Abstract
Hemorrhagic transformation (HT) is a common complication of ischemic stroke that is exacerbated by thrombolytic therapy. Methods to better prevent, predict, and treat HT are needed. In this review, we summarize studies of HT in both animals and humans. We propose that early HT (<18 to 24 hours after stroke onset) relates to leukocyte-derived matrix metalloproteinase-9 (MMP-9) and brain-derived MMP-2 that damage the neurovascular unit and promote blood–brain barrier (BBB) disruption. This contrasts to delayed HT (>18 to 24 hours after stroke) that relates to ischemia activation of brain proteases (MMP-2, MMP-3, MMP-9, and endogenous tissue plasminogen activator), neuroinflammation, and factors that promote vascular remodeling (vascular endothelial growth factor and high-moblity-group-box-1). Processes that mediate BBB repair and reduce HT risk are discussed, including transforming growth factor beta signaling in monocytes, Src kinase signaling, MMP inhibitors, and inhibitors of reactive oxygen species. Finally, clinical features associated with HT in patients with stroke are reviewed, including approaches to predict HT by clinical factors, brain imaging, and blood biomarkers. Though remarkable advances in our understanding of HT have been made, additional efforts are needed to translate these discoveries to the clinic and reduce the impact of HT on patients with ischemic stroke.
Keywords: blood–brain barrier, hemorrhagic transformation, intracerebral hemorrhage, ischemic stroke, oxidative stress, reperfusion, tissue plasminogen activator
Introduction
Hemorrhagic transformation (HT) is bleeding into an area of ischemic brain after stroke. It occurs in as many as 10% to 40% of patients with ischemic stroke,1, 2 and is associated with increased stroke morbidity and mortality.3, 4, 5 It is the major complication of tissue plasminogen activator (tPA), the only FDA-approved therapy for acute ischemic stroke. Thus, improved understanding of HT is essential to reduce its impact on patients with ischemic stroke and improve our ability to restore blood flow to ischemic brain without producing this complication.
The severity of HT can vary from microscopic bleeding to large hemorrhages. Clinical studies frequently divide HT into four groups: small petechial hemorrhagic infarction (HI1), confluent petechial hemorrhagic infarction (HI2), small parenchymal hemorrhage (PH1) (<30% of infarct, mild mass effect), and large parenchymal hemorrhage (PH2, >30% of infarct, marked mass effect).3 Hemorrhagic transformation is also often divided into symptomatic or asymptomatic groups based on the deterioration in neurologic status, defined as an increase in the National Institutes of Health Stroke Scale by >4 points within the first 36 hours of stroke onset.6 These clinical classifications are useful in that larger hemorrhages are more likely symptomatic, more likely to negatively affect stroke outcomes, and thus are important to prevent.3, 5, 7, 8 However, even so-called ‘asymptomatic' HT can worsen stroke outcomes, particularly when cognition and neurologic function are assessed weeks to months after stroke onset.6, 9 This may relate in part to exacerbation of cerebral edema and other toxic effects of blood produced by HT.10 Thus, reducing smaller HT may also benefit patients with stroke.
Hemorrhagic transformation occurs when cerebral blood flow is restored to damaged vasculature. There remains some uncertainty as to whether the mechanisms that cause smaller petechial hemorrhages are the same as larger parenchymal hemorrhages. Some have argued that they differ since petechial hemorrhage relates to the duration and severity of ischemia, whereas parenchymal hemorrhage may not.11 In addition, petechial hemorrhage is often thought to be a marker of good outcome, possibly because it indicates early reperfusion to still viable brain tissue. Nonetheless, in either petechial or parenchymal hemorrhage the cause of bleeding is injury to and/or remodeling of blood vessels which form the blood–brain barrier (BBB) and are part of the neurovascular unit (vessel-glia-neuron).12
In the following sections, we summarize the factors that contribute to HT in animals and humans. We highlight that reperfusion alone without tPA is a key factor to generate HT. However, when tPA is administered the risk of HT is increased through a number of discussed mechanisms. We present evidence suggesting that there is a difference between HT that occurs early after stroke compared with HT that is delayed. The key roles of reactive oxygen species (ROS), metalloproteinases, and other proteases are discussed, including the relationship between leukocyte-derived and brain-derived factors. We summarize potential methods to restore BBB integrity after stroke and reduce HT. Finally, we discuss the clinical factors and predictors that have been associated with HT.
