Abstract
BACKGROUND:
Helicobacter pylori infection occurs more frequently in Arctic Aboriginal settings than elsewhere in North America and Europe. Research aimed at reducing health risks from H pylori infection has been conducted in the Aboriginal community of Aklavik, Northwest Territories.
OBJECTIVE:
To compare the effectiveness of the Canadian standard therapy with an alternative therapy for eliminating H pylori infection in Aklavik.
METHODS:
Treatment-naive H pylori-positive individuals were randomly assigned to a 10-day regimen (oral twice-daily doses) with rabeprazole (20 mg): standard triple therapy (proton pump inhibitor, added clarithromycin [500 mg] and amoxicillin [1 g] [PPI-CA]); sequential therapy (ST) added amoxicillin (1 g) on days 1 to 5, and metronidazole (500 mg) and clarithromycin (500 mg) on days 6 to 10. Participants with clarithromycin-resistant H pylori were randomly assigned to ST or quadruple therapy. Treatment effectiveness was estimated as per cent (95% CI) with a negative urea breath test at least 10 weeks after treatment.
RESULTS:
Of 104 (53 PPI-CA, 51 ST) randomized participants, 89 (49 PPI-CA, 40 ST) had post-treatment results. Per-protocol treatment effectiveness was 59% (95% CI 45% to 73%) for PPI-CA and 73% (95% CI 58% to 87%) for ST. Based on intention to treat, effectiveness was 55% (95% CI 41% to 69%) for PPI-CA and 57% (95% CI 43% to 71%) for ST. Of 77 participants (43 PPI-CA, 34 ST) with 100% adherence, effectiveness was 63% (95% CI 43% to 82%) for PPI-CA and 81% (95% CI 63% to 99%) for ST.
CONCLUSIONS:
While additional evidence is needed to confirm that ST is more effective for Arctic Aboriginal communities than the Canadian standard H pylori treatment, these results show standard PPI-CA treatment to be inadequate for communities such as Aklavik.
Keywords: Aboriginal health; Arctic, Canada; Community-based participatory research; Helicobacter pylori; Randomized controlled trial
Abstract
HISTORIQUE :
L’infection par le Helicobacter pylori est plus fréquente dans les communautés autochtones de l’Arctique qu’ailleurs en Amérique du Nord et en Europe. La recherche visant à réduire les risques de l’infection par le H pylori pour la santé a été menée dans la communauté autochtone d’Aklavik, dans les Territoires du Nord-Ouest.
OBJECTIF :
Comparer l’efficacité d’une thérapie canadienne standard à celle d’une autre thérapie pour éliminer à l’infection par le H pylori à Aklavik.
MÉTHODOLOGIE :
Les chercheurs ont réparti au hasard des per-sonnes positives auH pylori naïves au traitement à un schéma thérapeu-tique de dix jours (deux doses quotidiennes par voie orale) au rabéprazole (20 mg) : trithérapie standard (inhibiteur de la pompe à protons, associé à de la clarithromycine [500 mg] et à de l’amoxicilline [1 g] [IPP-CA]); thérapie séquentielle (TS) associée à de l’amoxicilline (1 g) les jours 1 à 5 et à du métronidazole (500 mg) et de la clarithromycine (500 mg) les jours 6 à 10. Ils ont réparti au hasard les participants ayant un H pylori résistant à la clarithromycine entre la TS ou une quadrithérapie. Ils ont estimé l’efficacité du traitement en qualité de pourcentage (95 % IC) ayant obtenu un test respiratoire à l’urée négatif au moins dix semaines après le traitement.
RÉSULTATS :
Sur les 104 participants répartis au hasard (53 IPP-CA, 51 TS), 89 (49 IPP-CA, 40 TS) présentaient des résultats après la thérapie. L’efficacité de la thérapie conforme au protocole s’établissait comme suit : 59 % (95 %IC 45 % à 73 %) pour l’IPP-CA et 73 % (95 % IC 58 % à 87 %) pour la TS. D’après l’intention de traiter, l’efficacité s’établissait à 55 % (95 %IC 41 % à 69 %) pour l’IPP-CA et 57 % (95 % IC 43 % à 71 %) pour la TS. Sur les 77 participants (43 IPP-CA, 34 TS) ayant adhéré à 100 % à la thérapie, l’efficacité s’élevait à 63 % (95 % IC 43 % à 82 %) pour l’IPP-CA et à 81 % (95 % IC 63 % à 99 %) pour la TS.
CONCLUSIONS :
Il faudra d’autres données pour confirmer que la TSest plus efficace dans les communautés autochtones de l’Arctique pour le traitement systématique contre le H pylori au Canada, mais ces résultats démontrent que le traitement aux IPP-CA ne convient pas à des communautés comme celle d’Aklavik.
