Abstract
Objective
We test the hypothesis that clinically significant depression, severe depression in particular, increases the risk of Alzheimer’s Disease (AD).
Design
A longitudinal, three-wave epidemiological enquiry was implemented in a sample of individuals aged ≥55 years (n = 4,803) followed-up at 2.5 years and 4.5 years.
Setting
Population-based cohort drawn from the ZARADEMP Project, in Zaragoza, Spain.
Participants
Cognitively intact individuals at baseline (n = 3,864).
Main outcome measures
Depression was assessed by a standardized diagnostic interview (Geriatric Mental State, GMS-AGECAT). A panel of research psychiatrist diagnosed AD according to DSM-IV criteria. Fine and Gray multivariate regression model was used in the analysis, accounting for mortality.
Results
At baseline, clinically significant depression was diagnosed in 452 participants (11.7%). Among the depressed, 16.4% had severe depression. Seventy incident cases of AD were found at follow-up. Compared with non-depressed individuals, the incidence rate of AD was significantly higher in the depressed (incidence rate ratio, IRR = 1.91 (95%CI: 1.04–3.51) and particularly in the severely depressed (IRR = 3.59 (95%CI: 1.30–9.94). A consistent, significant association was observed between severe depression at baseline and incident AD in the multivariate model (hazard ratio, HR = 4.30 (95%CI: 1.39–13.33). Untreated depression was associated with incident AD in the unadjusted model, although in the final model this association was attenuated and non-significant.
Conclusions
Severe depression increases the risk of AD, even after controlling for the competing risk of death. This finding may stimulate studies about the effect of treating depression in relation to the risk of AD.
INTRODUCTION
The epidemic nature of both Alzheimer’s disease (AD) and depression in the elderly pose major public health problems (1)(2), and the complex relationships between the two conditions have been emphasized. (3)
The most consistent risk factors for dementia and Alzheimer’s (AD) are age (1) and apoE genotype. (4) Potentially modifiable risk factors have also been reported, such as vascular risk factors, (5) and possibly dietary and lifestyle factors (6) or low educational background. (7)(8) Several reports have also suggested that depression is a risk factor for AD (9)(10)(11) and a meta-analysis estimated an odds ratio of 1.90 (95%CI: 1.55–2.33). (12) However, some studies found no association between both conditions. (13)(14) Moreover, some authors have suggested that depressive symptoms may be an early manifestation rather than a risk factor for AD. (15) These inconsistencies reflect variation in several factors, such as length of observation, rate of follow-up participation, potentially modifying variables controlled, and/or the approach to assessment of both depression and AD. (9) Furthermore, most previous studies on the association between depression and AD did use non-optimal methods to assess depression, such as symptom-based scales, (9)(10)(13)(14)(16)(17) and therefore do not provide enough information on a relevant clinical subject such as treatable depression.
Few studies have assessed whether characteristics of depression affect the risk of AD, and those that have show rather unexpected results. Fuhrer et al. reported an increased risk of AD in treated, as opposed to untreated, depression. (16) Becker et al. found that persistent depression did not increase risk for AD, (14) and a Dutch Study found an increased risk of AD only in those with a history of early-onset depression. (13) Since clinically significant, severe depression has been reported to increase the risk of all cause dementia, (18)(19) it is important to examine for association specifically with AD. Furthermore, some studies reporting a positive association between depressive symptoms and AD show a linear increase of risk with the number of symptoms. (9)(11)
In light of inconclusive evidence, we tested the hypothesis that clinically significant depression, severe depression in particular, increases the risk of AD by studying a general population cohort. Depression and AD were diagnosed according to standardized clinical criteria. Since death may prevent the occurrence of AD in the aged population, we used a competing risk regression model, a novel method in this area of research. In addition, we examined the effect of the following characteristics of depression as predictors of AD: first-ever depression, persistent depression and untreated depression.
METHOD
I. Design Overview and Study Population
The sample for the present study was drawn from the Zaragoza Dementia and Depression Project (ZARADEMP), (7)(20) a longitudinal three-wave epidemiological enquiry, conducted in Zaragoza, Spain, estimating the incidence and risk factors for dementia and depression, as well as their link to general medical morbidity, in the adult population aged 55 or more years. The Helsinki convention principles of written informed consent, privacy, and confidentiality have been maintained throughout the project. The Ethics Committe of the University of Zaragoza and the Fondo de Investigación Sanitaria (FIS) approved the project according to Spanish law.
