118–124. doi: 10.1006/geno.1994.1466. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Borgmeyer U., Heyman R. A., Zhou J. Y., Ong E. S., Oro A. E., Kakizuka A., Evans R. M. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992 Mar;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- Milner C. M., Campbell R. D. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32(4):242–251. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- Milstone D. S., Shaw S. K., Parker K. L., Szyf M., Seidman J. G. An element regulating adrenal-specific steroid 21-hydroxylase expression is located within the slp gene. J Biol Chem. 1992 Oct 25;267(30):21924–21927. [PubMed] [Google Scholar]
- Nagasue N., Ito A., Yukaya H., Ogawa Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology. 1985 Sep;89(3):643–647. doi: 10.1016/0016-5085(85)90463-9. [DOI] [PubMed] [Google Scholar]
- Nagasue N., Ogawa Y., Yukaya H., Ohta N., Ito A. Serum levels of estrogens and testosterone in cirrhotic men with and without hepatocellular carcinoma. Gastroenterology. 1985 Mar;88(3):768–772. doi: 10.1016/0016-5085(85)90149-0. [DOI] [PubMed] [Google Scholar]
- Nagata T., Weiss E. H., Abe K., Kitagawa K., Ando A., Yara-Kikuti Y., Seldin M. F., Ozato K., Inoko H., Taketo M. Physical mapping of the retinoid X receptor B gene in mouse and human. Immunogenetics. 1995;41(2-3):83–90. doi: 10.1007/BF00182317. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Oomen L. C., van der Valk M. A., Hart A. A., Demant P., Emmelot P. Influence of mouse major histocompatibility complex (H-2) on N-ethyl-N-nitrosourea-induced tumor formation in various organs. Cancer Res. 1988 Dec 1;48(23):6634–6641. [PubMed] [Google Scholar]
- Parkin D. M., Pisani P., Ferlay J. Estimates of the worldwide incidence of eighteen major cancers in 1985. Int J Cancer. 1993 Jun 19;54(4):594–606. doi: 10.1002/ijc.2910540413. [DOI] [PubMed] [Google Scholar]
- Poole T. M., Chiaverotti T. A., Carabeo R. A., Drinkwater N. R. Genetic analysis of multistage hepatocarcinogenesis. Prog Clin Biol Res. 1996;395:33–45. [PubMed] [Google Scholar]
- Poole T. M., Drinkwater N. R. Hormonal and genetic interactions in murine hepatocarcinogenesis. Prog Clin Biol Res. 1995;391:187–194. [PubMed] [Google Scholar]
- Poole T. M., Drinkwater N. R. Strain dependent effects of sex hormones on hepatocarcinogenesis in mice. Carcinogenesis. 1996 Feb;17(2):191–196. doi: 10.1093/carcin/17.2.191. [DOI] [PubMed] [Google Scholar]
- Qian G. S., Ross R. K., Yu M. C., Yuan J. M., Gao Y. T., Henderson B. E., Wogan G. N., Groopman J. D. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People's Republic of China. Cancer Epidemiol Biomarkers Prev. 1994 Jan-Feb;3(1):3–10. [PubMed] [Google Scholar]
- Seldin M. F., Kruh G. D. Mapping of Abll within a conserved linkage group on distal mouse chromosome 1 syntenic with human chromosome 1 using an interspecific cross. Genomics. 1989 Feb;4(2):221–223. doi: 10.1016/0888-7543(89)90305-4. [DOI] [PubMed] [Google Scholar]
- Seldin M. F. Mouse chromosome 1. Mamm Genome. 1994;5(Spec No):S1–21. [PubMed] [Google Scholar]
- Smith G. S., Walford R. L., Mickey M. R. Lifespan and incidence of cancer and other diseases in selected long-lived inbred mice and their F 1 hybrids. J Natl Cancer Inst. 1973 May;50(5):1195–1213. doi: 10.1093/jnci/50.5.1195. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch S. D., Itze L., Mihailovich N., Rao K. V. Modifying role of partial hepatectomy and gonadectomy in ethylnitrosourea-induced hepatocarcinogenesis. Cancer Res. 1980 May;40(5):1538–1542. [PubMed] [Google Scholar]
- Vesselinovitch S. D., Mihailovich N. The effect of gonadectomy on the development of hepatomas induced by urethan. Cancer Res. 1967 Oct;27(10):1788–1791. [PubMed] [Google Scholar]
- Yamamoto R., Iishi H., Tatsuta M., Tsuji M., Terada N. Roles of ovaries and testes in hepatocellular tumorigenesis induced in mice by 3'-methyl-4-dimethylaminoazobenzene. Int J Cancer. 1991 Aug 19;49(1):83–88. doi: 10.1002/ijc.2910490116. [DOI] [PubMed] [Google Scholar]