Helicobacter pylori, a bacterium identified in 1982, causes gastritis, peptic ulcer disease and gastric cancer (1,2). The infection may also play a role in dyspepsia. H pylori infection is acquired primarily in childhood and is often a lifelong disease. The risk of acquiring this infection is associated with lower socioeconomic status, household crowding and having family members with the infection (3). The Canadian Helicobacter Study Group identified three groups at high risk for H pylori infection and subsequent disease: elderly individuals; immigrants; and First Nations and Inuit populations (4).
Approximately 80% of Canadians live within 150 km of the United States border (5), and a similar proportion are concentrated in the metropolitan areas of Quebec and Ontario, the British Columbia lower mainland, and the Calgary-Edmonton corridor in Alberta (6). The prevalence of H pylori infection in southern Canada, where most of the population resides, is generally ≤30% (7).
A few reports from Aboriginal populations across the circumpolar north (Alaska, northern Canada, Greenland, Arctic Russia) show a substantially higher prevalence of H pylori infection than elsewhere in North America and Europe (7). The Inuvialuit (Inuit) and Gwich’in (First Nations) in Aklavik, Northwest Territories (NWT), have been the focus of recent efforts toward investigating the burden of disease from H pylori in Arctic Aboriginal communities of Canada. Recent work by the Canadian North Helicobacter pylori (CANHelp) Working Group found that the prevalence of H pylori infection is 62% among participants in the Aklavik H pylori Project, a community-based, participatory research project focused on community-identified research goals (8). Aklavik is a hamlet (population approximately 600) located at the Arctic circle on the Mackenzie River, approximately 100 km south of the Beaufort Sea. There are no published reports of the effectiveness of H pylori therapy in Canada’s Arctic populations.
Successful treatment of H pylori infection has generally required the combination of a proton pump inhibitor (PPI) with two or three different antibiotics. According to published reports, the best available regimens typically achieve success in 80% to 90% of patients, depending on population characteristics (9); cure rates <80% have been deemed unacceptable for use in patient care (10). In general, treatment failure is more common in high-prevalence populations (9). The Canadian standard for treatment to eliminate H pylori infection has been triple therapy consisting of a PPI, 500 mg of clarithromycin and 1 g of amoxicillin (PPI-CA) or, in the case of penicillin allergy, 500 mg of metronidazole (PPI-CM), all given twice daily for seven to 14 days (11). A meta-analysis of results of Canadian treatment trials reported an average efficacy of PPI-CA and PPI-CM triple therapy regimens of 82% to 84% (12). Although the success rate with this therapy was reported initially to be as a high as 90% in some populations, it is clear that over the past few years, the treatment success rate in many countries has dropped below 75% (13). Among proposed reasons for why this triple therapy has lost efficacy is the increasing prevalence of resistance to clarithromycin and, to a lesser extent, metronidazole (13). Relatively few data regarding patterns of resistance to antibiotics are available. Canadian data show that the frequency of clarithromycin resistance has risen substantially from <2% in the early 1990s to approximately 15% in 2005, while metronidazole resistance has remained relatively stable at approximately 20% to 25% (14,15).
Sequential therapy (ST) consisting of five days of treatment with twice-daily doses of a PPI and amoxicillin (1 g) followed by five days of triple therapy with twice-daily doses of a PPI, clarithromycin (500 mg) and metronidazole (500 mg) has emerged as a new successful therapy (13,16). Most data regarding this regimen originate from Europe, with reports that the success rate is 10% to 20% higher than conventional triple therapy. One recent study provided evidence that the ST cure rate was 10% higher than the standard triple therapy because ST was better able to overcome clarithromycin resistance (17). There are no Canadian clinical trial data on the success of ST. A recent case series report from Edmonton, Alberta, estimated an ST success rate of 89%, while the rate for PPI-CA was 55% (18). The goal of the treatment phase of the Aklavik H pylori Project was to compare PPI-CA with ST to determine which treatment regimens are optimal in the Arctic Aboriginal setting.
METHODS
Population
The Aklavik H pylori Project was conducted by the CANHelp Working Group, which links University of Alberta investigators with northern Canadian community groups and health officials to address community concerns about health risks from H pylori infection and identify solutions for reducing these risks in northern Canada. As its initial project, the research in Aklavik aimed to describe the H pylori disease burden in the community, identify determinants of effective therapy, involve community members in planning to keep the focus on community concerns, and develop effective strategies for knowledge exchange between researchers and the community (19). Aklavik is located at a traditional Inuvialuit and Gwich’in trading site, and is currently home to members of both groups. Many residents follow a traditional lifestyle of trapping, hunting and fishing, while the hamlet provides access to basic technological advances of modern life. Aklavik is accessible by winter ice road and air (20).