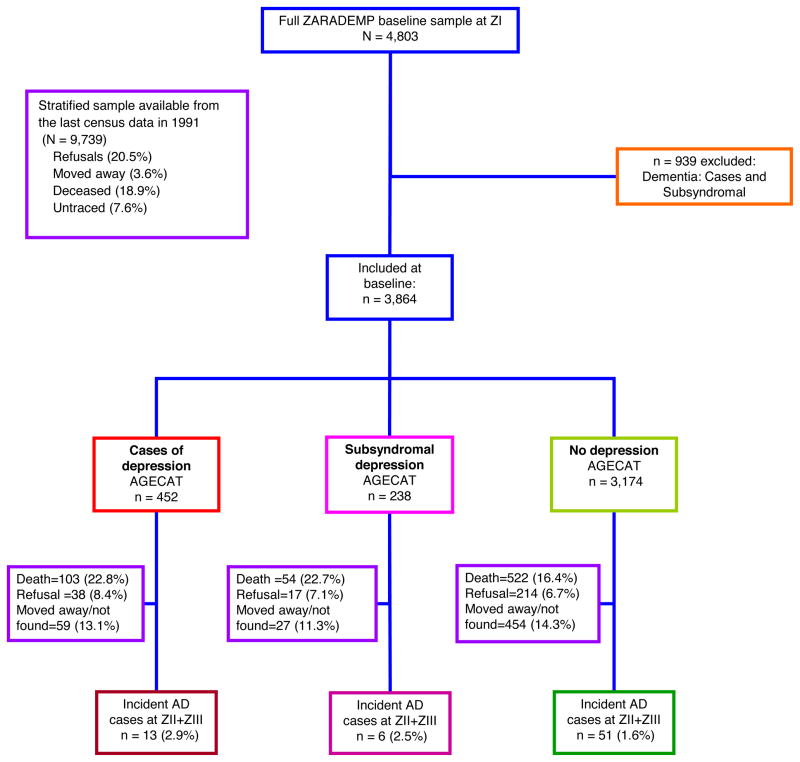
A random sample of community dwelling elders, stratified with proportional allocation by age and sex, was drawn from the 1991 census. Sample size was driven by the project’s main objective, which was to study risk factors for incident dementia, taking in account information on attrition from a previous Zaragoza Study. (8) At enrolment, the refusal rate was 20.5%; 4,803 individuals were ultimately interviewed at baseline (Wave I, 1994). Individuals with all cause dementia were excluded from follow-up evaluations (Wave II, 1997; Wave III, 1999). To include in the follow-up cohort only cognitively intact individuals, stringent criteria were applied. “Subsyndromal” dementia (see definition below) at baseline according to Geriatric Mental State (GMS) -Automated Geriatric Examination for Computer Assisted Taxonomy package (AGECAT) criteria (21) were also excluded, leaving a final sample of 3,864 assessed at the baseline of this project. Additional details about design and objectives have been published previously. (20)
Figure 1 illustrates the flow diagram of the present study and the ZARADEMP Project.
Figure 1.
Study flow chart
II. Procedure
A two-phase epidemiological case-finding process focused on dementia and depression was implemented at one baseline (Wave I) and two follow-up waves (Waves II and III). In Phase I, well-trained and regularly supervised lay interviewers conducted the 25–90 minute ZARADEMP interview at each participants’ residence. This incorporated standardized Spanish versions of the following: Mini-Mental State Examination (MMSE); (22) GMS; (21) History and Aetiology Schedule (HAS); (23) Lawton & Brody scale (24) and Katz’s Index (25) to assess instrumental and basic activities of daily living (ADLs), respectively; and a series of standardized questions regarding medical and psychiatric history coming from the Risk Factors Questionnaire (RFQ) used in EURODEM. (26) In phase II, triggered by predetermined criteria, utilizing the same interview, a trained research psychiatrist reassessed all participants suspected of dementia or depression to confirm the diagnosis. These interviews were also conducted in participants’ homes. The validity of this approach has been established. (8) A similar procedure was implemented in waves II and III, 2.5 and 4.5 years later respectively, with the interviewers unaware of the results of the baseline interview.
III. Clinical Measurements
The diagnosis of depression was based on staged GMS-AGECAT approach. This is a valid approach for the detection of “depression requiring clinical attention” in community samples. (2) After symptom assessment (Stage I), a diagnosis of depression emerges from the stage II. In this stage, a computer program compares syndrome clusters (dementia, depression, anxiety, etc.) to reach a final diagnosis, recorded as either a diagnostic “subsyndromal” (confidence levels 1 and 2) or a diagnostic “case” (confidence levels ≥ 3). AGECAT “caseness” implies the “desirability of intervention”. (2) (27) Furthermore, “cases” of depression are classified as “severe depression” (which includes melancholic symptoms) and “nonsevere depression”. “Subsyndromal” in this system imply that psychopathological symptoms are not severe enough to merit an intervention. Information from the HAS was used to define onset of depression and use of antidepressant treatment (classified according to the Anatomical Therapeutic Chemical Classification system (28) codes: N06A* and/or N06C1A). We defined depression as persistent if it was present at baseline and the first follow-up evaluation (Wave II).