Hemorrhagic transformation and reperfusion without tissue plasminogen activator
Many animal studies have focused on HT related to tPA. From a mechanistic point of view, this complicates interpretation of results as the effects of brain ischemia and reperfusion are difficult to separate from the effects of tPA. To address the effects of reperfusion alone, we compared permanent and temporary mechanical cerebral artery occlusions without tPA.13 In rats, the rate of HT can be increased by increasing the duration of time from stroke onset to reperfusion of ischemic tissue. For example, in Sprague Dawley rats with a middle cerebral artery occlusion (MCAO), reperfusion at 5 hours results in 81.8% of rats having HT. In contrast, with permanent MCAO the rate of HT is only 18.2% (P<0.05). Mortality rates are also increased in the group reperfused at 5 hours (54.5%) compared with the permanent MCAO group (18.1%). Thus, delayed reperfusion after prolonged ischemia increases the rate of HT and worsens stroke outcome.13, 14 These rodent studies are consistent with ischemic stroke in humans where reperfusion by mechanical means increases the risk of HT.15
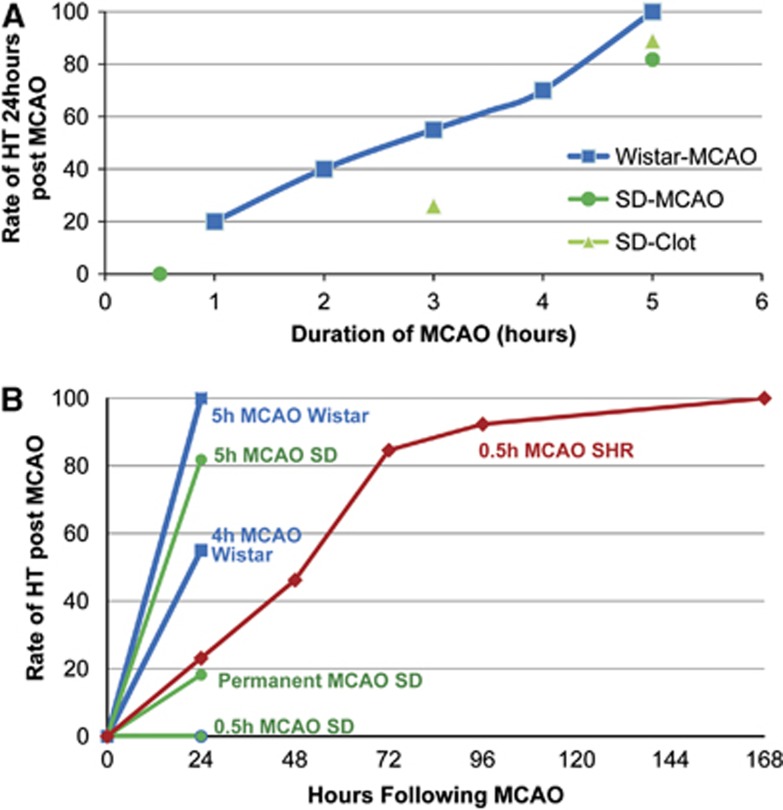
There is a strong relationship between duration of ischemia and reperfusion induced HT. In rats, duration of ischemia increases the rate of HT increase (Figure 1A). In Wistar rats MCAO lasting 1.5, 2.5, 3.5, and 5 hours the rate of HT at 24 hours after stroke is 25%, 50%, 75%, and 100%, respectively.16 Similar rates of HT have been observed in mice and Sprague Dawley rats in both the MCAO and clot injection stroke models.13, 17, 18 If reperfusion does not occur, then the risk of HT is lower (18.2%) (Figure 1B).13, 14 In humans, increased time from ischemia onset to reperfusion has also been associated with an increase in HT risk in both tPA-treated and -untreated patients.17 Recanalization that occurs beyond >6 hours of stroke onset is an independent predictor of HT in human stroke.19 In contrast, early reperfusion is associated with a reduced risk of HT.20 Thus, time from stroke onset to reperfusion is a key factor in determining the rate of HT, with longer durations of ischemia increasing the likelihood of HT when blood flow is restored.