Fieldwork began in November 2007 and a large proportion of residents (384 of approximately 600) participated as follows: 345 completed clinical surveys; 333 underwent 13C-urea breath tests (UBT); and risk-factor surveys were collected from 285 individuals and 145 households. Participants were offered upper gastrointestinal endoscopy to obtain biopsies for pathology and bacterial culture. In February 2008, gastroenterologists performed endoscopies at the Aklavik Health Centre in temporary endoscopy units. Of 307 participants screened for H pylori using UBT by November 2008 when the treatment trial started, 59% were positive, with high prevalence from an early age (51% of 57 children <15 years of age). Of 194 participants with gastric biopsies, the pathologist detected H pylori in 129 (67%). This H pylori-positive group had a high prevalence of pathologies associated with a high risk of gastric cancer: 43% with severe inflammation; 21% with atrophy; and 11% with intestinal metaplasia (21–23). The prevalence of H pylori resistance to metronidazole was 23% (28 of 120), resistance to clarithromycin was 3% (four of 120), and resistance to both metronidazole and clarithromycin was 5% (six of 120). H pylori-positive individuals were invited to participate in the treatment trial in October 2008, and treatments were dispensed from November 2008 to February 2009.
Participant selection
Participants were invited to participate in the trial if they met eligibility criteria. Participants were eligible for inclusion in the trial if they were >15 years of age, and had been identified as H pylori infected with a positive UBT and/or biopsy-based evidence of H pylori infection. Exclusion criteria were as follows: allergy to amoxicillin, metronidazole or clarithromycin; antibiotic therapy within four weeks before randomization (unless there was a positive UBT in the interim); pregnant or breastfeeding; and severe cardiorespiratory, pulmonary, endocrine, hepatic or renal disease. All participants underwent an interview with a nurse practitioner or physician to determine safety of enrollment. All Aklavik H pylori Project participants who tested positive for H pylori infection but were not eligible for the treatment trial, including children, were offered appropriate H pylori therapy outside of the trial.
Treatment
Three 10-day treatment regimens were used:
PPI-CA: oral twice-daily doses of rabeprazole (20 mg), amoxicillin (1 g) and clarithromycin (500 mg);
ST: oral twice-daily doses of rabeprazole (20 mg) and amoxicillin (1 g) on days 1 to 5 and, on days 6 to 10, rabeprazole (20 mg), metronidazole (500 mg) and clarithromycin (500 mg); and
Quadruple therapy: oral doses of rabeprazole (20 mg) twice daily, metronidazole (500 mg) four times daily, bismuth subsalicylate (Pepto-Bismol, Procter & Gamble, USA) (two tablets) four times daily and tetracycline (500 mg) four times daily
Participants were randomly assigned to one of these regimens with equal probability after considering treatment history and antibiotic susceptibilities if known. Antimicrobial susceptibility data were available for participants whose H pylori strains were isolated from gastric biopsies and tested for antimicrobial susceptibility before the start of the trial. Treatment-naive participants whose H pylori isolates tested susceptible to clarithromycin or whose H pylori antimicrobial susceptibility was unknown were randomly assigned to PPI-CA or ST. Participants whose isolates were found to be resistant to clarithromycin were randomly assigned to ST or quadruple therapy, based on published reports indicating that the efficacy of PPI-CA therapy markedly diminishes if a patient harbours a clarithromycin-resistant strain (13). Because it is known that resistance to metronidazole can be partially overcome by metronidazole-containing regimens of sufficient duration, patients whose isolates were resistant to metronidazole were randomly assigned to PPI-CA or ST (24). Participants who had taken PPI-CA before this trial and remained H pylori positive were randomly assigned to ST or quadruple therapy. All medications were blister packaged to group the doses to be taken each morning, lunch, dinner and evening during the 10-day treatment. Participants were asked to return the blister packs after their 10-day treatment. Treatment adherence was assessed by pill count of returned blister packs and by an interviewer-administered post-treatment questionnaire, which also ascertained side effects and problems encountered in taking the medication.
Randomization and blinding
Drug treatment was allocated to each participant at random using a table of random numbers (generated by AM using Excel [Microsoft Corporation, USA]), with treatment allocation based on whether an odd or an even number was generated. Allocation was concealed to the patient and prescriber but once treatment was assigned (by AM), participants and prescriber (assignments were implemented by project staff in Aklavik) were not blinded to treatment type. Post-treatment test results for H pylori infection status were read by a technician who was unaware of the assigned treatment.
