Abstract
Background:
Circulating concentrations of the cytokines interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF) and chemokines monocyte chemotatic protein 1 (MCP-1)/CCL2 and growth-regulator oncogene α (GROα)/chemokine C-X-C motif ligand 1 are commonly increased in cancer patients and they are increasingly recognised as important promoters, via divergent mechanisms, of cancer progression and metastasis.
Methods:
The effect of galectins-2, -4 and -8, whose circulating levels are highly increased in cancer patients, on endothelial secretion of cytokines was assessed in vitro by cytokine array and in mice. The relationship between serum levels of galectins and cytokines was analysed in colon and breast cancer patients.
Results:
Galectins-2, -4 and -8 at pathological concentrations induce secretion of G-CSF, IL-6, MCP-1 and GROα from the blood vascular endothelial cells in vitro and in mice. Multiple regression analysis indicates that increased circulation of these galectins accounts for 41∼83% of the variance of these cytokines in the sera of colon and breast cancer patients. The galectin-induced secretion of these cytokines/chemokines is shown to enhance the expression of endothelial cell surface adhesion molecules, causing increased cancer-endothelial adhesion and increased endothelial tubule formation.
Conclusion:
The increased circulation of galectins -2, -4 and -8 in cancer patients contributes substantially to the increased circulation of G-CSF, IL-6 and MCP-1 by interaction with the blood vascular endothelium. These cytokines and chemokines in turn enhance endothelial cell activities in angiogenesis and metastasis.
Keywords: galectins, chemokines, angiogenesis, metastasis
Galectins are a family of at least 15 galactoside-binding proteins that share similar carbohydrate recognition domains (CRD). They are widely expressed by various types of human cells. Altered expressions of galectins, usually increased, is commonly seen in various cancers (Liu and Rabinovich, 2005; Newlaczyl and Yu, 2011; Barrow et al, 2011b) and is increasingly shown to be involved in the regulation of various stages of cancer development, progression and metastasis (Liu and Rabinovich, 2005; Newlaczyl and Yu, 2011).
Research over the past few years has shown that the concentrations of several galectins, notably galectins -2, -3, -4 and -8 are markedly increased, typically 5- to 30-fold, in the bloodstream of various types of cancers including colorectal (Watanabe et al, 2011; Barrow et al, 2011a, 2013), breast (Iurisci et al, 2000), pancreatic (Senapati et al, 2011; Xie et al, 2012), melanoma (Vereecken et al, 2009), bladder (Sakaki et al, 2008), head and neck cancer (Saussez et al, 2008). Patients with metastasis have even higher levels of circulating galectins than those with only localised tumours (Barrow et al, 2011a). Our recent studies have revealed that increased circulation of galectin-3, a key member of the galectin family, is an important promoter of metastasis (Zhao et al, 2009). We have shown that part of the metastasis-promoting effect of circulating galectin-3 is related to its binding to the oncofetal Thomsen-Friedenreich carbohydrate Galβ1, 3GalNAc- (TF) antigen that commonly exists as an O-glycan on the extracellular domain of the cancer-associated mucin protein MUC1 (Yu et al, 2007). The galectin-3-MUC1 interaction induces MUC1 cell surface polarisation resulting in exposure of smaller cell surface adhesion molecules, leading to increased cancer cell adhesion to the vascular endothelium (Zhao et al, 2009) and increased cancer cell homotypic aggregation for the formation of tumour emboli that prolongs the survival of tumour cells in the circulation (Zhao et al, 2010). A similar effect on cancer cell adhesion and aggregation is also observed with circulating galectins -2, -4 and -8 (Barrow et al, 2011a).
More recently, we have revealed that the metastasis-promoting effects of circulating galectin-3 are also contributed to by its interaction with the blood vascular endothelium, inducing the endothelial cells to secret cytokines granulocyte colony-stimulating factor (G-CSF), GM-CSF, interleukin-6 (IL-6) and sICAM-1 (Chen et al, 2013). Pro-inflammatory cytokines such as IL-6 are well known for their roles in promoting cancer progression and metastasis via divergent mechanisms such as stimulation of cancer cell proliferation, angiogenesis, cell adhesion and by the local and systemic suppression of host anti-tumour immunity (Ara and Declerck, 2010; Tsujimoto et al, 2010). We have previously analysed the circulating levels of all the galectins (galectins -1, -2, -3, -4, -7 and -8) whose recombinant forms are commercially available and this revealed increase of circulating galectins -2, -3, -4 and -8 in cancer patients (Barrow et al, 2011a). As members of the galectin family share similar CRD, the effect of increased circulation of galectin-3 on endothelial secretion of the metastasis-promoting cytokines led us to speculate that the increased levels of circulating galectins -2, -4 and -8 in cancer might also, like that of galectin-3, induce secretion of cytokines from the vascular endothelium and thus contribute to cancer metastasis.
This study therefore investigated the effects of increased circulating galectins -2, -4 and -8 on cytokine secretion by the vascular endothelium in vitro and in mice and analysed the relationship between these circulating galectins and cytokine concentrations in the sera of colon and breast cancer patients.
Materials and methods
Materials
Recombinant human galectins -2, -4 and -8 (residual endotoxin levels <1.0 EU μg−1 protein), and human Cytokine Protein Array, antibodies against CD44, E-selectin, VCAM-1 and integrinαvβ1 were purchased from R&D Systems (Abingdon, UK). Calcein AM Cell Labeling Solution was from Invitrogen (Paisley, UK). Human recombinant cytokine IL-6, G-CSF, growth-regulator oncogene α (GROα) and monocyte chemotatic protein 1 (MCP-1), mouse recombinant IL-6, G-CSF, GROα, MCP-1 and mouse cytokine ELISA kits were from PeproTech (London, UK). Mouse cytokine GROα ELISA kit was from PromoKine (Heidelberg, Germany). In Vitro Angiogenesis Tube Formation kits were from AMS Biotechnology Ltd (Abingdon, UK). Non-Enzymatic Cell Dissociation Solution (NECDS) and all other chemicals were from Sigma (Dorset, UK).
Cell lines
The MUC1-negative HCT116 human colon cancer cells (Ren et al, 2004) were obtained from the European Cell Culture Collections via the Public Health Laboratory Services (Porton Down, Wiltshire, UK) and cultured in McCoy's 5a medium. The MUC1-negative ACA19− cells selected from human melanoma A375 cells (Wesseling et al, 1996) were kindly provided by Dr John Hilkens (The Netherland Cancer Institute) and cultured in Dulbecco's modified Eagle's medium (No authentication of the cell lines was done by the authors other than verification of appropriate presence/absence of MUC1). Human micro-vascular lung endothelial cells (HMVEC-Ls) and human umbilical vein endothelial cells (HUVECs) were obtained from Lonza (Wokingham, UK) and cultured in EGM-2 and EGM endothelial growth media (Lonza), respectively. Less than five passages of the endothelial cells were used in all experiments.
Cytokine array
Human micro-vascular lung endothelial cells (2 × 105 per well) were cultured in six-well plates at 37 °C for 24 h before introduction of 1.5 μg ml−1 recombinant galectins -2, -4, -8 or BSA for 24 h. The culture media were collected and the concentrations of cytokines in the culture media were analysed by the Human Cytokine Protein Arrays exactly as in the instruction of the kit. These arrays assayed 36 cytokines (C5/C5a, CD40 Ligand, G-CSF, GM-CSF, GROα, I-309, sICAM-1, IFN-γ, IL-1α, IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12 p70, IL-13, IL-16, IL-17, IL-17E, IL-23, IL-27, IL-32α, IP-10, I-TAC, MCP-1, MIF, MIP-1α, MIP-1β, Serpin E1, RANTES, SDF-1, TNF-α and sTREM-1), each in duplicate. The arrays were quantified with Bio-Rad Image Lab software (Image Lab 2.0, Hercules, CA, USA).
Cytokine determination
Human micro-vascular lung endothelial cells (1 × 105 per well) were cultured in 12-well plates at 37 °C for 24 h before introduction of control BSA or recombinant galectins -2, -4 or -8 for 24 h. The culture media were collected and the concentrations of G-CSF, IL-6, GROα and MCP-1 in the culture media were analysed by ELISA.
Determination of cancer cell-endothelial adhesion
Human micro-vascular lung endothelial cells (4 × 104 per well) were cultured in 96-well plates for 24 h for the formation of endothelial cell monolayers. Recombinant galectins -2, -4 or -8 (1.5 μg ml−1) or control BSA were then added with or without further addition of lactose (final concentration 200 μg ml−1) for 24 h. The endothelial cells were either washed and used for subsequent assessment of cancer cell adhesion, or the culture media (conditioned media) were collected and used for assessment of their effects on cancer cell adhesion to fresh, untreated HMVEC-Ls monolayers.
