Abstract
Introduction
Dietary fats influence intestinal inflammation and regulate mucosal immunity. Data on the association between dietary fat and risk of Crohn’s disease (CD) and ulcerative colitis (UC) are limited and conflicting.
Methods
We conducted a prospective study of women enrolled in the Nurses’ Health Study cohorts. Diet was prospectively ascertained every four years using a validated semi-quantitative food frequency questionnaire. Self-reported CD and UC were confirmed through medical record review. We examined the effect of energy-adjusted cumulative average total fat intake as well as specific types of fat and fatty acids on the risk of CD and UC using Cox proportional hazards models adjusting for potential confounders.
Results
Among 170,805 women, we confirmed 269 incident cases of CD (incidence 8/100,000 person-years) and 338 incident cases of UC (incidence 10/100,000 person-years) over 26 years and 3,317,338 person-years of follow-up. Cumulative energy-adjusted intake of total fat, saturated fats, unsaturated fats, n-6 and n-3 polyunsaturated fatty acids (PUFA) were not associated with risk of CD or UC. However, greater intake of long-chain n-3 PUFA was associated with a trend towards lower risk of UC (Hazard ratio (HR) 0.72, 95% CI 0.51 – 1.01). In contrast, high long-term intake of trans-unsaturated fatty acids was associated with a trend towards an increased incidence of UC (HR 1.34, 95% CI 0.94 – 1.92).
Conclusion
A high intake of dietary long-chain n-3 PUFA may be associated with a reduced risk of UC. In contrast, high intake of trans-unsaturated fats may be associated with an increased risk of UC.
INTRODUCTION
The key mechanism underlying the development of Crohn’s disease (CD) and ulcerative colitis (UC) is a dysregulated immune response to commensal flora in a genetically susceptible host1, 2. A confluence of genetic susceptibility, intestinal microbial composition, immune dysregulation, and the extrinsic environmental triggers is essential for the development of disease. Advances in genetics have led to identification of 163 distinct susceptibility alleles for CD or UC3. Studies have also documented dysbiosis in the microbiome of patients with IBD with reduced bacterial diversity and a diminished proportion of firmicutes2, 4–6.
Diet has been hypothesized to play an important role in the pathogenesis of inflammatory bowel diseases (IBD)7. Epidemiologic trends have demonstrated a rising incidence of IBD in countries in which the diseases were previously uncommon8. This increase has paralleled a “westernization” of life style. In particular, dietary changes in such countries have demonstrated increasing intake of dietary fat, particularly n-6 polyunsaturated fatty acids (PUFA) and reduced intake of n-3 PUFA9. Considerable biological plausibility supports a role of low n-3 PUFA intake, particularly long-chain PUFAs, in the pathogenesis of these diseases. Dietary n-3 PUFA competitively inhibits formation of pro-inflammatory prostaglandins and leukotrienes through the arachidonic acid pathway9, and inhibits vascular adhesion and migration, angiogenesis, and adaptive immune responses through peroxisome proliferator-activated receptor (PPARγ) and NF-κB mediated pathways10–12. In contrast while some have proposed that dietary n-6 PUFA or linoleic acid is pro-inflammatory, several prospective cohort studies and randomized controlled trials have failed to demonstrate an association between n-6 PUFA intake and inflammatory markers13, 14.
Nonetheless, specific data linking dietary factors with IBD incidence in human populations have been limited and conflicting 15–17. Most prior studies of diet and IBD have been retrospective and case-control in design16, 17. The few prospective studies that have examined an association between dietary fat and IBD have been limited by an assessment of diet at a single time point, a small number of cases, lack of adjustment for potentially important confounders, or examination of only UC but not CD18–21. To overcome these limitations, we performed this prospective study using two large well-characterized cohorts of women that have provided long-term, validated, and updated data on their intake of total fat and specific fatty acids and have been followed for incidence of physician-confirmed cases of CD and UC.
METHODS
Study Population
Our study included participants from the Nurses Health Study I, a prospective cohort of 121,700 female registered nurses between the ages of 30–55 years at enrollment in 1976, and the Nurses Health Study II, a parallel prospective cohort of 116,686 female registered nurses between the ages of 25–42 years initiated in 1989. Women completed biennial questionnaires reporting medical history including new diagnoses, and detailed measures of diet and environmental exposure with a follow-up rate > 90%. All women who first completed a detailed dietary assessment in 1984 (NHS I) or 1991 (NHS II) were included in this study. Women who were deceased prior to the first dietary questionnaire, reported a diagnosis of IBD prior to the baseline dietary assessment, or had a history of cancer (excluding non-melanoma skin cancer) were excluded. The study was approved by the Institutional Review Board at Partners Healthcare.
Dietary assessment
Detailed assessments of diet were conducted prospectively using a validated semi-quantitative food frequency questionnaire (FFQ). A 61-item questionnaire was first administered in 1980 and expanded to a 121-item questionnaire in 1984 and a 136-item questionnaire in 1986. Subsequent questionnaires in NHS I were administered in 1990, 1994, 1998, 2002, and 2006 while questionnaires in NHS II were administered first in 1991 and subsequently in 1995, 1999, 2003, and 2007. Participants were asked on an average how often they had consumed specific types of beverage or food items during the past year with nine possible responses (never to six or more times per day). The methodology detailing assessment of total dietary fat as well as individual fatty acids have been reported22–27. Intake of total fat, types of fat (saturated fats [SFA), trans-unsaturated fats, mono-unsaturated fats [MUFA), poly-unsaturated fats [PUFA)) and specific fatty acids were calculated based on U.S. Department of Agriculture food composition data incorporating margarine and fats used in cooking and baking. All measures were adjusted for total energy intake. The reproducibility and validity of fat intake has been validated. Correlation between energy-adjusted intake and two 1-week dietary records was good for total fat (0.57), saturated fats (0.68), trans-unsaturated fat (0.51), poly-unsaturated fatty acids (0.48), mono-unsaturated fats (0.58), and n-3 fatty acids (0.48)23, 27. Long-term dietary intake from multiple FFQs correlated well with erythrocyte measures of linoleic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)28, and fatty acid composition from subcutaneous fat aspirates29. Women who did not complete the baseline food frequency questionnaire were similar in age and smoking status to those who completed the SFFQ, but less likely to be obese, or currently use post-menopausal hormone or aspirin.
Ascertainment of CD and UC
Diagnosis of CD and UC in the Nurses’ Health Study cohorts has been described previously30–36. Since 1976 in NHS I and 1989 in NHS II, 2,735 and 2,541 women each respectively self-reported a diagnosis of CD or UC through 2010 in NHS I and 2009 in NHS II. Figure 1 represents the flow of ascertainment of cases. Among 4,330 women with an initial self-report of IBD who were alive, 1,447 denied the diagnosis on the subsequent supplementary questionnaire with a more detailed description of the diseases, 820 could not be contacted, and 716 women either denied permission for medical record review, or the appropriate records could not be obtained. Among the remaining 1,347 women with sufficient medical information, records were reviewed by two board-certified gastroenterologists blinded to exposure status. A diagnosis of CD or UC was established based on standard clinical criteria including duration of typical symptoms of 4 weeks or longer, and confirmatory endoscopic, histologic, and radiographic findings37–39. Disagreements were infrequent and resolved through consensus. Based on this review, a diagnosis of chronic colitis was rejected in 312 women, and 192 women were diagnosed with non-IBD chronic colitis (microscopic colitis). After excluding women with missing date of diagnosis, or dietary information, our final case population included 269 incident CD and 338 incident UC cases. Baseline characteristics, including age, body mass index, smoking, oral contraceptive use, menopausal status, use of aspirin or NSAIDs, and fat intake among women who initially self-reported IBD but whose diagnosis of CD or UC was not subsequently confirmed through medical record review were largely similar to the characteristics of women for whom we did confirm CD or UC (p > 0.3 for all).