Outcome
The outcome for estimating treatment effectiveness was H pylori status as determined by a post-treatment UBT. All participants were actively encouraged to return for a follow-up UBT a minimum of 10 weeks after completing therapy (between February 2009 and July 2010). The samples were analyzed using the InfraRed Isotope Analyser System (Wagner Analysen Technik, Germany). The UBT is the optimal non-invasive method for diagnosing H pylori infection, and validation studies have shown it to be robust to a variety of test protocols (25). Agreement between histology and UBT results in this population was 95% (data not shown). The effectiveness of each treatment was estimated as the proportion with a negative post-treatment UBT of total participants in each treatment group, and presented with a 95% CI as a measure of statistical precision. For intention-to-treat analysis, it was assumed that participants who did not return for a post-treatment UBT did not take most of the assigned treatment and remained H pylori positive. For per-protocol analysis, each treatment group was limited to those for whom follow-up UBT results were available.
RESULTS
Among Aklavik H pylori Project participants, 202 had either a positive UBT test result (n=180) or H pylori-positive histopathology (n=22); 82 had antibiotic susceptibility results. Of the H pylori-positive participants, 176 were adults, of whom 124 (70%) consented to participate in the trial (Figure 1). Of those who consented, 113 were eligible and stratified according to previous treatment status before random assignment of therapy. Because only seven participants had received previous treatment for H pylori infection, presented results are restricted to treatment-naive participants. Of the 106 treatment-naive participants, H pylori antimicrobial susceptibility results were available for 50; five were classified as clarithromycin resistant and randomized separately, with three assigned to ST and two assigned to quadruple therapy (further testing led to reclassification of one in each of these groups as susceptible). Of the remaining 101 participants, 53 received PPI-CA (20 confirmed susceptible to all assigned antibiotics, seven resistant to metronidazole and 26 with unknown susceptibility) and 48 received ST (including 13 confirmed susceptible to all assigned antibiotics; six resistant to metronidazole and 29 with unknown susceptibility). In all, PPI-CA was assigned to 53 participants and ST to 51 (including the three resistant to clarithromycin who were randomized separately).
Figure 1).
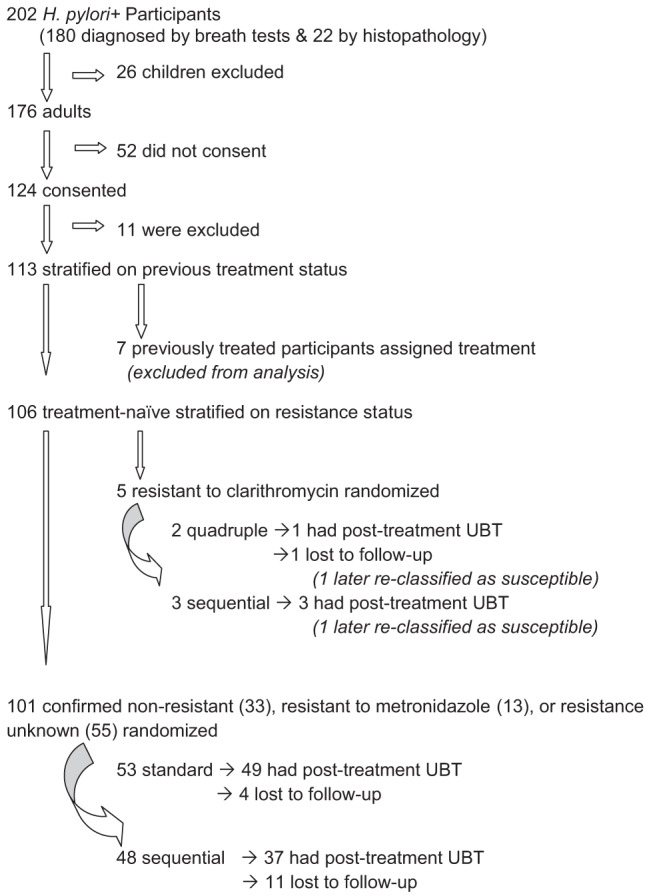
Aklavik Helicobacter pylori Project treatment trial participation. UBT Urea breath test
Of the treatment-naive participants who received either PPI-CA or ST, 86% (89 of 104) underwent a post-treatment UBT; the proportion with follow-up data according to treatment group was 92% (49 of 53) on PPI-CA and 78% (40 of 51) on ST. Table 1 summarizes participant characteristics and Table 2 summarizes antimicrobial susceptibility status, with both showing this information according to treatment group among all randomized participants and separately for the subgroup of those with post-treatment infection status. Based on per-protocol analysis among those with a post-treatment test result, treatment success was achieved in 29 of 49 (59% [95% CI 45% to 73%]) participants assigned to PPI-CA and 28 of 40 (70% [95% CI 53% to 83%]) assigned to ST (Table 2). Two individuals in the ST group were classified as not treated successfully due to inadequate UBT samples. The per-protocol analysis shows substantially higher treatment effectiveness of ST compared with PPI-CA, but the treatment group sizes were too small for a high degree of confidence that ST confers a clinically meaningful advantage. Intention-to-treat analysis estimated treatment effectiveness in 29 of 53 (55% [95% CI 40% to 68%]) for PPI-CA and 28 of 51 (55% [95% CI 40% to 69%]) for ST. The large decrease in ST effectiveness when analyzing by intention-to-treat rather than per-protocol analysis reflects the relatively large loss to follow-up in the ST group. Of the two participants assigned to quadruple therapy, one underwent a negative post-treatment UBT and the other was lost to follow-up.