ACA19− and HCT116 cancer cells were detached from the culture plates with NECDS, washed and suspended at 5 × 106 cells per ml in serum-free DMEM. The cells were labelled with 10 μl ml−1 Calcein AM Cell Labeling Solution at 37 °C for 30 min. After two washes with PBS, the cells were resuspended into 5 × 104 ml−1 with serum-free DMEM and applied (5 × 103 per well) to monolayers of fresh or galectin-treated HMVEC-Ls for 1 h at 37 °C. The HMVEC-Ls monolayer was washed twice with PBS and the endothelial cell-associated fluorescence was measured using a TECAN infinite F200 fluorescent microplate reader at 485 nm excitation/520 nm emission.
Analysis of endothelial cell surface adhesion molecule expression
Human micro-vascular lung endothelial cells were treated with galectins -2, -4, -8 or control BSA (all 1.5 μg ml−1) for 24 h before released by NECDS. The cells were washed with PBS and fixed with 2% paraformaldehyde for 20 min before incubation with 5% goat serum/PBS for 30 min. After removal of the supernatant, the cells were suspended in 1% goat serum/PBS (106 cells ml−1) and introduced with antibodies against cell surface adhesion molecules (integrinαvβ1, CD44, E-selectin or VCAM, all at 1 : 400 dilutions in 1% BSA in PBS) for 1 h. The cells were washed twice with PBS before application of FITC-conjugated secondary antibodies (1 : 400 dilutions) for 1 h. After three washes, the cells were resuspended in PBS and analysed by flow cytometry.
Measurement of endothelial tubule formation
Human micro-vascular lung endothelial cells cultured in 12-well plates were incubated with 1.5 μg ml−1 BSA, galectins -2, -4 or -8 for 24 h. The culture media were collected and used for assessment of their ability to stimulate HUVEC tubule formation assessed using the in vitro Angiogenesis kit with or without the addition of a combination of antibodies against G-CSF (5 μg ml−1), IL-6 (3 μg ml−1), GROα (20 μg ml−1) or MCP-1(20 μg ml−1). Endothelial tube formation was visualised by fluorescence microscopy. The tubule length and branch points were quantified using ImageJ.
In vivo measurement of the galectin effect on cytokine secretion in mice
Twenty-seven 6–8 weeks old female C57BL/6 mice, obtained from Charles River Laboratories (Margate, Kent, UK) and maintained and used in accordance with the animal care protocol approved by University of Liverpool, were randomly divided into nine equal groups and 5 μg galectins -2, -4, -8 or a combination of 5 μg each galectins -2, -4 and 8 was injected by intravenous tail vein injection. The blood was obtained by cardiac puncture at 0, 24 and 48 h and the serum concentrations of G-CSF, GM-CSF, IL-6 and sICAM-1 were determined by ELISAs.
Human sera
Serum samples from 40 breast and 50 colorectal cancer patients were obtained from the CTBRC cancer tissue bank (Liverpool, UK) (Supplementary Table S1). The serum samples had been obtained by CTBRC from patients at the time of primary tumour resection at the Royal Liverpool University Hospital with informed consents from the patients. The study was approved by the Liverpool (Adult) Local Research Ethics Committee (REC number: 07/Q1505/14).
Serum galectin assays
The galectins -2, -4 and -8 concentrations in the serum of breast and colorectal cancer patients were determined by ELISA as described in our previous study (Barrow et al, 2011a).
Statistical analysis
Unpaired t-test was used for single comparison, one-way ANOVA followed by Bonferroni correction for multiple comparisons was used where appropriate. The associations between serum levels of galectins and cytokines in cancer patients were tested using both simple and multiple regression models. Multiple regression analysis was used to test whether there was evidence of an overall correlation between each cytokine outcome and a combination of galectins and the F-test results were reported. The beta estimates, which represent the change in cytokines outcomes (ng) as the galectin level is increased by 1 ng, were reported. R2 values were also reported in the multiple regression analysis as a measure of the extent to which alterations in serum concentrations of galectins -2, -4 and -8 together account for the observed variance in cytokine concentration. P<0.05 from two-sided tests were considered significant in all the analyses.
Results
Galectins -2, -4 and -8 each induce secretion of cytokines from HMVEC-Ls
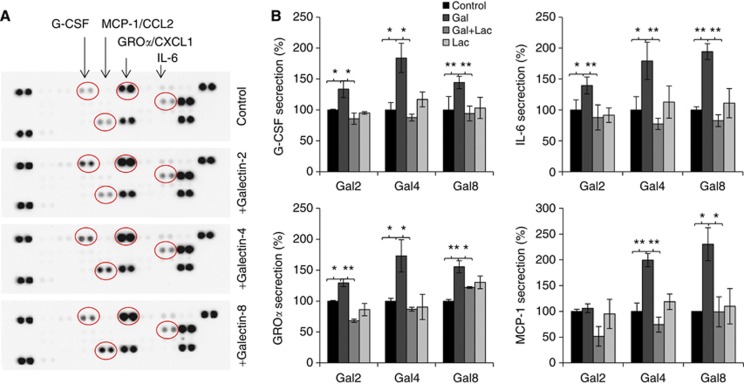
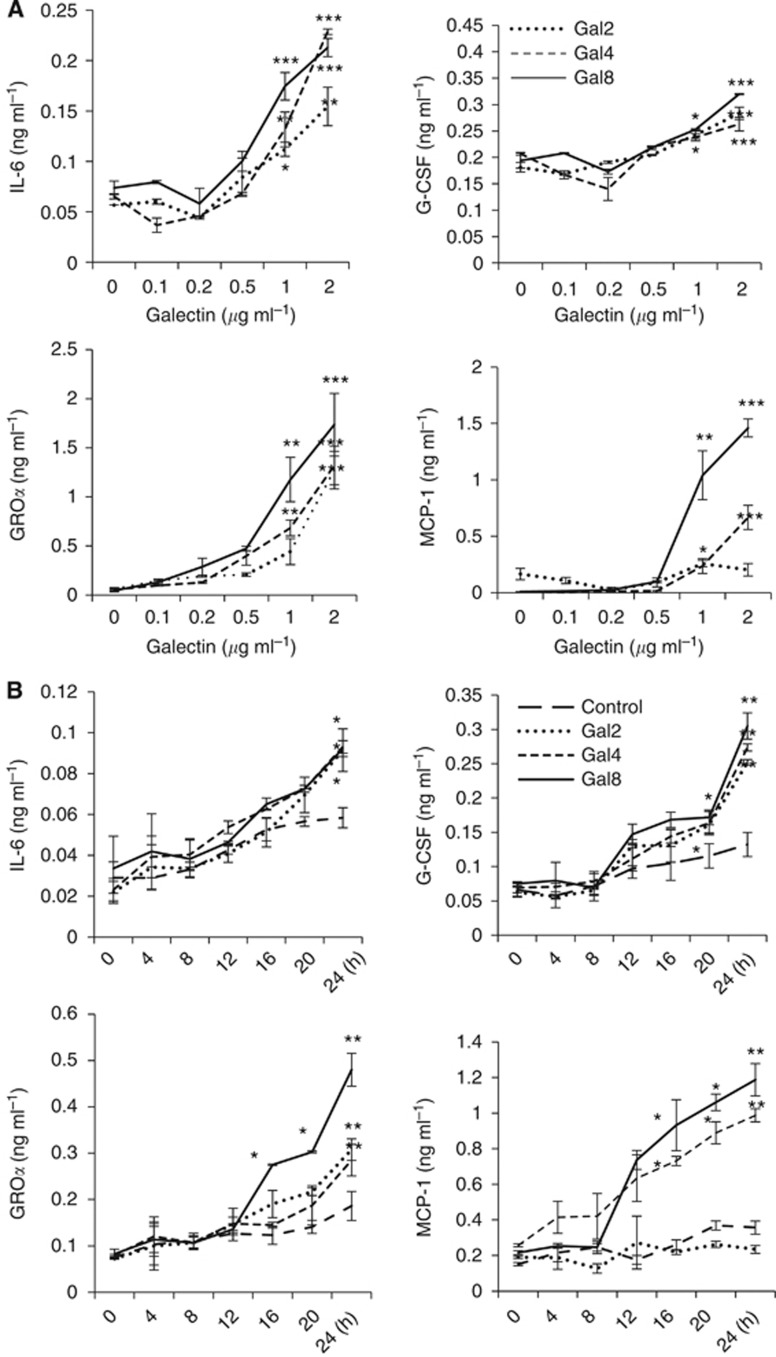
To investigate the influence of galectins -2, -4 and -8 on endothelial secretion of cytokines, we first tested the cytokine secretion profile of HMVEC-Ls in their response to recombinant galectins -2, -4 or -8 at a concentration (1.5 μg ml−1) typically found for each of these galectins in cancer sera. Treatment of HMVEC-Ls with galectins -2, -4 or -8 resulted in increased cell release of G-CSF, IL-6, GROα/chemokine C-X-C motif ligand 1 (CXCL1) and MCP-1/CCL2. Galectin-2 treatment resulted in a 3.1-fold increase of G-CSF, 1.5-fold IL-6 and 1.7-fold GROα. Galectin-4 treatment caused a 2.6-fold increase of G-CSF, 1.4-fold IL-6, 1.7-fold GROα and 3.0-fold MCP-1, whereas galectin-8 induced 2.4-fold increase of G-CSF, 1.5-fold IL-6, 1.7-fold GROα and 3.0-fold MCP-1 (Figure 1A). These effects of galectins were inhibited by the presence of lactose (Figure 1B) and showed to be time-dependent and occurred dose-dependently at various pathological galectin concentrations seen in cancer patients (Barrow et al, 2011a) (Figure 2).
