Abstract
Background:
This study evaluated the efficacy and safety of ziv-aflibercept in combination with cisplatin and pemetrexed in non-small cell lung cancer (NSCLC).
Methods:
This single arm, multicentre phase II trial enrolled patients with previously untreated, locally advanced or metastatic non-squamous NSCLC. Patients received intravenous ziv-aflibercept 6 mg kg−1, pemetrexed 500 mg m−2, and cisplatin 75 mg m−2, every 21 days for up to six cycles. Maintenance administration of ziv-aflibercept was to continue until disease progression, intolerable toxicity or other cause for withdrawal. The co-primary end points were objective response rate (ORR) and progression-free survival (PFS). Planned sample size was 72 patients.
Results:
The study was closed prematurely because of three confirmed and two suspected cases of reversible posterior leukoencephalopathy syndrome (RPLS). A total of 42 patients were enrolled. Median age was 61.5 years; 55% were male, 86% Caucasian and 50% had Eastern Cooperative Oncology Group performance status (ECOG PS)=0. A median of four cycles of ziv-aflibercept was administered. The most common treatment-emergent adverse events (TEAEs) of any grade were nausea (69%) and fatigue (67%), with hypertension (36%) as the most common grade 3/4 TEAE. Of the 38 evaluable patients, ORR was 26% and median PFS was 5 months.
Conclusion:
Cases of RPLS had been observed in other studies in the ziv-aflibercept clinical development programme but the rate observed in this study was higher than previously observed. This might be related to declining renal function and/or hypertension. Although ORR and PFS were in accordance with most historical first-line NSCLC studies, this combination of ziv-aflibercept/cisplatin/pemetrexed will not be further explored in NSCLC.
Keywords: ziv-aflibercept, non-small cell lung cancer, reversible posterior leukoencephalopathy syndrome, anti-angiogenesis
Cancer growth is dependent upon angiogenesis to maintain a source of nutrition and oxygen (Folkman, 1995), and vascular endothelial growth factor (VEGF) has a key role in tumour angiogenesis (Ferrara and Davis-Smyth, 1997). Non-small cell lung cancer (NSCLC) produces VEGF, and high serum levels of VEGF are correlated with poor prognosis (Korpanty et al, 2010). Anti-angiogenic therapy thus aims to disrupt blood supply to tumours and has proven clinical benefit in non-squamous NSCLC (Jain, 2001).
Combination chemotherapy is used for the first-line treatment of advanced/metastatic NSCLC (Schiller et al, 2002). The addition of the anti-VEGF antibody, bevacizumab, to carboplatin/paclitaxel in this setting improved response rate, progression-free survival (PFS), and overall survival (OS; Sandler et al, 2006). Similarly, bevacizumab improved PFS when added to cisplatin/gemcitabine, although OS was not significantly prolonged as a secondary end point in this case (Reck et al, 2009). For non-squamous histology, cisplatin/pemetrexed is a very active combination chemotherapy (Scagliotti et al, 2008), and thus combinations of platinum/pemetrexed with bevacizumab or other anti-angiogenics are of strong interest for the first-line treatment of advanced/metastatic non-squamous NSCLC (Patel et al, 2009b).
Ziv-aflibercept (ZALTRAP, Sanofi, Bridgewater, NJ, USA and Regeneron Pharmaceuticals, Tarrytown, NY, USA) is a recombinant fusion protein consisting of portions of human VEGF receptor extracellular domains fused to the Fc portion of human immunoglobulin (Gaya and Tse, 2012). Ziv-aflibercept binds VEGF-A by acting as a high-affinity ligand trap to prevent binding to its endogenous receptor VEGFR-2, thereby inhibiting VEGF-induced angiogenesis in preclinical models (Lassoued et al, 2010). Endothelial cells expressing high levels of VEGFR-2 were highly susceptible to blockade by ziv-aflibercept (Sitohy et al, 2011). In addition, ziv-aflibercept binds PIGF (placental growth factor) and VEGF-B, which could potentially inhibit cancer invasion (Dowlati, 2010). Studies have investigated ziv-aflibercept as a single agent or in combination with other chemotherapeutic agents in treatment of various types of cancers (Lockhart et al, 2010; Tew et al, 2010; de Groot et al, 2011; Isambert et al, 2012). In August 2012, ziv-aflibercept was approved by the US FDA for use in metastatic colorectal cancer based on the results of VELOUR trial (Van Cutsem et al, 2012). A phase II study using ziv-aflibercept as monotherapy demonstrated objective responses in heavily pretreated patients with advanced adenocarcinoma of the lung (Leighl et al, 2010), and improvement in response and PFS (but not OS) was observed in combination with docetaxel as second-line treatment of NSCLC (Ramlau et al, 2012).
