Abstract
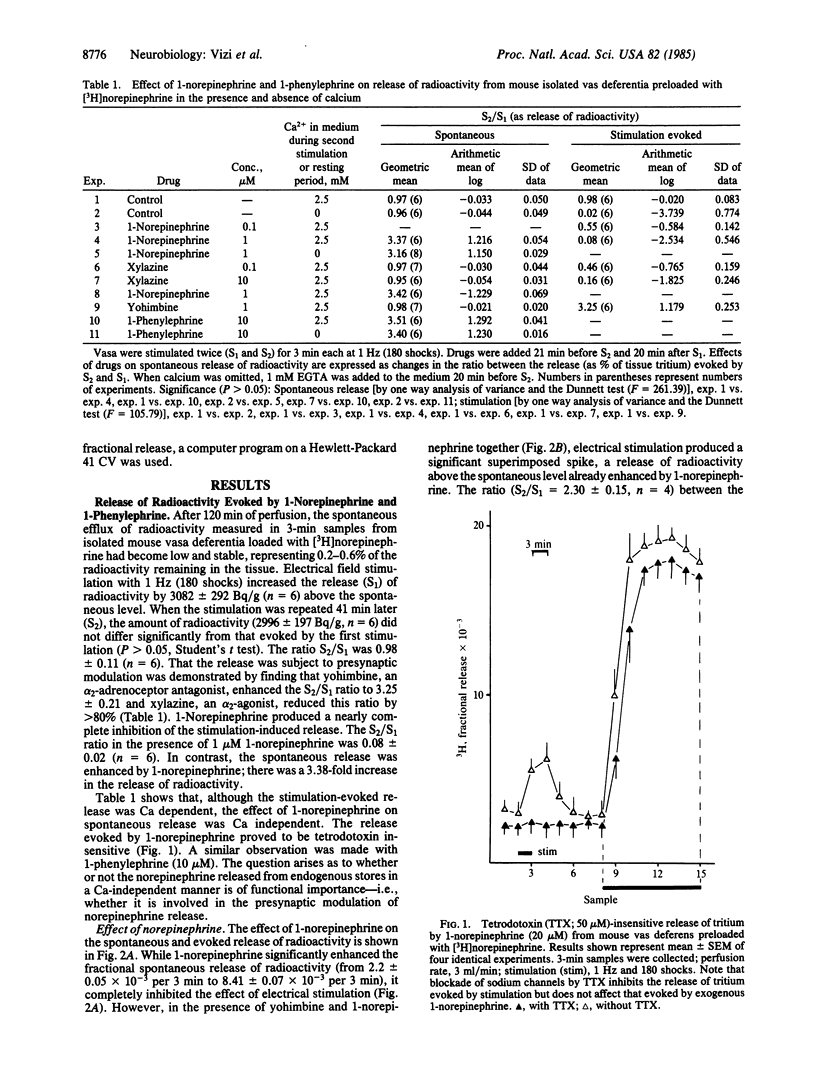
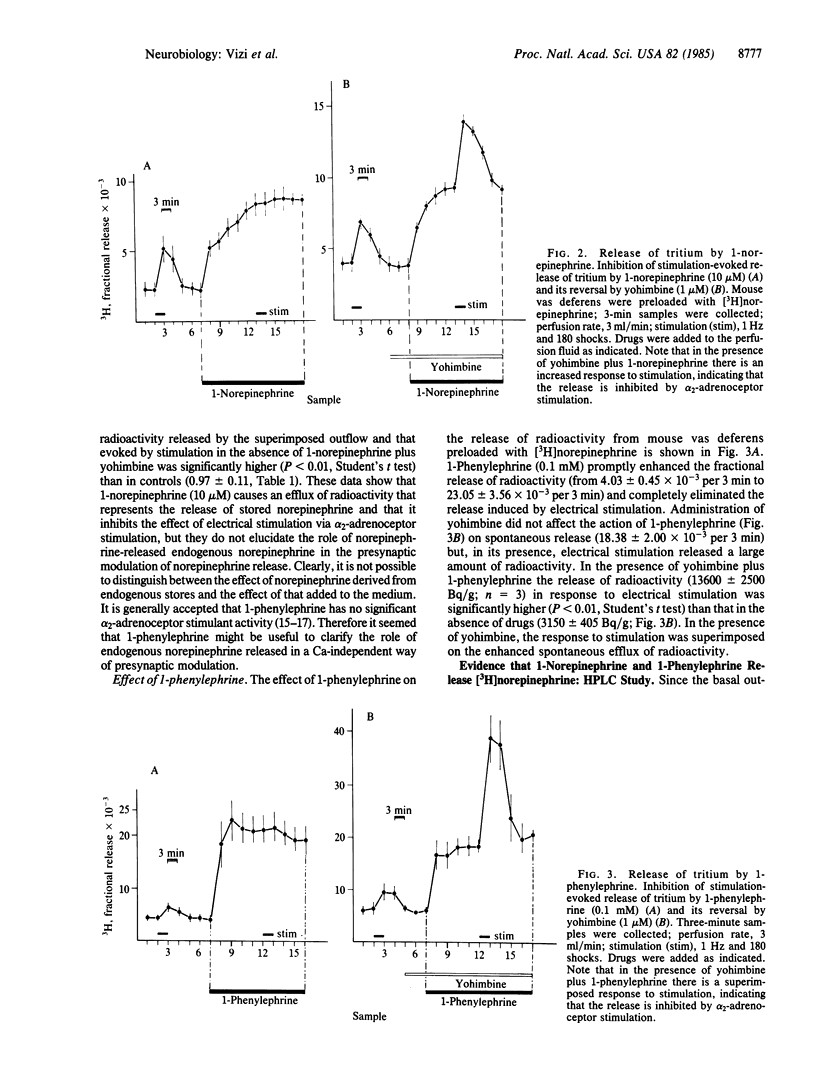
The release of [3H]norepinephrine from isolated mouse vas deferens has been measured. 1-Phenylephrine and 1-norepinephrine significantly enhanced the spontaneous release of radioactivity. As shown by a combination of HPLC and scintillation spectrometry, the release of total radioactivity in response to 1-phenylephrine or 1-norepinephrine consisted mainly of [3H]norepinephrine. Evidence has been obtained that the release of endogenous norepinephrine by exogenous norepinephrine and 1-phenylephrine is independent of the external Ca2+ concentration. The released endogenous norepinephrine in turn inhibits the release of norepinephrine in response to electrical field stimulation. In the presence of yohimbine, the enhancement of spontaneous release due to 1-phenylephrine (or to 1-norepinephrine) was not affected, whereas there was a significant superimposed release of [3H]norepinephrine in response to field stimulation, indicating that the inhibition of stimulation-induced norepinephrine release is an alpha 2-adrenoceptor-mediated process. An important consequence of these findings is to question previous interpretations that the effects of administration of 1-norepinephrine or 1-phenylephrine are due exclusively to their direct effects on the effector cells. The Ca-independent release of endogenous norepinephrine might partly initiate their pharmacological responses. It is concluded that this Ca-independent release is of functional importance, since norepinephrine may accumulate in a concentration sufficient to modulate the release of norepinephrine from varicosities in response to electrical stimulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J., GORDON E., HERTTING G., KOPIN I. J., POTTER L. T. On the mechanism of tachyphylaxis to tyramine in the isolated rat heart. Br J Pharmacol Chemother. 1962 Aug;19:56–63. doi: 10.1111/j.1476-5381.1962.tb01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler-Graschinsky E., Langer S. Z. Possible role of a beta-adrenoceptor in the regulation of noradrenaline release by nerve stimulation through a positive feed-back mechanism. Br J Pharmacol. 1975 Jan;53(1):43–50. doi: 10.1111/j.1476-5381.1975.tb07328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler-Graschinsky E., Langer S. Z., Rubio M. C. Metabolism of norepinephrine released by phenoxybenzamine in isolated guinea-pig atria. J Pharmacol Exp Ther. 1972 Feb;180(2):286–301. [PubMed] [Google Scholar]