For the diagnosis of dementia, assessments were complemented by medical reports and laboratory data, frequently available at most people’s homes in Spain. Outside caregivers were interviewed when the participant was considered to be unreliable (in “cases” of dementia and approximately 10% of “subsyndromal” dementia). Participants were considered “probable cases” on the basis of GMS threshold “global” score (1/2) and/or Mini-Mental (23/24) standard cut-off points. The Hachinski scale (29) and a neurological examination were used in the diagnostic process to differentiate AD from other causes of dementia (e.g., vascular dementia). The validity of this process has been previously established. (8) Identified cases of dementia were presented to a panel of 4 research psychiatrists who made the final diagnosis. Variables in the ZARADEMP interview were operationalized to conform to the DSM-IV criteria used to diagnose cases. For a diagnosis of DSM-IV “incident” dementia and “type of dementia” (AD, other), agreement by at least 3 of the 4 psychiatrists was required. To document the accuracy of the panel diagnosis of dementia, individuals considered to be cases of AD by the panel were invited for a hospital diagnostic work-up, which included neuroimaging and a complete neuropsychological diagnostic battery, and NINCDS-ADRDA (30) criteria were then used to diagnose AD. Only 15 subjects diagnosed as AD by the panel of research psychiatrists accepted the invitation, and the diagnosis of AD was confirmed in 13 of them (86.7%) (7).
Potential confounders assessed at baseline included demographic variables, MMSE score, functional disability, and vascular risk factors or diseases. The variable “education” was categorized in three levels: none, primary school and secondary school or higher. The Katz Index (Basic ADLs) (25) and Lawton & Brody Scale (Instrumental ADLs) (24) were dichotomized, distinguishing between some disability (disability for at least one ADL) and no disability. The presence of vascular risk factors (hypertension, diabetes) and/or diseases was based on the medical history obtained by EURODEM RFQ. (26) Vascular diseases were dichotomized, distinguishing between vascular disease (angina and/or myocardial infarct and/or stroke) and no history of vascular disease.
VI. Statistical Analysis
Depressed and non-depressed individuals were compared at baseline on a number of variables using two-tailed chi-square tests for categorical data and two-tailed t tests for continuous variables. Incidence rate (IR) was calculated using standard procedures and rates were compared using incidence rate ratios (IRR). Multivariate models were estimated to calculate the risk of developing AD over time. We used the cumulative incidence function (CIF) approach to display the risk of patients experiencing the event of interest (AD), taking into account the competing event (death) as time progressed. (31) To estimate the effect of baseline predictors on the CIF, we used Fine and Gray (32) regression model to estimate the subdistribution hazard. This model modifies the Cox proportional hazard model allowing for competing risks. To examine the assumption of proportional distribution hazards, we visually inspected Schoenfeld residuals, and confirmed by testing the time-varying effect of each covariate using the Scheike and Zhang test. (33) Confidence intervals were computed by bootstrap resampling using the Kalbsfleisch and Prentice method. (34) For all analyses, “R” program, version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria), with cmprsk and timereg libraries, was applied.
To explore mechanisms explaining the association, we used a series of models in which we gradually controlled for potential modifiers. Model 1 included terms for age, sex and educational studies. Model 2 included the terms in model 1 plus terms for cognitive status at baseline (MMSE score) and functional disability. Model 3 included the terms in model 2 plus vascular risk factors and diseases.
The same models were used to study the effect of different clinical characteristics of depression on the risk of AD, adjusted for the same variables.
RESULTS
Of the initial cohort (n = 3,864), 452 participants (11.7%) were diagnosed with depression and 238 (6.2%) were classified with subsyndromal depression (Figure 1). Baseline characteristics by depression status are in Table 1. Compared with non-depressed participants, depressed persons were older, as well as more likely to be female, have functional disabilities, have diabetes, or have vascular disease. Depressed participants were less educated and performed worse cognitively. Compared with subjects with subsyndromal depression, depressed participants were more likely to be female and performed worse cognitively.
Table 1.
Characteristics of participants at baseline by depression status
| Characteristics of participants | No Depression n = 3,174 | Subsyndromal Depression n = 238 | Depression n = 452 |
|---|---|---|---|
| Age, mean (SD) | 71.5 (8.9) | 73.6 (9.3)b | 73.5 (9.4)a |
| Women, n (%) | 1,576 (49.7) | 168 (70.6)b | 359 (79.4)a,c |
| Education | |||
| Primary school, n (%) | 2,346 (74.5) | 185 (77.7) | 359 (80.3) |
| Secondary/higher school, n (%) | 578 (19) | 33 (13.9) | 46 (10.3)a |
| MMSE, mean (SD) | 27.5 (2.4) | 27 (2)b | 26.6 (2.8)a,c |
| Disability | |||
| Basic ADLs, n (%) | 159 (5) | 30 (12.6)b | 61 (13.5)a |
| Instrumental ADLs, n (%) | 313 (9.9) | 37 (15.6)b | 104 (23.2)a,c |
| Vascular risk factors | |||
| Hypertension, n (%) | 1,974 (67.5) | 146 (68.2) | 299 (69.7) |
| Diabetes, n (%) | 358 (11.4) | 40 (17.1)b | 73 (16.2)a |
| Vascular disease, n (%) | 339 (11.0) | 33 (14.3)b | 64 (15)a |
Significant differences (p < 0.05) between “cases” of depression and “no cases” of depression in either t (degrees of freedom, df = 3,624) test or X2 (df=1) test;
Significant differences (p < 0.05) between “subsyndromal depression” and “no cases” of depression in either t (df=3,410) test or X2 (df=1) test;
Significant differences (p < 0.05) between “cases” of depression and “subsyndromal depression” in either t (df=688) test or X2 (df=1) test;
Among the depressed, 16.4% at baseline (n = 74) had severe depression; 9.9% (n = 45) had history of depression, and 16.8% (n = 76) were taking antidepressant medication. At first wave follow-up (Wave II), 28.1% (n = 127) continued to be depressed (persistent depression) (Table 2).