We report the results of a phase II trial of ziv-aflibercept in combination with cisplatin and pemetrexed in patients with advanced or metastatic non-squamous NSCLC. This study was conducted after a phase I trial using the same regimen of ziv-aflibercept/cisplatin/pemetrexed (Diaz-Padilla et al, 2012). That phase I trial determined the recommended dose of ziv-aflibercept (6 mg kg−1 every 21 days) to be used in the current phase II trial, which aimed to evaluate the efficacy and safety of ziv-aflibercept in combination with cisplatin and pemetrexed in the first-line treatment of advanced/metastatic NSCLC.
Materials and methods
Eligibility
Patients eligible for this study had histologically/cytologically confirmed, untreated, locally advanced/metastatic NSCLC, and they had to have measurable disease as per the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (Therasse et al, 2000). Patients with squamous histology and/or cavitating lesions were excluded. Patients were 18 years of age or older, and had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, with adequate bone marrow, renal, and hepatic functions and calculated creatinine clearance (CrCL) ⩾60 ml min−1. Patients were excluded from the study if they had brain or central nervous system metastases; systolic blood pressure (BP) >150 mm Hg and/or diastolic blood pressure >100 mm Hg; bleeding diathesis or evidence of active bleeding; or recent significant cardiovascular, cerebrovascular, or thromboembolic conditions.
The protocol was approved by the Institutional Review Boards at each participating institution. Informed consent was obtained from each patient.
Study design
This is a single arm, open label, multicentre phase II study (ClinicalTrials.gov identifier: NCT00794417). Patients received the three-drug combination intravenously on day 1 of every 21 days, with ziv-aflibercept (6 mg kg−1) first, followed by pemetrexed (500 mg m−2) and cisplatin (75 mg m−2). Premedications consisted of folic acid, vitamin B12, and dexamethasone as a prophylactic measure to reduce pemetrexed-related toxicities and standard anti-emetics. Patients could receive up to six cycles of combination therapy. For patients who completed the combined chemotherapy, maintenance ziv-aflibercept every 21 days was to continue until disease progression, intolerable toxicity, or withdrawal from the study.
End points and assessments
The two co-primary end points were objective response rate (ORR) and PFS. The ORR was defined as the proportion of patients with complete response plus partial response (CR+PR). The PFS was defined as the time interval from the first dose of combination chemotherapy to tumour progression or death, whichever occurred first. Secondary variables were the determination of the adverse events (AEs), pharmacokinetics (PK), and pharmacodynamic profiles (including anti-ziv-aflibercept antibody and hematopoiesis). Pharmacokinetic end points included the area under the concentration curve, maximum concentration (Cmax), clearance, and terminal half-life (t1/2).
Tumour imaging (CT or MRI) was performed at screening, on day 21 (±7 days) of every even numbered cycle (every 6 weeks), and when disease progression was suspected. Responses were assessed using RECIST, version 1.0 (Therasse et al, 2000). Safety and tolerability were assessed at baseline and at least every 21 days, as evaluated by AEs and changes in laboratory parameters graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 (National Cancer Institute, 2006). Ziv-aflibercept (free or bound to VEGF) in plasma samples was quantified using a validated, direct enzyme-linked immunosorbent assay. A validated, non-quantitative, titre-based, bridging assay was used to detect anti-ziv-aflibercept antibodies in serum samples.
Correlative studies
The exploratory objective of the correlative studies was to evaluate changes in erythropoiesis in response to VEGF inhibition. It was hypothesised that VEGF inhibition would result in an increase in haemoglobin via increased hepatic erythropoietin production.
Statistical analysis
Statistical testing was done to determine whether the ORR was larger than 20% or whether the PFS was greater than 4.5 months. Exact test (one-sided) was used to test the null hypothesis that ORR was ⩽20% versus the alternative hypothesis that ORR was ⩾35%. Assuming type I error was not >2.5%, a sample of 72 patients would provide >80% power to test the hypothesis using exact binomial test. The calculated sample size of 72 patients would also provide >90% power to test the null hypothesis that median PFS was ⩽4.5 months versus the alternative hypothesis that PFS was ⩾6.5 months at one-sided alpha of 2.5% using one sample log-rank test.
Safety data were to be summarised. Concentrations of free ziv-aflibercept and adjusted bound ziv-aflibercept: VEGF complex were to be summarised every 21 days over the duration of the study by nominal time point. Noncompartmental parameters were calculated using WinNonlin (version 5.3, Pharsight Corporation, Mountain View, CA, USA) and model 202 (constant infusion) using nominal time points after a single dose of ziv-aflibercept. The noncompartmental analysis was performed over the dosing interval, 21 days following the first dose. All analyses used statistical software SAS (version 9.1.3, Cary, NC, USA).
Results
Patients
This study was closed prematurely because of three confirmed and two suspected but unconfirmed cases of reversible posterior leukoencephalopathy syndrome (RPLS). A total of 42 patients were enrolled from 17 participating sites across the United States and Canada between January, 2009 and December, 2010. Table 1 summarises the patient demographics. Median age was 61.5 years; 55% were male, 86% were Caucasian and 50% had ECOG PS of 0.