TABLE 1.
Characteristics of participants randomly assigned to standard or sequential therapy and among those with post-treatment status for per-protocol analysis
| Standard therapy (PPI-CA) group | Sequential therapy group | |||
|---|---|---|---|---|
|
|
|
|||
| Randomized* (n=53) | Per-protocol† (n=49) | Randomized* (n=51) | Per-protocol† (n=40) | |
| Age, years, mean (95% CI) | 42 (38–47) | 43 (38–48) | 38 (34–43) | 38 (33–43) |
| Male sex | 49 (35–63) | 49 (35–63) | 49 (35–63) | 55 (39–71) |
| Aboriginal ethnicity | 91 (82–99) | 90 (81–98) | 94 (87–100) | 92 (84–100) |
| Nonsmoker‡ | 38 (25–52) | 42 (27–56) | 35 (21–49) | 42 (26–58) |
| Any alcohol intake | 67 (54–80) | 65 (51–78) | 58 (44–73) | 58 (42–74) |
| No epigastric symptoms‡ | 31 (19–46) | 30 (17–45) | 40 (26–56) | 39 (24–57) |
| No comorbidities | 87 (77–96) | 88 (78–97) | 77 (65–89) | 76 (62–90) |
| On PPI treatment‡ | 6 (1–17) | 6 (1–17) | 2 (0–11) | 0 (–) |
Data presented as % (95% CI) unless otherwise indicated.
Includes participants lost to follow-up;
Restricted to participants with post-treatment urea breath test result;
Missing data: One in the proton pump inhibitor (PPI)-clarithromycin amoxicillin (PPI-CA) group, two in the standard therapy group
TABLE 2.
Antimicrobial susceptibility of Helicobacter pylori strains among participants randomly assigned to standard or sequential therapy and among those with post-treatment status for per-protocol analysis
| H pylori antimicrobial susceptibility result | Standard therapy (n=49) | Sequential therapy (n=40) |
|---|---|---|
| Susceptibility results not available | 25 (51.0) | 21 (52.0) |
| Susceptibility results available | 24 (49.0) | 19 (48.0) |
| Confirmed susceptible* | 17 (70.8) | 13 (68.4) |
| Resistant to metronidazole only | 7 (29.2) | 4 (21.0) |
| Resistant to clarithromycin only | 0 (0) | 1 (5.3) |
| Resistant to metronidazole and clarithromycin | 0 (0) | 1 (5.3) |
| Resistant to amoxicillin | 0 (0) | 0 (0) |
Data presented n (%).
Susceptible to amoxicillin, clarithromycin and metronidazole
Pretreatment antimicrobial susceptibility results were available for 43 of the 89 participants with post-treatment UBT results, 24 on PPI-CA and 19 on ST; 30% (13 of 43) were infected with resistant H pylori strains (Table 3). In the PPI-CA group, seven participants had metronidazole-resistant strains, and treatment was successful in four of the seven (57%). In the ST group, six participants had resistant H pylori: four were resistant to metronidazole only and all were successfully treated; one was resistant to clarithromycin only and failed treatment; and one was resistant to both clarithromycin and metronidazole and was successfully treated. H pylori from one of the participants assigned to ST was initially classified clarithromycin resistant but later determined to be susceptible. Among participants with confirmed susceptible strains, treatment was effective in eight of 17 (47%) on PPI-CA and nine of 13 on (69%) on ST. Among participants with H pylori of unknown antimicrobial susceptibility, treatment was effective in 17 of 25 (68%) on PPI-CA and 14 of 21 (67%) on ST.
TABLE 3.
