Abstract
Background:
Sedentary behaviour is ubiquitous in modern society. Emerging studies have focused on the health consequences of sedentary behaviour, including colorectal cancer, but whether sedentary behaviour is associated with the risks of colon and rectal cancer remains unclear. No systematic reviews have applied quantitative techniques to independently compute summary risk estimates. We aimed to conduct a meta-analysis to investigate this issue.
Methods:
We searched PubMed, Embase, and Google Scholar databases up to May 2013 to identify cohort and case–control studies that evaluated the association between sedentary behaviour and colon or rectal cancer. A random-effect model was used to pool the results of included studies. Publication bias was assessed by using Begg's funnel plot.
Results:
Twenty-three studies with 63 reports were included in our meta-analysis. These groups included 4 324 462 participants (27 231 colon cancer cases and 13 813 rectal cancer cases). Sedentary behaviour was significantly associated with colon cancer (relative risk (RR): 1.30, 95% confidence interval (CI): 1.22–1.39) but did not have a statistically significant association with rectal cancer (RR 1.05, 95% CI, 0.98–1.13). Subgroup analyses suggested that the odds ratio (OR) of colon cancer was 1.46 (95% CI: 1.22–1.68) in the case–control studies, and the RR was 1.27 (95% CI: 1.18–1.36) in the cohort studies, the OR of rectal cancer was 1.06 (95% CI: 0.85–1.33) in the case–control studies, and the RR was 1.06 (95% CI, 1.01–1.12) in the cohort studies.
Conclusion:
Sedentary behaviour is associated with an increased risk of colon cancer. Subgroup analyses suggest a positive association between sedentary behaviour and risk of rectal cancer in cohort studies. Reducing sedentary behaviour is potentially important for the prevention of colorectal cancer.
Keywords: sedentary behaviour, colon cancer, rectal cancer, meta-analysis
Colorectal cancer is the third most common cancer in men and the second in women worldwide and accounts for eight percent of all cancer deaths (Fredriksson et al, 1989; Organization, 2011). It has been proposed that the risk associated with a sedentary lifestyle could be explained by hyperinsulinism or insulin resistance, which might stimulate the growth of colonic cancer cells (McKeown-Eyssen, 1994; Giovannucci, 1995).
Sedentary behaviour, which is distinctly different from physical inactivity, is defined as activities that are done sitting or in reclining posture that expend <1.5 times the basal metabolic rate. It is characterised by prolonged sitting or lying down and absence of whole body movement, such as watching television, desk-bound work, using computer and game-consoles, sitting at work, and sitting in automobiles. (Pate et al, 2008; Owen et al, 2010; Wilmot et al, 2012). Several studies have demonstrated that sedentary behaviour is an independent risk factor for health problems and diseases. Sedentary behaviour may reduce overall energy expenditure (Brown et al, 2009), and it has been positively associated with obesity (Wijndaele et al, 2010), weight gain (Blanck et al, 2007), diabetes, cardiovascular disease (Katzmarzyk et al, 2009; Dunstan et al, 2010; Wijndaele et al, 2011), and prostate, ovarian, breast, and endometrial cancers (Mathew et al, 2009; George et al, 2010; Lynch, 2010). In 2012, a meta-analysis showed that people who spend the higher amounts of time in sedentary behaviours have greater odds of having metabolic syndrome (Edwardson et al, 2012). Another review focused on the association between prolonged TV viewing in adults and risk of type 2 diabetes and cardiovascular disease(Grontved and Hu, 2011).
Some likely biological mechanisms have been put forth to suggest why sedentary behaviour may contribute to the development and progression of colorectal cancer risk, which included adiposity accumulation and metabolic dysfunction. Sedentary behaviour might result in adiposity, which is likely an independent contributor to colorectal cancer and a mediating variable on the other pathways. Colorectal carcinogenesis may be promoted by increased levels of sex hormones, insulin resistance, chronic inflammation, and altered secretion of adipokines (Neilson et al, 2009; Lynch, 2010), which are also mediating pathways of adiposity facilitating colorectal carcinogenesis.
Nowadays, people spend 7.7 h in sedentary behaviours every day, and this number may continue to rise (Matthews et al, 2008). Recently, there has been an increase in the number of studies assessing the association between sedentary behaviour and colon or rectal cancer. However, the results are inconsistent. Two previous systematic reviews (Wolin et al, 2009; Boyle et al, 2012) focused only on the association between physical activity and colon cancer and did not independently analyse the association between sedentary behaviour and colon and rectal cancers. Another review(Boyle, 2012) summarised the association between sedentary behaviour and colon cancer but did not use quantitative techniques to compute summary risk estimates. Thus, we conducted a meta-analysis to evaluate the relationship between sedentary behaviour and colon and rectal cancer.
Materials and methods
Search strategy
Our meta-analysis was conducted according to the checklist of the Meta-analysis of Observational Studies in Epidemiology(Stroup et al, 2000). PubMed, Embase, and Google Scholar were searched up to May 2013. Text word, title word, abstract and subject headings were searched to cover sedentary behaviours and colon and rectal cancer. We used ‘sedentary' or ‘sitting' or ‘occupational sitting time' or ‘occupational work' or ‘TV' ‘television' or ‘screen time' or ‘computer and game-console use' or ‘car driving' combined with ‘colon' or ‘colorectal' or ‘rectum' or ‘rectal' or ‘bowel' and ‘cancer' or ‘neoplasm' or ‘carcinoma' as the search terms. In addition, we reviewed the reference lists of retrieved articles to identify any studies that were not identified from the preliminary literature searches.
Inclusion criteria
Studies were considered eligible if they met the following criteria: (1) the study was a case–control or cohort study design, (2) was published in English, (3) was self-reported or reported a job title-based or objective measure of sedentary behaviour, (4) the study reported the relative risk (RR) or odds ratio (OR) with 95% confidence intervals (CIs) for the association between sedentary behaviour and colon and/or rectal cancer.