Figure 1.
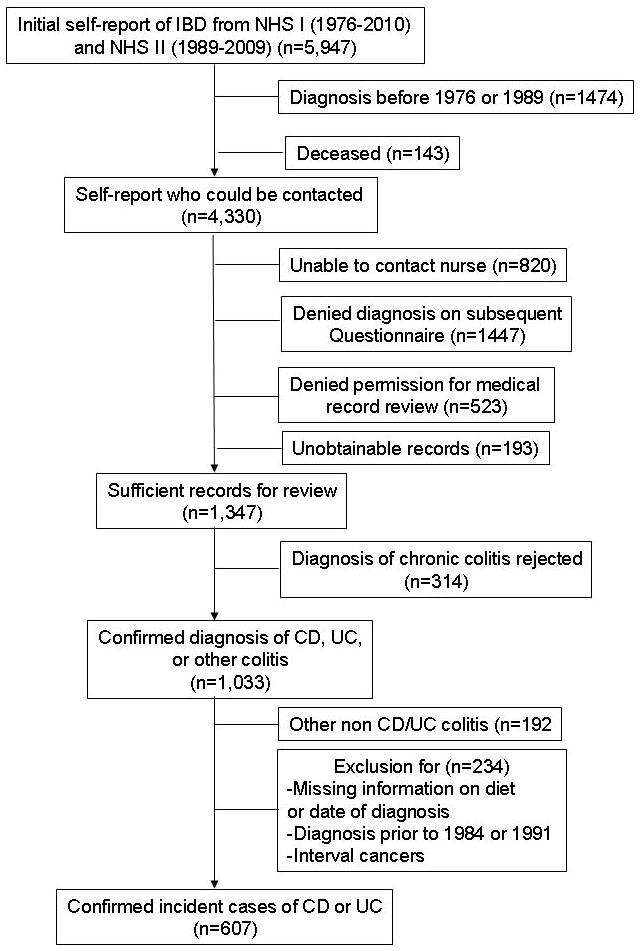
Flow of potential cases of incident Crohn’s disease or ulcerative colitis
Covariates
Covariates were selected for inclusion in the multivariate model based on known associations with IBD30, 33–35. Cigarette smoking (current, past, or never)33, oral contraceptive use (ever or never)35, post-menopausal hormone use (premenopausal, never, current, or past use)34 were modeled as time-varying covariates, updated biennially with each questionnaire. Body mass index (BMI) was calculated based on self-reported weight and height at study entry and expressed as kilograms per square meter (kg/m2). Consistent with prior analyses, we used BMI at baseline to avoid the potential influence of pre-diagnosis symptoms on body weight. Regular use of aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) was also assessed biennially and defined as use on 5 or more days per month30.
Statistical Analysis
Women contributed person time from the date of completion of the baseline questionnaire (1984 for NHS I and 1991 for NHS II) until the date of diagnosis of CD or UC, death, or till the date of return of the last questionnaire. Cox proportional hazards models adjusting for potential confounders was used to identify the independent effect of the exposures of interest. Dietary fat, types of fat, and intake of specific fatty acids were modeled as cumulative averages of intake up to the questionnaire immediately preceding the year of diagnosis of CD or UC. Cumulative average intake was adjusted for total energy intake and modeled as quintiles consisted with prior analysis22, 23, 27. Cumulative average intake incorporates all available data from repeated measures, thereby providing the most stable long-term estimate of adult diet 40. We also examined the ratio of n-3 PUFA and n-6 PUFA intake based on prior work demonstrating the possible importance of the relative balance of these fatty acids with risk of chronic disease41, 42. Tests for linear trend were conducted using the median value for each quintile as a continuous variable in the regression models based on the assumption on a monotonic dose-response relationship between fat intake and risk of disease. As we observed no heterogeneity in the associations between the cohorts (NHS I and II) (P>0.30), all analyses were performed pooling individual-level data from both cohorts while adjusting for cohort in our multivariate model. To account for pre-diagnosis symptoms potentially modifying diet in the 2 years prior to diagnosis, we performed a lag analysis extending the interval between exposure and diagnosis to 4 years. All models satisfied the proportionality of hazards assumptions. P-values < 0.05 indicated independent statistical significance. All statistical analysis was performed using SAS 9.1 (SAS Institute, Cary, NC).
Systematic Review
We performed a systematic review of the literature examining the association between overall dietary fat intake or intake of specific fatty acids and risk of CD and UC. We conducted a search on Pubmed for articles from 1966 to 2013 using the medical subject heading (MeSH) terms “dietary fat” “dietary fatty acids” “dietary n-3” “dietary n-6” or “dietary linoleic acid” and “ulcerative colitis” “Crohn’s disease” “inflammatory bowel disease”. We reviewed all eligible studies and hand-searched reference lists of original publications as well as relevant review articles.
RESULTS
Our study comprised 3,317,338 person-years of follow-up over 26 years among 76,738 women in NHS I and 94,067 women in NHS II. We documented 269 incident cases of CD (incidence 8 per 100,000 person-years) and 338 of UC (incidence 10 per 100,000 person-years), diagnosed at a median age of 54 and 52 years respectively. Table 1 presents the characteristics of the cohort stratified by quintile of total energy-adjusted dietary fat intake. Women in the highest quintile of cumulative intake of dietary fat were more likely to be current smokers than those in the lowest quintile (p < 0.001) and were more likely to have a BMI ≥ 30kg/m2. Dietary fat intake ranged from a mean of 47 grams (g)/day in the lowest quintile to 77 g/day in the highest quintile. Similar trends across quintiles were also seen for consumption of saturated fats, MUFA, PUFA, or trans-unsaturated fats. The differences were less striking for arachidonic acid, EPA, and DHA, which comprised a smaller fraction of total dietary fat intake.
Table 1.