- Daly J. W., Creveling C. R., Witkop B. The chemorelease of norepinephrine in mouse hearts. Structure-activity relationships. II. Drugs affecting the sympathetic and central nervous systems. J Med Chem. 1966 May;9(3):280–284. doi: 10.1021/jm00321a002. [DOI] [PubMed] [Google Scholar]
- Flavahan N. A., McGrath J. C. alpha 1-adrenoceptors can mediate chronotropic responses in the rat heart. Br J Pharmacol. 1981 Jul;73(3):586–588. doi: 10.1111/j.1476-5381.1981.tb16791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govier W. C. A positive inotropic effect of phenylephrine mediated through alpha adrenergic receptors. Life Sci. 1967 Jul 1;6(13):1361–1365. doi: 10.1016/0024-3205(67)90182-8. [DOI] [PubMed] [Google Scholar]
- Govier W. C. Myocardial alpha adrenergic receptors and their role in the production of a positive inotropic effect by sympathomimetic agents. J Pharmacol Exp Ther. 1968 Jan;159(1):82–90. [PubMed] [Google Scholar]
- HERTTING G., AXELROD J., KOPIN I. J., WHITBY L. G. Lack of uptake of catecholamines after chronic denervation of sympathetic nerves. Nature. 1961 Jan 7;189:66–66. doi: 10.1038/189066a0. [DOI] [PubMed] [Google Scholar]
- Kirpekar S. M., Prat J. C., Puig M., Wakade A. R. Modification of the evoked release of noradrenaline from the perfused cat spleen by various ions and agents. J Physiol. 1972 Mar;221(3):601–615. doi: 10.1113/jphysiol.1972.sp009770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefèvre F., Fénard S., Cavero I. Vascular beta-adrenoceptor stimulating properties of phenylephrine. Eur J Pharmacol. 1977 May 1;43(1):85–88. doi: 10.1016/0014-2999(77)90163-7. [DOI] [PubMed] [Google Scholar]
- Lindmar R., Löffelholz K., Muscholl E. Unterschiede zwischen Tyramin und Dimethylphenylpiperzin in der Ca-Abhangigkeit und im zeitlichen Verlauf der Noradrenalin-Freisetzung am isolierten Kaninchenherzen. Experientia. 1967 Nov 15;23(11):933–934. doi: 10.1007/BF02136230. [DOI] [PubMed] [Google Scholar]
- Luchelli-Fortis M. A., Langer S. Z. Reserpine-induced depletion of the norepinephrine stores: is it a reliable criterion for the classification of the mechanism of action of sympathomimetic amines? J Pharmacol Exp Ther. 1974 Mar;188(3):640–653. [PubMed] [Google Scholar]