Data extraction
We extracted the following information from each retrieved article: name of the first author, date and country of study, study design, characteristics of study participants at baseline, duration of follow-up, number of cases, definition and measurement of sedentary behaviour, outcomes, RR/OR (corresponding 95% CI), and confounding factors that were adjusted in the analysis. Data extraction was conducted independently by two authors (YJC and YG), and any disagreements were resolved by discussion.
Quality assessment
We used a quality assessment tool with reference to MOOSE (Stroup et al, 2000), QATSO (Wong et al, 2008), and STROBE (von Elm et al, 2007). The total score available was six points (one point for a prospective study design; two points if a self-reported measure of time spent in sedentary behaviour or classification of job title-based was used; one point if two or more demographic confounders were controlled for in the analysis; one point if analyses controlled for physical activity; and one point for an objective measure of the health outcome). We assigned scores of 0–2, 3–4, and 5–6 for low, moderate, and high quality of studies, respectively. Each study was rated independently by two authors (YJC and YG), with ratings reported in Table 1.
Table 1. Characteristics of studies included in meta-analysis.
| Source | Design and Study Location | Study participants | No. cases | Age at baseline, years | Sedentary behaviour measurement mode | Sedentary measure used in meta-analysis | Adjustment for confounders | Quality assessment |
|---|---|---|---|---|---|---|---|---|
|
Garabrant et al, 1984 |
Cohort, USA |
4163 men |
326 C; 104 R |
20–64 |
Job title-based |
Sedentary work vs high occupational activity |
Adjusted uniformly within site for cases with unreported occupation |
4 |
|
Weiderpass et al, 2003 |
Cohort, Finland |
892 591 women |
NA |
25–65 |
Job title-based |
Sedentary work vs physical work |
Turnover rate |
4 |
|
Moradi et al, 2008 |
Cohort, Sweden |
922 266 men and women |
2000 C(W); 5900 C(M); 1122 R(W); 4206 R(M) |
NA |
Self-reported |
Sedentary work vs very high/high occupational activity |
Age, place of residence and socioeconomic status |
4 |
|
Howard et al, 2008 |
Cohort, USA |
488 720 men and women |
3240 C(M); 1482 C(W) |
50–71 |
Self-reported |
>9+vs<3 h in spent watching TV or videos (hours/day) |
Age, smoking, alcohol consumption, education, race, family history of colon cancer, total energy,fruit and vegetables intake, total physical activity, BMI |
6 |
|
Gerhardsson et al, 1986 |
Cohort, Sweden |
1 223 908 men |
5100 C; 4533 R |
20–64 |
Job title-based |
>50% vs <50% time in sitting work |
Age, density, social class |
5 |
|
Fraser and Pearce, 1993 |
Cohort, New Zealand |
2503 men |
180 C; 430 R |
15–64 |
Job title-based |
Sedentary work vs physical work |
Unadjusted |
3 |
|
Thune and Lund, 1996 |
Cohort, Norway |
81 243 men and women |
99 C(W); 236 C(M); 58 R(W); 170 R(M) |
Men median 58.1 women median 54.6 |
Self-reported |
Sedentary vs standing work occupational |
Age, geographic region and BMI |
5 |
|
Thune and Lund, 1996 |
Cohort, Norway |
81 243 men and women |
99 C(W); 236 C(M); 58 R(W); 170 R(M) |
Men median 58.1 women median 54.6 |
Self-reported |
Sedentary vs moderate activity recreational |
Age, geographic region and BMI |
5 |
|
Colbert et al, 2001 |
Cohort, Finland |
29 133 men |
152 C; 104 R |
50–69 |
Self-administered |
Sedentary work vs light work occupational |
Age, supplement group, BMI, and smoking |
5 |
|
Colbert et al, 2001 |
Cohort, Finland |
29 133 men |
152 C; 104 R |
50–69 |
Self-administered |
Sedentary work vs active recreational |
Age, supplement group, BMI, and smoking |
5 |
|
Johnsen et al, 2006 |
Cohort, Danish |
54 478 men and women |
140 C(W); 157 C(M) |
50–64 |
Self-reported |
sitting work vs standing work |
Sports, cycling, walking, gardening, housework, do-it-self, BMI, education, NSAID, present use of HRT, smoking and intake of total energy, fat, dietary fibre, red meat and alcohol |
6 |
|
Friedenreich et al, 2006 |
Cohort, International |
413 044 men and women |
1094 C; 599 R |
51.9 (10.00) |
Self-administered |
Sitting work vs standing work |
Age and centre and energy, education, smoking, height, weight ), fibre, and fish intake |
5 |
|
Simons et al, 2013 |
Cohort, The Netherlands |
4416 men and women |
1109 C(W); 1165 C(M); 464 R(M) |
Men:61.3 (4.2) women: 61.4 (4.3) |
Self-reported |
Occupational sitting hours of <2 vs 6–8 h/day |
Age, family history of colorectal cancer, smoking status, alcohol intake, BMI, meat intake, processed meat intake, and total energy intake |
5 |
|
Campbell et al, 2013 |
Cohort, USA |
184 194 men and women |
1664 C; 598 R |
NA |
Self-reported |
Leisure time spending sitting hours <3vs ⩾6 h/day |