Baseline characteristics‡ of the study population according to quintile of dietary fat intake
| Quintile 1 (n = 34,861) | Quintile 2 (n=34,819) | Quintile 3 (n=34,508) | Quintile 4 (n=32,042) | Quintile 5 (n=34,580) | |
|---|---|---|---|---|---|
| Mean age (in years) (standard deviation) | 43.8(9.8) | 43.2(9.4) | 42.9(9.2) | 42.3(8.8) | 42.8(8.8) |
| White race (%) | 95 | 97 | 97 | 98 | 98 |
| Smoking status (%) | |||||
| Never Smoker | 56 | 57 | 57 | 56 | 53 |
| Past Smoker | 28 | 27 | 26 | 26 | 25 |
| Current Smoker | 16 | 16 | 17 | 18 | 22 |
| Ever oral contraceptive use (%) | 83 | 84 | 84 | 85 | 86 |
| Pre-menopausal (%) | 68 | 69 | 69 | 69 | 69 |
| Post-menopausal hormone use (%) † | |||||
| Never Users | 50 | 53 | 53 | 53 | 53 |
| Past Users | 23 | 20 | 20 | 20 | 20 |
| Current Users | 27 | 27 | 27 | 27 | 27 |
| Body Mass Index (%) | |||||
| < 20.0 kg/m2 | 18 | 14 | 14 | 13 | 13 |
| 20.0 – 24.9 kg/m2 | 55 | 54 | 53 | 51 | 48 |
| 25.0 – 29.9 kg/m2 | 19 | 21 | 22 | 23 | 24 |
| > 30.0 kg/m2 | 8 | 10 | 11 | 13 | 17 |
| Regular aspirin use ± (%) | 17 | 18 | 18 | 18 | 18 |
| Regular NSAID use ± (%) | 10 | 10 | 10 | 10 | 11 |
| Mean total fat intake (g/day) (SD) | 47.3(5.6) | 57.0(1.8) | 62.6(1.6) | 68.1(2.0) | 77.4(5.9) |
| Mean saturated fat intake (g/day) (SD) | 16.9(2.8) | 20.4(2.2) | 22.3(2.4) | 24.1(2.8) | 27.3(4.0) |
| Mean trans-saturated fat intake (g/day) (SD) | 2.3(0.8) | 3.0(0.8) | 3.4(0.9) | 3.7(1.0) | 4.3(1.2) |
| Mean mono-unsaturated fat intake (g/day) (SD) | 17.1(2.5) | 21.1(1.5) | 23.4(1.6) | 25.7(1.8) | 29.4(3.1) |
| Mean poly-unsaturated fat intake (g/day) (SD) | 9.2(2.0) | 10.7(2.1) | 11.5(2.2) | 12.3(2.4) | 13.8(3.4) |
| Mean intake of specific fatty acids (g/day) (SD) | |||||
| Linoleic acid | 7.8(1.8) | 9.1(1.9) | 10.0(2.0) | 10.7(2.3) | 12.1(3.2) |
| Oleic acid | 15.6(2.4) | 19.3(1.6) | 21.5(1.7) | 23.6(1.9) | 27.0(3.1) |
| Arachidonic acid | 0.1(0.1) | 0.1(0.1) | 0.2(0.1) | 0.2(0.1) | 0.2(0.1) |
| n-3 PUFA | 1.1(0.4) | 1.2(0.3) | 1.3(0.4) | 1.3(0.4) | 1.4(0.5) |
| n-6 PUFA | 7.9(1.9) | 9.3(2.1) | 10.1(2.2) | 10.8(2.5) | 12.3(3.5) |
| Long-chain n-3 PUFA|| | 0.3(0.2) | 0.2(0.2) | 0.2(0.2) | 0.2(0.2) | 0.2(0.2) |
| Mean total fiber intake (g/day) (SD) | 20.5(7.1) | 18.3(5.0) | 17.2(4.3) | 16.3(3.9) | 14.8(3.7) |
| Mean carbohydrate intake (g/day)(SD) | 243(39) | 219(30) | 206(28) | 194(26) | 173(28) |
| Mean total protein intake (g/day) (SD) | 77.7(18.4) | 79.8(16.4) | 80.2(15.8) | 80.4(15.1) | 80.7(14.9) |
SD – standard deviation; NSAID – non-steroidal anti-inflammatory drugs;
Baseline characteristics according to the 1984 questionnaire for Nurses Health Study I and 1991 questionnaire for Nurses Health Study II. Dietary fat categories according to energy-adjusted intake.
Percentages among postmenopausal women
regular use was defined as intake of 5 or more times per month
Long-chain n-3 PUFA comprises docosapentaenoic acid, eicosapentaenoic acid, docosahexaenoic acid
We did not observe any significant association in total fat intake and incidence of UC (Table 2) or CD (Table 3). Compared to women in the lowest quintile of cumulative intake of dietary fat, women in the highest quintile had a similar incidence of UC (multivariate HR 0.96, 95% CI 0.68 – 1.35) (Table 2) or CD (HR 0.98, 95% CI 0.66 – 1.45) (Table 3). We also observed no difference in the incidence of UC or CD across quintiles of intake of saturated fats, MUFA or total PUFA. However, higher trans-unsaturated fatty acid intake was associated with a trend towards increased incidence of UC (multivariate HR 1.34, 95% CI 0.94 – 1.46 for the highest quintile, p(trend) = 0.07) but not CD (p (trend) = 0.19).
Table 2.
Risk of Ulcerative Colitis according to intake of dietary fat, types of fat, and specific fatty acids‡
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P(linear trend) | |
|---|---|---|---|---|---|---|
| Total Fats | ||||||
| Number of cases | 74 | 66 | 64 | 69 | 65 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.91 (0.65 – 1.27) | 0.88 (0.63 – 1.23) | 0.98 (0.71 – 1.37) | 0.96 (0.69 – 1.35) | 0.94 |
| Multivariate HR (95% CI) † | 1.0 | 0.91 (0.65 – 1.28) | 0.89 (0.63 – 1.25) | 1.00 (0.71 – 1.39) | 0.96 (0.68 – 1.35) | 0.94 |
| Saturated Fats | ||||||
| Number of cases | 76 | 60 | 61 | 81 | 60 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.79 (0.56 – 1.11) | 0.83 (0.59 – 1.16) | 1.09 (0.79 – 1.49) | 0.85 (0.60 – 1.19) | 0.82 |
| Multivariate HR (95% CI) † | 1.0 | 0.80 (0.57 – 1.12) | 0.84 (0.60 – 1.18) | 1.10 (0.80 – 1.52) | 0.84 (0.59 – 1.19) | 0.82 |
| Trans Unsaturated Fats | ||||||
| Number of cases | 60 | 64 | 68 | 72 | 74 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.08 (0.75 – 1.53) | 1.15 (0.81 – 1.63) | 1.23 (0.87 – 1.75) | 1.31 (0.92 – 1.86) | 0.09 |
| Multivariate HR (95% CI) † | 1.0 | 1.09 (0.76 – 1.55) | 1.18 (0.83 – 1.68) | 1.26 (0.89 – 1.79) | 1.34 (0.94 – 1.92) | 0.07 |
| Mono-unsaturated Fats | ||||||
| Number of cases | 69 | 61 | 77 | 64 | 67 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.90 (0.64 – 1.28) | 1.13 (0.82 – 1.57) | 0.96 (0.68 – 1.35) | 1.05 (0.74 – 1.47) | 0.59 |
| Multivariate HR (95% CI) † | 1.0 | 0.91 (0.64 – 1.29) | 1.15 (0.82 – 1.59) | 0.97 (0.69 – 1.37) | 1.04 (0.74 – 1.46) | 0.61 |
| Poly-unsaturated Fats | ||||||
| Number of cases | 64 | 75 | 64 | 76 | 59 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.17 (0.83 – 1.63) | 1.06 (0.75 – 1.49) | 1.30 (0.93 – 1.81) | 1.03 (0.72 – 1.47) | 0.70 |
| Multivariate HR (95% CI) † | 1.0 | 1.19 (0.85 – 1.67) | 1.02 (0.72 – 1.45) | 1.28 (0.91 – 1.79) | 1.02 (0.72 – 1.47) | 0.69 |
| Long-chain n-3 PUFA|| | ||||||
| Number of cases | 75 | 73 | 58 | 72 | 60 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.88 (0.64 – 1.22) | 0.74 (0.53 – 1.05) | 0.91 (0.66 – 1.26) | 0.75 (0.54 – 1.07) | 0.22 |
| Multivariate HR (95% CI) † | 1.0 | 0.88 (0.63 – 1.21) | 0.75 (0.52 – 1.04) | 0.89 (0.64 – 1.24) | 0.72 (0.51 – 1.01) | 0.13 |
| n-3 PUFA | ||||||