Table 1. Baseline demographics (N=42).
|
Age (years) |
|
| Median | 61.5 |
| Range (years) |
38–77 |
|
Age (years) by category |
N
(%) |
| <65 | 23 (54.8) |
| ⩾65 |
19 (45.2) |
|
Gender |
N
(%) |
| Male | 23 (54.8) |
| Female |
19 (45.2) |
|
Race |
N
(%) |
| African American | 2 (4.8) |
| Asian American | 4 (9.5) |
| Caucasian American |
36 (85.7) |
|
ECOG performance status |
N
(%) |
| 0 | 21 (50.0) |
| 1 | 21 (50.0) |
Abbreviation: ECOG=Eastern Cooperative Oncology Group.
Safety evaluation
Treatment exposure and dose modifications
All 42 patients received at least one dose of each of the three study drugs, with a median of 92 days of treatment (range 21–288 days). Twenty-seven (64%) patients completed four or more cycles of the combination treatment. A median of 4.5 (range 1–6) cycles of pemetrexed and 4 (range 1–6) cycles of cisplatin were administered. The median dose intensity was 163.9 (range 110.1–175.5) mg m−2 per week for pemetrexed and 24.6 (range 15.3–26.3) mg m−2 per week for cisplatin. The delivered dose intensities were 98.3% for pemetrexed and 98.5% for cisplatin. Seventeen (40%) completed six or more cycles of ziv-aflibercept. The median dose intensity of ziv-aflibercept was 1.97 mg kg−1 per week, close to the planned intensity of 2 mg kg−1 per week. Reasons for treatment discontinuation were disease progression (33%), AEs (33%), and others (34%, including withdrawal of consent and investigator request). Seventeen patients had at least one cycle delayed. Eleven patients (26%) had at least 1 dose modification of ziv-aflibercept, 6 (14%) of pemetrexed, and 11 of cisplatin.
Adverse events
Thirty-five patients (83%) experienced a treatment-emergent adverse event (TEAE) of grade 3 or 4 (3/4), and 16 patients (38%) experienced a serious TEAE. The most common TEAEs were nausea (69%), fatigue (67%), and hypertension (57%). Hypertension, neutropaenia, and hypokalaemia were the most common grade 3/4 TEAEs in 36%, 14%, and 10% of patients, respectively. Table 2 summarised the most common TEAEs. Thirty-nine patients (93%) experienced at least one haematologic abnormality, with grade 3/4 in eight patients (19%), mostly neutropaenia. Every patient experienced at least one abnormal chemistry value, with grade 3/4 in 15 (36%), most commonly hyponatraemia.
Table 2. Summary of most common (>10%) TEAEs by NCI CTCAE grade (N=42).
| All grades N (%) | Grade 3/4 N (%) | |
|---|---|---|
| Number of patients with most common TEAE with NCI grade |
40 (95.2) |
27 (64.3) |
| Nausea |
29 (69.0) |
0 |
| Fatigue |
28 (66.7) |
3 (7.1) |
| Hypertension |
24 (57.1) |
15 (35.7) |
| Constipation |
21 (50.0) |
0 |
| Diarrhoea |
16 (38.1) |
1 (2.4) |
| Dysgeusia |
15 (35.7) |
0 |
| Stomatitis |
15 (35.7) |
0 |
| Dysphonia |
14 (33.3) |
0 |
| Headache |
14 (33.3) |
1 (2.4) |
| Proteinuria |
14 (33.3) |
1 (2.4) |
| Anorexia |
13 (31.0) |
0 |
| Dehydration |
12 (28.6) |
3 (7.1) |
| Dyspepsia |
12 (28.6) |
0 |
| Epistaxis |
11 (26.2) |
0 |
| Vomiting |
10 (23.8) |
0 |
| Weight loss |
10 (23.8) |
0 |
| Blood creatinine increased |
8 (19.0) |
0 |
| Cough |
8 (19.0) |
0 |
| Dyspnoea |
7 (16.7) |
1 (2.4) |
| Hyponatraemia |
7 (16.7) |
2 (4.8) |
| Neutropaenia |
7 (16.7) |
6 (14.3) |
| Thrombocytopaenia |
7 (16.7) |
3 (7.1) |
| Dizziness |
6 (14.3) |
0 |
| Hyperglycaemia |
6 (14.3) |
1 (2.4) |
| Hypokalaemia |
6 (14.3) |
4 (9.5) |
| Hypomagnesaemia |
6 (14.3) |
0 |
| Insomnia |
6 (14.3) |
0 |
| Rhinorrhaea |
6 (14.3) |
0 |
| Anaemia |
5 (11.9) |
2 (4.8) |
| Dry mouth |
5 (11.9) |
0 |
| Hiccups |
5 (11.9) |
0 |
| Hyperkalaemia |
5 (11.9) |
2 (4.8) |
| Hypocalcaemia |
5 (11.9) |
1 (2.4) |
| Musculoskeletal pain |
5 (11.9) |
0 |
| Oedema peripheral |
5 (11.9) |
0 |
| Pain in extremity |
5 (11.9) |
0 |
| Pneumonia | 5 (11.9) | 2 (4.8) |
Abbreviations: CTCAE=Common Terminology Criteria for Adverse Events; TEAE=treatment-emergent adverse events.