Age,education, BMI, smoking, red meat intake, recreational physical activity, and tumour stage |
6 |
|
Vetter et al, 1992 |
Case–control, Turkey |
471 men and women |
87 C |
14–97 |
Job title-based |
<2 h vs >6 h in spent sitting work |
Age, smoking |
4 |
|
Arbman et al, 1993 |
Case–control, Sweden |
1172 men and women |
98 C; 79 R |
40–75 |
Self-reported |
0 vs ⩾20years in sedentary work |
Age |
3 |
|
Peters et al, 1989 |
Case–control, USA |
294 men and women |
41 R(M) |
25–45 |
Job title-based |
More than 80% of the time on the occupational job |
Age, education |
4 |
|
Whittemore et al, 1990 |
Case–control, USA, |
1665 men and women in America |
179 C(M); 105 R(M); 114 C(W); 75 R(W); |
20–79 |
Interview |
⩾10 h vs<5 h sitting per day |
Unadjusted |
3 |
|
Whittemore et al, 1990 |
Case–control, China |
1728 men and women in China |
95 C(M); 131 R(M); 78 C(W); 128 R(W) |
20–80 |
Interview |
⩾10 h vs<6 h sitting per day |
Unadjusted |
3 |
|
Boyle et al, 2011 |
Case–control, Australia |
1848 men and women |
534 C; 318 R |
40–79 |
Job title-based |
0 vs ⩾10years in sedentary work |
Age, sex, lifetime recreational physical activity level, cigarette smoking (pack-year tertiles), diabetes, educational level, energy intake from food, alcohol intake, BMI and socioeconomic status |
5 |
|
Dosemeci et al, 1993 |
Case–control, Turkey |
6236 men and women |
93 C; 102 R |
<55 |
Job title-based |
<2 h vs >6 h/day in sitting work |
Age, smoking, socioeconomic status |
4 |
|
Levi et al, 1999 |
Case–control, Sweden |
714 men and women |
119 C; 104 R |
27–74 |
Interview |
Sitting work vs standing work |
Sex age education, and intake of total alcohol and energy |
4 |
|
Tavani et al, 1999 |
Case–control, Italian |
5379 men and women |
688 C(M); 537 C(W) |
19–74 |
Interview |
Sitting work vs standing work |
Terms for centre, age, education and intake of total alcohol and energy |
4 |
|
Tang et al, 1999 |
Case–control, Taiwan |
326 men and women |
27 C(W); 43 C(M); 44 R(W); 49 R(M) |
33–81 |
Interview |
Sedentary vs active leisure-time physical activity |
Total calories, dietary fibre, total vegetable protein and water intake, smoking(men only) alcohol drinking (men only) |
4 |
| Parent et al, 2011 | Case–control, Canada | 4264 men | 496 C; 249 R | Case 58.9(8.01) control 59.6(7.92) | Interview | 75%+s vs <75% of work years was spent in sedentary job | Age, socio-economic status, educational level, ethnicity, respondent status, smoking, BMI, sports and outdoor activities, coffee, tea, beer, alcohol, farming, β-carotene, asbestos, silica, aromatic amines | 5 |
Abbreviations: BMI=body mass index; C=colon; M=man; NA=not available; R=rectal; W=women; USA=United States.Age presented the range with mean (s.d.)
Statistical analysis
We used random-effect meta-analyses to estimate the summary RRs for the associations between sedentary behaviour and the risks of colon and rectal cancers. We combined the case–control and cohort studies in the primary meta-analysis because ORs and RRs provide similar estimates of risk when the outcome is rare (Greenland, 1987). If a study reported results for occupational and recreational physical activity separately, these were considered as separate reports. One study (Whittemore et al, 1990) was conducted in North America and China and was considered as two independent studies. Any studies stratified by sex and sedentary behaviour domain were also treated as separate reports.
Statistical heterogeneity among studies was evaluated using the I2 statistic, where values of 25%, 50%, and 75% represent cutoff points for low, moderate, and high degrees of heterogeneity, respectively (Higgins and Thompson, 2002). In sensitivity analyses, we conducted leave-one-out analyses (Wallace et al, 2009) for each study to examine the magnitude of influence of each study on pooled risk estimates. Subgroup analyses for sex, study design, ethnicity, sedentary behaviour domain, study quality, body mass index (BMI), and physical activity were conducted to examine the robustness of the primary results. For publication bias, we used the Begg test (Begg and Mazumdar, 1994),the Egger test (Egger et al, 1997), and visual inspection of a funnel plot to assess it. All statistical analyses were performed using STATA version 11.0 (Stata Corp, College Station, Texas, USA). All tests were two sided with a significance level of 0.05.
Results
Literature search
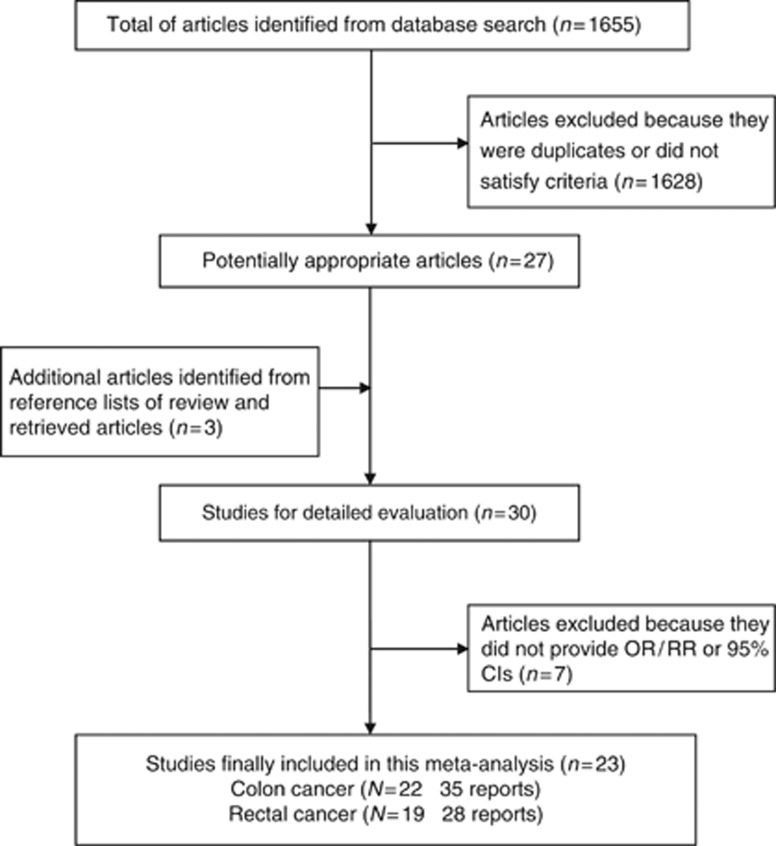
The search identified 1655 articles from the PUBMED, EMBASE, and Google Scholar databases, of which 108 articles were identified as potentially relevant. After retrieving the full text for detailed evaluation, 23 studies examining the association between sedentary behaviour and colon or rectal cancer were identified. A flow chart showing the study selection is presented in Figure 1.