Figure 1.
(A) The rate of hemorrhagic transformation (HT) increases with longer durations of cerebral ischemia followed by reperfusion. Middle cerebral artery occlusions (MCAOs) lasting 1 to 5.5 hours were induced in Wistar and Sprague Dawley (SD) rats. After reperfusion, the rate of HT was determined 24 hours after stroke onset.13, 14, 16, 17 (B) The rate of HT as a function of MCAO duration. Increased duration of MCAO increases the rate of HT in several rat stroke models. Evaluation beyond 24 hours of stroke onset identifies additional HT in the spontaneously hypertensive rat (SHR). Note that a 0.5-hour MCAO in an SD rat does not produce HT at 24 hours, whereas a 0.5-hour MCAO in the SHR does produce HT with a 100% HT rate at 7 days after stroke onset.
Though time to reperfusion is a key factor in determining the risk of HT in both animals and humans, other aspects important to HT in patients with stroke may not be reproduced in animal stroke models.21 Patients often have multiple vascular risk factors and comorbid disease that are associated with HT but are not usually modeled in animals (Table 1). In animal MCAO models, full reperfusion occurs rapidly as the suture is withdrawn. However in humans, reperfusion may be a more dynamic process that can occur gradually over hours with periods of partial reperfusion. The timing of HT after stroke onset and its relationship may also warrant consideration. Many of the animal models have evaluated HT that occurs within the first 24 hours of stroke onset. However, in patients with stroke the timing of HT can be quite variable, occurring within the first 24 hours in some22 but also occurring at later time points several days after stroke.23 Many of the symptomatic intracranial hemorrhages recorded in human tPA stroke trials have been noted within the first 24 to 36 hours of stroke onset.8, 24, 25, 26 However, HT can occur as late as 1 week after stroke.24, 27 Thus, several factors present in human stroke likely warrant consideration in animals to better model reperfusion and the complexity of HT that occurs in patients.
Table 1. Factors associated with hemorrhagic transformation in ischemic stroke patients.
| Factor | Reference | |
|---|---|---|
| Clinical features | Age | 50, 143, 144, 145, 146, 147, 148 |
| Stroke severity/NIHSS | 50, 143, 144, 146, 147, 148, 170 | |
| Systolic blood pressure | 144, 146, 152, 153 | |
| Hypertension history | 144, 145 | |
| Glucose | 50, 143, 144, 146, 148, 170 | |
| Diabetes | 170 | |
| Body weight | 144 | |
| Gender | 146 | |
| Congestive heart failure | 50, 148 | |
| Atrial fibrillation | 50, 148 | |
| Renal impairment | 50, 148 | |
| Antiplatelet use | 8, 50, 144, 167, 168, 169 | |
| Platelet count | 143 | |
| Anticoagulant/international normalized ratio/partial thromboplastin time | 162 | |
| Time to reperfusion | 144 | |
| Blood marker | MMP-9 | 60, 61, 122 |
| Fibronectin | 62 | |
| Fibrinogen | 180 | |
| S100B | 181 | |
| Ferritin | 182 | |
| Activated protein C | 183 | |
| Thrombin activatable fibrinolysis inhibitor (TAFI) | 184 | |
| Plasminogen activator inhibitor-1 (PAI-1) | 184 | |
| Vascular adhesion protein-1 (VAP-1) | 185 | |
| Tight junction proteins: CLDN5, OCLN, ZO1 | 75 | |
| Platelet-derived growth factor-CC (PDGF-CC) | 37 | |
| Genetics | Leukocyte mRNA (AREG, MARCH7, SMAD4, INPP5D, MCFD2, VEGI) | 76 |
| α-2-macroglobulin | 164 | |
| Factor XII | 164 | |
| Factor XIII | 165 | |
| Neuroimaging | Infarct size/diffusion weighted imaging infarct volume | 152, 186 |
| Early infarct sign | 50, 145, 170 | |
| Dense cerebral artery sign | 145 | |
| Leukoaraiosis | 50 | |
| MRI enhancement pattern | 172 | |
| BBB permeability | 174 | |