Table 2.
Incident cases and incidence rates of Alzheimer’s disease by depression status at baseline
| Depression status at baseline | AD at Z III | ||||
|---|---|---|---|---|---|
|
| |||||
| Cases | Person-Years | Incidence Rate (95% CI) | IRR (95% CI)§ | p | |
|
|
|||||
| No depression (n = 3,174) | 51 | 12,758 | 4.00 (2.98–5.26) | ||
|
| |||||
| Subsyndromal depression (n = 238) | 6 | 910 | 6.59 (2.42–14.35) | 1.65 (0.71–3.84) | 0.246 |
|
| |||||
| Depression (n = 452) | 13 | 1,703 | 7.63 (4.06–13.04) | 1.91 (1.04–3.51) | 0.037 |
|
| |||||
| Depression type | |||||
| Nonsevere (n = 378) | 9 | 1,425 | 6.32 (2.89–11.99) | 1.58 (0.78–3.21) | 0.206 |
| Severe (n = 74) | 4 | 279 | 14.36 (3.91–36.76) | 3.59 (1.30–9.94) | 0.014 |
| History of depression* | |||||
| First-ever episode (n = 367) | 12 | 1,357 | 8.84 (4.57–15.44) | 2.21 (1.18–4.15) | 0.013 |
| Previous episodes (n = 45) | 0 | 178 | 0.00 (0.00–20.69) | -- ¥ -- | |
| Persistence of depression | |||||
| Only at baseline (n = 325) | 9 | 1,135 | 7.93 (3.62–15.05) | 1.98 (0.98–4.03) | 0.060 |
| At baseline and first follow-up evaluation (n = 127) | 4 | 568 | 7.04 (1.92–18.02) | 1.76 (0.64–4.87) | 0.275 |
| Antidepressant treatment | |||||
| Treated depression (n = 76) | 1 | 291 | 3.43 (0.09–19.12) | 0.86 (0.12–6.22) | 0.881 |
| Untreated depression (n = 376) | 12 | 1,412 | 8.50 (4.39–14.85) | 2.13 (1.13–3.99) | 0.019 |
Reported incidence rate ratio (IRR) of AD is related to no depression; Confidence intervals (CI) are shown for both, incidence rate and IRR; and p-values (p) are related to IRR; AD: Alzheimer’s Disease; Z III: ZARADEMP III, at 4.5 years follow-up;
Information on history of depression was missing in 40 cases of depression; ¥ No incident cases of AD in this group; Bold entries in the table mean that the IRR is statistically significant
As seen in Table 2, 70 incident cases of AD were found at Wave II (n = 3,237 interviewed) and Wave III (n = 2,403). Person-years of follow-up are also shown. Mean age of incident cases of AD when diagnosed was 86.5 years (SD = 6.9). At baseline, as expected, mean age of individuals that eventually developed AD (83.7 years, SD = 7.1) was higher than in the subjects that did not develop AD (70.0 years, SD = 7.8), the differences being statistically significant (t = 14.58, df = 3,862, p<0.001). Thirteen (18.6%) of all cases of dementia occurred in people with depression at baseline. When compared with the incidence rate (IR) of AD among the non-depressed, the IR was higher in subsyndromal depression and was almost double and significantly higher in cases of depression (IRR = 1.91, 95%CI: 1.04–3.51).
Table 2 also shows that, compared with the non-depressed, the IR of AD was high among cases on non-severe depression, but was particularly high among the severe cases, the IRR being 3.59 (95%CI: 1.30–9.94). When compared with their counterparts, the IR of AD was higher among the cases of first-ever depression, non-persistent depression or untreated depression, but the differences were not statistically significant. However, when compared with the non-depressed, the IR was significantly higher among the cases of both first-ever depression and untreated depression.
In Fine and Gray multivariate regression model, the association between clinical significant depression at baseline and incident AD was close to statistical significance in the unadjusted model, but was attenuated in multivariate models (Table 3). However, and contrary to non-severe depression, a consistent, significant association was observed between severe depression at baseline and incident AD (in model 3, HR = 4.30 (95%CI: 1.39–13.33).
Table 3 also shows hazard ratios (HR) for the development of AD in relation to other characteristics of depression. In the unadjusted model, both first-ever depression and untreated depression were significantly associated with AD. However, in the final multivariate model these associations were attenuated. Persistent depression was not associated with AD.
Table 3.