Figure 1.
Flow chart of study selection.
Study characteristics
Table 1 showed the main characteristics of the included studies that were published between 1984 and 2013. The sample size of studies ranged from 294 to 1 223 908, with a total of 4 324 756. For colon cancer, the number of cancer cases ranged from 70 to 7900, with 27 231 reported colon cancer outcomes. For rectal cancer, the number of cancer cases ranged from 41 to 5328, with 13 813 reported rectal cancer outcomes. With regard to study location, one study was conducted in Australia (Boyle et al, 2011), one in New Zealand (Fraser and Pearce, 1993), two in Turkey (Vetter et al, 1992; Dosemeci et al, 1993), one in China (Whittemore et al, 1990), one in Taiwan (Tang et al, 1999), five in the United States of America (Garabrant et al, 1984; Peters et al, 1989; Whittemore et al, 1990; Howard et al, 2008; Campbell et al, 2013),one in Canada (Parent et al, 2011), and eleven in Europe (Gerhardsson et al, 1986; Arbman et al, 1993; Thune and Lund, 1996; Levi et al, 1999; Tavani et al, 1999; Colbert et al, 2001, Weiderpass et al, 2003; Friedenreich et al, 2006; Johnsen et al, 2006; Moradi et al, 2008; Simons et al, 2013). Five studies reported results for both men and women, three studies reported results for men and women separately, eight studies reported results for men only, and one study reported results for women only. The major adjustment confounding factors included age, density, social class, education, smoking, alcohol use, BMI, and physical activity. There were ten high quality studies and thirteen moderate quality studies in our meta-analysis. The average score for all included studies was 4.4.
Sedentary behaviour and the risk of colon cancer
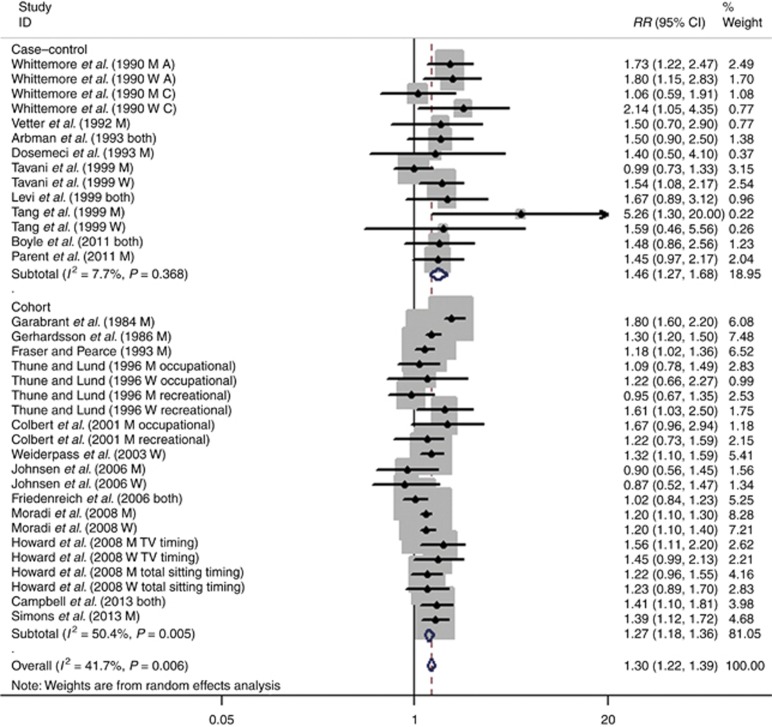
The results from the random-effect meta-analysis of sedentary behaviour and the risk of colon cancer are shown in Figure 2. The pooled RR was 1.30 (95% CI: 1.22–1.39). Subgroup analysis by study design shows that the combined RR was 1.27 (95% CI: 1.18–1.36) in cohort studies, and the combined OR was 1.46 (95% CI: 1.27–1.68) in case–control studies. A low heterogeneity was detected with an I2=7.7% across case–control studies, and a moderate heterogeneity was observed in cohort studies (I2=50.4% P=0.005).
Figure 2.
Forest plot of sedentary behaviour and risk for colon cancer.
Sedentary behaviour and the risk of rectal cancer
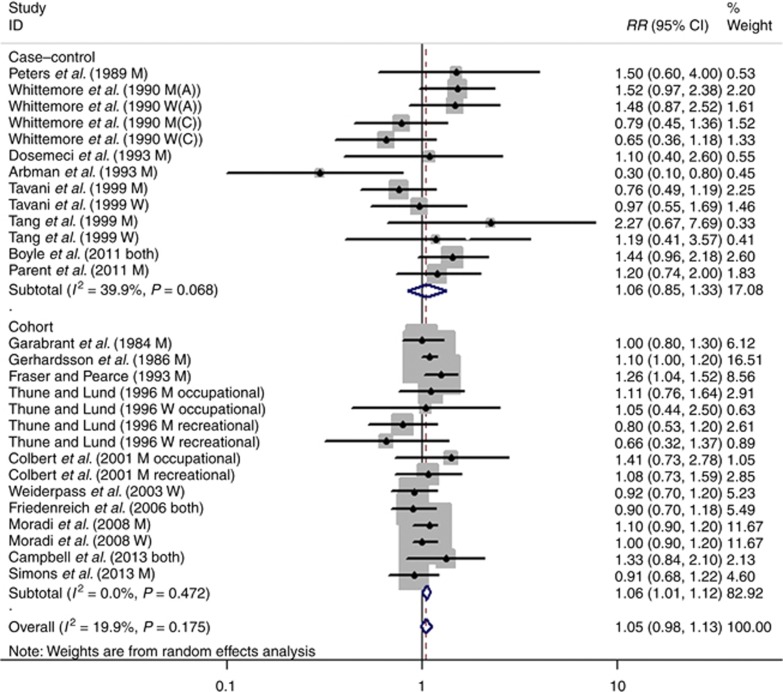
The results from the random-effect meta-analysis of sedentary behaviour and the risk of rectal cancer are shown in Figure 3. The pooled RR was 1.05 (95% CI: 0.98–1.13). Subgroup analysis by study design shows that the combined RR for cohort studies was 1.06 (95% CI: 1.01–1.12), and there was no evidence of a significant heterogeneity (I2=0.0%, P=0.472). For case–control studies, the combined OR was 1.06 (95% CI: 0.85–1.33), and there was a medium heterogeneity (I2=39.9%, P=0.068).