Seven patients (17%) died before clinical cutoff of the study. Five died due to disease progression and two due to TEAEs: 1 pneumonia and 1 sepsis.
Neurological toxicities
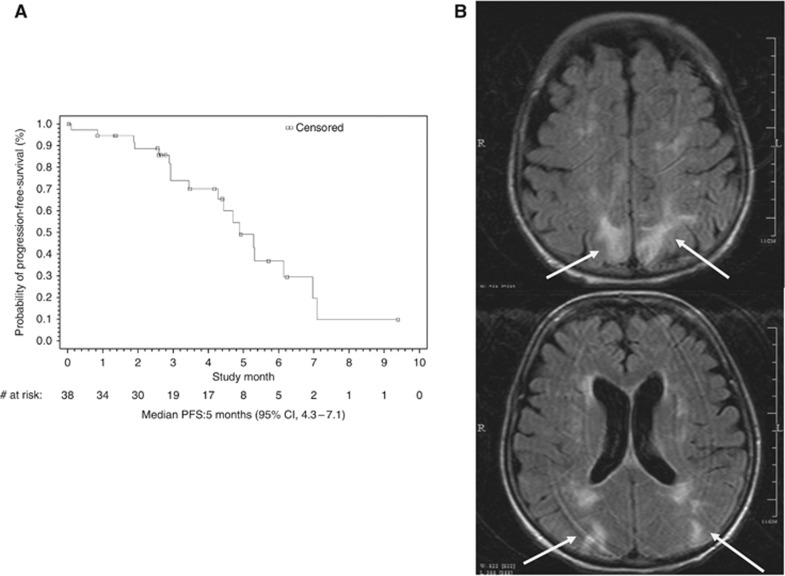
Between 26 September, 2010 and 30 December, 2010, five patients experienced neurological symptoms, including altered mental status in four, slurred speech in one, seizure in two, and headache in four patients. Three patients had a brain MRI that was consistent with RPLS (Figure 1B). Brain MRI was negative for RPLS in two other patients. The three patients diagnosed with RPLS were all Caucasian women, aged 38, 51, and 72 years, respectively. One of the three patients with RPLS entered the study with a history of hypertension. All three patients experienced elevated BP and two patients had reduced CrCL during the therapy: one patient's CrCL decreased by 45% from baseline (141 to 78 ml min−1) after one cycle, and the other's decreased by 20% (64 to 51 ml min−1) after four cycles. They were diagnosed with RPLS after one, two, and five cycles of ziv-aflibercept, respectively. Two patients recovered from RPLS, and one died due to disease progression before RPLS resolution. Pharmacokinetic information was available for two of the three RPLS patients and for one suspected case: systemic concentrations of free ziv-aflibercept were within the range of other patients in the treatment cohort.
Figure 1.
(A) The Kaplan–Meier curve of PFS (N=38). Median PFS: 5 months (95% CI, 4.3–7.1). (B) Brain MRI of a patient diagnosed with RPLS. Extensive increased T2 signal of a few scattered areas of restricted diffusion and vasogenic oedema in the frontal cortex/subcortical regions near the vertex, and the parietal and occipital regions bilaterally (arrows). No focal enhancing lesions to suggest metastatic disease.
In the phase I study using the same regimen (N=18), five patients experienced a mild neurocognitive disturbance but no RPLS was diagnosed (Diaz-Padilla et al, 2012). Rare cases of RPLS have been observed in the ziv-aflibercept clinical development programme but the rate observed in this study (3 out of 42=7%) was much higher than previously reported (i.e., 0.5% of 3795 patients treated with ziv-aflibercept as monotherapy or in combination with chemotherapy, ZALTRAP product insert). As a result, this study was permanently closed to enrolment. Patients who remained on study were re-consented with updated safety information regarding RPLS in addition to continued close monitoring.
Hypertension and renal insufficiency are two risk factors for RPLS. Twenty-four patients (57%) experienced hypertension (15 grade 3 but no grade 4) during the study, 15 of whom had a history of hypertension and 12 had taken antihypertensive medications before entering the study. Eight patients with hypertension also experienced proteinuria. Fourteen patients (33%) experienced proteinuria (all grades 1 or 2 except a single grade 3), none of whom had a history of renal disease. Fourteen patients experienced CrCL decreases during treatment, with six patients having CrCL decreases below 60 ml min−1 after treatment cycle 4.