| Number of cases | 75 | 72 | 67 | 62 | 62 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.98 (0.71 – 1.36) | 0.90 (0.64 – 1.25) | 0.88 (0.63 – 1.23) | 0.90 (0.64 – 1.26) | 0.49 |
| Multivariate HR (95% CI) † | 1.0 | 0.98 (0.71 – 1.36) | 0.90 (0.64 – 1.25) | 0.87 (0.62 – 1.22) | 0.88 (0.63 – 1.24) | 0.45 |
| n-6 PUFA | ||||||
| Number of cases | 69 | 70 | 67 | 66 | 66 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.04 (0.74 – 1.45) | 1.00 (0.71 – 1.41) | 1.04 (0.74 – 1.46) | 1.07 (0.76 – 1.51) | 0.61 |
| Multivariate HR (95% CI) † | 1.0 | 1.05 (0.75 – 1.46) | 1.02 (0.72 – 1.43) | 1.04 (0.74 – 1.47) | 1.08 (0.77 – 1.52) | 0.59 |
| n-3/n-6 PUFA ratio | ||||||
| Number of cases | 77 | 70 | 69 | 66 | 56 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.91 (0.65 – 1.25) | 0.89 (0.64 – 1.24) | 0.85 (0.61 – 1.18) | 0.71 (0.50 – 1.00) | 0.04 |
| Multivariate HR (95% CI) † | 1.0 | 0.91 (0.66 – 1.26) | 0.90 (0.65 – 1.24) | 0.85 (0.61 – 1.18) | 0.69 (0.49 – 0.98) | 0.03 |
| Arachidonic acid | ||||||
| Number of cases | 75 | 66 | 69 | 62 | 66 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.92 (0.66 – 1.29) | 0.98 (0.71 – 1.37) | 0.87 (0.62 – 1.21) | 0.93 (0.67 – 1.30) | 0.71 |
| Multivariate HR (95% CI) † | 1.0 | 0.92 (0.66 – 1.28) | 0.98 (0.70 – 1.36) | 0.86 (0.61 – 1.21) | 0.90 (0.65 – 1.27) | 0.63 |
| Linoleic acid | ||||||
| Number of cases | 66 | 66 | 73 | 68 | 65 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.98 (0.70 – 1.38) | 1.09 (0.78 – 1.52) | 1.05 (0.74 – 1.47) | 1.04 (0.73 – 1.47) | 0.72 |
| Multivariate HR (95% CI) † | 1.0 | 0.97 (0.69 – 1.37) | 1.11 (0.79 – 1.55) | 1.08 (0.77 – 1.52) | 1.04 (0.73 – 1.48) | 0.71 |
| Oleic acid | ||||||
| Number of cases | 68 | 61 | 71 | 74 | 64 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.92 (0.65 – 1.30) | 1.06 (0.76 – 1.48) | 1.13 (0.81 – 1.58) | 1.00 (0.71 – 1.41) | 0.54 |
| Multivariate HR (95% CI) † | 1.0 | 0.92 (0.65 – 1.31) | 1.07 (0.77 – 1.50) | 1.14 (0.82 – 1.59) | 1.00 (0.70 – 1.41) | 0.55 |
NSAID – non-steroidal anti-inflammatory drugs, HR – hazard ratio, CI – confidence interval
Cumulatively average energy-adjusted intake from 1984 (NHS I) or 1991 (NHS II)
per 100,000 person-years
Adjusted for age, cohort, smoking (never, past, current), body mass index (<20 kg/m2, 20–24.9kg/m2, 25–29 kg/m2, > 30kg/m2), oral contraceptive use (never, ever), use of post menopausal hormone therapy (premenopausal, postmenopausal hormone never user, past user, current user), regular use of NSAIDs (yes, no), regular use of aspirin (yes, no), total energy intake (quintile).
Long-chain n-3 PUFA comprises docosapentaenoic acid, eicosapentaenoic acid, docosahexaenoic acid
Table 3.
Risk of Crohn’s disease according to intake of dietary fat, types of fat, and specific fatty acids‡
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P(linear trend) | |
|---|---|---|---|---|---|---|
| Total Fats | ||||||
| Number of cases | 51 | 62 | 48 | 55 | 53 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.20 (0.83 – 1.74) | 0.94 (0.63 – 1.40) | 1.12 (0.76 – 1.65) | 1.11 (0.75 – 1.64) | 0.75 |
| Multivariate HR (95% CI) † | 1.0 | 1.17 (0.81 – 1.70) | 0.90 (0.60 – 1.34) | 1.05 (0.71 – 1.55) | 0.98 (0.66 – 1.45) | 0.71 |
| Saturated Fats | ||||||
| Number of cases | 55 | 63 | 49 | 54 | 48 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.13 (0.78 – 1.62) | 0.87 (0.59 – 1.29) | 0.98 (0.67 – 1.43) | 0.90 (0.61 – 1.34) | 0.44 |
| Multivariate HR (95% CI) † | 1.0 | 1.09 (0.76 – 1.58) | 0.83 (0.56 – 1.22) | 0.91 (0.62 – 1.34) | 0.79 (0.53 – 1.19) | 0.16 |
| Trans Unsaturated Fats | ||||||
| Number of cases | 55 | 61 | 51 | 58 | 44 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.08 (0.75 – 1.55) | 0.89 (0.61 – 1.31) | 1.01 (0.69 – 1.47) | 0.80 (0.53 – 1.21) | 0.24 |
| Multivariate HR (95% CI) † | 1.0 | 1.05 (0.73 – 1.52) | 0.86 (0.58 – 1.26) | 0.95 (0.65 – 1.39) | 0.75 (0.50 – 1.13) | 0.18 |
| Mono-unsaturated Fats | ||||||
| Number of cases | 52 | 61 | 48 | 52 | 56 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.15 (0.79 – 1.66) | 0.93 (0.62 – 1.37) | 1.02 (0.69 – 1.50) | 1.13 (0.77 – 1.65) | 0.70 |
| Multivariate HR (95% CI) † | 1.0 | 1.12 (0.77 – 1.62) | 0.89 (0.60 – 1.32) | 0.96 (0.65 – 1.41) | 1.02 (0.69 – 1.49) | 0.82 |
| Poly-unsaturated Fats | ||||||
| Number of cases | 50 | 67 | 52 | 54 | 46 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.35 (0.93 – 1.95) | 1.06 (0.72 – 1.56) | 1.12 (0.76 – 1.66) | 0.98 (0.65 – 1.47) | 0.56 |
| Multivariate HR (95% CI) † | 1.0 | 1.35 (0.93 – 1.94) | 1.05 (0.71 – 1.55) | 1.10 (0.74 – 1.62) | 0.95 (0.63 – 1.42) | 0.41 |
| n-3/n-6 PUFA ratio | ||||||
| Number of cases | 47 | 65 | 56 | 62 | 39 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.38 (0.95 – 2.00) | 1.18 (0.80 – 1.74) | 1.35 (0.92 – 1.98) | 0.84 (0.55 – 1.28) | 0.27 |
| Multivariate HR (95% CI) † | 1.0 | 1.38 (0.95 – 2.01) | 1.19 (0.80 – 1.75) | 1.36 (0.93 – 1.99) | 0.85 (0.55 – 1.30) | 0.33 |
| Arachidonic acid | ||||||
| Number of cases | 61 | 54 | 52 | 53 | 49 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.90 (0.62 – 1.30) | 0.90 (0.62 – 1.31) | 0.89 (0.61 – 1.29) | 0.87 (0.59 – 1.27) | 0.48 |
| Multivariate HR (95% CI) † | 1.0 | 0.89 (0.61 – 1.28) | 0.88 (0.60 – 1.28) | 0.85 (0.58 – 1.23) | 0.80 (0.55 – 1.18) | 0.18 |
| Linoleic acid | ||||||
| Number of cases | 49 | 62 | 50 | 58 | 50 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.23 (0.85 – 1.80) | 1.01 (0.68 – 1.50) | 1.20 (0.82 – 1.77) | 1.08 (0.72 – 1.61) | 0.78 |
| Multivariate HR (95% CI) † | 1.0 | 1.24 (0.85 – 1.81) | 1.00 (0.68 – 1.49) | 1.18 (0.80 – 1.73) | 1.05 (0.70 – 1.56) | 0.97 |
| Oleic acid | ||||||
| Number of cases | 52 | 62 | 49 | 48 | 58 | |
| Age-adjusted HR (95% CI) | 1.0 | 1.18 (0.81 – 1.70) | 0.95 (0.64 – 1.40) | 0.94 (0.63 – 1.40) | 1.17 (0.80 – 1.70) | 0.73 |
| Multivariate HR (95% CI) † | 1.0 | 1.14 (0.79 – 1.65) | 0.91 (0.61 – 1.35) | 0.88 (0.59 – 1.31) | 1.06 (0.72 – 1.55) | 0.84 |
| Long-chain n-3 PUFA|| | ||||||
| Number of cases | 57 | 59 | 59 | 45 | 49 | |