Figure 3.
Forest plot of sedentary behaviour and risk for rectal cancer.
Subgroup analyses
Table 2 showed the results from subgroup analyses examining the stability of the primary results and exploring the resource of potential heterogeneity. The associations between sedentary behaviour and the risk of colon and rectal cancer were similar in most subgroup analyses. Given the influence of physical activity on the association between sedentary behaviour and colon cancer, we also conducted stratified analysis by physical activity. The results remained materially unchanged after adjusting for physical activity (RR: 1.30, 95% CI: 1.16–1.46, P=0.505).
Table 2. Subgroup analyses of relative risk of colon and rectal cancer.
| No. of reports | Relative risk | (95%CI) | I2 | P-value for heterogeneity | |
|---|---|---|---|---|---|
|
Colon cancer | |||||
| Primary meta-analysis |
35 |
1.30 |
1.22–1.39 |
41.70% |
0.006 |
|
Sex | |||||
| Men | 19 | 1.30 | 1.18–1.42 | 55.80% | 0.002 |
| Women |
11 |
1.28 |
1.19–1.41 |
2.30% |
0.426 |
|
Study design | |||||
| Case–control studies | 14 | 1.46 | 1.27–1.68 | 7.70% | 0.368 |
| Cohort studies |
21 |
1.27 |
1.18–1.36 |
50.40% |
0.005 |
|
Ethnicity | |||||
| Asia population | 6 | 1.58 | 1.11–2.26 | 8.90% | 0.36 |
| Western population |
29 |
1.29 |
1.21–1.38 |
45.50% |
0.005 |
|
Sedentary behaviour domain | |||||
| Occupational | 25 | 1.30 | 1.20–1.40 | 49.60% | 0.003 |
| Recreational |
10 |
1.32 |
1.17–1.49 |
13.00% |
0.323 |
|
Study quality | |||||
| ⩾5 | 18 | 1.25 | 1.17–1.35 | 9.80% | 0.338 |
| <5 |
17 |
1.38 |
1.23–1.54 |
59.10% |
0.001 |
|
Controlling BMI in models | |||||
| Yes | 17 | 1.24 | 1.14–1.35 | 12.60% | 0.306 |
| No |
18 |
1.36 |
1.24–1.49 |
56.50% |
0.002 |
|
Controlling physical activity in models | |||||
| Yes | 9 | 1.30 | 1.16–1.46 | 0.00% | 0.505 |
| No |
26 |
1.31 |
1.21–1.42 |
50.90% |
0.002 |
|
Rectal cancer | |||||
| Primary meta-analysis |
28 |
1.05 |
0.98–1.13 |
19.90% |
0.175 |
|
Sex | |||||
| Men | 16 | 1.09 | 1.03–1.16 | 0.00% | 0.454 |
| Women |
8 |
0.98 |
0.87–1.10 |
0.00% |
0.579 |
|
Study design | |||||
| Case–control studies | 13 | 1.06 | 0.85–1.33 | 39.90% | 0.068 |
| Cohort studies |
15 |
1.06 |
1.01–1.12 |
0.00% |
0.472 |
|
Ethnicity | |||||
| Asia population | 5 | 0.87 | 0.62–1.22 | 0.00% | 0.411 |
| Western population |
23 |
1.06 |
0.99–1.14 |
22.40% |
0.164 |
|
Sedentary behaviour domain | |||||
| Occupational | 22 | 1.05 | 0.98–1.14 | 24.30% | 0.148 |
| Recreational |
6 |
1.03 |
0.80–1.31 |
14.30% |
0.323 |
|
Study quality | |||||
| ⩾5 | 12 | 1.07 | 1.00–1.15 | 0.00% | 0.474 |
| <5 |
16 |
1.04 |
0.93–1.17 |
34.70% |
0.084 |
|
Controlling BMI in models | |||||
| Yes | 11 | 1.02 | 0.91–1.16 | 0.00% | 0.459 |
| No |
17 |
1.06 |
0.97–1.16 |
31.80% |
0.102 |
|
Controlling physical activity in models | |||||
| Yes | 3 | 1.34 | 1.03–1.73 | 0.00% | 0.857 |
| No | 25 | 1.03 | 0.96–1.11 | 21.00% | 0.173 |
Sensitivity analyses
Exclusion of a study (Garabrant et al, 1984) that analysed sedentary behaviour and colon cancer yielded a pooled RR of 1.26 (95% CI: 1.20–1.33), and the statistical heterogeneity was forcefully attenuated (P=0.213, I2=15.7%). We excluded any single study in turn and pooled the results of remaining included studies, which did not change the overall combined RR, with a range from 1.26 (95% CI: 1.20–1.33; P=0.213) to 1.32 (95% CI: 1.24–1.41; P=0.016).
For rectal cancer, we found that exclusion of any single study recalculated the pooled OR for the remainder of the studies and showed that none of them was identified as a possible source of heterogeneity among all the included studies. A low heterogeneity of available data on sedentary behaviour and rectal cancer was observed (P>0.05).