Efficacy evaluation
As the study was closed prematurely, there was no statistical power to test the primary hypothesis. Of the 42 patients enrolled, 4 patients discontinued early from the study due to AEs (2), consent withdrawal (1), and investigator decision (1). As they did not have a post-baseline tumour assessment, they were excluded from the efficacy assessment per predefined statistical analysis plan. Of the 38 patients evaluable for efficacy, the median PFS was 5 months (95% CI, 4.3–7.1; Figure 1A), and ORR was 26% (95% CI, 12–40%), all of which (10/38) were PR. The disease control rate (PR+stable disease) was 89% (26%+63%). A mean reduction of 20% was observed in average percentage changes over time in tumour burden (sum of largest diameters of target lesions) from baseline.
Of the 38 patients evaluable for efficacy, 22 (58%) developed hypertension as AE during the study. Seven (7 out of 22=32%) had a PR compared with only three with no hypertension (3 out of 16=18%), suggesting that patients who developed hypertension may have had a higher likelihood of response to this treatment.
Correlative studies
Participation in the correlative studies was optional. Erythropoietin levels were obtained from 16 patients. No trend towards increase in haemoglobin or change in erythropoietin level was seen over time.
Pharmacokinetic and antibody evaluation
Twenty-three patients participated in blood sampling for PK analysis. Mean observed noncompartmental PK parameters for free ziv-aflibercept are presented in Table 3. The concentration–time profiles and PK of free ziv-aflibercept and adjusted bound ziv-aflibercept: VEGF were consistent with results in the phase I study (Diaz-Padilla et al, 2012). Mean trough concentrations after the second ziv-aflibercept dose plateaued and remained at ∼10 mg l−1. The mean adjusted bound ziv-aflibercept:VEGF complex Cmax was 7.81 mg l−1. Trough concentrations plateaued after day 42 and remained constant for the remainder of the study.
Table 3. Mean (s.d.) observed noncompartmental pharmacokinetic parameters for free ziv-aflibercept.
| |
Ziv-aflibercept |
|||
|---|---|---|---|---|
| Parameter | N | Mean | s.d. | |
|
t1/2 |
Day |
7 |
4.62 |
1.46 |
| CL |
l per day per kg |
7 |
0.016 |
0.004 |
|
Vss |
l kg−1 |
7 |
0.081 |
0.015 |
|
Cmax |
mg l−1 |
21 |
104 |
26.2 |
|
Clast |
mg l−1 |
21 |
65.2 |
50.8 |
|
tmax |
Day |
21 |
0.0695 |
0.019 |
|
tlast |
Day |
21 |
7.02 |
9.32 |
| AUCinf |
Day × mg l−1 |
7 |
402 |
100 |
| MRTinf | Day | 7 | 5.35 | 1.28 |
Abbreviations: AUCinf=area under the concentration curve from time zero extrapolated to infinity; CL=clearance; Clast=last positive plasma concentration; Cmax=maximal plasma concentration; MRTinf=mean residence time extrapolated to infinity; tlast=time of the last positive plasma concentration; tmax=time required to reach maximal plasma concentration; t1/2=observed half-life; Vss=volume of distribution at steady state.
Two patients had one sample each that was positive in the anti-drug antibody (ADA) assay. One was positive at baseline, but did not have an antibody titre drawn at the end of treatment (EOT) visit. This patient completed all six cycles of combination treatment without dose delay/reduction or grade 3/4 AEs and had stable disease. The other one was negative at baseline but positive at the EOT visit. This patient experienced anaphylaxis 20 minutes after start of the ziv-aflibercept infusion on day 1 of the second cycle. Study drug was permanently withdrawn. This patient had PK parameters and a concentration–time profile different from ADA-negative patients, probably because of the ADA formation.
Discussion
Ziv-aflibercept has been tested as a single agent and in combination with chemotherapy in the treatment of NSCLC (Leighl et al, 2010; Ramlau et al, 2012). On the basis of activity and safety profile, we conducted the current phase II study to explore the efficacy of ziv-aflibercept in the first-line setting. Similar to ECOG 4599, AVAiL, and PointBreak trials (Sandler et al, 2006; Patel et al, 2009a; Reck et al, 2009), this study was designed to test a three-drug regimen including an anti-angiogenesis agent, in this case ziv-aflibercept/cisplatin/pemetrexed. In addition, maintenance therapy with single-agent ziv-aflibercept was intended to prolong the benefits and delay the development of resistance. This approach was first tested in a phase I dose-escalation study that used the same regimen of ziv-aflibercept/cisplatin/pemetrexed in 18 patients with advanced solid tumours (Diaz-Padilla et al, 2012).