| Age-adjusted HR (95% CI) | 1.0 | 0.98 (0.68 – 1.42) | 1.02 (0.71 – 1.48) | 0.78 (0.52 – 1.15) | 0.85 (0.57 – 1.24) | 0.20 |
| Multivariate HR (95% CI) † | 1.0 | 0.98 (0.68 – 1.41) | 1.01 (0.70 – 1.46) | 0.77 (0.52 – 1.13) | 0.83 (0.57 – 1.23) | 0.11 |
NSAID – non-steroidal anti-inflammatory drugs, HR – hazard ratio, CI – confidence interval
Cumulatively averaged energy-adjusted intake from 1984 (NHS I) or 1991 (NHS II)
per 100,000 person-years
Adjusted for age, cohort, smoking (never, past, current), body mass index (<20 kg/m2, 20–24.9kg/m2, 25–29 kg/m2, > 30kg/m2), oral contraceptive use (never, ever), use of post menopausal hormone therapy (premenopausal, postmenopausal hormone never user, past user, current user), regular use of NSAIDs (yes, no), regular use of aspirin (yes, no), total energy intake (quintile).
Long-chain n-3 PUFA comprises docosapentaenoic acid, eicosapentaenoic acid, docosahexaenoic acid
We then examined whether dietary intake of individual fatty acids influenced risk of CD and UC. We observed no difference in risk of UC across quintiles of intake of n-3 PUFA (HR 0.88, 95% CI 0.63 – 1.24), n-6 PUFA (HR 1.08, 95% CI 0.77 – 1.52), oleic acid (HR 1.00, 95% CI 0.70 – 1.41), arachidonic acid (HR 0.90, 95% CI 0.65 – 1.27), and linoleic acid (HR 1.04, 95% CI 0.73 – 1.48). However, intake of long-chain n-3 fatty acids (docosapentaenoic acid, eicosapentaenoic acid, docosahexaenoic acid) was inversely associated with risk of UC. Compared to those with the lowest quintile of long-chain n-3 PUFA intake, those with the highest intake had reduced incidence of UC (HR 0.72, 95%CI 0.51 – 1.02, p(trend) 0.13). Consequently, the incidence of UC decreased across quintiles of the ratio of total n-3/n-6 PUFA intake. Compared to the lowest quintile of n-3/n-6 PUFA ratio, women in the highest quintile of n-3/n-6 PUFA intake had a multivariate HR 0.69 (95% CI 0.49 – 0.98; p(linear trend) 0.03) for UC (Table 2). In contrast, intake of each of the individual fatty acids was not associated with risk of CD (Table 3).
In a sensitivity analysis, our findings were robust to introduction of a lag of 2 questionnaire cycles (4 years) between dietary exposure and development of disease. We did not observe a statistically significant interaction between dietary fat intake and the other known risk factors including smoking, oral contraceptive, post-menopausal hormone use, or body mass index. Our findings were also not materially altered after adjustment for total carbohydrate, protein, and dietary fiber intake in the multivariate model.
Supplementary Figure 1 describes the flow chart of records reviewed for the systematic review. A total of 266 records were initially identified. After exclusion of studies that were deemed not relevant based upon review of the title or abstract, or did not include original epidemiologic data (e.g. review articles, editorials, pre-clinical laboratory studies), we reviewed a total of 21 full-text articles. Among these articles, we included 15 in our systematic review (Supplemental Tables 1 and 2). There was substantial heterogeneity between the studies. Nearly all were case-control studies with retrospective ascertainment of pre-illness diet, which is more prone to bias. Only one study, the EPIC cohort, was prospective in assessment of diet and incident disease. Furthermore, the different studies used varying groupings, particularly for individual fatty acids, and employed different cut-offs for definition of high compared to low intake. Due to this substantial variation in study quality, exposure, and outcomes, we did not quantitatively synthesize the data using a meta-analytic approach.
DISCUSSION
In two large cohorts of women with prospectively collected, updated dietary data using a validated FFQ, we demonstrate that long-term intake of total fat, saturated fats, unsaturated fats (mono or polyunsaturated) were not associated with risk of CD or UC. However, we did observe an inverse association between greater long-term intake of long-chain n-3 PUFA and risk of UC, and a trend towards increased risk of UC associated with high intake of trans-unsaturated fatty acids.
To our knowledge, our study is the first to prospectively examine the association between dietary fat and risk of CD. There has been conflicting data on the association between dietary fat and UC as summarized by recent reviews 16, 17. Most prior studies have been retrospective, including a small number of cases. Consistent with our findings, most of these studies did not observe a statistically significant elevation in disease risk with total fat intake19, 43–45. Although a few small studies have suggested an association between MUFA intake and UC, 43, 44, 46 these results were not corroborated by larger studies 19. Similarly, previous studies have variably demonstrated an association with intake of arachidonic acid18, linoleic acid21, or total fat intake46. In our analysis, we found no association between total PUFA, MUFA, saturated fat intake and risk of UC. However, trans-unsaturated fatty acid intake appeared to be associated with an increased risk of UC but not CD. Considerable biologic plausibility supports an association between trans-unsaturated fat intake and systemic inflammation47. The association with various adverse cardiovascular outcomes, coronary heart disease and diabetes has been well recognized48. However the risk attributable to trans-fat intake is higher than that suggested by effect on lipid profile alone. In healthy women, trans-unsaturated fat intake is associated with increased tumor necrosis factor (TNF) α levels, higher levels of TNF receptors 1 and 247, 49, C-reactive protein, and interleukin-6 (IL-6)50, all key factors in the pathogenesis of IBD. In addition, trans-unsaturated fat intake has been associated with increased levels of soluble adhesion molecules, which are markers of endothelial dysfunction and are associated with the pathogenesis of IBD47, 50.