Figure 1.
Galectins -2, -4 and -8 induce cytokine secretion from endothelial cells. Human micro-vascular lung endothelial cells were incubated with 1.5 μg ml−1 galectins -2, -4, -8 or BSA for 24 h before the cytokine levels in the culture media were analysed by the cytokine array (A). In (B), HMVEC-Ls cells were treated with 1.5 μg ml−1 galectins -2, -4, -8 or BSA in the presence or absence of 200 μg ml−1 lactose for 24 h before the G-CSF, IL-6, GROα and MCP-1 levels in the cultured media were determined by ELISA. The data are expressed as percentage compared with BSA-treated controls from three independent experiments, each in triplicate. *P<0.05, **P<0.01 (ANOVA, Bonferroni).
Figure 2.
Galectins -2, -4 and -8-induced endothelial secretions of cytokines are time- and dose-dependent. Human micro-vascular lung endothelial cells were treated with various concentrations of galectins -2, -4 or -8 for 24 h (A), or with 1.5 μg ml−1 galectins -2, -4, -8 or BSA for various times (B), before the G-CSF, IL-6, GROα and MCP-1 levels in the cultured media were determined. The data are expressed as mean±s.d. of triplicate determinations *P<0.05, **P<0.01, ***P<0.001 (ANOVA, Bonferroni).
It was found that the presence of each of these four cytokines/chemokines, either alone or in combination at concentrations (0.3 ng ml−1 G-CSF, 0.2 ng ml−1 IL-6, 1 ng ml−1 GROα or 1 ng ml−1 MCP-1) similar to that induced from HMVEC-Ls by 24-h treatment with 1.5 μg ml−1 galectin, did not show any significant effect on the levels of galectins -2, -4 or -8 in the medium of HMVEC-Ls after 24 and 48 h culture (Data not shown). This indicates that the galectin-induced secretion of these cytokines does not form a feedback loop to enhance endothelial secretion of galectins.
Galectin-induced cytokine secretion enhances cancer cell-endothelial adhesion
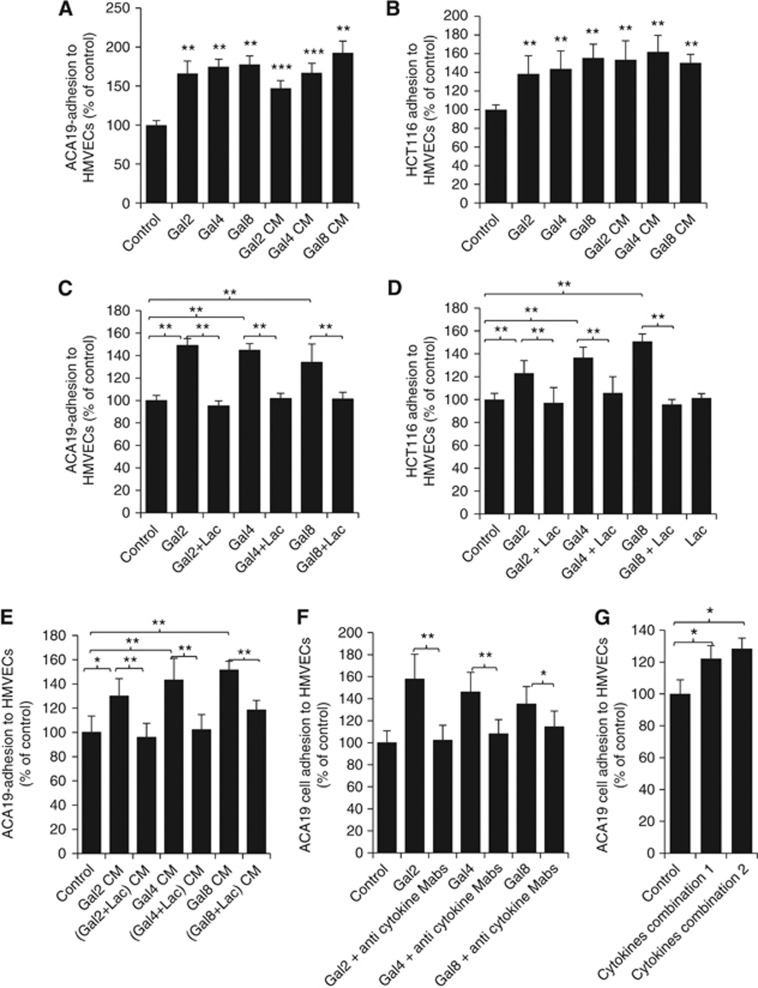
Our previous studies have shown that circulating galectin-3 induces endothelial secretion of G-CSF, IL-6, GM-CSF and ICAM-1 that enhances cancer cell adhesion to the vascular endothelium (Chen et al, 2013). As G-CSF and IL-6 were also seen to be induced from the vascular endothelium by galectins -2, -4 and -8, we then investigated the influence of the galectin-induced secretion of these cytokines on cancer cell adhesion to endothelium. Co-culture of HMVEC-Ls with 1.5 μg ml−1 galectins -2, -4 or -8 for 24 h each increased 70∼82% adhesion of human melanoma ACA19− (Figure 3A) and 39∼60% adhesion of colon cancer HCT116 cells (Figure 3B). When the culture medium (conditioned medium) from 24 h galectin-treated HMVEC-Ls was used as culture medium to assess ACA19− and HCT116 cell adhesion to fresh HMVEC-Ls, similar increases of cell adhesion were observed. The increased cell adhesion induced by the galectins (Figure 3C and D) or the conditioned medium (Figure 3E) was largely prevented by the presence of lactose in the initial galectin-HMVEC-Ls culture. The presence of lactose alone did not have any effect on adhesion of ACA19− and HCT116 cells to HMVECs. Addition to the conditioned medium of neutralising antibodies against G-CSF, IL-6, GROα and MCP-1 almost completely negated the conditioned medium-induced cancer cell adhesion (Figure 3F). Furthermore, introduction of a combination of recombinant G-CSF, IL-6, GROα and MCP-1 or G-CSF, IL-6 and GROα at concentrations similar to that induced from HMVEC-Ls after 24 h galectin-treatment (Figure 2), to the conditioned medium from BSA-treated control HMVEC-Ls induced a similar increase of ACA19− cell adhesion as that from the conditioned medium from the galectin-treated HMVEC-Ls (Figure 3G). Collectively, these results indicate that the increased secretion of these cytokines by galectins -2, -4 or -8 enhances cancer cell adhesion to endothelium.
Figure 3.
Galectin-induced cytokine secretion enhances cancer cell-endothelial adhesion. (A and B) The presence of galectins -2, -4 or -8 increase cancer cell adhesion to HMVEC-Ls. Human micro-vascular lung endothelial cells were treated with 1.5 μg ml−1 galectins -2, -4 or -8 for 24 h. The cells were either washed and used for subsequent assessment of ACA19− (A) or HCT116 (B) cell adhesion, or the culture medium (CM) were collected and used for subsequent assessment of ACA19− (A) or HCT116 (B) cell adhesion. (C and D) The galectin-induced cancer cell adhesion is inhibited by lactose. Human micro-vascular lung endothelial cells were treated with 1.5 μg ml−1 galectins -2, -4 or -8 in the presence or absence of 200 μg ml−1 lactose for 24 h. The HMVEC-Ls were then used for assessment of ACA19− (C) and HCT116 (D) cell adhesion or the culture medium were collected and used for assessment of ACA19− cell adhesion to fresh HMVEC-Ls (E). (F) Galectin-mediated cancer cell-endothelial adhesion is inhibited by neutralising anti-cytokine antibodies. Human micro-vascular lung endothelial cells were treated with or without 1.5 μg ml−1 galectins -2, -4 or -8 in the presence or absence of antibodies against G-CSF (5 ng ml−1), IL-6 (3 ng ml−1), GROα (20 ng ml−1) and MCP-1(20 ng ml−1) in combination for 24 h before ACA19− adhesion to the HMVEC-Ls was assessed. (G) Recombinant cytokines induce cancer cell adhesion to HMVEC-Ls. Human micro-vascular lung endothelial cells were treated without or with a combination of G-CSF (0.25 ng ml−1), IL-6 (0.15 ng ml−1) and GROα (1 ng ml−1) (combination 1) or G-CSF (0.25 ng ml−1), IL-6 (0.15 ng ml−1), GROα (1 ng ml−1) and MCP-1 (1 ng ml−1) (combination 2) for 24 h before ACA19− adhesion to HMVEC-Ls was assessed. All the data are expressed as percentage compared with BSA-treated controls from at least three independent experiments, each in triplicate. *P<0.05, **P<0.01, ***P<0.001 (ANOVA, Bonferroni).
