Abstract
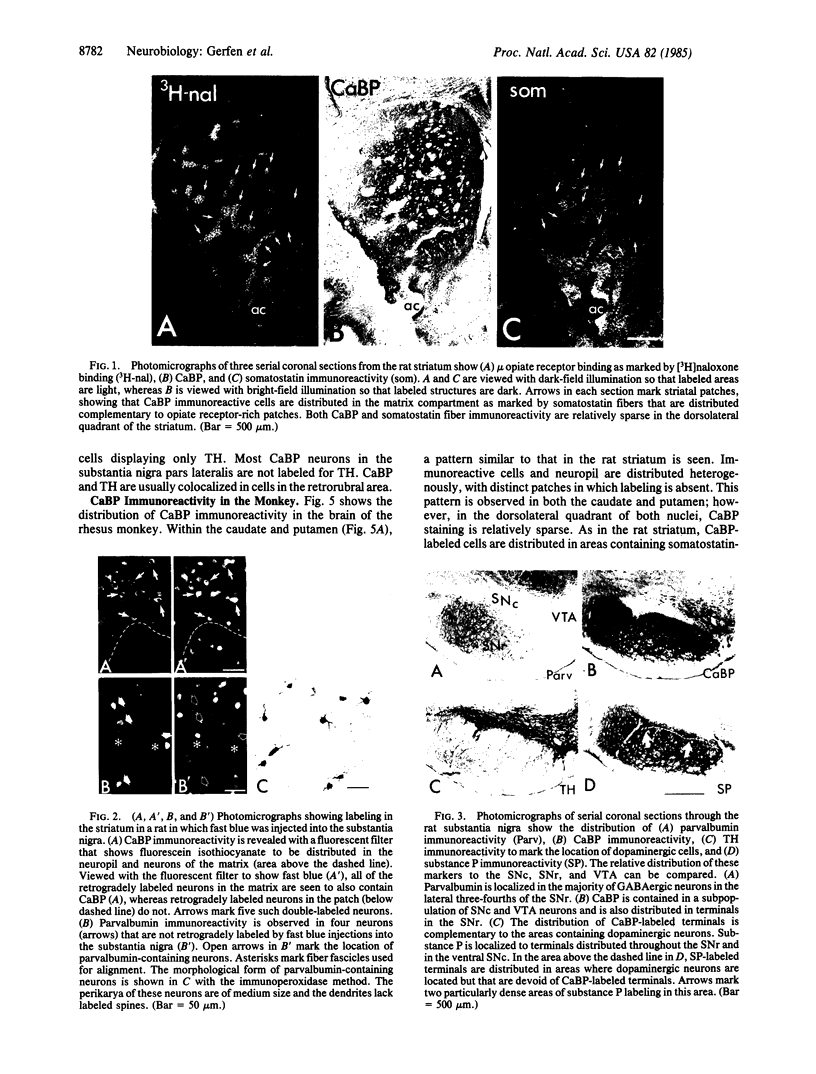
Calcium-binding protein (CaBP) and parvalbumin are two proteins that are expressed in brain and bind calcium in the micromolar range. The immunohistochemical distribution of these two proteins was examined in the basal ganglia of rats and rhesus monkeys. In the striatum, CaBP immunoreactivity is localized to a subset of striatonigral projection neurons; CaBP-positive neurons are distributed in areas containing somatostatin-immunoreactive fibers and not in the complementary areas containing dense mu opiate-receptor binding. These biochemical labels mark, respectively, the matrix and patch compartments of the striatum. Previous studies have shown that striatal matrix neurons project to the substantia nigra pars reticulata, whereas striatal patch neurons project to the substantia nigra pars compacta. Consistent with the restricted localization of CaBP in the matrix projection neurons is the confinement of CaBP-immunoreactive afferent fibers to the pars reticulata. CaBP is also localized to a portion of dopaminergic and a few nondopaminergic neurons in the substantia nigra pars compacta and in most dopaminergic neurons in the ventral tegmental area. Parvalbumin immunoreactivity is localized to a subset of substantia nigra pars reticulata neurons and their axons. In the lateral striatum, some medium-sized aspiny interneurons are also parvalbumin immunoreactive. The distinct distributions of CaBP and parvalbumin in the basal ganglia are discussed in terms of their possible roles as intracellular calcium buffer systems related to the physiologic response properties of the neurons in which they are contained.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arluison M., Dietl M., Thibault J. Ultrastructural morphology of dopaminergic nerve terminals and synapses in the striatum of the rat using tyrosine hydroxylase immunocytochemistry: a topographical study. Brain Res Bull. 1984 Aug;13(2):269–285. doi: 10.1016/0361-9230(84)90128-x. [DOI] [PubMed] [Google Scholar]
- Aronin N., Difiglia M., Graveland G. A., Schwartz W. J., Wu J. Y. Localization of immunoreactive enkephalins in GABA synthesizing neurons of the rat neostriatum. Brain Res. 1984 May 23;300(2):376–380. doi: 10.1016/0006-8993(84)90850-3. [DOI] [PubMed] [Google Scholar]
- Baimbridge K. G., Miller J. J. Immunohistochemical localization of calcium-binding protein in the cerebellum, hippocampal formation and olfactory bulb of the rat. Brain Res. 1982 Aug 12;245(2):223–229. doi: 10.1016/0006-8993(82)90804-6. [DOI] [PubMed] [Google Scholar]
- Baimbridge K. G., Miller J. J., Parkes C. O. Calcium-binding protein distribution in the rat brain. Brain Res. 1982 May 13;239(2):519–525. doi: 10.1016/0006-8993(82)90526-1. [DOI] [PubMed] [Google Scholar]
- Bolam J. P., Clarke D. J., Smith A. D., Somogyi P. A type of aspiny neuron in the rat neostriatum accumulates [3H]gamma-aminobutyric acid: combination of Golgi-staining, autoradiography, and electron microscopy. J Comp Neurol. 1983 Jan 10;213(2):121–134. doi: 10.1002/cne.902130202. [DOI] [PubMed] [Google Scholar]