Risk of Alzheimer’s disease in clinical significant depression, according to characteristics of depression.
| Model 1 | Model 2 | Model 3 | Model 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Depression* | 1.82 | 0.97–3.42 | 0.062 | 1.22 | 0.65–2.32 | 0.540 | 1.13 | 0.58–2.18 | 0.710 | 1.11 | 0.57–2.15 | 0.750 |
| Subsyndromal depression* | 1.68 | 0.72–3.92 | 0.230 | 1.14 | 0.48–2.67 | 0.770 | 1.21 | 0.50–2.91 | 0.700 | 1.23 | 0.50–3.02 | 0.640 |
| Type of depression | ||||||||||||
| Severe depression* | 3.63 | 1.32–9.96 | 0.013 | 4.08 | 1.44–11.53 | 0.008 | 4.07 | 1.34–12.34 | 0.013 | 4.30 | 1.39–13.33 | 0.011 |
| Nonsevere depression* | 1.46 | 0.69–3.08 | 0.320 | 0.91 | 0.43–1.93 | 0.800 | 0.83 | 0.38–1.81 | 0.640 | 0.81 | 0.37–1.76 | 0.590 |
| History of depression | ||||||||||||
| First-ever episode* | 2.09 | 1.09–4.02 | 0.027 | 1.32 | 0.67–2.58 | 0.420 | 1.20 | 0.59–2.41 | 0.620 | 1.20 | 0.60–2.40 | 0.610 |
| Previous episodes* | ¥ | ¥ | ¥ | ¥ | ||||||||
| Persistence of depression | ||||||||||||
| Depression at baseline and first f/u evaluation* | 1.78 | 0.84–3.75 | 0.130 | 1.15 | 0.55–2.49 | 0.710 | 1.03 | 0.48–2.22 | 0.940 | 1.02 | 0.47–2.21 | 0.960 |
| Depression only at baseline* | 2.13 | 0.77–5.91 | 0.150 | 1.55 | 0.54–4.51 | 0.420 | 1.54 | 0.52–4.58 | 0.440 | 1.53 | 0.54–4.39 | 0.420 |
| Antidepressant treatment | ||||||||||||
| Untreated depression* | 2.04 | 1.06–3.92 | 0.033 | 1.23 | 0.63–2.40 | 0.540 | 1.13 | 0.57–2.26 | 0.730 | 1.51 | 0.21–10.98 | 0.740 |
| Treated depression* | 1.02 | 0.14–7.44 | 0.990 | 1.66 | 0.22–12.42 | 0.620 | 1.51 | 0.20–11.19 | 0.690 | 1.12 | 0.56–2.23 | 0.680 |
Fine and Gray regression model, hazard ratios (HR), confidence intervals (CI) and p-values (p) based on “normal approximation” of Wald chi-square test with 1 degree of freedom are shown for all variables analyzed;
Reported risk of AD is related to no depression; Bold entries in the table mean that the HR is statistically significant; Model 0: bivariate analysis; Model 1: included terms for age, sex and educational studies; Model 2: included the terms in model 1 plus terms for cognitive status at baseline (MMSE score) and functional disability; Model 3: included the terms in model 2 plus vascular risk factors and diseases. Vascular disease includes history of angina and/or myocardial infarctation and/or stroke; ¥ No incident cases of AD were found in the group of depressed with previous episodes of depression.
COMMENT
This 5-year longitudinal study of community dwelling adults aged 55 or more years supports the hypothesis that clinically significant, severe depression increases the risk of AD. The incidence rate of AD was almost double in subjects with clinically significant, overall depression when compared with the non-depressed, and was almost four times higher in cases of severe depression. While the incidence rate was also higher in cases of non-severe depression, the differences were not statistically significant. It is conceivable that age at baseline has influenced the results, since we have shown, as expected, that mean age of individuals that eventually developed AD was significantly higher than in the subjects that did not develop AD. Similarly, cognitive status at baseline might have influenced the results, since we have observed that mean MMSE score at baseline for subjects developing AD (24.6, SD = 2.2) was also significantly lower than in subjects non-demented at follow-up (27.7, SD = 2.6; t = 9.91, df = 3,862, p<0.001). However, and importantly, when controlling in Fine and Gray regression model for age and MMSE at baseline, as well as for other accepted risk factors of AD such as sex, educational level, functional disability and vascular risk factors, and contrary to non-severe depression, severe depression was associated with an increased risk of AD (HR = 4.30 (95%CI: 1.39–13.33). Furthermore, in support of the impact of depression severity in the increased risk of AD, subjects with severe depression (mean age 71.1 (SD = 8.1) were significantly younger at baseline than subjects with non-severe depression (mean age 74.1 years, SD = 9.5; t = 2.54, df = 450, p = 0.011). However, the increased risk was only observed in the severely depressed subjects.
