Abstract
Purpose
Reoperations after gastrectomy for gastric cancer are performed for many types of complications. Unexpected reoperations may cause mental, physical, and financial problems for patients. The aim of the present study was to evaluate the causes of reoperations and to develop a strategic decision-making process for these reoperations.
Materials and Methods
From September 2002 through August 2010, 6,131 patients underwent open conventional gastrectomy operations at Samsung Medical Center. Of these, 129 patients (2.1%) required reoperation because of postoperative complications. We performed a retrospective analysis of the patients using an electronic medical record review. Statistical data were analyzed to compare age, sex, stage, type of gastrectomy, length of operation, size of tumor, and number of lymph node metastasis between patients who had been operated and those who had not.
Results
The variables of age, sex, tumor stage, type of gastrectomy, length of operation, and number of lymph node metastases did not differ between the 2 groups. However, the mean tumor size in the reoperation group was greater than that in the non-reoperation group (5.0±3.7 [standard deviation] versus 4.1±2.9, P=0.007). The leading cause of reoperation was surgical-site infection (n=49, 0.79%). Patients with intra-abdominal bleeding were operated on again in the shortest period after the initial gastrectomy (6.3±4.2 days). Patients with incisional hernia were not reoperated on until after 208.3±81.0 days, the longest postoperative period.
Conclusions
Tumor size was the major variable leading to reoperation after gastrectomy for gastric cancer. The most common complication requiring the reoperation was a surgical site-related complication.
Keywords: Stomach neoplasms, Postoperative complications, Gastrectomy, Reoperation
Introduction
According to GLOBALCAN, in 2008, gastric cancer was the fourth most commonly diagnosed cancer in men and the fifth most commonly diagnosed in women worldwide.1 In that year in Korea, gastric cancer was the second most common cancer leading to death.2 Gastrectomy with lymph node dissections has been accepted as the only curative treatment for gastric cancer.3 Although the rate of complications after gastrectomy has decreased over the last decade because of improved surgical techniques, nutritional support, antibiotics, and anesthesia,4 complications requiring reoperation have not decreased; the rate of these complications ranges from 2% to 10%.4-7 Reports about reoperation after gastrectomy have several flaws: excessively long observation periods, limited number of patients observed, and variations in surgeons or surgical techniques. The operations reviewed in our study were performed in a single center, by the same surgeons during the period of observation, with the same surgical techniques, and with a sufficient number of patients. The aim of the study was to evaluate the causes and frequency of reoperation. We also wanted to demonstrate when and why the surgeons decided to perform the reoperations.
Materials and Methods
1. Population
Electronic medical records of 6,131 patients who had undergone an open conventional gastrectomy for gastric cancer at Samsung Medical Center, Korea, from September 2002 through August 2010 were reviewed retrospectively. Within the first 12 months after the initial gastrectomy, 159 patients underwent reoperation. Among them, 129 patients were reoperated because of complications related to the initial gastrectomy.
2. Definition of terms
Surgical site-related complication was defined as a complication interfering with the healing process on the abdominal wall, including wound seroma, bleeding, or infection, within 14 days after operation. An event leading to wound dehiscence after 14 days also was considered to be a surgical site-related complication. Leaks were defined as leakage from anastomotic sites, with no time limit. When we found pneumoperitoneum in the imaging studies or definite holes during the operation, we called it perforation, with no time limit.
3. Surgical technique
Five surgeons (MG Choi, JH Noh, TS Sohn, JM Bae, S Kim) performed all the operations. Depending on the location of the gastric cancer, total gastrectomy or subtotal gastrectomy was performed, with D2 or more lymph node dissection (even in the patients with early gastric cancer), according to the lymph node station of the Japanese Research Society for Gastric Cancer.8,9
4. Statistical analysis
Data were collected retrospectively by reviewing the patients' electronic medical records. Statistical analysis was performed using the IBM SPSS Statistics for Windows ver. 20.0 (IBM Co., Armonk, NY, USA). The clinicopathological variables evaluated were age, sex, body mass index (BMI), tumor size, number of dissected lymph nodes, location of tumor, depth of invasion, status of lymph node metastasis, length of operation, and type of surgery performed. Statistical analysis was performed using the independent t-test, chi-square test, or ANOVA, if the normality of the data was tested and the data set was well modeled by a normal distribution. If the data set was not modeled by a normal distribution, a Mann-Whitney test, Fisher's exact test, or Kruskal-Wallis test was performed. A P-value<0.05 was considered significant.