Publication bias
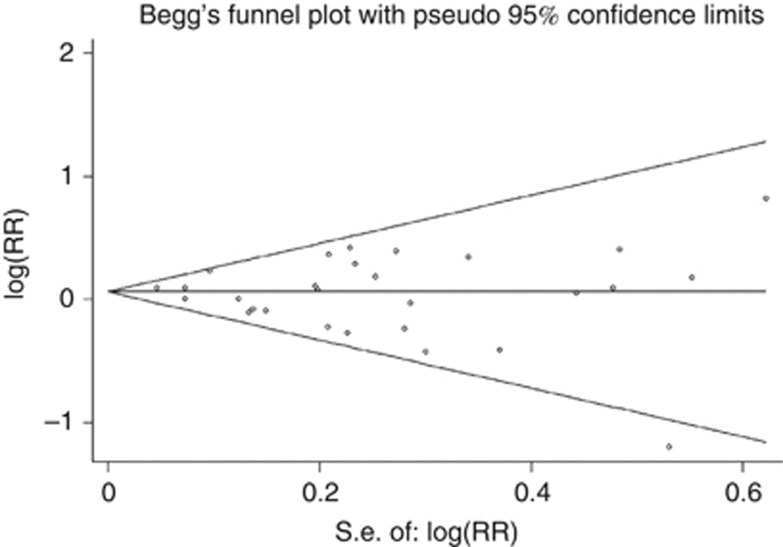
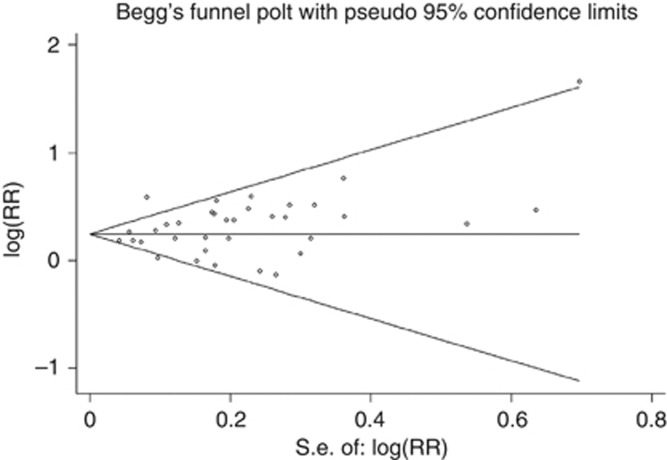
Visual inspection of the funnel plot did not reveal any asymmetry (see Figure 4). We found no evidence of significant publication bias for rectal cancer (Egger test, P=0.083, Begg test, P=0.722,). Visual inspection of the funnel plot showed a weak indication of publication bias in colon cancer (see Figure 5), and there was evidence of asymmetry for association with colon cancer risk (Egger test, P<0.001), whereas the Begg test did not identify evidence of substantial publication bias.
Figure 4.
Funnel plot of sedentary behaviour and rectal cancer.
Figure 5.
Funnel plot of sedentary behaviour and colon cancer.
Discussion
The results of our meta-analysis suggest that the risk of colon cancer is 30% higher among sedentary people. Null reverse association exists between sedentary behaviour and rectal cancer, but, in cohort studies, sedentary behaviour is associated with rectal cancer. Similar results were obtained in most subgroup analyses.
Our research result is consistent with a large cohort study, the NOCCA study (Pukkala et al, 2009), for which the investigators found that the risk of colon and rectal cancer was related to some jobs. However, we excluded the study because it focused on the link between occupation and colon and rectal cancer and did not clearly define the occupations as sedentary work. Our included studies either clearly defined the kind of sedentary work or reported the numbers of sedentary hours per day.
Our subgroups analyses identify three findings. A major finding is that the relationship between sedentary behaviour and colon cancer may be independent of physical activity, which is important because it suggests that sedentary behaviour could be an independent determinant of colon cancer distinct from that of physical inactivity. Another finding is that sedentary behaviour increased the risk of colon cancer both in case–control and cohort studies, and the sedentary behaviour was associated with a significantly increased risk for rectal cancer in cohort studies, but not in case–control studies. Finally, we also found that the pooled RR for participants from the United States of America (1.51; 95% CI: 1.33–1.71) was higher than the pooled RR for European participants (1.22; 95% CI: 1.15–1.29). There was null statistically significant risk of colon cancer in Asian participants (1.79; 95% CI: 0.98–3.25), which may result from the limited number of included studies (two case–control studies comprising 2054 participants). On the basis of the above findings, we should pay close attention to the sedentary behaviour risk in different countries. As we know, the more developed the county, the higher the economic level, which may frequently give rise to sedentary behaviour, such as prolonged TV viewing, workplace sitting, and time spent in automobiles. Thus, developed countries should take actions to discourage sedentary behaviour, and developing countries should pay attention to the connection between sedentary behaviour and health consequences.
There are several biologic mechanisms through which sedentary behaviour in general could increase the risk of colorectal cancer. Sedentary behaviour has been shown to increase blood glucose levels and to decrease insulin sensitivity (Healy et al, 2007). Increased blood glucose and decreased insulin resistance are both thought to promote colorectal cancer carcinogenesis (Giovannucci, 2001; Giovannucci, 2007). Sedentary behaviour has also been linked to an increased risk of diabetes and obesity (Hu et al, 2003), both of which are established risk factors for colorectal cancer (Lynch, 2010). Other proposed mechanisms by which sedentary behaviour may increase the risk of colorectal cancer include increasing levels of proinflammatory factors, decreasing levels of anti-inflammatory factors, and vitamin D (Feskanich et al, 2004).
Our meta-analysis has several strengths. First, this is the first meta-analysis to systematically quantify the strength of association between sedentary behaviour and colon and rectal outcomes. Second, our study supports the hypothesis that sedentary behaviour is probably an independent risk factor for the colon cancer. Third, as sedentary behaviours are increasingly prevalent in modern society, the results of our study not only can act as an aetiology explanation but also can increase public awareness.
A few limitations of our meta-analysis should be acknowledged. First, the included studies were conducted among different population groups, and the measurement and categorisation of the sedentary behaviour were highly heterogeneous. Thus, the results of this meta-analysis should be interpreted cautiously. Second, the study relied on job title-based and self-reported engagement in sedentary behaviours, which was likely to cause the misclassification of exposure, and may underestimate the reported associations. Third, the limited information provided in the included studies precluded the possibility of dose-response analysis.