Increased expression of the cell surface adhesion molecules is responsible for galectin-induced-, cytokine-mediated cancer cell-endothelial adhesion
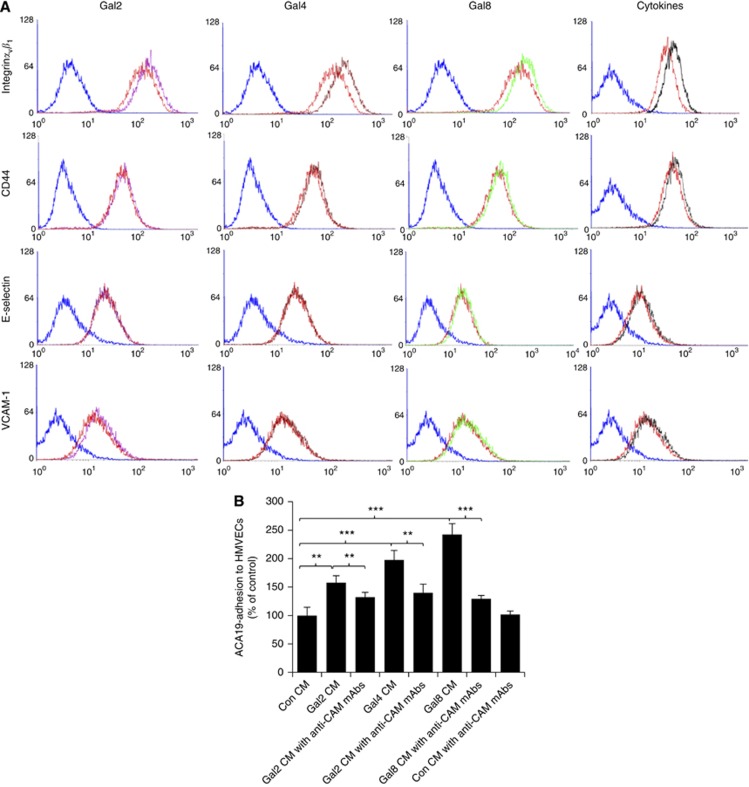
We next investigated whether the galectin-induced, cytokine-mediated increase of cancer cell adhesion was associated with change in the expression of endothelial cell surface adhesion molecules. Twenty-four hour treatment of HMVEC-Ls with each of these galectins enhanced the expression of several cell surface adhesion molecules in particular integrinαvβ1 (Figure 4A). Treatment with galectin-2 resulted in 34.8% increase of cell surface integrinαvβ1, a small increase of VCAM-1 (9.8%) but no effect on CD44 and E-selectin, whereas galectins -4 or -8 treatment induced 41.0% and 32.4%, respectively, increase of integrinαvβ1 and a small increase of CD44 (8.9 and 5.4%) but no effect on E-Selectin and VCAM-1 expressions.
Figure 4.
Galectin-induced cytokine secretion enhances expression of the endothelial cell surface adhesion molecules, which are responsible for galectin-mediated cancer cell-endothelial adhesion. (A) The presence of galectins induces expressions of cell surface adhesion molecules. Human micro-vascular lung endothelial cells were treated with control 1.5 μg ml−1 BSA (red colour) or 1.5 μg ml−1 galectins -2 (purple), -4 (brown), -8 (green), a combination of G-CSF (0.25 ng ml−1), IL-6 (0.15 ng ml−1), GROα (1 ng ml−1), MCP-1 (1 ng ml−1) (black) for 24 h before the expressions of the HMVEC surface integrinαvβ1, VCAM-1, CD44 and E-selectin were analysed by flow cytometry. (B) The presence of neutralising antibodies against cell surface adhesion molecules inhibits galectins -2, -4 or -8-mediated cancer cell adhesion. Human micro-vascular lung endothelial cells were treated without or with 1.5 μg ml−1 galectins -2, -4 or -8 for 24 h. The culture media were collected and used to assess ACA19− cell adhesion to fresh HMVEC-Ls without or with addition of a combination of neutralising antibodies against integrinαvβ1 (10 μg ml−1), CD44 (10 μg ml−1), VCAM-1(10 μg ml−1) and E-selectin (10 μg ml−1). **P<0.01, ***P<0.001 (ANOVA, Bonferroni).
To investigate whether the increased expression of these cell surface adhesion molecules was responsible for the galectin induced, cytokine-mediated cancer cell adhesion, adhesion of ACA19− cells to HMVEC-Ls was assessed with the conditioned medium from control- or galectin-treated HMVEC-Ls with and without addition of neutralising antibodies against integrinαvβ1, CD44, VCAM-1and E-selectin. The addition of neutralising antibodies against these cell adhesion molecules in the conditioned medium from galectin-treated HMVEC-Ls reduced the conditioned medium-mediated ACA19− cell adhesion (Figure 4B). Together, these results suggest that the increased expression of endothelial cell surface adhesion molecules is largely responsible for the galectin-induced, cytokine-mediated cancer cell adhesion.
Galectin-induced cytokine secretion promotes endothelial cell tube formation
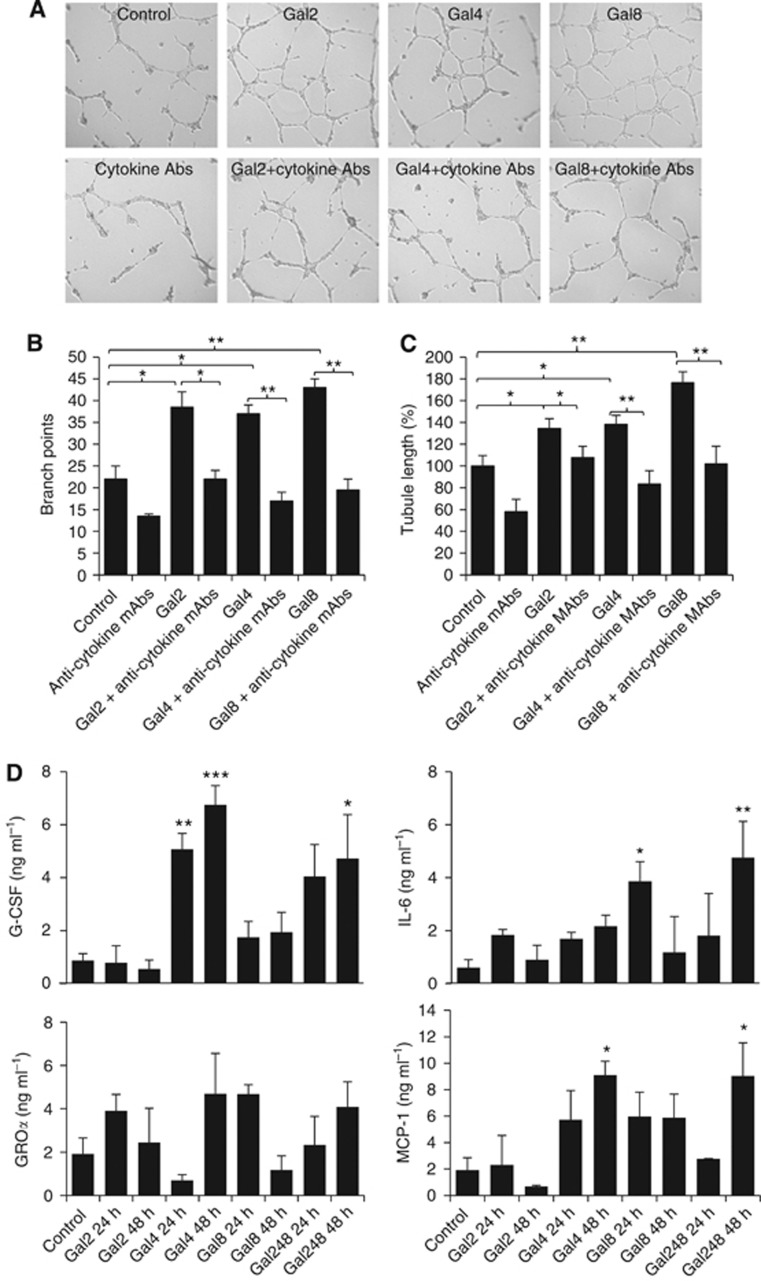
As many pro-inflammatory cytokines and chemokines are angiogenesis promoters (Ara and Declerck, 2010), we then assessed the effect of galectin-induced secretion of these cytokines/chemokines on endothelial tubule formation, an important component of angiogenesis. Culture of HUVECs with the conditioned medium obtained from 24 h galectin-treated HMVEC-Ls increased the ability of HUVECs to form microtubule structures (67∼87% increase of branch points and 34∼78% increase of tubule length) in comparison with the HUVECs cultured in the conditioned medium from BSA-treated control HMVEC-Ls (Figure 5A–C). These effects of galectins were prevented by the addition to the conditioned medium of a combination of neutralising anti-cytokine antibodies. These results indicate that the galectin-induced secretion of cytokines from endothelium enhances endothelial angiogenesis.