It might be argued that only 4 cases of severe depression developed AD but, in fact, 74 such cases were followed up, amounting to 279 person-years. Furthermore, and contrary to previous, similar studies, this may be the first report using a competing risk model in studying depression as a risk factor for AD. Traditional models (such as KM and Cox regression) do not take into account competing risks, and may overestimate the risk of the disease (AD) in the presence of high rates of mortality, since death prevents the occurrence of the event of interest. In view of the expected high mortality in the elderly, the competing risk approach to accurately determining disease risk for individuals in this age group is preferred. (35) This approach is particularly relevant in studying the outcome of depression in the elderly, since depression in this age group has been associated with increased mortality (36) as previously reported in this same population. (37)
Some recent reports document the association between depression and all cause dementia (38)(39), and some found an specific association for severe forms of depression (18)(19). However, these reports did not address specifically the association with AD. While the relationship between depression and AD has been addressed previously, most studies used symptom-based scales and different threshold levels to identify depression, and the results were inconsistent. Devanand et al. (11) documented an increased risk of AD for depression measured with the Hamilton Rating Scale for Depression (HRSD), and similar results were reported by Wilson et al., (9) Saczynski et al. (10) and Li et al. (40) using the Center for Epidemiologic Studies Depression Scale (CES-D) In contrast, Becker et al. (14) did not find a significant association when using the CES-D, and both Fuhrer et al. (16) and Dal Forno et al. (17) used the same instrument and found that the increased risk of AD was limited to men.
By using AGECAT diagnostic criteria of depression, this study has advantages over previous studies, since “caseness” implies the “desirability of intervention”. (2) A previous, 4.5 year follow-up study in Zaragoza (37) supports using this system in the assessment of community elderly, as it predicted mortality. While agreement between AGECAT and DSM criteria for depression is only moderate, (27) cases of depression considered to be “severe” using AGECAT criteria may be similar to those considered “major” by some investigators. (41)
Geerlings et al. (42) found an association between depression documented with GMS-AGECAT criteria and incident AD, but this was limited to individuals with more than 8 years of education (OR = 5.31). The relevance of our findings should be emphasized, since most elderly living in the community in countries such as Spain have lower educational levels. (43) Furthermore, Geerlings et al. limited their analysis of AGECAT data to “syndrome clusters”, emerging from phase I of the computer program. (42) Therefore, they could not complete the differential diagnosis emerging from phase II in AGECAT, as we did, and could not differentiate between “severe” and “nonsevere” depression.
In relation to characteristics of depression, and similar to previous authors, (14) we found no association between persistent depression and AD. In untreated depression, we did find a significantly increased incidence rate of AD, and also the risk of AD was increased in the bivariate model. While the strength and significance of this association was attenuated after adjustment, its direction was maintained. Contrary to Fuhrer et al., (16) we found no association between depression treated with antidepressants and AD risk. Other studies in the literature did not find that antidepressant intake modified the association between depression and dementia. (19) Nevertheless, sparse antidepressant use limits statistical power and precludes definitive conclusions. (37)
Regarding first-ever depression, we also found a significantly increased incidence rate of AD, and a trend for an increased risk of AD. Panza et al. (15) concluded in their review that first-ever depression in late life is associated with incident AD. Very recently, Li et al. (40) reported this association in late-onset depression, and argued with others that depression might be a prodrome to AD rather than a risk factor. (15) However, this is controversial, as several support the idea that depression increases the risk of AD. (10)(12)(19) Our findings support the notion of increased risk as opposed to prodrome for the following reasons. First, to minimize the possibility of including in the cohort individuals with cognitive deficits at baseline, we excluded all those with very mild deficits (“subsyndromal” dementia). Furthermore, cognitive status at baseline was controlled in the analysis. Second, it might be argued that individuals with nonsevere depression were in fact incipient, prodromal cases of AD. (44) However, “severe” depression, but not its milder counterpart, was associated with AD. Finally, the specificity of the finding supports the real risk interpretation, since incident AD, but not incident dementia was associated with depression in this study (data not shown). To explain the findings supporting an increased risk of AD, we tend to favor the “brain reserve” and/or “cognitive reserve” hypothesis as the final common pathway, suggested by authors such as Butters et al.: (45) depression might alter the reserve through different mechanisms, both biological and psychosocial.
Our study has several strengths, such as use of a representative population sample, including institutionalized individuals; longitudinal design; high sensitivity and specificity of case finding, with instruments validated within the study; and inclusion of actual mortality data in the Fine and Gray model to study depression as a risk factor for AD.
While this study follows state of the art epidemiological approaches in psychiatry, some limitations must be noted, including the limited number of cases of severe depression that developed AD. There was significant attrition from sampling to enrolment. However, the attrition rate was expected by design (8)(20) and we previously argued that our investigation is comparable to other several two-stage epidemiological studies. (20) Another limitation is that we do not have data on ApoE, and that hospital-based diagnosis was not completed in all cases of dementia. In fact, in 2 cases (13.3% of cases considered to be AD by the panel of psychiatrists), the final, hospital-based diagnosis was “mixed dementia” (AD type and vascular dementia type). We trust this does not affect importantly the main conclusions, particularly in view that we controlled for vascular risk-factors in the association found between depression and incident AD.
In studying risk of AD, few reports assess the effect of characteristics of depression, (13)(14) (40) and none simultaneously studies several characteristics of depression, as we did here. However, some of the subgroups of depression were small, and therefore the statistical power to detect small differences was weak.
In conclusion, clinically significant, severe depression increases the risk of AD, even after controlling for the competing risk of death. This finding as well as the finding that untreated depression might be associated with incident AD may stimulate studies about the effect of treating depression in relation to the risk of AD.