On the basis of our findings, we put forward some suggestions for future studies. First, further research using more objective measures of sedentary behaviour and energy expenditure may make observed effects more accurate and conceivable. In addition, more prospective and interventional studies are needed to explore the underlying mechanisms and to determine the cause and effect relationships that link sedentary behaviour and colorectal cancer.
In conclusion, our meta-analysis suggests that sedentary behaviour is associated with an increased risk of colon cancer. The increased risk of rectal cancer associated with sedentary behaviour in subgroup analyses warrants further studies. Given that sedentary behaviours are increasingly prevalent and pervasive in modern society, the result of our meta-analysis is greatly important to both cancer aetiology and public health education.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Arbman G, Axelson O, Fredriksson M, Nilsson E, Sjodahl R. Do occupational factors influence the risk of colon and rectal cancer in different ways. Cancer. 1993;72:2543–2549. doi: 10.1002/1097-0142(19931101)72:9<2543::aid-cncr2820720906>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Blanck HM, Mccullough ML, Patel AV, Gillespie C, Calle EE, Cokkinides VE, Galuska DA, Khan LK, Serdula MK. Sedentary behavior, recreational physical activity, and 7-year weight gain among postmenopausal U.S. women. Obesity. 2007;15:1578–1588. doi: 10.1038/oby.2007.187. [DOI] [PubMed] [Google Scholar]
- Boyle T. Physical Activity and Colon Cancer: Timing, Intensity, and Sedentary Behavior. Am J Lifestyle Med. 2012;6:204–215. [Google Scholar]
- Boyle T, Fritschi L, Heyworth J, Bull F. Long-Term Sedentary Work and the Risk of Subsite-specific Colorectal Cancer. Am J Epidemiol. 2011;173:1183–1191. doi: 10.1093/aje/kwq513. [DOI] [PubMed] [Google Scholar]
- Boyle T, Keegel T, Bull F, Heyworth J, Fritschi L. Physical activity and risks of proximal and distal colon cancers: a systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1548–1561. doi: 10.1093/jnci/djs354. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Bauman AE, Owen N. Stand up, sit down, keep moving: turning circles in physical activity research. Br J Sports Med. 2009;43:86–88. doi: 10.1136/bjsm.2008.055285. [DOI] [PubMed] [Google Scholar]
- Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–885. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Hartman TJ, Malila N, Limburg PJ, Pietinen P, Virtamo J, Taylor PR, Albanes D. Physical activity in relation to cancer of the colon and rectum in a cohort of male smokers. Cancer Epidemiol Biomarkers Prev. 2001;10:265–268. [PubMed] [Google Scholar]
- Dosemeci M, Hayes RB, Vetter R, Hoover RN, Tucker M, Engin K, Unsal M, Blair A. Occupational physical activity, socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes Control. 1993;4:313–321. doi: 10.1007/BF00051333. [DOI] [PubMed] [Google Scholar]
- Dunstan DW, Barr EL, Healy GN, SalmoN J, Shaw JE, Balkau B, Magliano DJ, CameroN AJ, Zimmet PZ, Owen N. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Circulation. 2010;121:384–391. doi: 10.1161/CIRCULATIONAHA.109.894824. [DOI] [PubMed] [Google Scholar]
- Edwardson CL, Gorely T, Davies MJ, Gray LJ, Khunti K, Wilmot EG, Yates T, Biddle SJ. Association of sedentary behaviour with metabolic syndrome: a meta-analysis. PLoS One. 2012;7:e34916. doi: 10.1371/journal.pone.0034916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feskanich D, Ma J, Fuchs CS, Kirkner GJ, Hankinson SE, Hollis BW, Giovannucci EL. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- Fraser G, Pearce N. Occupational physical activity and risk of cancer of the colon and rectum in New Zealand males. Cancer Causes Control. 1993;4:45–50. doi: 10.1007/BF00051713. [DOI] [PubMed] [Google Scholar]
- Fredriksson M, Bengtsson NO, Hardell L, Axelson O. Colon cancer, physical activity, and occupational exposures. A case-control study. Cancer. 1989;63:1838–1842. doi: 10.1002/1097-0142(19900501)63:9<1838::aid-cncr2820630930>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman M, Johnsen NF, Tjonneland A, Overvad K, Mendez M, Quiros JR, Martinez C, Dorronsoro M, Navarro C, Gurrea AB, Bingham S, Khaw KT, Allen N, Key T, Trichopoulou A, Trichopoulos D, Orfanou N, Krogh V, Palli D, Tumino R, Panico S, Vineis P, Bueno-De-Mesquita HB, Peeters PH, Monninkhof E, Berglund G, Manjer J, Ferrari P, Slimani N, Kaaks R, Riboli E. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15:2398–2407. doi: 10.1158/1055-9965.EPI-06-0595. [DOI] [PubMed] [Google Scholar]
- Garabrant DH, Peters JM, Mack TM, Bernstein L. Job activity and colon cancer risk. Am J Epidemiol. 1984;119:1005–1014. doi: 10.1093/oxfordjournals.aje.a113805. [DOI] [PubMed] [Google Scholar]
- George SM, Irwin ML, Matthews CE, Mayne ST, Gail MH, Moore SC, AlbaneS D, Ballard-Barbash R, Hollenbeck AR, Schatzkin A, LeitzmanN MF. Beyond recreational physical activity: examining occupational and household activity, transportation activity, and sedentary behavior in relation to postmenopausal breast cancer risk. Am J Public Health. 2010;100:2288–2295. doi: 10.2105/AJPH.2009.180828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardsson M, Norell SE, Kiviranta H, Pedersen NL, Ahlbom A. Sedentary jobs and colon cancer. Am J Epidemiol. 1986;123:775–780. doi: 10.1093/oxfordjournals.aje.a114306. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–179. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120SS. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–s842. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- Grontved A, Hu FB. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA. 2011;305:2448–2455. doi: 10.1001/jama.2011.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care. 2007;30:1384–1389. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- Howard RA, Freedman DM, Park Y, Hollenbeck A, Schatzkin A, LeitzmanN MF. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19:939–953. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- Johnsen NF, Christensen J, Thomsen BL, Olsen A, Loft S, Overvad K, Tjonneland A. Physical activity and risk of colon cancer in a cohort of Danish middle-aged men and women. Eur J Epidemiol. 2006;21:877–884. doi: 10.1007/s10654-006-9076-z. [DOI] [PubMed] [Google Scholar]