Treatment effectiveness among trial participants randomly assigned to standard or sequential therapy, stratified according to Helicobacter pylori antimicrobial susceptibility status
| Antimicrobial susceptibility | Assigned treatment, n | Tested post-treatment, n | Negative post-treatment status, n | Treatment effectiveness, % (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Intention to treat | Per protocol | ||||
| Standard therapy (PPI-CA) | 53 | 49 | 29 | 55 (40–68) | 59 (45–73) |
| Metronidazole resistant | 7 | 7 | 4 | † | † |
| Susceptible* | 20 | 17 | 8 | 40 (19–64) | 47 (23–72) |
| Susceptibility unknown | 26 | 25 | 17 | 65 (44–83) | 68 (46–85) |
| Sequential therapy | 51 | 40 | 28 | 55 (40–69) | 70 (53–83) |
| Metronidazole resistant | 7 | 4 | 4 | † | † |
| Clarithromycin resistant | 1 | 1 | 0 | † | † |
| Metronidazole and clarithromycin resistant | 1 | 1 | 1 | † | † |
| Susceptible* | 13 | 13 | 9 | 69 (39–91) | 69 (39–91) |
| Susceptibility unknown | 29 | 21 | 14 | 48 (29–67) | 74 (49–91) |
Susceptible to amoxicillin, clarithromycin and metronidazole;
Estimates not reported for subgroups with fewer than 10 observations. PPI-CA Proton pump inhibitor-clarithromycin and amoxicillin
Treatment was generally well tolerated, with no major side effects presenting to the local health centre or reported to project staff. Treatment adherence information was obtained from 77 of the 89 (43 PPI-CA, 34 ST) participants with follow-up results (40 returned blister packs so that skipped doses could be counted; 37 did not return blister packs but responded to a structured questionnaire about their adherence to the regimen). Among the 77 with adherence data (using pill counts when possible and self-report otherwise), 26 of 43 (60% [95% CI 44% to 75%]) completed all doses in the PPI-CA group and 20 of 34 (58% [95% CI 41% to 75%) did so in the ST group. Among those with 100% adherence, treatment effectiveness was 65% (95% CI 44% to 83%) on PPI-CA and 75% (95% CI 51% to 91%) on ST.
DISCUSSION
We conducted a treatment trial in a small hamlet located in the Arctic region of Canada which, similar to communities investigated elsewhere in the circumpolar north, has an elevated prevalence of H pylori infection (19). Despite our small study size, our findings make a notable contribution to the literature, given the scant information available for the people of this region. Based on per-protocol analysis, we observed an 11% improvement in treatment success with ST compared with PPI-CA (the standard used across Canada), although our small sample precluded precise estimates of the size of this treatment effect. While per-protocol and intention-to-treat analyses yielded similar estimates of treatment effectiveness for PPI-CA, the intention-to-treat analysis resulted in a much lower estimate of effectiveness of ST than did per-protocol analysis because a much larger number of people assigned to ST were lost to follow-up. It is unclear why more ST participants were lost to follow-up. Arranging for post-treatment follow-up can be difficult, especially during the summer months when many individuals move to camps outside the village for fishing, hunting and other activities. Furthermore, because our team members are present in the village for limited periods of time, it is difficult to coordinate follow-up visits because people may be away. Because both regimens had a 10-day duration, are given twice a day and were provided as blister packs, we do not believe the higher complexity of the ST regimen is an explanation. The low success rate of the PPI-CA regimen is in accordance with the summary data for this regimen by Graham and Fischbach (13) and confirms that its effectiveness is unacceptably low. In fact, partly based on these data, ST has now become the first-line regimen for treatment of H pylori infection in this area.
Resistance to antibiotics used in H pylori treatment regimens does reduce the success of the corresponding regimens (13,26,27). In particular, regimens containing clarithromycin have substantially reduced effectiveness in treating clarithromycin-resistant strains; however, evidence shows that metronidazole-resistant strains can be partially overcome by regimens that still contain metronidazole. The reason for the difference between clarithromycin and metronidazole resistance is that clarithromycin shows a bimodal distribution (ie, is an all-or-none phenomenon) whereas resistance to metronidazole shows a much wider range and does not have a bimodal distribution (26,28). For metronidazole resistance, it has also been shown that resistance can be overcome more if the drug is administered at a higher dose or the regimen is taken for a longer duration (26). For clarithromycin resistance, there are data that show that ST is better able to overcome clarithromycin resistance than PPI-CA regimens (17). There are few published data regarding resistance of H pylori to antibiotics. Interestingly, the reported frequencies of resistance in Aklavik are similar to those found in the rest of Canada, although resistance to clarithromycin (8%) is lower than the reported rate of 15% (15). Reports from Alaska suggest that H pylori treatment failure related to clarithromycin resistance is a problem in Alaska Native communities (29).
Although our results suggest ST is better than PPI-CA it is noteworthy to point out that this was an enriched population in that patients with known resistance (n=8 in the present study) were not randomized. Our success rate for ST of 70% was still well below what is generally accepted to be a desirable level (13). We are now further testing ST and comparing it with quadruple therapy in other Northern communities.