| HARM | 173 | |
| Apparent diffusion coefficient value | 176 | |
| Collateral flow | 20 | |
| Cerebral blood flow or volume | 177 | |
| Rating scores | HAT (NIHSS, glucose, early infarct sign, diabetes) | 170 |
| Multicenter Stroke Survey (age, NIHSS, glucose, platelet count) | 143 | |
| SITS-SICH (age, NIHSS, glucose, systolic BP, hypertension history, body weight, time to thrombolysis, antiplatelet) | 144 | |
| SEDAN score (NIHSS, glucose, early infract sign, dense artery sign) | 145 | |
| GRASPS score (age, NIHSS, glucose, systolic BP, sex, asian race) | 146 | |
| SPAN-100 index (age, NIHSS) | 147 | |
| iScore (age, NIHSS, glucose, sex, stroke subtype, atrial fibrillation, congestive heart failure, prior myocardial infarction, smoker, cancer, dialysis, prior disability) | 148 |
MMP-9, matrix metalloproteinase-9; AREG, amphiregulin; MARCH7, membrane-associated ring finger (C3HC4) 7; SMAD4, SMAD family member 4; INPP5D, inositol polyphosphate-5-phosphatase; MCFD2, multiple coagulation factor deficiency 2; VEGI, vascular endothelial growth inhibitor; MRI, magnetic resonance imaging; BBB, blood–brain barrier; HARM, Hyperintense Acute Injury Marker; HAT, Hemorrhage After Thrombolysis; BP, blood pressure; SEDAN, blood Sugar, Early infarct signs and hyperDense cerebral artery sign, Age, and NIHSS; SITS-SICH, Safe Implementation of Thrombolysis in Stroke Symptomatic Intracerebral Hemorrhage; GRASPS, Glucose Race Age Sex Pressure Stroke Severity; SPAN, Stroke Prognostication using Age and NIHSS; NIHSS, National Institutes of Health Stroke Scale.
Hemorrhagic transformation and tissue plasminogen activator
Hemorrhagic transformation is a major concern in patients treated with tPA. Tissue plasminogen activator increases the rate of HT by as much as 10-fold.24, 28 Given that tPA remains the only FDA-approved medical therapy for acute ischemic stroke, methods to reduce HT in tPA-treated patients are of critical importance. An improved understanding of HT could aid in the prediction and/or prevention of tPA-related HT, thus improving its safety profile. Preventing tPA-related HT might make it possible to extend the therapeutic window and increase the eligibility criteria for thrombolysis. In addition, strategies to prevent tPA-related HT may also be applicable to other reperfusion strategies such as endovascular therapy.
Tissue plasminogen activator increases the risk of HT through a number of mechanisms.29, 30 One reason is that tPA promotes reperfusion by degrading fibrin-based blood clots. As presented in the preceding section, reperfusion is a key factor in HT formation. However, tPA promotes HT through mechanisms beyond its role in thrombolysis and reperfusion.31 It does this in part by increasing matrix metalloproteinase-9 (MMP-9),32 MMP-2,33 and MMP-334 and through effects mediated by specific receptors (Figure 2). Tissue plasminogen activator acts on the protease activated receptor 1 to increase the MMP-9 expression via NFκB.35 It also binds the platelet-derived growth factor receptor alpha (PDGFRα) to activate PDGF-CC.36 Of note, tPA binding to the PDGFRα on astrocyte end feet increases the BBB permeability36 and increased levels of PDGF-CC are associated with HT in tPA-treated stroke patients37 (Figure 2). Tissue plasminogen activator also binds the lipoprotein receptor protein (LRP) receptor on endothelial cells to increase MMP-3 and MMP-9 expression.32, 34, 38 Finally, tPA can promote neutrophil degranulation and the release of MMP-9 into the blood.39 Thus, in tPA-treated patients HT may occur not only as a result of increased reperfusion, but also through tPA's effects on metalloproteinase activity, PDGFRα and LRP receptor signaling.