The only prior prospective analysis of dietary fat and IBD was a case-control study of UC nested with the EPIC cohort. Tjonneland et al. suggested an inverse association between higher intake of linoleic acid and DHA, a specific n-3 PUFA, and UC21. Other n-3 PUFAs, including α-linoleic acid, EPA and oleic acid were not associated with risk of UC. In contrast to these findings, in our analysis total n-3 PUFA or linoleic acid intake alone did not influence risk of UC. However, we found that the effect of n-3 PUFA was primarily driven by long-chain n-3 fatty acids including EPA and DHA. Consequent to the inverse association with long-chain n-3 PUFA, we observed that the ratio of n-3/n-6 PUFA intake was inversely associated with disease risk. However, the lack of association with n-6 PUFA intake quintiles, and the wealth of evidence supporting the absence of pro-inflammatory effect of n-6 PUFA13, 14 suggest that the primary factor behind this positive association is the protective effect of long-chain n-3 PUFA intake.
Several reasons could account for the differences in our results with those of the EPIC cohort. First, our analysis assessed diet using a validated FFQ whereas EPIC used a 7 day diet recall, with the former being a more stable estimate of long-term diet. Second, we updated our dietary assessment with FFQs administered every 4 years, which likely better accounts for changes in diet over time than assessment at a single time point. Third, the EPIC analyses were limited by a small number of cases18, 20 with relatively low power to detect weaker associations. Fourth, the median age of diagnosis in our cohort was nearly a decade younger than the EPIC cohort. Finally, we simultaneously collected detailed information on a wider spectrum of potentially significant confounding variables than prior analyses that were only able to account for the influence of smoking and body mass index.
The results of the present study are largely consistent with the results of our systematic review, and further strengthen some associations. Two cohort studies including ours support the inverse association between total n-3 PUFA or specifically long-chain n-3 PUFA and risk of ulcerative colitis20. In contrast, the studies that found no effect of n-3 PUFA on UC risk were small case control studies subject to biases in ascertainment of pre-illness diet46. Other weak associations proposed by prior studies including total fat, mono-unsaturated or poly-unsaturated fat all failed to meet statistical significance in high quality prospective cohort studies, suggesting that total intake of fat, MUFA, or PUFA is unlikely to play a role in the pathogenesis of CD or UC. We identified conflicting results in the literature regarding the possible role of linoleic (or total n-6 PUFA intake) with studies both supporting and refuting this association. Nevertheless, we failed to find a statistically significant effect in our cohort, the largest reported to date examining this association in women.
Several mechanisms may explain how long-chain n-3 PUFA may influence risk of UC9, 51. First, long-chain n-3 PUFA acting as a competitive substrate, decreases production of the eicosanoids from arachidonic acid, and reduces membrane levels of leukotriene B4. Furthermore, long-chain n-3 PUFA affects cell membrane structure and inhibits dimerization and activation of the toll-like receptor (TLR-4) which is important in mediating intestinal inflammation52. In mouse models, administration of n-3 PUFA before induction of colitis appears to protect against the development of colitis51. Second, dietary long-chain n-3 PUFA inhibits vascular adhesion molecule expression in contrast to arachidonic acid which increases ICAM-1 expression9. Third, long-chain n-3 PUFA also modulates the adaptive immune response by inhibiting T-cell proliferation and antigen presentation and binding to nuclear receptors which function as lipid sensors regulates PPARγ mediated NF-κB activation9, 51.
In contrast to our results demonstrating an inverse association between long-chain n-3 PUFA intake and incidence of UC, randomized controlled trials of fish oil or n-3 PUFA in patients with established IBD have mostly failed to demonstrate a benefit in inducing or maintaining remission53, 54. Although the CD trials have generally included a larger number of patients, UC trials have been mostly limited to small cohorts15, 42. In addition, many trials did not account for consumption of other fatty acids and/or utilized oleic acid as a placebo control which itself may have a protective effect against inflammation. In support of this explanation, a recent Japanese study showed that a dietary intervention focused on lowering n-6/n-3 PUFA ratio was effective in maintain disease remission in patients with IBD55, possibly through increasing n-3 PUFA intake. Finally, it is plausible that n-3 PUFAs could influence disease onset yet have no effect on disease remission or progression. In experimental models, the timing of exposure to n-3 PUFA on pro-inflammatory phenotypes appears to be critical; pre-treatment of cultured human endothelial cells with DHA reduced eventual IL-1 production whereas treatment of the activated endothelial cells with DHA did not have the same effect56.
There are several strengths to our study. First, we used prospective assessment of diet through a validated FFQ, perhaps the best validated measure of long-term diet. Second, we updated our dietary information every 4 years, accounting for the inevitable temporal changes in diet that occurred over 26 years of follow-up. Third, we confirmed all cases of CD and UC through detailed medical record review by board certified gastroenterologists blinded to the exposures. Fourth, owing to the large number of cases of CD and UC included in our study, we had sufficient power to adjust for a number of potentially relevant confounding variables and demonstrate the robustness of our results. Fifth, the medical background of our participants likely increases the accuracy of self-reported exposures and confounders. Last, the prospective cohort design of our study allowed us to estimate the absolute as well as relative risk associated with intake of total fat and individual fatty acids.
There are a few limitations to our study. First, our cohort consisted entirely of female health professionals, most of whom were Caucasian. However, there are limited data to support a differential effect of diet on risk of IBD according to gender, race, or profession. Prior analyses of our cohorts have yielded associations consistent with those identified in other cohorts, and the overall incidence of disease is comparable to other population-based cohorts36. Second, although we were able to account for several potential confounding factors, our study is observational and unable to confirm causality. However, the consistency of our results and the wealth of laboratory data supporting plausible biologic mechanisms for the protective influence of long-chain n-3 PUFA and the ratio of n-3/n-6 PUFA intake and UC suggest our findings are unlikely to be fully explained by unmeasured confounding. Given the relatively low incidence of UC in the general population and the modest effects observed in our cohort for long-chain n-3 PUFA intake, it is unlikely that recommendations to increase consumption of long-chain n-3 PUFA for primary prevention of UC will have a substantial public health impact.
In conclusion, using two large prospective cohorts of women, we demonstrate that total fat, saturated or unsaturated fat, or individual PUFA did not influence risk of CD. However, our results suggest that women in the highest quintile of long-term dietary intake of long-chain n-3 PUFA may have a significantly reduced risk while those with high trans-saturated fat intake may have an increased risk of UC. Our findings support experimental data demonstrating the importance of n-3 PUFA in modulating the production of inflammatory mediators such as prostaglandins and leukotrienes, maintenance of the intestinal barrier, regulation of the adaptive immune response, and immune cell adhesion and trafficking. Further studies are needed to confirm our results and explore the potential of modifying fatty acid intake in the prevention or treatment of UC.
Supplementary Material
What is already known about the subject?
The pathogenesis of inflammatory bowel diseases is influenced by genetics, environment and the gut microbiome.
Diet may influence risk of Crohn’s disease (CD) and ulcerative colitis (UC).
Literature examining this association has been limited by retrospective studies, small sample sizes, and conflicting results.
What are the new findings?
Our study supports an association of higher long-term intake of long chain n-3 fatty acids with a lower risk of UC.
Greater intake of trans-unsaturated fatty acids is associated with an increased risk of UC.