Results
After the first operation, 129 (2.1%) patients underwent reoperation because of postoperative complications. We compared the complication rate of the five surgeons; the rates were 1.89%, 1.95%, 2.12%, 2.28%, and 2.32%, respectively, a statistically insignificant difference among them (P=0.353). The patients' clinicopathological characteristics are shown in Table 1. Statistically, there was no significant difference in variables except for tumor size. The mean tumor size in the reoperation group was approximately 20% greater than that in the non-reoperation group (5.0±3.7 [standard deviation, SD] vs. 4.1±2.9 [SD]; P=0.007, t-test).
Table 1.
Patient characteristics
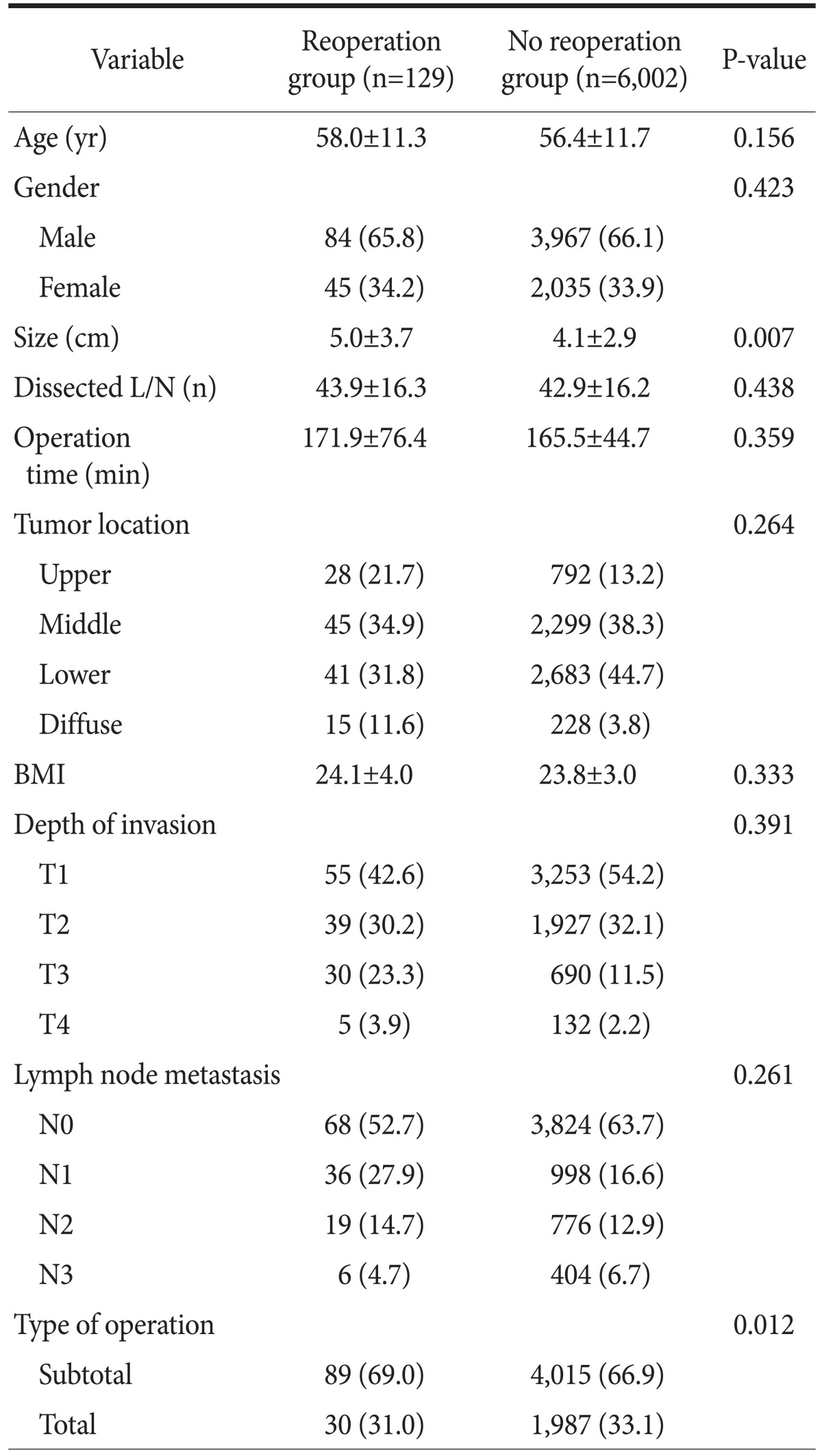
Values are presented as mean±standard deviation or number (%). L/N = lymph node; BMI = body mass index.