Figure 5.
Galectins induce cytokine secretion in vivo and the galectin-induced cytokine secretion enhances endothelial tubule formation. Human umbilical vein endothelial cells cultured on matrix proteins were incubated with conditioned medium (CM) obtained from HMVEC-Ls treated with BSA, galectins -2, -4 or -8 (1.5 μg ml−1) for 24 h, with or without introduction to the CM of a combination of neutralising antibodies against G-CSF (5 ng ml−1), IL-6(3 ng ml−1), GROα (20 ng ml−1) and MCP-1(20 ng ml−1) for 24 h at 37 °C. Representative images are shown in (A). The tubule length (B) and branch points (C) were quantified. Data are expressed from three independent experiments, each in triplicate. (D) Shows in vivo effect of tail vein injection of 5 μg galectins -2, -4, -8, individually, or in combination (5 μg each) on serum levels of the cytokines/chemokines at 0, 24 and 48 h in mice. *P<0.05, **P<0.01, ***P<0.001 (ANOVA, Bonferroni).
Galectin-3 induces cytokine secretion in vivo
Galectins from human and mouse origins all bind to galactoside-terminated glycans through their CRD. The galectin-mediated secretion of cytokines from human vascular endothelial cells was shown to be inhibited by lactose (Figure 3C and D), indicating the importance of galectin CRD in their actions on cytokine secretion from the vascular endothelium. As not all recombinant mouse galectins were commercially available, we used recombinant human galectins, which showed induced secretion of these cytokines from both human (Figures 1 and 2) and mouse vascular endothelial cells (Supplementary Figure S1) in vitro, in the subsequent animal experiments. When 5 μg per human galectins -2, -4 or -8, equating approximately to circulating galectin concentrations seen in cancer patients with metastasis (Barrow et al, 2011a), was injected intravenously into the animal tail vein, a 490.7±68.6% and 683.7±83.7% increase of serum G-CSF were seen at 24 and 48 h by galectin-4 (Figure 5D). A 375.9±54.9% increase of MCP-1/CCL2 was also observed after 48 h by galectin-4. Galectin-8 injection caused a 543.3±123.3% increase of serum IL-6 after 48 h. Interestingly, galectin-2 injection did not show any significant effect on serum levels of any of these cytokines/chemokines. Serum GROα/CXCL1 levels were not affected by injection of any of these galectins. Injection of a combination of 5 μg per mouse of each galectins -2, -4 and -8 increased serum G-CSF (448.8±191.9%), IL-6 (691.7±228.3%) and MCP-1 (373.3±130.4%) after 48 h and the increase of each of these cytokines/chemokines was equivalent to that produced by the most influential galectin member (galectin-4) when injected individually. These results provide evidence of a direct impact of circulating galectins on secretion of these cytokines in vivo. They also indicate that these galectin members may share some of the endothelial receptors to exert their effects on endothelial secretion of these cytokines/chemokines, and thus showing lack of an additive effect when they are present in combination.
Relationship between serum levels of galectins and cytokines/chemokines in breast and colon cancer patients
To see whether the relationship between these galectins and cytokines/chemokines observed in vitro and in mice occurred in cancer patients, serum levels of circulating galectins -2, -4 and -8 and G-CSF, IL-6, GROα/CXCL2, and MCP-1/CCL1 were analysed in breast and colon cancer patients (Supplementary Table S2). Simple regression analysis showed significant correlation of G-CSF and IL-6 levels, but not GROα and MCP-1, with each of the galectins in both breast and colon cancer patients (Table 1, Supplementary Figure 2). Multiple regression analysis demonstrated significant correlation of G-CSF (P<0.001) and IL-6 levels (P<0.001) with galectin-2 in breast and colon cancers. Granulocyte colony-stimulating factor and IL-6 levels were significantly (P=0.048) or nearly significantly (P=0.056) correlated with galectin-4 in colon cancer but not in breast cancer. Both G-CSF and IL-6 showed close to significant correlation with galectin-8 in breast (P=0.06 and 0.079) and colon cancers (P=0.051 and 0.069). In the fitted multiple regression model, galectins -2, -4 and -8 in combination was seen to account for 82 and 83% of the variance (R2 values), respectively, of G-CSF and IL-6 in the sera of breast cancer patients and 69 and 51% in colon cancer (Table 1). They were also accountable for 41% variance of MCP-1 in the sera of colon cancer patients and 6% in breast cancer patients. A small (8%) variance in GROα in the breast cancer sera, but none in colon cancer sera, was seen to be accountable by these galectins. The combined impact of galectins -2, 4- and -8 on these cytokines in the sera is large for most of these cytokines as manifested by the high coefficient of multiple correlation (R) values (0.91 for G-CSF, 0.91 for IL-6 in breast cancer and 0.78 for G-CSF, 0.71 for IL-6 and 0.64 for MCP-1/CCL2 in colon cancer patients). These observations provide further support to an important role of these galectins on secretion of these cytokines in the presence of cancer. It should be mentioned that as circulating galectin levels are correlated with each other in the presence of cancer, the multiple regression analysis is likely to identify only the significance of the strongest association and may mask weaker associations between the galectins and cytokines/chemokines.
Table 1. Simple and multiple regression analysis of serum galectins -2, -4, and -8 and GCS-F, IL-6, GROα and MCP-1 levels in human breast and colon cancer patients.
|
Simple regression |
Multiple regression |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
G-CSF |
IL-6 |
GROα/CXCL-1 |
MCP1/CCL-2 |
G-CSF |
IL-6 |
GROα/CXCL-1 |
MCP1/CCL-2 |
|||||||||
| Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | Beta (95%CI) | P | |
|
Breast cancer (n=40) | ||||||||||||||||
| Gal-2 | 16.02 (13.42–18.62 | <0.001 | 4.56 (3.84–5.27) | <0.001 | −0.002 (−0.007–0.004) | 0.59 | 0.000 (−0.001–0.001) | 0.71 | 15.97 (12.56–19.39) | <0.001 | 4.56 (3.62–5.51) | <0.001 | −0.003 (−0.01–0.005) | 0.51 | −0.0002 (−0.002–0.001) | 0.75 |
| Gal-4 | 16.28 (7.93–24.62) | <0.001 | 4.63 (2.28–6.99) | <0.001 | −0.002 (−0.012–0.007) | 0.61 | 0.001 (−0.002–0.003) | 0.60 | −2.87 (−8.53–2.79) | 0.31 | −0.79 (−2.36–0.78) | 0.32 | −0.003 (−0.015–0.001) | 0.64 | 0.0002 (−0.002–0.003) | 0.88 |
| Gal-8 | 19.96 (8.32–31.60) | 0.001 | 5.55 (2.24–8.86) | 0.002 | 0.008 (−0.005–0.020) | 0.22 | 0.002 (−0.001–0.005) | 0.13 | 6.162 (−0.269–12.59) | 0.06 | 1.59 (−0.19–3.37) | 0.079 | 0.012 (−0.003–0.026) | 0.11 | 0.002 (−0.001–0.005) | 0.16 |
| F-test |
|
NA |
|
NA |
|
NA |
|
NA |
|
<0.001 |
|
<0.001 |
|
0.40 |
|
0.50 |
|
R2 |
NA |
NA |
NA |
NA |
0.82 |
0.83 |
0.08 |
0.06 |
||||||||
|
Colon cancer (n=50) | ||||||||||||||||
| Gal-2 | 1.64 (1.28–2.01) | <0.001 | 0.13 (0.09–0.17) | <0.001 | 0.00 (−0.011–0.11) | 0.96 | 0.001 (0.003–0.008) | <0.001 | 1.45 (1.08–1.82) | <0.001 | 0.11 (0.064–0.15) | <0.001 | −0.000 (−0.012–0.12) | 0.99 | 0.005 (0.0024–0.007) | <0.001 |
| Gal-4 | 1.34 (0.50–2.18) | 0.002 | 0.14 (0.04–0.2) | 0.003 | −0.002 (−0.019–0.015) | 0.82 | 0.003 (−0.001–0.008) | 0.15 | 0.56 (0.006–1.12) | 0.048 | 0.064 (−0.002–0.13) | 0.056 | −0.002 (−0.02–0.16) | 0.82 | 0.0002 (−0.003–0.004) | 0.91 |
| Gal-8 | 2.07 (0.39–3.76) | 0.017 | 0.20 (0.04–0.36) | 0.017 | 0.002 (−0.031–0.035) | 0.90 | 0.13 (0.005–0.20) | 0.002 | 1.04 (0.005–2.10) | 0.050 | 0.11 (−0.01–0.24) | 0.069 | 0.002 (−0.031–0.037) | 0.88 | 0.0095 (0.002–0.16) | <0.001 |
| F-test |
|
NA |
|
NA |
|
NA |
|
NA |
|
<0.001 |
|
<0.001 |
|
0.99 |
|
<0.001 |
| R2 | NA | NA | NA | NA | 0.69 | 0.51 | 0.00 | 0.41 | ||||||||
Abbreviations: CI=confidence interval; CXCL-1=chemokine C-X-C motif ligand 1; G-CSF=granulocyte colony-stimulating factor; GRO-α=growth-regulator oncogene α IL-6=interleukin-6; MCP-1=monocyte chemotatic protein 1.