Acknowledgments
The authors acknowledge the contribution of the lay interviewers, senior medical students, and members of the ZARADEMP Workgroup who participated in the study.
Funding/Support: Supported by Grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Health, Madrid, Spain (grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128). Dr. Lyketsos’ effort was supported by grant P50AG005146 to the Johns Hopkins Alzheimer’s Disease Research Center.
Footnotes
Previous presentation: None preliminary results have been previously presented.
Financial disclosure: We declare that Dr. Gracia-García has received Grant support from Janssen, AstraZeneca and the Ilustre Colegio de Médicos de Zaragoza; she has received Honorarium from AstraZeneca and Lilly; and she has received travel support from Lilly, Almirall, Lundbeck, Rovi, Pfizer and Janssen. Dr De-la-Cámara has received financial support to attend scientific meetings from Janssen-Cilag, Almirall, Eli Lilly, Lundbeck, Rovi, Esteve, Novartis and Astrazeneca. Dr. Quintanilla has been funded for acting as associated researcher by Janssen-Cilag; he has received travel support from Lundbeck. Dr. Ventura has received Grant support from Fondo de Investigación Sanitaria (FIS); Ministry of Sciences and Innovation, Spain and the Banco de Instruments of CIBERSAM; He has received travel support from Pfizer and Lumbeck. Dr Campayo has received Grant support (Research or EACCME) from FIS and Rovi; and literature or travel support from Pfizer and Sanofi-Aventis. Dr. Lyketsos has received Grant support (research or CME) from NIMH, NIA, Associated Jewish Federation of Baltimore, Weinberg Foundation, Forest, Glaxo-Smith-Kline, Eisai, Pfizer, Astra-Zeneca, Lilly, Ortho-McNeil, Bristol-Myers, Novartis, National Football League, Elan, Functional Neuromodulation Inc; He has been a consultant or advisor to Astra-Zeneca, Glaxo-Smith Kline, Eisai, Novartis, Forest, Supernus, Adlyfe, Takeda, Wyeth, Lundbeck, Merz, Lilly, Pfizer, Genentech, Elan, NFL Players Association, NFL Benefits Office, Avanir, Zinfandel, and has received Honorarium or travel support from Pfizer, Forest, Glaxo-Smith Kline, Health Monitor. Dr. Lobo had a consultancy with Janssen, and he has received Honorarium or travel support from Eli Lilly and Bial. None of these activities is related to the current project. The other authors report no financial relationships with commercial interest.
References
- 1.Galik EM, Rabins P, Lyketsos CG. Dementia. In: Blumenfield M, Strain JJ, editors. Psychosomatic Medicine. Lippincott Williams & Wilkins; 2006. pp. 513–535. [Google Scholar]
- 2.Copeland JR, Beekman AT, Braam AW, et al. Depression among older people in Europe: the EURODEP studies. World Psychiatry. 2004;3(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- 3.Meyers BS, Bruce ML. The depression-dementia conundrum: integrating clinical and epidemiological perspectives. Arch Gen Psychiatry. 1998;55(12):1082–1083. doi: 10.1001/archpsyc.55.12.1082. [DOI] [PubMed] [Google Scholar]
- 4.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 5.Mielke MM, Rosenberg PB, Tschanz J, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69(19):1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- 6.Daviglus ML, Bell CC, Berrettini W, Bowen PE, et al. National Institutes of Health State-of-the-Science Conference statement: preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153(3):176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 7.Lobo A, Lopez-Anton R, Santabarbara J, et al. Incidence and lifetime risk of dementia and Alzheimer’s disease in a Southern European population. Acta Psychiatr Scand. 2011;124(5):372–383. doi: 10.1111/j.1600-0447.2011.01754.x. [DOI] [PubMed] [Google Scholar]
- 8.Lobo A, Saz P, Marcos G, et al. The Prevalence of Dementia and Depression in the Elderly Community in a Southern European Population. The Zaragoza Study. Arch Gen Psychiatry. 1995;52:497–506. doi: 10.1001/archpsyc.1995.03950180083011. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 10.Saczynski JS, Beiser A, Seshadri S, et al. Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology. 2010;75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53(2):175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 12.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geerlings MI, den Heijer T, Koudstaal PJ, et al. History of depression, depressive symptoms, and medial temporal lobe atrophy and the risk of Alzheimer disease. Neurology. 2008;70(15):1258–1264. doi: 10.1212/01.wnl.0000308937.30473.d1. [DOI] [PubMed] [Google Scholar]
- 14.Becker JT, Chang YF, Lopez OL, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. 2009;17(8):653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panza F, Frisardi V, Capurso C, et al. Late-life depression, mild cognitive impairment, and dementia: possible continuum? Am J Geriatr Psychiatry. 2010;18(2):98–116. doi: 10.1097/JGP.0b013e3181b0fa13. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrer R, Dufouil C, Dartigues JF. Exploring sex differences in the relationship between depressive symptoms and dementia incidence: prospective results from the PAQUID Study. J Am Geriatr Soc. 2003;51(8):1055–1063. doi: 10.1046/j.1532-5415.2003.51352.x. [DOI] [PubMed] [Google Scholar]