The causes and frequency of reoperation are shown in Table 2. The most common complication for reoperation was surgical-site related. Eight patients had perforation; two of the patients underwent reoperation on day three, just after starting an oral diet. In these 2 patients, we found the small bowel sutured. Three patients had a perforated stomach, which we theorized was caused by manipulation during the initial operation. Duodenal stump perforation was diagnosed in three patients. During the second operation in these patients, we found obstruction of the duodenum and jejunum, perhaps due to acute afferent loop syndrome.
Table 2.
Causes and incidence including mean interval to reoperation after gastrectomy with mortality
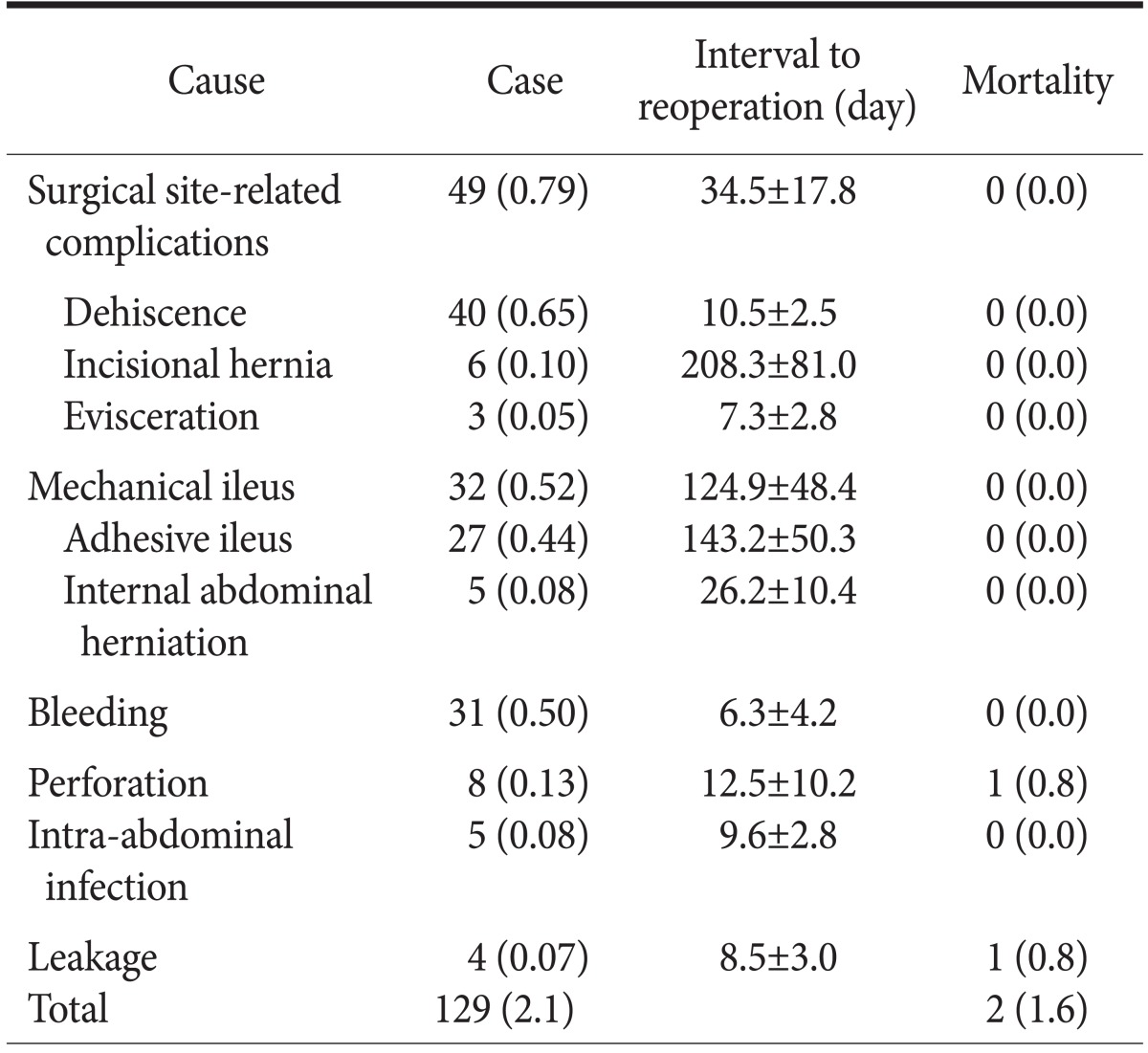
Values are presented as number (%) or mean±standard deviation.