Discussion
This study shows that galectins -2, -4 and -8, at concentrations found in the bloodstream of cancer patients, induce secretion of G-CSF, IL-6, MCP-1/CCL2 and GROα/CXCL1 from the vascular endothelium in vitro and in mice. Such a relationship between circulating galectin-2, -4, -8 and G-CSF, IL-6 and MCP-1/CCL2 was further confirmed in the sera of breast and colon cancer patients. Multiple regression analysis shows that these galectins in combination account for 51–83% of the changes in G-CSF and IL-6 in the sera of breast and colon cancer patients and 41% of the changes in MCP1/CCL2 in colon cancer sera. The galectin-induced secretion of these cytokines/chemokines was shown to enhance the expression of endothelial cell surface adhesion molecules, which causes increased cancer cell-endothelial adhesion, and also to increase endothelial tubule formation, a component of angiogenesis. Thus, the increased circulation of galectins -2, -4 and -8 found in cancer makes important contribution to the elevated circulating concentrations of these cytokines/chemokines that are frequently observed in cancer patients.
Interleukin-6 and G-CSF are pro-inflammatory cytokines and GROα, also known as CXCL1, NAP-3 (neutrophil-activating protein 3) or MSGAα (melanoma growth stimulating activity, alpha) and MCP-1, also known as CCL2 (chemokine c-c motif ligand 2) or small inducible cytokine A2 are chemoattractant cytokines, or chemokines. All these cytokines/chemokines are known to have very important roles in cancer progression and metastasis by regulating divergent tumour cell behaviours and the tumour microenvironment. Interleukin-6 interaction with its receptor IL-6Rα on cancer cells induces activation of cell survival signalling pathways such as JAK/STAT and Ras/ERK leading to increased cell proliferation and growth (Ara et al, 2009; Ara and Declerck, 2010). Interleukin-6 also induces the release of angiogenesis-promoting factors such as VEGF and bFGF (Wei et al, 2003). Interleukin-6 can activate STAT-3 signalling in regulator T cells and help tumour cells to escape immune surveillance (Mantovani et al, 2008). Interleukin-6 also promotes recruitment of circulating tumour cells back into their primary tumour sites, a process called ‘tumour self-seeding' that accelerates tumour growth and angiogenesis (Kim et al, 2009a). Elevated concentrations of circulating IL-6 are common in cancer patients and correlate with metastasis and poor prognosis (Salgado et al, 2003; Michalaki et al, 2004; Ikeguchi et al, 2009; Knupfer and Preiss, 2010).
Interaction of G-CSF with its receptor G-CSFR increases cancer cell proliferation, invasion and migration (Wang et al, 2012). Higher serum concentrations of G-CSF are associated with poor prognosis in urothelial cancer (Mizutani et al, 1995). The presence of G-CSF modifies the distant microenvironment by mobilising Ly6G+Ly6C+ granulocytes to create the so called ‘pre-metastatic niche' before the arrival of metastatic tumour cells and facilitates subsequent tumour cell homing, migration, angiogenesis and metastasis at this newly created pre-metastatic environment (Kowanetz et al, 2010). Direct injections of G-CSF into the tail vein of nude mice increases lung metastasis of animals injected not only with metastatic but also with otherwise non-metastatic breast cancer cells (Kowanetz et al, 2010).
Growth-regulator oncogene α/CXCL1 and MCP-1/CCL2 are chemoattractant cytokines and, like many other chemokines, promote cancer progression and metastasis (Verbeke et al, 2011; Acharyya et al, 2012; Viola et al, 2012) by several mechanisms such as increased angiogenesis, activation of tumour-specific immune responses to weaken the host anti-tumour immunity, stimulation of tumour cell proliferation and metastasis (Payne and Cornelius, 2002; Verbeke et al, 2011). Interaction of GROα/CXCL1 with its receptor CXCR2 promotes cancer cell invasion and migration by activation of cellular AKT/NF-κB signalling (Kuo et al, 2012). Administration of anti- GROα/CXCL1 antibodies inhibits tumour formation and angiogenesis in vitro and in mice (Luan et al, 1997). Suppression of GROα/CXCL1 expression in human colon cancer LS174T cells by shRNA before cell inoculation into the spleens of nude mice almost completely prevents liver metastasis in comparison with those inoculated with parental LS174T cells (Bandapalli et al, 2012). Higher serum concentrations of GRO/CXCL1 are seen in cancer and, it, like IL-6, promotes colonisation of circulating tumour cells to their tumour of origins (self-seeding), thus accelerating tumour growth and angiogenesis of human breast, colon and melanoma tumours (Kim et al, 2009b). Recently, GROα/CXCL1 has been reported to be at the centre of chemo-resistance triggered by chemotherapeutic agents of breast cancer by helping the tumour cells to recruit pro-survival factor S100A8/9 (Acharyya et al, 2012).
Monocyte chemotatic protein 1/CCL2, through interaction with its receptor CCR2 on cancer cells, increases cancer cell invasion and migration by activation of protein kinase C and protein tyrosine phosphorylation (Monti et al, 2003; Chiu et al, 2012). Inhibition of MCP-1/CCL2 biosynthesis by MCP-1/CCL2 inhibitor bindarit inhibits cancer cell proliferation and migration in vitro and significantly impairs metastasis of prostate cancer in mouse xenografts (Zollo et al, 2012). More lung metastases were formed in MCP-1(−/−) mice than in wild-type mice when 4T1 breast cancer cells were transplanted into the mammary pads (Yoshimura et al, 2013). Monocyte chemotatic protein 1/CCL2 concentrations are frequently elevated in cancer patients and it, like IL-6, is involved in driving the ‘stemness' of tumour-initiating cells (Chin and Wang, 2013).
Thus, IL-6, G-CSF, GROα/CXCL1 and MCP-1/CCL2 are all critical regulators of cancer progression and metastasis via divergent mechanisms that act locally or remotely. The galectin-mediated increase of these cytokines/chemokines in cancers therefore likely makes an important contribution to the impact of these cytokines/chemokines on cancer progression and metastasis.
It is noted that, although galectins-2, -4 and -8 have each been shown to induce secretion of IL-6, G-CSF, GROα/CXCL1 and MCP-1/CCL2 from vascular endothelial cells in vitro, direct correlation of serum galectin concentrations in cancer patients was observed predominately with G-CSF and IL-6 and less with MCP-1/CCL2 and no correlation with GROα. It is possible that the galectin-mediated secretion of MCP-1/CCL2 and GROα/CXCL1 may be a secondary effect consequent to galectin stimulation of IL-6 and G-CSF secretion. Both GROα/CXCL1 and MCP-1/CCL2 have been shown previously to be inducible by pro-inflammatory cytokines. For example, IL-1 or TNFα can induce the secretion of GROα/CXCL1 from cancer as well as cancer stromal cells (Son et al, 2007; Kogan-Sakin et al, 2009), and IL-6 can induce the secretion of MCP-1/CCL2 from tumour cells (Lederle et al, 2011). The observation that these galectins had no significant effect on GROα/CXCL1 levels when injected directly into the animal tail vein is in keeping with this possibility. Further investigation to determine the identity and nature of the galectin-binding ligands on endothelial cells that are responsible for the galectin-mediated secretion of these cytokines should help to understand the actions of these galectins. It will also help to understand whether the galectin-induced increase in cytokine concentration shown in mice were entirely from the galectin-endothelium interaction or also contributed to by galectin interaction with non-endothelial cells.