- Bolam J. P., Wainer B. H., Smith A. D. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984 Jul;12(3):711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin as a neuronal marker. Nature. 1981 Sep 24;293(5830):300–302. doi: 10.1038/293300a0. [DOI] [PubMed] [Google Scholar]
- Celio M. R., Heizmann C. W. Calcium-binding protein parvalbumin is associated with fast contracting muscle fibres. Nature. 1982 Jun 10;297(5866):504–506. doi: 10.1038/297504a0. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., Aronin N. Ultrastructural features of immunoreactive somatostatin neurons in the rat caudate nucleus. J Neurosci. 1982 Sep;2(9):1267–1274. doi: 10.1523/JNEUROSCI.02-09-01267.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Staines W. A., Arbuthnott G. W., Fibiger H. C. Crossed connections of the substantia nigra in the rat. J Comp Neurol. 1982 May 20;207(3):283–303. doi: 10.1002/cne.902070308. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985 Jun 22;236(4):454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984 Oct 4;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. S. Cytoarchitectonic heterogeneity of the primate neostriatum: subdivision into Island and Matrix cellular compartments. J Comp Neurol. 1982 Mar 10;205(4):398–413. doi: 10.1002/cne.902050408. [DOI] [PubMed] [Google Scholar]
- Graybiel A. M., Chesselet M. F. Compartmental distribution of striatal cell bodies expressing [Met]enkephalin-like immunoreactivity. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7980–7984. doi: 10.1073/pnas.81.24.7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A. M., Ragsdale C. W., Jr Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5723–5726. doi: 10.1073/pnas.75.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann C. W. Parvalbumin, an intracellular calcium-binding protein; distribution, properties and possible roles in mammalian cells. Experientia. 1984 Sep 15;40(9):910–921. doi: 10.1007/BF01946439. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Edley S. M., Stuart J. Cell clusters in the nucleus accumbens of the rat, and the mosaic relationship of opiate receptors, acetylcholinesterase and subcortical afferent terminations. Neuroscience. 1984 Mar;11(3):561–593. doi: 10.1016/0306-4522(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Light microscopic localization of brain opiate receptors: a general autoradiographic method which preserves tissue quality. J Neurosci. 1982 Aug;2(8):1129–1149. doi: 10.1523/JNEUROSCI.02-08-01129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M., Pert C. B. Mosaic distribution of opiate receptors, parafascicular projections and acetylcholinesterase in rat striatum. Nature. 1981 Jun 4;291(5814):415–418. doi: 10.1038/291415a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Inagaki S., Parent A. Distribution of substance P and enkephalin-like immunoreactivity in the substantia nigra of rat, cat and monkey. Brain Res Bull. 1984 Aug;13(2):319–329. doi: 10.1016/0361-9230(84)90133-3. [DOI] [PubMed] [Google Scholar]