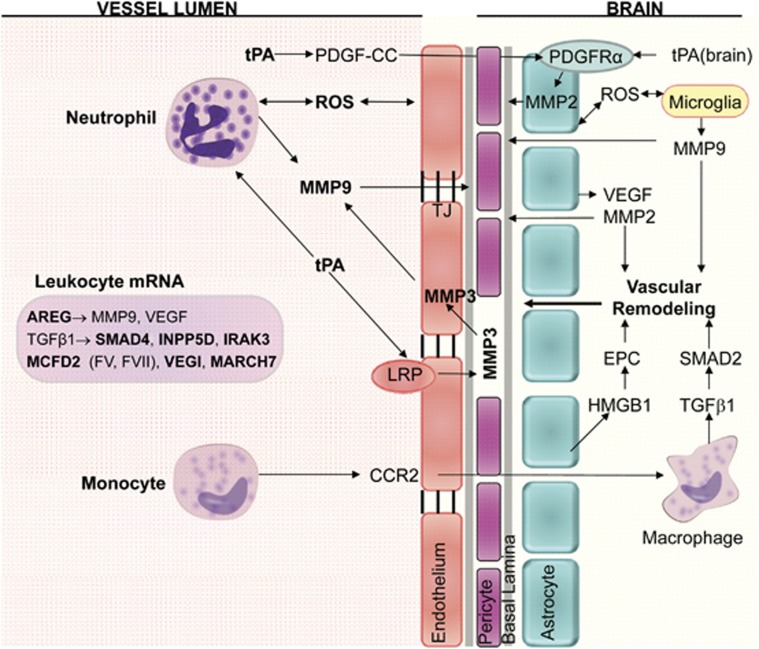
Figure 2.
Diagram of cell types and molecules associated with hemorrhagic transformation (HT) after ischemic stroke. Neutrophils are a major source of MMP-9 within the first 18 to 24 hours of stroke onset, and brain is a major source of MMP-9 at >18 to 24 hours after stroke onset. Exogenous tPA can act on neutrophils to increase MMP-9. Endogenous tPA can act on endothelial cell lipoprotein receptor protein (LRP) to increase MMP-3, and on PDGF-CC to increase MMP-2 via astrocyte PDGFRα receptors. Monocytes bind CCR2, enter brain where TGFβ1 induction of SMAD2 stabilizes the blood–brain barrier (BBB) and prevents HT for 1 to 7 days after stroke. AREG, amphiregulin; CCR2, C-C chemokine receptor type 2; EPC, endothelial progenitor cell; HMGB1, high-mobility-group-box-1; INPP5D, phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase 1; IRAK3, interleukin-1 receptor-associated kinase 3; MARCH7, membrane-associated ring finger (C3HC4) 7; MCFD2, multiple coagulation factor D2; MMP, matrix metalloproteinase; PDGF-CC, platelet-derived growth factor CC; PDGFRα, platelet-derived growth factor receptor alpha; ROS, reactive oxygen species; SMAD, mothers against decapentaplegic homolog; TGFβ1, transforming growth factor β1; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor; VEGI, vascular endothelial growth inhibitor.