The source of the increased circulation of these galectins in cancer patients is not yet known. Earlier studies have reported reduction of serum levels of galectin-3 (Iurisci et al, 2000), -1 and -4 (Watanabe et al, 2011) following surgical removal of the primary tumours in colorectal cancer patients, indicating that tumour cells may make significant contributions to the increased circulation of those galectin members. On the other hand, cellular expressions of galectin-8 and -4 have been reported to be lower in colorectal cancer than in healthy colonic epithelium (Rechreche et al, 1997; Nagy et al, 2002), although their circulating levels are both higher in cancer. This suggests that non-cancer cells likely make important contribution to the increased circulation of these galectins. Some galectins, e.g., galectin-1 are shown to be expressed more strongly in the peri-tumour stromal cells than in the tumours (Sanjuan et al, 1997), indicating the possible contribution of the peri-tumour stromal cells to the increased circulation of these galectins in cancer. All immune cells express galectins and such expression is known to be influenced by inflammatory regulators (Nangia-Makker et al, 1993). Many pro-inflammatory cytokines, such as TNF-α, IL-1, IL-8 and GM-CSF, are upregulated in cancerous conditions, and their presence may cause the immune cells to secrete more galectins into the bloodstream. Thus, the increased circulation of the members of galectins in cancer patients likely comes from the peri-tumour stromal tissues, the immune cells as well as the tumour cells themselves. Simultaneous determination of serum galectin-3 and -4 levels has shown to provide good sensitivity and specificity in predicting metastasis in colorectal cancer patients (Barrow et al, 2013).
The half-life of circulating galectins in the body is unknown. An earlier study has shown that galectin-3C, an N-terminally truncated form of galectin-3, had a 3 h half-life in the serum when injected intramuscularly into nude mice (John et al, 2003). This suggests that the half-life of circulating galectins in the body is likely to be a few hours.
It should be mentioned that the presence of serum glycoproteins (or specific antibodies) is likely, by competitive binding, to influence the biological availability of these galectins in the circulation. Members of the galectin family bind serum glycoproteins with very different affinities. A systemic analysis of the galectin-binding proteins in human serum has shown that galectin-3 and -8 both recognised a broad range of serum proteins, whereas galectin-2 and -4 showed binding to only trace or no serum ligands (Cederfur et al, 2008). For example, IgGA1 is shown to bind strongly to galectin-1 (Sangeetha and Appukuttan, 2005), whereas a haptoglobin-related serum glycoprotein (Bresalier et al, 2004) and the Mac-2-binding protein (Iacovazzi et al, 2010) are serum ligands of galectin-3. Our own study has shown that, although circulating galectin-2, -3, -4 and -8 levels were all elevated in the circulation of cancer patients and all were shown to induce cancer cell adhesion by interaction with the TF disaccharide on the cancer-associated MUC1 in vitro, only serum galectin-2 level was found to be directly associated with a significantly increased mortality in patients with colorectal cancer (Barrow et al, 2011a). Moreover, the galectin-2-associated reduction in patients' survival was found to be significantly reduced by the presence of autoantibodies against the TF epitope of MUC1 in the serum (Barrow et al, 2011a). Thus, the biological impact of the galectin-induced cytokine secretion and consequently cytokine-mediated metastasis and angiogenesis will be significantly influenced by the presence of galectin-binding glycoproteins and autoantibodies in the circulation and a net influence is likely to be contributed more by those galectins that have minimal binding competitors in the circulation (e.g., galectins -2 and -4). No significant correlation between cancer stages and the levels of circulating galectins -2, -4, and -8 was observed in the serum of breast and colorectal cancer patients (Barrow et al, 2011a).
Thus, the increased circulation of galectins -2, -4 and -8 in cancer enhances endothelial secretion of G-CSF, IL-6, GROα/CXCL1 and MCP-1/CCL2 into the blood circulation and makes important contribution to the increased circulation of these cytokines/chemokines frequently seen in cancer patients. As these cytokines/chemokines are important promoters in cancer progression and metastasis via divergent mechanisms locally and remotely, the galectin-mediated increase of these cytokines/chemokines in cancer patients likely has a profound influence on cancer progression and metastasis and represents a good target for cancer therapy.
Acknowledgments
We thank Dr John Hilkens (The Netherlands Cancer Institute) for the ACA19− cells. This work was supported, in part, by a Medical Research Council grant G1000772.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, Norton L, Brogi E, Massague J. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150 (1:165–178. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46 (7:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Song L, Shimada H, Keshelava N, Russell HV, Metelitsa LS, Groshen SG, Seeger RC, DeClerck YA. Interleukin-6 in the bone marrow microenvironment promotes the growth and survival of neuroblastoma cells. Cancer Res. 2009;69 (1:329–337. doi: 10.1158/0008-5472.CAN-08-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandapalli OR, Ehrmann F, Ehemann V, Gaida M, Macher-Goeppinger S, Wente M, Schirmacher P, Brand K. Down-regulation of CXCL1 inhibits tumor growth in colorectal liver metastasis. Cytokine. 2012;57 (1:46–53. doi: 10.1016/j.cyto.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Barrow H, Guo X, Wandall HH, Pedersen JW, Fu B, Zhao Q, Chen C, Rhodes JM, Yu LG. Serum galectin-2, -4, and -8 are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011;17 (22:7035–7046. doi: 10.1158/1078-0432.CCR-11-1462. [DOI] [PubMed] [Google Scholar]
- Barrow H, Rhodes JM, Yu L-G. The role of galectins in colorectal cancer progression. Int J Cancer. 2011;129:1–8. doi: 10.1002/ijc.25945. [DOI] [PubMed] [Google Scholar]
- Barrow H, Rhodes JM, Yu LG. Simultaneous determination of serum galectin-3 and -4 levels detects metastases in colorectal cancer patients. Cell Oncol. 2013;36 (1:9–13. doi: 10.1007/s13402-012-0109-1. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Byrd JC, Tessler D, Lebel J, Koomen J, Hawke D, Half E, Liu KF, Mazurek N, Great Lakes-New England C, Epidemiology Center of the Early Detection Research N A circulating ligand for galectin-3 is a haptoglobin-related glycoprotein elevated in individuals with colon cancer. Gastroenterology. 2004;127 (3:741–748. doi: 10.1053/j.gastro.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Cederfur C, Salomonsson E, Nilsson J, Halim A, Oberg CT, Larson G, Nilsson UJ, Leffler H. Different affinity of galectins for human serum glycoproteins: galectin-3 binds many protease inhibitors and acute phase proteins. Glycobiology. 2008;18 (5:384–394. doi: 10.1093/glycob/cwn015. [DOI] [PubMed] [Google Scholar]
- Chen C, Duckworth CA, Zhao Q, Pritchard DM, Rhodes JM, Yu LG. Increased circulation of galectin-3 in cancer induces secretion of metastasis-promoting cytokines from blood vascular endothelium. Clin Cancer Res. 2013;19 (7:1693–1704. doi: 10.1158/1078-0432.CCR-12-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AR, Wang SE. Cytokines driving breast cancer stemness. Mol Cell Endocri. 2013;382 (1:598–602. doi: 10.1016/j.mce.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Chiu HY, Sun KH, Chen SY, Wang HH, Lee MY, Tsou YC, Jwo SC, Sun GH, Tang SJ. Autocrine CCL2 promotes cell migration and invasion via PKC activation and tyrosine phosphorylation of paxillin in bladder cancer cells. Cytokine. 2012;59 (2:423–432. doi: 10.1016/j.cyto.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Iacovazzi PA, Notarnicola M, Caruso MG, Guerra V, Frisullo S, Altomare DF. Serum levels of galectin-3 and its ligand 90k/mac-2bp in colorectal cancer patients. Immunopharmacol Immunotoxicol. 2010;32 (1:160–164. doi: 10.1080/08923970902936880. [DOI] [PubMed] [Google Scholar]
- Ikeguchi M, Hatada T, Yamamoto M, Miyake T, Matsunaga T, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S. Serum interleukin-6 and-10 levels in patients with gastric cancer. Gastric Cancer. 2009;12 (2:95–100. doi: 10.1007/s10120-009-0509-8. [DOI] [PubMed] [Google Scholar]
- Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6 (4:1389–1393. [PubMed] [Google Scholar]
- John CM, Leffler H, Kahl-Knutsson B, Svensson I, Jarvis GA. Truncated galectin-3 inhibits tumor growth and metastasis in orthotopic nude mouse model of human breast cancer. Clin Cancer Res. 2003;9 (6:2374–2383. [PubMed] [Google Scholar]