1. Surgical site-related complications
Surgical site-related complications were found in 49 (0.79%) patients who underwent reoperation. The decision to perform the reoperation was made depending on whether the integrity of the fascia was intact. When a probe did not reach the base of the cavity in the lesion, the integrity of the fascia was evaluated further. For five patients, computerized axial tomography (CAT) was required to check the lesion. Reoperations for surgical site-related complication were performed electively, except that evisceration received immediate action.
2. Mechanical ileus
Exploratory laparotomy was performed in 32 (0.52%) patients diagnosed with mechanical ileus on mean day 124.9 (±48.4 [SD]). These patients had visited the emergency department with various symptoms such as abdominal pain, vomiting, and abdominal distension. These patients were diagnosed using CAT. Of the 27 patients with adhesive ileus, 7 underwent reoperation on the day of the emergency department (ED) visit. Other patients with internal abdominal herniation also underwent reoperations on the day of the ED visit. The decision to operate was made based on the patients' vital signs or symptoms, not on the basis of laboratory test results. Obstruction occurred in various sites of the small bowel, including obstruction in the Roux limb in 2 patients. Most of the patients with mechanical ileus were simply treated with adhesiolysis. However, 7 patients underwent small bowel resection and anastomosis. On CAT, strangulation of the small bowel was seen in 7 of the 32 patients. One patient with strangulation underwent an exploratory laparotomy; the small bowel was found viable, so the surgeon ended the operation. Two days later, the surgeon performed a laparotomy and found the small bowel viable, so he completed the operation.
3. Bleeding
Intra-abdominal bleeding led to reoperation on mean day 6.3 (± 4.2 [SD]) in 31 (0.50%) patients after the first operation. Surgeons suspected bleeding because of color change in closed-suction drainage fluid, change in patients' vital signs, decrease in hemoglobin or hematocrit levels, or patients' additional symptoms. Before the second operation, the average hemoglobin and hematocrit drop was 4.27 (±0.78 [SD]) g/dl and 13.33 (±3.45 [SD]) percent, respectively, a decrease of more than 30% in both measurements. However, surgeons decided to reoperate based on laboratory test results in only 6 patients; the rest of the patients underwent reoperation when there were changes in vital signs, especially heart rate. Surgeons were unable to find the site of bleeding in some cases. The bleeding sites found during the second operations are listed in Table 3. Among 17 known bleeding sites, 10 were located around the spleen.
Table 3.