Dietary fat as well as intake of specific fatty acids do not appear associated with risk of CD.
How might it impact on clinical practice in the foreseeable future?
Intervention studies specifically focusing on increasing intake of long-chain n-3 fatty acids in modulation of disease activity in ulcerative colitis are warranted.
Acknowledgments
Grant support:
This work was supported by a Research Scholars Award of the American Gastroenterological Association (A.N.A.), Crohn’s and Colitis Foundation of America (H.K.), the Broad Medical Research Program of the Broad Foundation (A.T.C), and the National Institutes of Health (K24 DK098311, P01 CA87969, P30 DK043351, K08 DK064256, K23 DK091742, K23 DK099681, UM1 CA176276).
The authors thank the dedication of the Nurses’ Health Study I and II participants and members of Channing Division of Network Medicine.
Footnotes
The research presented in this manuscript is original. The contents of this article are solely the responsibility of the authors. The American Gastroenterological Association the Broad Medical Research Foundation, and the NIH had no role in the collection, management, analysis, or interpretation of the data and had no role in the preparation, review, or approval of the manuscript.
Disclosures
Ashwin N. Ananthakrishnan – Scientific advisory board for Prometheus Inc. and Janssen Inc.
Hamed Khalili – none
Gauree G Konijeti - none
Leslie M. Higuchi- none
Charles S. Fuchs- none
Walter C. Willett-none
James M. Richter- Consultant for Policy Analysis, Inc.
Andrew T. Chan- Consultant for Bayer HealthCare, Millennium Pharmaceuticals, Pfizer Inc., Pozen Inc.
Author Contributions
Ashwin N. Ananthakrishnan - study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision.
Hamed Khalili - acquisition of data; analysis and interpretation of data; critical revision of the manuscript for important intellectual content.
Gauree G Konijeti - acquisition of data; critical revision of the manuscript for important intellectual content
Leslie M. Higuchi- acquisition of data; critical revision of the manuscript for important intellectual content.
Punyanganie de Silva - acquisition of data; critical revision of the manuscript for important intellectual content.
Charles S. Fuchs- study concept and design; critical revision of the manuscript for important intellectual content; study supervision.
Walter C. Willett - study concept and design; acquisition of data; critical revision of the manuscript for important intellectual content;
James M. Richter- study concept and design, acquisition of data; critical revision of the manuscript for important intellectual content.
Andrew T. Chan- study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis; obtained funding; study supervision
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307–17. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern D, Hun KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amineinejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Cohain A, Cichon S, D’Amato M, De Jong DJ, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Gieger C, Karlsen J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro MD, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor K, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, SZ, Zhang B, Zhang CK, Zhao H, Consortium TIIG, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly M, Franke A, Parkes M, Vermeire S, Barret JC, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012 doi: 10.1038/nature11582. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentschew L, Ferguson LR. Role of nutrition and microbiota in susceptibility to inflammatory bowel diseases. Mol Nutr Food Res. 2012;56:524–35. doi: 10.1002/mnfr.201100630. [DOI] [PubMed] [Google Scholar]
- 5.Bibiloni R, Mangold M, Madsen KL, Fedorak RN, Tannock GW. The bacteriology of biopsies differs between newly diagnosed, untreated, Crohn’s disease and ulcerative colitis patients. J Med Microbiol. 2006;55:1141–9. doi: 10.1099/jmm.0.46498-0. [DOI] [PubMed] [Google Scholar]
- 6.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asakura H, Suzuki K, Kitahora T, Morizane T. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? J Gastroenterol Hepatol. 2008;23:1794–801. doi: 10.1111/j.1440-1746.2008.05681.x. [DOI] [PubMed] [Google Scholar]
- 8.Thia KT, Loftus EV, Jr, Sandborn WJ, Yang SK. An update on the epidemiology of inflammatory bowel disease in Asia. Am J Gastroenterol. 2008;103:3167–82. doi: 10.1111/j.1572-0241.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 9.Marion-Letellier R, Savoye G, Beck PL, Panaccione R, Ghosh S. Polyunsaturated Fatty Acids in Inflammatory Bowel Diseases: A Reappraisal of Effects and Therapeutic Approaches. Inflamm Bowel Dis. 2013 doi: 10.1097/MIB.0b013e3182810122. [DOI] [PubMed] [Google Scholar]
- 10.Hjerkinn EM, Seljeflot I, Ellingsen I, Berstad P, Hjermann I, Sandvik L, Arnesen H. Influence of long-term intervention with dietary counseling, long-chain n-3 fatty acid supplements, or both on circulating markers of endothelial activation in men with long-standing hyperlipidemia. Am J Clin Nutr. 2005;81:583–9. doi: 10.1093/ajcn/81.3.583. [DOI] [PubMed] [Google Scholar]
- 11.Yang Y, Lu N, Chen D, Meng L, Zheng Y, Hui R. Effects of n-3 PUFA supplementation on plasma soluble adhesion molecules: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;95:972–80. doi: 10.3945/ajcn.111.025924. [DOI] [PubMed] [Google Scholar]
- 12.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 13.Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–41. 1041 e1–15. doi: 10.1016/j.jand.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Fritsche KL. Too much linoleic acid promotes inflammation-doesn’t it? Prostaglandins Leukot Essent Fatty Acids. 2008;79:173–5. doi: 10.1016/j.plefa.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Cabre E, Domenech E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J Gastroenterol. 2012;18:3814–22. doi: 10.3748/wjg.v18.i29.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman-Kiddell CA, Davies PS, Gillen L, Radford-Smith GL. Role of diet in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:137–51. doi: 10.1002/ibd.20968. [DOI] [PubMed] [Google Scholar]
- 17.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–73. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 18.de Silva PS, Olsen A, Christensen J, Schmidt EB, Overvaad K, Tjonneland A, Hart AR. An association between dietary arachidonic acid, measured in adipose tissue, and ulcerative colitis. Gastroenterology. 2010;139:1912–7. doi: 10.1053/j.gastro.2010.07.065. [DOI] [PubMed] [Google Scholar]
- 19.Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T, Appleby P, Bergmann MM, Boeing H, Hallmans G, Danielsson A, Palmqvist R, Sjodin H, Hagglund G, Overvad K, Palli D, Masala G, Riboli E, Kennedy H, Welch A, Khaw KT, Day N, Bingham S. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77:57–64. doi: 10.1159/000121412. [DOI] [PubMed] [Google Scholar]
- 20.John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010;22:602–6. doi: 10.1097/MEG.0b013e3283352d05. [DOI] [PubMed] [Google Scholar]
- 21.Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G, Lindgren S, Grip O, Palli D, Day NE, Khaw KT, Bingham S, Riboli E, Kennedy H, Hart A. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut. 2009;58:1606–11. doi: 10.1136/gut.2008.169078. [DOI] [PubMed] [Google Scholar]