- Katzmarzyk PT, Church TS, Craig CL, Bouchard C. Sitting time and mortality from all causes, cardiovascular disease, and cancer. Med Sci Sports Exerc. 2009;41:998–1005. doi: 10.1249/MSS.0b013e3181930355. [DOI] [PubMed] [Google Scholar]
- Levi F, Pasche C, Lucchini F, Tavani A, La Vecchia C. Occupational and leisure-time physical activity and the risk of colorectal cancer. Eur J Cancer Prev. 1999;8:487–493. doi: 10.1097/00008469-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19:2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- Mathew A, Gajalakshmi V, Rajan B, Kanimozhi VC, Brennan P, Binukumar BP, Boffetta P. Physical activity levels among urban and rural women in south India and the risk of breast cancer: a case-control study. Eur J Cancer Prev. 2009;18:368–376. doi: 10.1097/CEJ.0b013e32832e1c46. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk. Cancer Epidemiol Biomarkers Prev. 1994;3:687–695. [PubMed] [Google Scholar]
- Moradi T, Gridley G, Bjork J, Dosemeci M, Ji BT, Berkel HJ, Lemeshow S. Occupational physical activity and risk for cancer of the colon and rectum in Sweden among men and women by anatomic subsite. Eur J Cancer Prev. 2008;17:201–208. doi: 10.1097/CEJ.0b013e3282b6fd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18:11–27. doi: 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- Organization WH.2011. Globocan2008: Cancer incidence and mortality worldwide in 2008:.
- Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38:105–113. doi: 10.1097/JES.0b013e3181e373a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent ME, Rousseau MC, El-Zein M, Latreille B, Desy M, Siemiatycki J. Occupational and recreational physical activity during adult life and the risk of cancer among men. Cancer Epidemiol. 2011;35:151–159. doi: 10.1016/j.canep.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Pate RR, O'Neill JR, Lobelo F. The evolving definition of ‘sedentary'. Exerc Sport Sci Rev. 2008;36:173–178. doi: 10.1097/JES.0b013e3181877d1a. [DOI] [PubMed] [Google Scholar]
- Peters RK, Garabrant DH, Yu MC, Mack TM. A case-control study of occupational and dietary factors in colorectal cancer in young men by subsite. Cancer Res. 1989;49:5459–5468. [PubMed] [Google Scholar]
- Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparen P, Tryggvadottir L, Weiderpass E, Kjaerheim K. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- Simons CC, Hughes LA, Van Engeland M, Goldbohm RA, Van Den Brandt PA, Weijenberg MP. Physical activity, occupational sitting time, and colorectal cancer risk in the Netherlands cohort study. Am J Epidemiol. 2013;177:514–530. doi: 10.1093/aje/kws280. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Tang R, Wang JY, Lo SK, Hsieh LL. Physical activity, water intake and risk of colorectal cancer in Taiwan: a hospital-based case-control study. Int J Cancer. 1999;82:484–489. doi: 10.1002/(sici)1097-0215(19990812)82:4<484::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Tavani A, Braga C, La Vecchia C, Conti E, Filiberti R, Montella M, Amadori D, Russo A, Franceschi S. Physical activity and risk of cancers of the colon and rectum: an Italian case-control study. Br J Cancer. 1999;79:1912–1916. doi: 10.1038/sj.bjc.6690304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73:1134–1140. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter R, Dosemeci M, Blair A, Wacholder S, Unsal M, Engin K, Fraumeni JF., Jr Occupational physical activity and colon cancer risk in Turkey. Eur J Epidemiol. 1992;8:845–850. doi: 10.1007/BF00145330. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiderpass E, Vainio H, Kauppinen T, Vasama-Neuvonen K, Partanen T, Pukkala E. Occupational Exposures and Gastrointestinal Cancers Among Finnish Women. J Occup Environ Med. 2003;45:305–315. doi: 10.1097/01.jom.0000052963.43131.44. [DOI] [PubMed] [Google Scholar]
- Whittemore AS, Wu-Williams AH, Lee M, Zheng S, Gallagher RP, Jiao DA, Zhou L, Wang XH, Chen K, Jung D, Chong-Ze T, Ling CD, Xu JY, Paffenbarger RS, Henderson BE. Diet, physical activity, and colorectal cancer among Chinese in North America and China. J Natl Cancer Inst. 1990;82:915–926. doi: 10.1093/jnci/82.11.915. [DOI] [PubMed] [Google Scholar]
- Wijndaele K, Brage S, Besson H, Khaw KT, Sharp SJ, Luben R, Wareham NJ, Ekelund U. Television viewing time independently predicts all-cause and cardiovascular mortality: the EPIC Norfolk study. Int J Epidemiol. 2011;40:150–159. doi: 10.1093/ije/dyq105. [DOI] [PubMed] [Google Scholar]
- Wijndaele K, Healy GN, Dunstan DW, Barnett AG, Salmon J, Shaw JE, Zimmet PZ, Owen N. Increased cardiometabolic risk is associated with increased TV viewing time. Med Sci Sports Exerc. 2010;42:1511–1518. doi: 10.1249/MSS.0b013e3181d322ac. [DOI] [PubMed] [Google Scholar]
- Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55:2895–2905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–616. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WC, Cheung CS, Hart GJ. Development of a quality assessment tool for systematic reviews of observational studies (QATSO) of HIV prevalence in men having sex with men and associated risk behaviours. Emerg Themes Epidemiol. 2008;5:23. doi: 10.1186/1742-7622-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]