Our study population was representative of patients with advanced NSCLC. Overall, the median ziv-aflibercept dose intensity was similar to the planned intensity. The delivered dose intensities of pemetrexed/cisplatin in this study were over 98%, higher than those in the pemetrexed/cisplatin arm (94.8% and 95.0%, respectively) of the phase III trial (Scagliotti et al, 2008). The PK of ziv-aflibercept in this study was characterised as nonlinear and similar to that observed in the phase I study. The mean observed terminal t1/2 was independent of ziv-aflibercept dose. Administration of ziv-aflibercept did not alter pemetrexed PK. Development of ADA was a rare event, leading to reduced drug concentration in one patient who experienced anaphylaxis. As with all therapeutic proteins, there is a potential for immunogenicity; however, severe hypersensitivity reactions are rare. Although this study was terminated early, the two co-primary end points, ORR of 26% and median PFS of 5 months, were in accordance with most historical first-line NSCLC studies (Schiller et al, 2002; Scagliotti et al, 2008) and slightly less than triplet regimens incorporating another anti-VEGF agent (Sandler et al, 2006; Reck et al, 2009). However, there was no statistical power to test the primary hypothesis that ziv-aflibercept would enhance the efficacy of standard chemotherapy in NSCLC.
Biomarkers that can reliably predict the degree of VEGF blockade in vivo are currently not available. Preclinical studies identified increased erythropoietin production and erythropoiesis as a possible surrogate marker of VEGF inhibition, as animal data indicate that stringent VEGF inhibition, including by ziv-aflibercept, modulates erythropoiesis via increased hepatic erythropoietin synthesis (Tam et al, 2006). Bevacizumab has also been associated with increased haemoglobin in NSCLC (Riess et al, 2012) and reduced anaemia (Sher and Wu, 2011). Therefore, this study explored whether the increase in haemoglobin observed previously could be reproduced in the presence of chemotherapy and would correlate with anti-angiogenic activity. No trend towards increase in haemoglobin or change in erythropoietin level was found in a small subset of patients. However, as in the phase I study, a stabilisation of median haemoglobin values for multiple cycles as well as low rate of all-grade anaemia was observed. The result provides some support for the hypothesis that VEGF is a negative regulator of erythropoiesis, and its inhibitors may have a role in the management of anaemia.
The toxicity profile of this trial was consistent with published data on cisplatin plus pemetrexed and with the known effects of ziv-aflibercept with the exception of a higher than anticipated rate of RPLS (Gadgeel, 2012). Hypertension was the third most frequent TEAE and is a known adverse effect of anti-VEGF therapies. However, higher response rate was observed among patients who developed hypertension during the treatment than among those who did not in a post hoc analysis. This observation is consistent with data from ECOG 4599 that suggested improved outcomes associated with bevacizumab in patients developing hypertension on therapy (Dahlberg et al, 2010).
Although cases of RPLS have been observed in other ziv-aflibercept studies, the 7% rate observed in this study was much higher. It should be noted that the dose and schedule of ziv-aflibercept in this study at 6 mg kg−1 every 21 days is different from the one approved in colorectal cancer at 4 mg kg−1 every 14 days (Van Cutsem et al, 2012), although the dose intensity is the same at 2 mg kg−1 per week. At the recommended phase II dose of 6 mg kg−1 for ziv-aflibercept, no RPLS was reported in the phase I study that used the same regimen (N=7 at that dose level; Diaz-Padilla et al, 2012), or in another phase I study of ziv-aflibercept/cisplatin/docetaxel (N=17 at that dose level; Freyer et al, 2012), nor in combination with docetaxel in the VITAL study (N=456 in the combination arm; Ramlau et al, 2012). A meta-analysis of safety data from three large placebo-controlled studies reported no RPLS among 1333 patients treated with ziv-aflibercept in combination with standard chemotherapy (Allegra et al, 2012). It is likely that the development of RPLS may be regimen dependent rather than dose or schedule dependent.
Reversible posterior leukoencephalopathy syndrome is described as a brain-capillary leak syndrome frequently related to hypertension, fluid retention, and possibly the cytotoxic effects of immunosuppressive agents on the vascular endothelium (Hinchey et al, 1996). Risk factors include female sex, hypertension, and renal dysfunction (Vaughn et al, 2008), as well as anticancer agents: 75% were diagnosed in women and 71% were associated with combination regimens (Marinella and Markert, 2009). Bevacizumab and gemcitabine have been most commonly associated with RPLS. Treatment including cisplatin without concomitant anti-VEGF therapy has been associated with RPLS (Ito et al, 1998), whereas pemetrexed, before this study, was not. Consistent with the literature, the three cases of RPLS were all diagnosed in women, which may be related to an anticancer drug–oestrogen interaction inducing altered cerebral vasoreactivity and endothelial dysfunction. Agents that decrease VEGF signalling increases the risk of RPLS (including bevacizumab, sunitinib, sorafenib, and ziv-aflibercept), suggesting a class effect toxicity (Glusker et al, 2006). Clinical features of RPLS are neurological symptoms characterized by headaches, altered mental status, visual disturbances, or seizures, and systemic signs such as hypertension. Onset is variable ranging from hours to 1 month after completing therapy (Lee et al, 2008). Characteristic findings in brain MRI demonstrate bilateral, symmetric parieto-occipital subcortical and cortical vasogenic oedema (Bartynski, 2008). Removal of the causative agent and treatment of hypertension and renal insufficiency are indicated for RPLS, which is usually, but not always, reversible clinically.