Early and delayed hemorrhagic transformation
Disruption of the BBB is central to HT formation in ischemic stroke. After ischemic brain injury, disruption of the BBB occurs early.40 Within 10 minutes of reperfusion, there is evidence of basal lamina degradation and BBB disruption.41, 42 In humans, early opening of the BBB has been documented within 2 to 6 hours (median 3.8 hours) of stroke onset.21, 43 The early disruption of the BBB in stroke may not be uniform over time. Indeed, periods of enhanced early BBB permeability have been observed at 4 to 8 hours and again at 12 to 16 hours.44, 45 These escalations in early BBB opening may relate to aspects of an evolving infarct and perfusion status such as hyperemia and hypoperfusion.45 Beginning at ∼24 hours, a persistent disruption of the BBB is present that lasts for weeks.
In this review, we suggest the possibility of two types of HT, an early HT that occurs within the first 18 to 24 hours of stroke onset and a delayed HT that occurs after 18 to 24 hours of stroke onset. We propose that the mechanisms contributing to early BBB disruption and early HT differ from those involved in delayed BBB disruption and delayed HT (Figure 3). In early HT, ROS, blood-derived MMP-9, and brain-derived MMP-2 have emerged as important mediators. In delayed HT, brain-derived factors including MMP-9, MMP-3, other proteases, vascular remodeling, and neuroinflammation begin to have a more prominent role. This is important as treatments designed to prevent HT may need to be tailored depending on the type of HT and causal factors. Evidence supporting this proposal is detailed below.
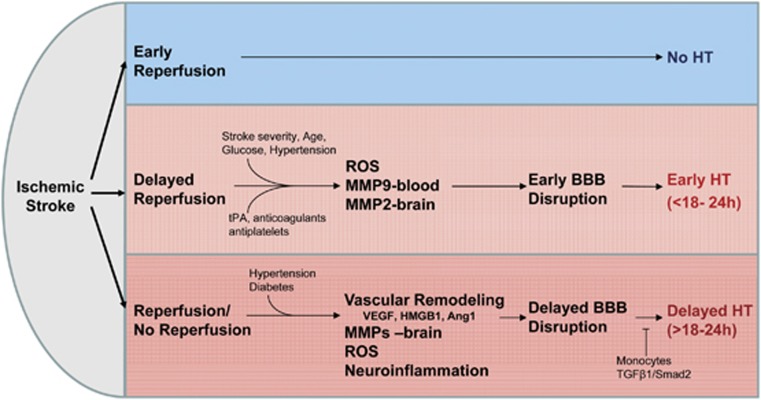
Figure 3.
Early reperfusion reduces the risk of hemorrhagic transformation (HT). Delayed reperfusion increases the BBB disruption and the risk of HT. ROS, leukocyte-derived MMP-9, and brain-derived MMP-2 have an important role in producing early BBB disruption and early HT. In contrast, brain-derived vascular remodeling, MMPs (MMP-9, MMP-2, and MMP-3), other brain proteases (plasmin, endogenous tPA, urokinase (uPA), and cathepsins), ROS, and neuroinflammation contribute to delayed BBB and delayed HT. A subset of monocytes that enter brain may prevent delayed HT. BBB, blood–brain barrier; MMP, matrix metalloproteinase; ROS, reactive oxygen species; SMAD, mothers against decapentaplegic homolog; TGFβ1, transforming growth factor β1; tPA, tissue plasminogen activator; VEGF, vascular endothelial growth factor; HMGB1, high-mobility-group-box-1.
Early Hemorrhagic Transformation: Role of Reactive Oxygen Species
Reactive oxygen species have an important role in early HT. Reperfusion of ischemic tissue results in the production of ROS from several sources, including intracellular mitochondria, nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase), xanthine oxidases, cellular membrane receptors, and inflammatory mediators29, 46 (Figure 3). Increased ROS produced by ischemia-reperfusion can disrupt the neurovascular unit through damage to endothelial cells, pericytes, smooth muscle cells, and astrocytes. This results in increased BBB permeability and increased likelihood of HT. Reactive oxygen species damage of the neurovascular unit at the capillary level might predispose to petechial hemorrhage, whereas at the small arteriolar level ROS injury to both endothelial and smooth muscle cells could produce larger parenchymal hemorrhages.