ST starts with five-day treatment of amoxicillin as the only antibiotic. This is believed to decrease the bacterial load and, in this way, increase the ability of the two antibiotics clarithromycin and metronidazole to eliminate the infection during the second phase of treatment. Use of a beta-lactam, such as amoxicillin, which compromises the cell wall in the initial phase of ST, has also been postulated to help to overcome clarithromycin resistance by preventing the formation of efflux channels (30). In fact, the study by Vaira et al (17) documented that 10-day sequential regimens, was superior to standard triple therapy (eg, PPI-CA) for eradication of H pylori (eg, 89% versus 77% in one trial) (17). This was further supported by a recent meta-analysis that estimated effectiveness of 93% with sequential therapy (31).
A more recent meta-analysis points out some limitations in the research on ST, a major concern being that most research on ST has been performed in Italian populations (32). Our research helps to broaden the generalizability of available data on this therapeutic approach. Although our study was small, we studied the treatment regimens in a real-world situation. We attempted to overcome the complexity of the ST regimen by having all medications blister packaged by the pharmacy to minimize pill counting and bottle confusion in the participants. It should be noted that current ST is not an option for patients allergic to penicillin.
Communities across the circumpolar north tend to be small and spread out, thus it is necessary to study multiple communities in the region for more precise estimates of the effectiveness of candidate treatment regimens. We have been asked to study H pylori infection in other NWT and Yukon communities, where we plan to perform further randomized controlled trials of promising alternatives to the standard Canadian therapy. Future research should help identify optimal first-line therapy for this region. Graham and Fischbach (13) have pointed out the need for practitioners to base H pylori therapy on what works best locally rather than follow guidelines based on data from other settings. While additional information is required to determine what works best for communities in the circumpolar north, our treatment effectiveness estimate of 59% shows that PPI-CA is not an appropriate first-line strategy in communities such as Aklavik. At a minimum, if standard triple therapy is used in Arctic populations, we recommend that post-UBT testing be performed to determine treatment success and whether there is a need for a second treatment.
Acknowledgments
CANHelp Working Group: Aklavik, NWT: Rachel Munday (Aklavik Health Centre); Robert Buckle, Glen Gordon, Annie Buckle, Jerome Gordon, Andrew Gordon, Billy Archie (Aklavik Health Committee). Inuvik, NWT: Leah Seaman (Inuvik Regional Hospital), Crystal Lennie (Inuvialuit Regional Corporation). Yellowknife, NWT: Kami Kandola (NWT Health and Social Services), John Morse (formerly, Stanton Territorial Health Authority), Susan Chatwood (Institute for Circumpolar Health Research). Edmonton, AB: Karen Goodman, Justin Cheung, Amy Morse, Richard Fedorak, Christopher Fletcher, Safwat Girgis, Monika Keelan, Sander Veldhuyzen van Zanten (University of Alberta investigators), Janis Geary, Katharine Fagan-Garcia, Hsiu-Ju Chang, Ashley Wynne (University of Alberta research staff); Robert Bailey (Northern Health Services Network).
Footnotes
DISCLOSURES: In the past three years, Sander van Zanten has received speaking fees from AstraZeneca, Nycomed (now Takeda) and Abbott Laboratories who market omeprazole, esomeprazole, pantoprazole, lansoprazole and Dexilant in Canada. He has served as member of advisory boards of AstraZeneca, Takeda, Shire and Abbott Laboratories. In the past, he has received research support from AstraZeneca, Abbott Laboratories and Janssen Pharmaceutica (rebeprazole). Amy Morse has served as a member of an advisory board for Shire. No other authors have any conflicts of interest to declare. All authors approved the final version of the manuscript.
FUNDING: This study was funded by grants from the Canadian Institutes of Health Research, Alberta Innovates-Health Solutions (formerly Alberta Heritage Foundation for Medical Research), ArcticNet Network Centres of Excellence, Canadian Association of Gastroenterology, and AstraZeneca.