- 22.Chiuve SE, Rimm EB, Manson JE, Whang W, Mozaffarian D, Stampfer MJ, Willett WC, Albert CM. Intake of total trans, trans-18:1, and trans-18:2 fatty acids and risk of sudden cardiac death in women. Am Heart J. 2009;158:761–7. doi: 10.1016/j.ahj.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiuve SE, Rimm EB, Sandhu RK, Bernstein AM, Rexrode KM, Manson JE, Willett WC, Albert CM. Dietary fat quality and risk of sudden cardiac death in women. Am J Clin Nutr. 2012;96:498–507. doi: 10.3945/ajcn.112.040287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui X, Rosner B, Willett WC, Hankinson SE. Dietary fat, fiber, and carbohydrate intake in relation to risk of endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:978–89. doi: 10.1158/1055-9965.EPI-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garland M, Sacks FM, Colditz GA, Rimm EB, Sampson LA, Willett WC, Hunter DJ. The relation between dietary intake and adipose tissue composition of selected fatty acids in US women. Am J Clin Nutr. 1998;67:25–30. doi: 10.1093/ajcn/67.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Lucas M, Mirzaei F, O’Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. Am J Clin Nutr. 2011;93:1337–43. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Missmer SA, Chavarro JE, Malspeis S, Bertone-Johnson ER, Hornstein MD, Spiegelman D, Barbieri RL, Willett WC, Hankinson SE. A prospective study of dietary fat consumption and endometriosis risk. Hum Reprod. 2010;25:1528–35. doi: 10.1093/humrep/deq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. doi: 10.1093/ajcn/86.1.74. [DOI] [PubMed] [Google Scholar]
- 29.London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54:340–5. doi: 10.1093/ajcn/54.2.340. [DOI] [PubMed] [Google Scholar]
- 30.Ananthakrishnan AN, Higuchi LM, Huang ES, Khalili H, Richter JM, Fuchs CS, Chan AT. Aspirin, Nonsteroidal Anti-inflammatory Drug Use, and Risk for Crohn Disease and Ulcerative Colitis: A Cohort Study. Ann Intern Med. 2012;156:350–9. doi: 10.1059/0003-4819-156-5-201203060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ananthakrishnan AN, Khalili H, Higuchi LM, Bao Y, Korzenik JR, Giovannucci EL, Richter JM, Fuchs CS, Chan AT. Higher predicted vitamin d status is associated with reduced risk of Crohn’s disease. Gastroenterology. 2012;142:482–9. doi: 10.1053/j.gastro.2011.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ananthakrishnan AN, Khalili H, Pan A, Higuchi LM, de Silva P, Richter JM, Fuchs CS, Chan AT. Association between depressive symptoms and incidence of Crohn’s disease and ulcerative colitis: results from the nurses’ health study. Clin Gastroenterol Hepatol. 2013;11:57–62. doi: 10.1016/j.cgh.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A Prospective Study of Cigarette Smoking and the Risk of Inflammatory Bowel Disease in Women. American Journal of Gastroenterology. 2012;107:1399–406. doi: 10.1038/ajg.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalili H, Higuchi LM, Ananthakrishnan AN, Manson JE, Feskanich D, Richter JM, Fuchs CS, Chan AT. Hormone Therapy Increases Risk of Ulcerative Colitis but not Crohn’s Disease. Gastroenterology. 2012;143:1199–206. doi: 10.1053/j.gastro.2012.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khalili H, Higuchi LM, Ananthakrishnan AN, Richter JM, Feskanich D, Fuchs CS, Chan AT. Oral Contraceptives, Reproductive Factors, and Risk of Inflammatory Bowel Disease. Gut. 2012 doi: 10.1136/gutjnl-2012-302362. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, Chan AT. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61:1686–92. doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 38.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–94. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 39.Sands BE. From symptom to diagnosis: clinical distinctions among various forms of intestinal inflammation. Gastroenterology. 2004;126:1518–32. doi: 10.1053/j.gastro.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 40.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 41.Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, Takeyama Y, Morino Y, Yamauchi T, Muramatsu T, Hibi K, Terashima M, Michishita I. Effects of serum n-3 to n-6 polyunsaturated fatty acids ratios on coronary atherosclerosis in statin-treated patients with coronary artery disease. Am J Cardiol. 2013;111:6–11. doi: 10.1016/j.amjcard.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Cabre E, Manosa M, Gassull MA. Omega-3 fatty acids and inflammatory bowel diseases - a systematic review. Br J Nutr. 2012;107 (Suppl 2):S240–52. doi: 10.1017/S0007114512001626. [DOI] [PubMed] [Google Scholar]
- 43.Geerling BJ, Dagnelie PC, Badart-Smook A, Russel MG, Stockbrugger RW, Brummer RJ. Diet as a risk factor for the development of ulcerative colitis. Am J Gastroenterol. 2000;95:1008–13. doi: 10.1111/j.1572-0241.2000.01942.x. [DOI] [PubMed] [Google Scholar]
- 44.Reif S, Klein I, Lubin F, Farbstein M, Hallak A, Gilat T. Pre-illness dietary factors in inflammatory bowel disease. Gut. 1997;40:754–60. doi: 10.1136/gut.40.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am J Gastroenterol. 2010;105:2195–201. doi: 10.1038/ajg.2010.192. [DOI] [PubMed] [Google Scholar]
- 46.Sakamoto N, Kono S, Wakai K, Fukuda Y, Satomi M, Shimoyama T, Inaba Y, Miyake Y, Sasaki S, Okamoto K, Kobashi G, Washio M, Yokoyama T, Date C, Tanaka H. Dietary risk factors for inflammatory bowel disease: a multicenter case-control study in Japan. Inflamm Bowel Dis. 2005;11:154–63. doi: 10.1097/00054725-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Mozaffarian D. Trans fatty acids - effects on systemic inflammation and endothelial function. Atheroscler Suppl. 2006;7:29–32. doi: 10.1016/j.atherosclerosissup.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 48.Teegala SM, Willett WC, Mozaffarian D. Consumption and health effects of trans fatty acids: a review. J AOAC Int. 2009;92:1250–7. [PubMed] [Google Scholar]
- 49.Mozaffarian D, Rimm EB, King IB, Lawler RL, McDonald GB, Levy WC. trans fatty acids and systemic inflammation in heart failure. Am J Clin Nutr. 2004;80:1521–5. doi: 10.1093/ajcn/80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–6. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 51.Shores DR, Binion DG, Freeman BA, Baker PR. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis. 2010;17:2192–204. doi: 10.1002/ibd.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J Immunol. 2005;174:5390–7. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 53.Feagan BG, Sandborn WJ, Mittmann U, Bar-Meir S, D’Haens G, Bradette M, Cohen A, Dallaire C, Ponich TP, McDonald JW, Hebuterne X, Pare P, Klvana P, Niv Y, Ardizzone S, Alexeeva O, Rostom A, Kiudelis G, Spleiss J, Gilgen D, Vandervoort MK, Wong CJ, Zou GY, Donner A, Rutgeerts P. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: the EPIC Randomized Controlled Trials. Jama. 2008;299:1690–7. doi: 10.1001/jama.299.14.1690. [DOI] [PubMed] [Google Scholar]
- 54.Turner D, Shah PS, Steinhart AH, Zlotkin S, Griffiths AM. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): a systematic review and meta-analyses. Inflamm Bowel Dis. 2011;17:336–45. doi: 10.1002/ibd.21374. [DOI] [PubMed] [Google Scholar]
- 55.Uchiyama K, Nakamura M, Odahara S, Koido S, Katahira K, Shiraishi H, Ohkusa T, Fujise K, Tajiri H. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:1696–707. doi: 10.1002/ibd.21251. [DOI] [PubMed] [Google Scholar]
- 56.Collie-Duguid ES, Wahle KW. Inhibitory effect of fish oil N-3 polyunsaturated fatty acids on the expression of endothelial cell adhesion molecules. Biochem Biophys Res Commun. 1996;220:969–74. doi: 10.1006/bbrc.1996.0516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