In conclusion, this phase II study was designed to evaluate ziv-aflibercept in combination with cisplatin and pemetrexed in patients with untreated, advanced/metastatic non-squamous NSCLC. However, three confirmed and two suspected but unconfirmed cases of RPLS led to the early termination of the trial. The reason for the increased incidence of RPLS might be related to declining CrCL and/or increased BP. Although ORR and median PFS were in accordance with most historical first-line NSCLC studies, this combination of ziv-aflibercept/cisplatin/pemetrexed will not be further pursued in NSCLC. Future efforts to identify predictive biomarkers of anti-VEGF agents are warranted.
Acknowledgments
This study was supported by Sanofi and Regeneron Pharmaceuticals. We thank all the patients who participated in this study. We also thank all the participating study sites, and the investigators and research staff.
Drs Liu, Gao and DiCioccio are employees of Regeneron Pharmaceuticals, Inc. The remaining authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Allegra CJ, Tabernero J, Rougier P, Scagliotti GV, Philip PA, Lakomy R, Ramlau R, Assadourian S, Chevalier S, Van Cutsem E. Meta-analysis of anti-VEGF class adverse events from three double-blind (Db) placebo (Pbo)-controlled phase III trials with IV aflibercept (Afl) J Clin Oncol. 2012;30 (Suppl 4:abstract 561. [Google Scholar]
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036–1042. doi: 10.3174/ajnr.A0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–954. doi: 10.1200/JCO.2009.25.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot JF, Lamborn KR, Chang SM, Gilbert MR, Cloughesy TF, Aldape K, Yao J, Jackson EF, Lieberman F, Robins HI, Mehta MP, Lassman AB, DeAngelis LM, Yung WKA, Chen A, Prados MD, Wen PY. Phase II study of aflibercept in recurrent malignant glioma: A North American Brain Tumor Consortium Study. J Clin Oncol. 2011;29:2689–2695. doi: 10.1200/JCO.2010.34.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Padilla I, Siu LL, San Pedro-Salcedo M, Razak ARA, Colevas AD, Shepherd FA, Leighl NB, Neal JW, Thibault A, Liu L, Lisano J, Gao B, Lawson EB, Wakelee HA. A phase I dose-escalation study of aflibercept administered in combination with pemetrexed and cisplatin in patients with advanced solid tumours. Br J Cancer. 2012;107:604–611. doi: 10.1038/bjc.2012.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati A. Hunting and trapping the vascular endothelial growth factor. J Clin Oncol. 2010;28:185–187. doi: 10.1200/JCO.2009.25.4359. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Folkman J. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Freyer G, Isambert N, You B, Zanetta S, Falandry C, Favier L, Trillet-Lenoir V, Assadourian S, Soussan-Lazard K, Ziti-Ljajic S, Fumoleau P. Phase I dose-escalation study of aflibercept in combination with docetaxel and cisplatin in patients with advanced solid tumours. Br J Cancer. 2012;107:598–603. doi: 10.1038/bjc.2012.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadgeel SM. Safety profile and tolerability of antiangiogenic agents in non-small-cell lung cancer. Clin Lung Cancer. 2012;13:96–106. doi: 10.1016/j.cllc.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Gaya A, Tse V. A preclinical and clinical review of aflibercept for the management of cancer. Cancer Treat Rev. 2012;38:484–493. doi: 10.1016/j.ctrv.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;354:980–982. doi: 10.1056/NEJMc052954. [DOI] [PubMed] [Google Scholar]
- Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, Pessin MS, Lamy C, Mas J-L, Caplan LR. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- Isambert N, Freyer G, Zanetta S, You B, Fumoleau P, Falandry C, Favier L, Assadourian S, Soussan-Lazard K, Ziti-Ljajic S, Trillet-Lenoir V. Phase I dose-escalation study of intravenous aflibercept in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2012;18:1743–1750. doi: 10.1158/1078-0432.CCR-11-1918. [DOI] [PubMed] [Google Scholar]
- Ito Y, Arahata Y, Goto Y, Hirayama M, Nagamutsu M, Yasuda T, Yanagi T, Sobue G. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 1998;19:415–417. [PMC free article] [PubMed] [Google Scholar]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- Korpanty G, Smyth E, Sullivan LA, Brekken RA, Carney DN. Antiangiogenic therapy in lung cancer: focus on vascular endothelial growth factor pathway. Exp Biol Med. 2010;235:3–9. doi: 10.1258/ebm.2009.009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassoued W, Murphy D, Tsai J, Oueslati R, Thurston G, Lee WMF. Effect of VEGF and VEGF Trap on vascular endothelial cell signaling in tumors. Cancer Biol Ther. 2010;10:1326–1333. doi: 10.4161/cbt.10.12.14009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VH, Wijdicks EFM, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–210. doi: 10.1001/archneurol.2007.46. [DOI] [PubMed] [Google Scholar]