Site of bleeding
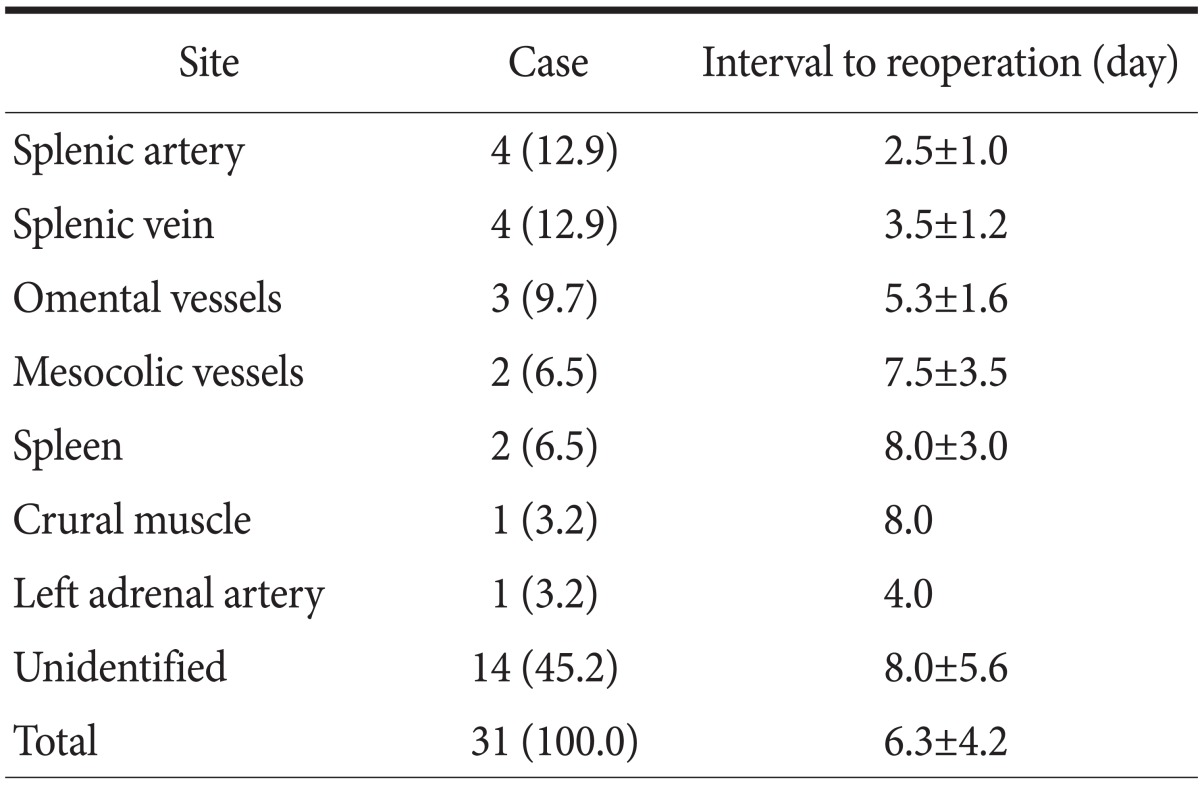
Values are presented as number (%), mean±standard deviation, or mean only.
4. Mortality cases
Reoperation mortality rate after gastrectomy was 1.56% (n=2). One patient had undergone subtotal gastrectomy with Billroth I reconstruction. That patient had bleeding around the spleen and underwent an exploratory laparotomy. Hemostasis of the bleeding site was performed successfully. However, chronic obstructive pulmonary disease, diagnosed before the operation, was aggravated, and the patient died of pulmonary failure. The other patient initially underwent a total gastrectomy. On day 18, the patient reported abdominal pain, and at reoperation was found to have an anastomotic leak between the esophagus and jejunum, which led to sepsis and death.
Discussion
The aim of this study was to evaluate the causes, frequency of reoperation, and decision-making process of reoperation within the first 12 months after an initial gastrectomy for gastric cancer. We found the rate of reoperation because of complications after gastrectomy to be 2.1%, which is similar to the reoperation rate of 2.0% to 10% reported in the literature.4-7,10,11 In general, mechanical ileus is the most common cause of reoperation. The reported rate of surgical site-related complications is 3% to 25%,12,13 with the frequency of reoperation due to wound dehiscence estimated at 1% to 3%.14,15 The rate of this complication was lower in our series than in some studies.4,7,13 Complications related to the surgical site were the leading cause of reoperation in this study. Unfortunately, we cannot determine why surgical wound-related complications were the most common. Wound dehiscence is affected by local, regional, and systemic factors such as infection, abdominal pressure, diabetes, age, and nutritional status. In this study, no difference was found in the variables of age and BMI. In a future study, it is necessary to compare variables affecting wound closure with those recorded in other studies. It is important to recognize that surgical site-related complications are serious, although they might be treated less seriously than other complications. In this study, 2 patients died because underlying medical conditions were aggravated after the reoperation. Therefore, surgical site-related complications should be treated similar to other serious complications.