- McGrath J. C. Evidence for more than one type of post-junctional alpha-adrenoceptor. Biochem Pharmacol. 1982 Feb 15;31(4):467–484. doi: 10.1016/0006-2952(82)90147-2. [DOI] [PubMed] [Google Scholar]
- NASH C. W., COSTA E., BRODIE B. B. THE ACTIONS OF RESERPINE, GUANETHIDINE AND METARAMINOL ON CARDIAC CATECHOLAMINE STORES. Life Sci. 1964 May;3:441–449. doi: 10.1016/0024-3205(64)90204-8. [DOI] [PubMed] [Google Scholar]
- Nash C. W., Wolff S. A., Ferguson B. A. Release of tritiated noradrenaline from perfused rat hearts by sympathomimetic amines. Can J Physiol Pharmacol. 1968 Jan;46(1):35–42. doi: 10.1139/y68-006. [DOI] [PubMed] [Google Scholar]
- POTTER L. T., AXELROD J. Studies on the storage of norepinephrine and the effect of drugs. J Pharmacol Exp Ther. 1963 May;140:199–206. [PubMed] [Google Scholar]
- Paton D. M. Mechanism of efflux of noradrenaline from adrenergic nerves in rabbit atria. Br J Pharmacol. 1973 Dec;49(4):614–627. doi: 10.1111/j.1476-5381.1973.tb08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke K. Alpha sympathomimetic inhibition of adrenergic and cholinergic transmission in the rabbit heart. Naunyn Schmiedebergs Arch Pharmacol. 1972;274(1):18–45. doi: 10.1007/BF00501004. [DOI] [PubMed] [Google Scholar]
- Starke K., Endo T., Taube H. D. Relative pre- and postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 1975;291(1):55–78. doi: 10.1007/BF00510821. [DOI] [PubMed] [Google Scholar]
- Starke K., Montel H. Influence of drugs with affinity for alpha-adrenoceptors on noradrenaline release by potassium, tyramine and dimethylphenylpiperazinium. Eur J Pharmacol. 1974 Aug;27(3):273–280. doi: 10.1016/0014-2999(74)90001-6. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Vizi E. S. Presynaptic modulation of neurochemical transmission. Prog Neurobiol. 1979;12(3-4):181–290. doi: 10.1016/0301-0082(79)90011-x. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Localization of adrenergic receptors in guinea pig ileum and rabbit jejunum to cholinergic neurons and to smooth muscle cells. Acta Physiol Scand. 1977 Feb;99(2):190–207. doi: 10.1111/j.1748-1716.1977.tb10370.x. [DOI] [PubMed] [Google Scholar]
- van Meel J. C., de Jonge A., Timmermans P. B., van Zwieten P. A. Selectivity of some alpha adrenoceptor agonists for peripheral alpha-1 and alpha-2 adrenoceptors in the normotensive rat. J Pharmacol Exp Ther. 1981 Dec;219(3):760–767. [PubMed] [Google Scholar]