- Leighl NB, Raez LE, Besse B, Rosen PJ, Barlesi F, Massarelli E, Gabrail N, Hart LL, Albain KS, Berkowitz L, Melnyk O, Shepherd FA, Sternas L, Ackerman J, Shun Z, Miller VA, Herbst RS. A multicenter, phase 2 study of vascular endothelial growth factor trap (aflibercept) in platinum- and erlotinib-resistant adenocarcinoma of the lung. J Thorac Oncol. 2010;5:1054–1059. doi: 10.1097/jto.0b013e3181e2f7fb. [DOI] [PubMed] [Google Scholar]
- Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L, Buzenet G, Koehler E, Sosman JA, Schwartz LH, Gultekin DH, Koutcher JA, Donnelly EF, Andal R, Dancy I, Spriggs DR, Tew WP. Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol. 2010;28:207–214. doi: 10.1200/JCO.2009.22.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinella MA, Markert RJ. Reversible posterior leucoencephalopathy syndrome associated with anticancer drugs. Intern Med J. 2009;39:826–834. doi: 10.1111/j.1445-5994.2008.01829.x. [DOI] [PubMed] [Google Scholar]
- National Cancer Institute 2006. Common Terminology Criteria for Adverse Events, Version 3.0. Available from http://ctep.cancer.gov (accessed 28 August 2013).
- Patel JD, Bonomi P, Socinski MA, Govindan R, Hong S, Obasaju C, Pennella EJ, Girvan AC, Guba SC. Treatment rationale and study design for the PointBreak study: A randomized, open-label phase III study of pemetrexed/carboplatin/bevacizumab followed by maintenance pemetrexed/bevacizumab versus paclitaxel/carboplatin/bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non–small-cell lung cancer. Clin Lung Cancer. 2009;10:252–256. doi: 10.3816/CLC.2009.n.035. [DOI] [PubMed] [Google Scholar]
- Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT, Bonomi PD. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer. J Clin Oncol. 2009;27:3284–3289. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- Ramlau R, Gorbunova V, Ciuleanu TE, Novello S, Ozguroglu M, Goksel T, Baldotto C, Bennouna J, Shepherd FA, Le-Guennec S, Rey A, Miller VA, Thatcher N, Scagliotti GV. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30:3640–3647. doi: 10.1200/JCO.2012.42.6932. [DOI] [PubMed] [Google Scholar]
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, Leighl N, Mezger J, Archer V, Moore N, Manegold C. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- Riess JW, Logan AC, Krupitskaya Y, Padda S, Clement-Duchene C, Ganjoo K, Colevas AD, Pedro-Salcedo MS, Kuo CJ, Wakelee HA. Maintenance bevacizumab is associated with increased hemoglobin in patients with advanced, nonsquamous, non-small cell lung cancer. Cancer Invest. 2012;30:231–235. doi: 10.3109/07357907.2012.656862. [DOI] [PubMed] [Google Scholar]
- Sandler AB, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- Schiller JH, Harrington DP, Belani CP, Langer CJ, Sandler AB, Krook JE, Zhu J, Johnson DH, the Eastern Cooperative Oncology Group Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- Sher A, Wu S. Anti-vascular endothelial growth factor antibody bevacizumab reduced the risk of anemia associated with chemotherapy–A meta-analysis. Acta Oncol. 2011;50:997–1005. doi: 10.3109/0284186X.2011.581689. [DOI] [PubMed] [Google Scholar]
- Sitohy B, Nagy JA, Jaminet S-CS, Dvorak HF. Tumor-surrogate blood vessel subtypes exhibit differential susceptibility to anti-VEGF therapy. Cancer Res. 2011;71:7021–7028. doi: 10.1158/0008-5472.CAN-11-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BYY, Wei K, Rudge JS, Hoffman J, Holash J, Park S, Yuan J, Hefner C, Chartier C, Lee J-S, Jiang S, Nayak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- Tew WP, Gordon M, Murren J, Dupont J, Pezzulli S, Aghajanian C, Sabbatini P, Mendelson D, Schwartz L, Gettinger S, Psyrri A, Cedarbaum JM, Spriggs DR. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin Cancer Res. 2010;16:358–366. doi: 10.1158/1078-0432.CCR-09-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors (RECIST Guidelines) J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, Ruff P, van Hazel GA, Moiseyenko V, Ferry D, McKendrick J, Polikoff J, Tellier A, Castan R, Allegra C. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- Vaughn C, Zhang L, Schiff D. Reversible posterior leukoencephalopathy syndrome in cancer. Curr Oncol Rep. 2008;10:86–91. doi: 10.1007/s11912-008-0013-z. [DOI] [PubMed] [Google Scholar]