- 17.Dal Forno G, Palermo MT, Donohue JE, et al. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol. 2005;57(3):381–387. doi: 10.1002/ana.20405. [DOI] [PubMed] [Google Scholar]
- 18.Palsson S, Aevarsson O, Skoog I. Depression, cerebral atrophy, cognitive performance and incidence of dementia. Population study of 85-year-olds. Br J Psychiatry. 1999;174:249–253. doi: 10.1192/bjp.174.3.249. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Hu Z, Wei L, et al. Severity of depression and risk for subsequent dementia: cohort studies in China and the UK. Br J Psychiatry. 2008;193(5):373–377. doi: 10.1192/bjp.bp.107.044974. [DOI] [PubMed] [Google Scholar]
- 20.Lobo A, Saz P, Día JL, et al. and the ZARADEMP Workgroup. The ZARADEMP Project on the incidence, prevalence and risk factors of dementia (and depression) in the elderly community. II. Methods and first results. Eur J Psychiatry. 2005;19(1):40–54. [Google Scholar]
- 21.Copeland JR, Dewey ME, Griffiths-Jones HM. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol Med. 1986;16(1):89–99. doi: 10.1017/s0033291700057779. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Dewey ME, Copeland JR. Diagnosis of dementia from the history and aetiology schedule. Int J Geriatr Psychiatry. 2001;16(9):912–917. doi: 10.1002/gps.446. [DOI] [PubMed] [Google Scholar]
- 24.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- 25.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: A standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 26.Launer LJ, Brayne C, Breteler MM. Epidemiologic approach to the study of dementing diseases: a nested case-control study in European incidence studies of dementia. Neuroepidemiology. 1992;11 (Suppl 1):S114–S148. doi: 10.1159/000111005. [DOI] [PubMed] [Google Scholar]
- 27.Schaub RT, Linden M, Copeland JR. A comparison of GMS-A/AGECAT, DSM-III-R for dementia and depression, including subthreshold depression (SD)--results from the Berlin Aging Study (BASE) Int J Geriatr Psychiatry. 2003;18(2):109–117. doi: 10.1002/gps.799. [DOI] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment, 2012. Oslo: WHO; 2011. [Google Scholar]
- 29.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(9):632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 31.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 32.Fine JPGR. A proportional hazard model for the sub-distribution of competing risks. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 33.Scheike TH, Zhang MJ. Flexible competing risks regression modeling and goodness-of-fit. Lifetime Data Anal. 2008;14(4):464–483. doi: 10.1007/s10985-008-9094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalbfleisch JDPR. The statistical analysis of failure time data. John Wiley & Sons Inc; 1980. [Google Scholar]
- 35.Berry SD, Ngo L, Samelson EJ, et al. Competing risk of death: an important consideration in studies of older adults. J Am Geriatr Soc. 2010;58(4):783–787. doi: 10.1111/j.1532-5415.2010.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma VK, Copeland JR, Dewey ME, et al. Outcome of the depressed elderly living in the community in Liverpool: a 5-year follow-up. Psychol Med. 1998;28(6):1329–1337. doi: 10.1017/s0033291798007521. [DOI] [PubMed] [Google Scholar]
- 37.De-la-Cámara C, Saz P, Lopez-Anton R, et al. Depression in the elderly community: II Outcome in a 4.5 years follow-up. Eur J Psychiatry. 2008;22 (3):141–150. [Google Scholar]
- 38.Byers AL, Covinsky KE, Barnes DE, et al. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20 (8):664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Köhler S, van Boxtel M, Jolles J, et al. Depressive symptoms and risk for dementia: a 9-year follow-up of the Maastricht Aging Study. Am J Geriatr Psychiatry. 2011;19 (10):902–905. doi: 10.1097/JGP.0b013e31821f1b6a. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Wang LY, Shofer JB, et al. Temporal relationship between depression and dementia: findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry. 2011;68(9):970–977. doi: 10.1001/archgenpsychiatry.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penninx BW, Geerlings SW, Deeg DJ, et al. Minor and major depression and the risk of death in older persons. Arch Gen Psychiatry. 1999;56(10):889–895. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- 42.Geerlings MI, Schmand B, Braam AW, et al. Depressive symptoms and risk of Alzheimer’s disease in more highly educated older people. J Am Geriatr Soc. 2000;48(9):1092–1097. doi: 10.1111/j.1532-5415.2000.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 43.Instituto Nacional de Estadística. [Accessed December 15, 2011.];INEbase: Demografía y población; Cifras de población y Censos demográficos. http://www.ine.es/inebmenu/mnu_cifraspob.htm.
- 44.Forsell Y, Jorm AF, Winblad B. Variation in psychiatric and behavioural symptoms at different stages of dementia: data from physicians’ examinations and informants’ reports. Dementia. 1993;4(5):282–286. doi: 10.1159/000107334. [DOI] [PubMed] [Google Scholar]
- 45.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]