In our series, the rate of reoperation caused by other complications, including mechanical ileus, intra-abdominal bleeding, intestinal perforation, intra-abdominal infection, and anastomotic leaks, was similar to that in previous studies.5-7,11,16,17 The mortality rate in the present study was 1.6%, whereas the reported rates are 6% to 10%.4,6,7
Determination of when and why the surgeon decided to reoperate was another objective of this study. This decision was different for each complication. For instance, change in vital signs, especially heart rate, was a factor in an intra-abdominal bleeding complication, whereas the results of CAT were a factor for mechanical ileus. The decision to reoperate was not made on the basis of numeric data but on patients' symptoms and changes in their vital signs.
The limitations of this current study are clear. First, this is a retrospective study, and second, every patient could not be traced through the period of observation. If we had been able to follow the patients, we believe the reoperation rate would have been higher, as would the number of fatalities. However, even though this study has limitations, it has 2 important qualities. The gastrectomy was performed by the same surgeons and with the same techniques during the period of the observation, during which annual rate of reoperation did not change dramatically. Furthermore, the study demonstrated that surgical site-related complication was the most common reason for reoperation.
The present study showed that the rate of reoperation because of complications after gastrectomy for gastric cancer was 2.1%. The most common complication requiring reoperation was surgical site-related complication (n=49, 0.79%). Less common complications were intra-abdominal bleeding and mechanical ileus. Tumor size was the most important variable leading to reoperation.
Acknowledgments
This manuscript was presented at the 15th World Congress on Gastrointestinal Cancer, June 22, 2011.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCulloch P, Niita ME, Kazi H, Gama-Rodrigues JJ. Gastrectomy with extended lymphadenectomy for primary treatment of gastric cancer. Br J Surg. 2005;92:5–13. doi: 10.1002/bjs.4839. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa D, Kurioka H, Yamaguchi T, Koike H, Okamoto K, Otsuji E, et al. Postoperative complications following gastrectomy for gastric cancer during the last decade. Hepatogastroenterology. 2004;51:613–617. [PubMed] [Google Scholar]
- 5.Aoki M, Saka M, Morita S, Fukagawa T, Katai H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World J Surg. 2010;34:2389–2392. doi: 10.1007/s00268-010-0602-5. [DOI] [PubMed] [Google Scholar]
- 6.Wu CW, Chang IS, Lo SS, Hsieh MC, Chen JH, Lui WY, et al. Complications following D3 gastrectomy: post hoc analysis of a randomized trial. World J Surg. 2006;30:12–16. doi: 10.1007/s00268-005-7951-5. [DOI] [PubMed] [Google Scholar]
- 7.Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH Yonsei Gastric Cancer Clinic. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastrointest Surg. 2009;13:239–245. doi: 10.1007/s11605-008-0716-3. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 9.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 10.Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol. 2013;19:4060–4065. doi: 10.3748/wjg.v19.i25.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly KJ, Allen PJ, Brennan MF, Gollub MJ, Coit DG, Strong VE. Internal hernia after gastrectomy for cancer with Roux-Y reconstruction. Surgery. 2013;154:305–311. doi: 10.1016/j.surg.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Luijendijk RW, Lemmen MH, Hop WC, Wereldsma JC. Incisional hernia recurrence following "vest-over-pants" or vertical Mayo repair of primary hernias of the midline. World J Surg. 1997;21:62–65. doi: 10.1007/s002689900194. [DOI] [PubMed] [Google Scholar]
- 13.Mäkelä JT, Kiviniemi H, Juvonen T, Laitinen S. Factors influencing wound dehiscence after midline laparotomy. Am J Surg. 1995;170:387–390. doi: 10.1016/s0002-9610(99)80309-2. [DOI] [PubMed] [Google Scholar]
- 14.Israelsson LA, Jonsson T. Suture length to wound length ratio and healing of midline laparotomy incisions. Br J Surg. 1993;80:1284–1286. doi: 10.1002/bjs.1800801020. [DOI] [PubMed] [Google Scholar]
- 15.Osther PJ, Gjøde P, Mortensen BB, Mortensen PB, Bartholin J, Gottrup F. Randomized comparison of polyglycolic acid and polyglyconate sutures for abdominal fascial closure after laparotomy in patients with suspected impaired wound healing. Br J Surg. 1995;82:1080–1082. doi: 10.1002/bjs.1800820824. [DOI] [PubMed] [Google Scholar]
- 16.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 17.Mittermair R, Sucher R, Perathoner A. Results and complications after laparoscopic sleeve gastrectomy. Surg Today. 2013 doi: 10.1007/s00595-013-0688-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