Experimental evidence supports a role of ROS in early HT. Superoxide and peroxynitrite have been found to disrupt microvascular integrity and thus may contribute to HT. After 2 hours of MCAO and 3 hours of reperfusion in a rat, both superoxide and peroxynitrite are increased in microvessels and astrocytic end feet.46 In this model, disruption of the BBB correlated with ROS levels, and was prevented by inhibiting nitric oxide. Levels of ROS are increased in reperfusion compared with permanent MCAO models.44 In permanent MCAO, levels of ROS are lower, with an initial decrease that is followed by a slow rise over the course of 3 hours. In contrast, reperfusion after 1 hour of MCAO results in a burst in ROS formation and a sustained increase in levels over the course of 3 hours. The time course of reperfusion induced ROS over the first 24 hours has not been studied.
Superoxide radicals have also been shown to be an important mediator of early reperfusion induced BBB disruption.30 In mice deficient in copper/zinc-superoxide dismutase (SOD1), ischemia induced BBB disruption was reduced by 88% (P<0.0001) after 3 hours of reperfusion and 73% (P<0.01) after 7 hours of reperfusion. Active in situ MMP mediated proteolysis was shown in ischemic leaking capillaries that produce ROS.30 Superoxide in ischemic reperfusion may arise from xanthine oxidase or nicotinamide adenine dinucleotide phosphate oxidase (NOX2) in immune cells.47 The rate of early HT and severity of BBB disruption are reduced in mice deficient in NOX2 and when NOX2 is inhibited.47
In humans, the oxidative stress marker F2-isoprostane is increased in blood early after stroke onset (median 6 hours)48 and plasma levels of 3-nitrotyrosine are elevated at 3 and 24 hours.49 Indeed, several clinical factors associated with HT in stroke patients may promote the generation of ROS, including age, glucose, diabetes, infarct size, congestive heart failure, and renal impairment50 (Figure 3; Table 1). The association of ROS with ischemia-reperfusion injury and HT has led to several studies evaluating pharmacological inhibition of ROS production in stroke. In animals inhibiting ROS can decrease the BBB disruption and reduce the rate of HT.51, 52 However, in humans inhibiting ROS was not found to reduce HT or improve stroke outcomes.53 These studies are discussed in detail below in the section titled Treatment of Hemorrhagic Transformation.
Early Hemorrhagic Transformation: Role of Leukocyte/Neutrophil Metalloproteinases
Ischemic stroke elicits a robust activation of the immune system. Within 30 minutes of focal cerebral ischemia, circulating leukocytes adhere to vascular endothelial cells.45 By 6 hours, neutrophils have begun to enter the brain of rats with temporary MCAO.54 Leukocyte adhesion and migration across the vasculature activates a number of signaling cascades (protein kinase C, focal adhesion kinase) that increase the BBB permeability.45 Metalloproteinases including MMP-9, MMP-2, and MMP-3 are key molecules involved in BBB opening and HT after ischemic stroke.40, 55, 56 Though the precise timing and cellular source of MMPs released after ischemia requires further study, circulating leukocytes are important contributors in early HT (Figures 2 and 3). Studies supporting this notion include the following:
(1) In rats and mice, plasma activity of MMP-9 and MMP-2 is increased within 3 to 8 hours of stroke onset.13, 14, 55 Use of MMP inhibitors in these small animals decreases the BBB opening and the rate of HT.13, 14 In primates, plasma MMP-9 activity is transiently increased within 2 hours of MCAO.57 In humans, MMP-9 activity increases early, with a peak at 6 to 8 hours after stroke and a return toward baseline by 24 to 26 hours.48 MMP-9 mRNA levels are also increased in human peripheral leukocytes at 3 and 5 hours after stroke with a return toward baseline by 24 hours58, 59 (Figure 4). Thus, there appears to be a transient increase in MMP-9 levels in circulating leukocytes and plasma, with a peak between 2and 8 hours of ischemic stroke onset. Importanlty, MMP-9 plasma levels measured at 3 hours are predictive of subsequent HT60, 61, 62 and early MMP-9 levels correlate with infarct severity and barrier injury.63 This suggests that MMP-9 in the blood may contribute to early opening of the BBB and early HT (Figures 2, 3, 4). It is important to note some studies have found MMP-9 to be increased in the plasma beyond the 24-hour time point in ischemic stroke.64 The reason for this increase in MMP-9 remains unclear, though it may relate to particular causes of stroke, comorbid disease, or size of infarct or a biphasic rise in MMP-9 with an initial early rise peaking by 6 to 8 hours followed by a second rise over the course of days. In addition, at least one study has suggested that neutrophils are not the primary source of MMP-9 involved in BBB disruption after stroke.65 In this study, however, brain MMP-9 was measured at 24 and 72 hours which is beyond the early HT period where we postulate leukocytes are the major source of MMP-9. This is discussed in greater detail in the Delayed Hemorrhagic Transformation section below.