- Kim M-Y, Oskarsson T, Acharyya S, Nguyen DX, Zhang XHF, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139 (7:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massague J. Tumor self-seeding by circulating cancer cells. Cell. 2009;139 (7:1315–1326. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knupfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients-a summary of published results. Int J Colorectal Dis. 2010;25 (2:135–140. doi: 10.1007/s00384-009-0818-8. [DOI] [PubMed] [Google Scholar]
- Kogan-Sakin I, Cohen M, Paland N, Madar S, Solomon H, Molchadsky A, Brosh R, Buganim Y, Goldfinger N, Klocker H, Schalken JA, Rotter V. Prostate stromal cells produce CXCL-1, CXCL-2, CXCL-3 and IL-8 in response to epithelia-secreted IL-1. Carcinogenesis. 2009;30 (4:698–705. doi: 10.1093/carcin/bgp043. [DOI] [PubMed] [Google Scholar]
- Kowanetz M, Wu XM, Lee J, Tan M, Hagenbeek T, Qu XP, Yu LL, Ross J, Korsisaari N, Cao T, Bou-Reslan H, Kallop D, Weimer R, Ludlam MJC, Kaminker JS, Modrusan Z, van Bruggen N, Peale FV, Carano R, Meng YG, Ferrara N. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA. 2010;107 (50:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo PL, Shen KH, Hung SH, Hsu YL. CXCL1/GROalpha increases cell migration and invasion of prostate cancer by decreasing fibulin-1 expression through NF-kappaB/HDAC1 epigenetic regulation. Carcinogenesis. 2012;33 (12:2477–2487. doi: 10.1093/carcin/bgs299. [DOI] [PubMed] [Google Scholar]
- Lederle W, Depner S, Schnur S, Obermueller E, Catone N, Just A, Fusenig NE, Mueller MM. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer. 2011;128 (12:2803–2814. doi: 10.1002/ijc.25621. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5 (1:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62 (5:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454 (7203:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Michalaki V, Odontiadis M, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;91 (6:1227–1227. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani Y, Okada Y, Terachi T, Kakehi Y, Yoshida O. Serum granulocyte-stimulating factor levels in patients with urinary-bladder tumor and various urological malignancies. Br J Urol. 1995;76 (5:580–586. doi: 10.1111/j.1464-410x.1995.tb07782.x. [DOI] [PubMed] [Google Scholar]
- Monti P, Leone BE, Marchesi F, Balzano G, Zerbi A, Scaltrini F, Pasquali C, Calori G, Pessi F, Sperti C, Di Carlo V, Allavena P, Piemonti L. The CC chemokine MCP-1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res. 2003;63 (21:7451–7461. [PubMed] [Google Scholar]
- Nagy N, Bronckart Y, Camby I, Legendre H, Lahm H, Kaltner H, Hadari Y, Van Ham P, Yeaton P, Pector JC, Zick Y, Salmon I, Danguy A, Kiss R, Gabius HJ. Galectin-8 expression decreases in cancer compared with normal and dysplastic human colon tissue and acts significantly on human colon cancer cell migration as a suppressor. Gut. 2002;50 (3:392–401. doi: 10.1136/gut.50.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangia-Makker P, Ochieng J, Christman JK, Raz A. Regulation of the expression of galactoside-binding lectin during human monocytic differentiation. Cancer Res. 1993;53 (20:5033–5037. [PubMed] [Google Scholar]
- Newlaczyl AU, Yu LG. Galectin-3—a jack-of-all-trades in cancer. Cancer Lett. 2011;313 (2:123–128. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. 2002;118 (6:915–922. doi: 10.1046/j.1523-1747.2002.01725.x. [DOI] [PubMed] [Google Scholar]
- Rechreche H, Mallo GV, Montalto G, Dagorn JC, Iovanna JL. Cloning and expression of the mRNA of human galectin-4, an S-type lectin down-regulated in colorectal cancer. Eur J Biochem. 1997;248 (1:225–230. doi: 10.1111/j.1432-1033.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- Ren J, Agata N, Chen D, Li Y, Yu WH, Huang L, Raina D, Chen W, Kharbanda S, Kufe D. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anticancer agents. Cancer Cell. 2004;5 (2:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Oka N, Nakanishi R, Yamaguchi K, Fukumori T, Kanayama HO. Serum level of galectin-3 in human bladder cancer. J Med Invest. 2008;55 (1-2:127–132. doi: 10.2152/jmi.55.127. [DOI] [PubMed] [Google Scholar]
- Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY. Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. Int J Cancer. 2003;103 (5:642–646. doi: 10.1002/ijc.10833. [DOI] [PubMed] [Google Scholar]
- Sangeetha SR, Appukuttan PS. IgA1 is the premier serum glycoprotein recognized by human galectin-1 since T antigen (Galbeta1—>3GalNAc-) is far superior to non-repeating N-acetyl lactosamine as ligand. Int J Biol Macromol. 2005;35 (5:269–276. doi: 10.1016/j.ijbiomac.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Sanjuan X, Fernandez PL, Castells A, Castronovo V, van den Brule F, Liu FT, Cardesa A, Campo E. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology. 1997;113 (6:1906–1915. doi: 10.1016/s0016-5085(97)70010-6. [DOI] [PubMed] [Google Scholar]
- Saussez S, Lorfevre F, Lequeux T, Laurent G, Chantrain G, Vertongen F, Toubeau G, Decaestecker C, Kiss R. The determination of the levels of circulating galectin-1 and -3 in HNSCC patients could be used to monitor tumor progression and/or responses to therapy. Oral Oncol. 2008;44 (1:86–93. doi: 10.1016/j.oraloncology.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Senapati S, Chaturvedi P, Chaney WG, Chakraborty S, Gnanapragassam VS, Sasson AR, Batra SK. Novel interaction of MUC4 and galectin: potential pathobiological implications for metastasis in lethal pancreatic cancer. Clin Cancer Res. 2011;17 (2:267–274. doi: 10.1158/1078-0432.CCR-10-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007;6 (8:1302–1312. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Ono S, Ichikura T, Matsumoto Y, Yamamoto J, Hase K. Roles of inflammatory cytokines in the progression of gastric cancer: friends or foes. Gastric Cancer. 2010;13 (4:212–221. doi: 10.1007/s10120-010-0568-x. [DOI] [PubMed] [Google Scholar]
- Verbeke H, Struyf S, Laureys G, Van Damme J. The expression and role of CXC chemokines in colorectal cancer. Cytokine Growth Factor Rev. 2011;22 (5-6:345–358. doi: 10.1016/j.cytogfr.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Vereecken P, Awada A, Suciu S, Castro G, Morandini R, Litynska A, Lienard D, Ezzedine K, Ghanem G, Heenen M. Evaluation of the prognostic significance of serum galectin-3 in American Joint Committee on Cancer stage III and stage IV melanoma patients. Melanoma Res. 2009;19 (5:316–320. doi: 10.1097/CMR.0b013e32832ec001. [DOI] [PubMed] [Google Scholar]
- Viola A, Sarukhan A, Bronte V, Molon B. The pros and cons of chemokines in tumor immunology. Trend Immunol. 2012;33 (10:496–504. doi: 10.1016/j.it.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Yao L, Zhao S, Zhang X, Yin J, Zhang Y, Chen X, Gao M, Ling EA, Hao A, Li G. Granulocyte-colony stimulating factor promotes proliferation, migration and invasion in glioma cells. Cancer Biol Ther. 2012;13 (6:389–400. doi: 10.4161/cbt.19237. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Takemasa I, Kaneko N, Yokoyama Y, Matsuo E, Iwasa S, Mori M, Matsuura N, Monden M, Nishimura O. Clinical significance of circulating galectins as colorectal cancer markers. Oncol Rep. 2011;25 (5:1217–1226. doi: 10.3892/or.2011.1198. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22 (10:1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7 (4:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Ni WK, Chen XD, Xiao MB, Chen BY, He S, Lu CH, Li XY, Jiang F, Ni RZ. The expressions and clinical significances of tissue and serum galectin-3 in pancreatic carcinoma. J Cancer Res Clin Oncol. 2012;138 (6:1035–1043. doi: 10.1007/s00432-012-1178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T, Howard OM, Ito T, Kuwabara M, Matsukawa A, Chen K, Liu Y, Liu M, Oppenheim JJ, Wang JM. Monocyte chemoattractant protein-1/ccl2 produced by stromal cells promotes lung metastasis of 4T1 murine breast cancer cells. PloS One. 2013;8 (3:e58791. doi: 10.1371/journal.pone.0058791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LG, Andrews N, Zhao Q, McKean D, Williams JF, Connor LJ, Gerasimenko OV, Hilkens J, Hirabayashi J, Kasai K, Rhodes JM. Galectin-3 interaction with Thomsen-Friedenreich disaccharide on cancer-associated MUC1 causes increased cancer cell endothelial adhesion. J Biol Chem. 2007;282 (1:773–781. doi: 10.1074/jbc.M606862200. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, Rhodes JM, Yu LG. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer. 2010;9:154. doi: 10.1186/1476-4598-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Guo X, Nash GB, Stone PC, Hilkens J, Rhodes JM, Yu LG. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009;69 (17:6799–6806. doi: 10.1158/0008-5472.CAN-09-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N, Vastolo V, Navas L, Garrone B, Mangano G, Biondi G, Guglielmotti A. Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis. 2012;29 (6:585–601. doi: 10.1007/s10585-012-9473-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.