- Kitai S. T., Wagner A., Precht W., Ono T. Nigro-caudate and caudato-nigral relationship: an electrophysiological study. Brain Res. 1975 Feb 21;85(1):44–48. doi: 10.1016/0006-8993(75)91002-1. [DOI] [PubMed] [Google Scholar]
- Kohno J., Shiosaka S., Shinoda K., Inagaki S., Tohyama M. Two distinct strio-nigral substance P pathways in the rat: an experimental immunohistochemical study. Brain Res. 1984 Aug 13;308(2):309–317. doi: 10.1016/0006-8993(84)91070-9. [DOI] [PubMed] [Google Scholar]
- Morrison J. H., Benoit R., Magistretti P. J., Bloom F. E. Immunohistochemical distribution of pro-somatostatin-related peptides in cerebral cortex. Brain Res. 1983 Mar 7;262(2):344–351. doi: 10.1016/0006-8993(83)91031-4. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Tappaz M. L., Berod A., Mugnaini E. Two-color immunohistochemistry for dopamine and GABA neurons in rat substantia nigra and zona incerta. Brain Res Bull. 1982 Jul-Dec;9(1-6):463–474. doi: 10.1016/0361-9230(82)90155-1. [DOI] [PubMed] [Google Scholar]
- Pechère J. F., Derancourt J., Haiech J. The participation of parvalbumins in the activation-relaxation cycle of vertebrate fast skeletal-muscle. FEBS Lett. 1977 Mar 15;75(1):111–114. doi: 10.1016/0014-5793(77)80064-1. [DOI] [PubMed] [Google Scholar]
- Pert C. B., Kuhar M. J., Snyder S. H. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale C. W., Jr, Graybiel A. M. The fronto-striatal projection in the cat and monkey and its relationship to inhomogeneities established by acetylcholinesterase histochemistry. Brain Res. 1981 Mar 16;208(2):259–266. doi: 10.1016/0006-8993(81)90556-4. [DOI] [PubMed] [Google Scholar]
- Sawchenko P. E., Swanson L. W. A method for tracing biochemically defined pathways in the central nervous system using combined fluorescence retrograde transport and immunohistochemical techniques. Brain Res. 1981 Apr 6;210(1-2):31–51. doi: 10.1016/0006-8993(81)90882-9. [DOI] [PubMed] [Google Scholar]
- Takagi H., Somogyi P., Somogyi J., Smith A. D. Fine structural studies on a type of somatostatin-immunoreactive neuron and its synaptic connections in the rat neostriatum: a correlated light and electron microscopic study. J Comp Neurol. 1983 Feb 10;214(1):1–16. doi: 10.1002/cne.902140102. [DOI] [PubMed] [Google Scholar]
- Vincent S., Hökfelt T., Christensson I., Terenius L. Immunohistochemical evidence for a dynorphin immunoreactive striato-nigral pathway. Eur J Pharmacol. 1982 Nov 19;85(2):251–252. doi: 10.1016/0014-2999(82)90477-0. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Taylor A. N. Vitamin d3-induced calcium-binding protein in chick intestinal mucosa. Science. 1966 May 6;152(3723):791–793. doi: 10.1126/science.152.3723.791. [DOI] [PubMed] [Google Scholar]
- Wright A. K., Arbuthnott G. W. The pattern of innervation of the corpus striatum by the substantia nigra. Neuroscience. 1981;6(10):2063–2067. doi: 10.1016/0306-4522(81)90044-0. [DOI] [PubMed] [Google Scholar]