REFERENCES
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Veldhuyzen van Zanten SJ, Sherman PM. Helicobacter pylori infection as a cause of gastritis, duodenal ulcer, gastric cancer and nonulcer dyspepsia: A systematic overview. CMAJ. 1994;150:177–85. [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman KJ, Correa P. The transmission of Helicobacter pylori. A critical review of the evidence. Int J Epidemiol. 1995;24:875–87. doi: 10.1093/ije/24.5.875. [DOI] [PubMed] [Google Scholar]
- 4.Jones N, Chiba N, Fallone C, et al. Helicobacter pylori in First Nations and recent immigrant populations in Canada. Can J Gastroenterol. 2012;26:97–103. doi: 10.1155/2012/174529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Custred G. Security threats on America’s borders. In: Moens A, editor. Immigration Policy and the Terrorist Threat in Canada and the United States. Vancouver: Fraser Institute; 2008. p. 96. [Google Scholar]
- 6.Statistics Canada Urban-rural population as a proportion of total population, Canada, provinces, territories and health regions. 2001 < www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo62a-eng.htm> (Accessed January 15, 2010) [Google Scholar]
- 7.Goodman KJ, Jacobson K, Veldhuyzen van Zanten S. Helicobacter pylori infection in Canadian and related Arctic Aboriginal populations. Can J Gastroenterol. 2008;22:289–95. doi: 10.1155/2008/258610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aplin L, Fagan-Garcia K, Geary J, Goodman KJ, CANHelp Working Group Household factors associated with H. pylori infection in Aklavik, Northwest Territories. Am J Epidemiol. 2011;173:S230. [Google Scholar]
- 9.Fischbach LA, Goodman KJ, Feldman M, Aragaki C. Sources of variation of Helicobacter pylori treatment success in adults worldwide: A meta-analysis. Int J Epidemiol. 2002;31:128–39. doi: 10.1093/ije/31.1.128. [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol. 2008;5:321–31. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt R, Fallone C, Veldhuyzan van Zanten S, et al. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori – an evidence-based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Can J Gastroenterol. 2004;18:547–54. doi: 10.1155/2004/326767. [DOI] [PubMed] [Google Scholar]
- 12.Rodgers C, Zanten SV van. A meta-analysis of the success rate of Helicobacter pylori therapy in Canada. Can J Gastroenterol. 2007;21:295–300. doi: 10.1155/2007/419784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 14.Best LM, Haldane DJ, Bezanson GS, Veldhuyzen van Zanten SJO. Helicobacter pylori: Primary susceptibility to clarithromycin in vitro in Nova Scotia. Can J Gastroenterol. 1997;11:298–300. doi: 10.1155/1997/159637. [DOI] [PubMed] [Google Scholar]
- 15.Best L, Cooper-Lesins G, Haldane D, Veldhuyzen van Zanten SJO. Helicobacter pylori antibiotic resistance in Canadian populations. Gastroenterology. 2004;126:S1293. A-189 (Abst). [Google Scholar]
- 16.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: Systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069–79. doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 17.Vaira D, Zullo A, Vakil N, et al. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: A randomized trial. Ann Intern Med. 2007;146:556–63. doi: 10.7326/0003-4819-146-8-200704170-00006. [DOI] [PubMed] [Google Scholar]
- 18.Pinchbeck M, Veldhuyzen van Zanten SJO. Comparison of triple therapy, quadruple therapy, and sequential therapy for the eradication of Helicobacter pylori infection. Can J Gastroenterol. 2012;26:138A. (Abst) [Google Scholar]
- 19.Cheung J, Goodman K, Munday R, et al. Helicobacter pylori infection in Canada’s arctic: searching for the solutions. Can J Gastroenterol. 2008;22:912–6. doi: 10.1155/2008/614538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwich’in Social & Cultural Institute The Gwich’in. 2006 < www.gwichin.ca/TheGwichin/aklavik.html> (Accessed September 15, 2006) [Google Scholar]
- 21.Zanten SJ Van, Dixon MF, Lee A. The gastric transitional zones: Neglected links between gastroduodenal pathology and helicobacter ecology. Gastroenterology. 1999;116:1217–29. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 22.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–9. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 23.Goodman KJ, Cheung J, Huntington J, Munday R, Fedorak RN, Veldhuyzen van Zanten SJO. Endoscopic and histopathologic characteristics of H. pylori infection in a Canadian Arctic hamlet. Helicobacter. 2009;14:314. [Google Scholar]
- 24.Bardhan K, Bayerdorffer E, Veldhuyzen Van Zanten SJ, et al. The HOMER Study: The effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter. 2000;5:196–201. doi: 10.1046/j.1523-5378.2000.00030.x. [DOI] [PubMed] [Google Scholar]
- 25.Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection – a critical review. Aliment Pharmacol Ther. 2004;20:1001–17. doi: 10.1111/j.1365-2036.2004.02203.x. [DOI] [PubMed] [Google Scholar]
- 26.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–57. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 27.Mégraud F, Lehn N, Lind T, et al. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: The MACH 2 study. Antimicrob Agents Chemother. 1999;43:2747–52. doi: 10.1128/aac.43.11.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mégraud F. Resistance of Helicobacter pylori to antibiotics. Aliment Pharmacol Ther. 1997;11(Suppl 1):43–53. doi: 10.1046/j.1365-2036.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 29.McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139:463–9. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 30.Francesco V De, Margiotta M, Zullo A, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94–100. doi: 10.7326/0003-4819-144-2-200601170-00006. [DOI] [PubMed] [Google Scholar]
- 31.Jafri NS, Hornung CA, Howden CW. Meta-analysis: Sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 32.Gisbert JP, Calvet X, O’Connor A, Megraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: A critical review. J Clin Gastroenterol. 2010;44:313–25. doi: 10.1097/MCG.0b013e3181c8a1a3. [DOI] [PubMed] [Google Scholar]