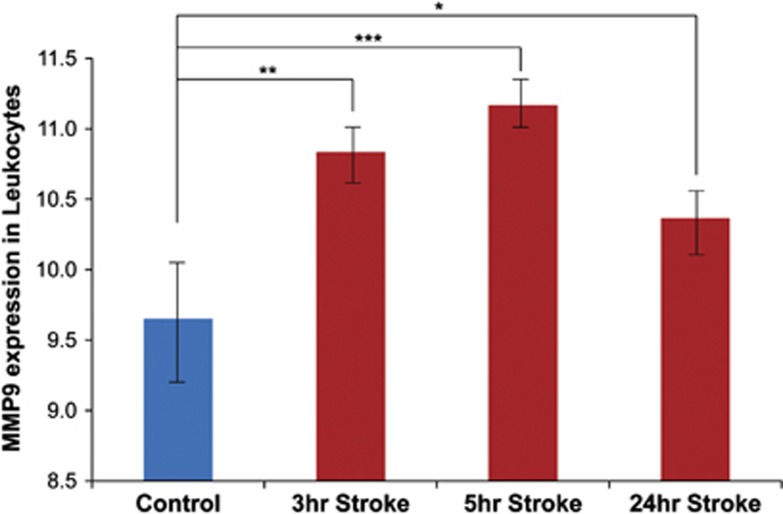
Figure 4.
Matrix metalloproteinase-9 (MMP-9) mRNA levels in blood of patients with ischemic stroke. MMP-9 mRNA was elevated at 3 and 5 hours after stroke compared with controls, with a return closer to baseline by 24 hours (*P=0.02; **P=9.9 × 10−5, ***P=8.6 × 10−8).
(2) Leukocytes are an important source of MMP-9 that contributes to early disruption of the BBB in ischemic stroke (Figure 2). This suggestion is based on two mouse studies that specifically looked at blood- versus brain-derived MMP-9 in relationship to BBB breakdown. Gidday et al.66 used MMP-9 knockout mice and chimeric knockouts lacking either MMP-9 in leukocytes or MMP-9 in resident brain cells. They found that early BBB breakdown is prevented in chimeric mice with leukocytes deficient in MMP-9. In fact, the reduction in BBB breakdown observed in the leukocyte MMP-9-deficient mouse was comparable to that observed in an MMP-9 knockout mouse. However, in chimeric mice where leukocytes had intact MMP-9, breakdown of the BBB did occur. This indicates that MMP-9 from leukocytes (mainly neutrophils) mediates BBB breakdown early after stroke in this model.66
These results were confirmed in an independent study by Wang et al.67 Matrix metalloproteinase-9 null mice that received wild-type (MMP-9 intact) bone marrow had comparable levels of brain MMP-9, BBB disruption, and infarct size at 24 hours after transient MCAO compared with wild-type controls. Furthermore, the BBB disruption in MMP-9 null mice receiving wild-type bone marrow occurred as early as 1 hour after reperfusion. In contrast, wild-type animals with MMP-9 null bone marrow showed barely detectable levels of MMP-9 in the brain, with considerable attenuation in BBB disruption and brain infarct size. These findings show that MMP-9 from bone marrow-derived cells is an important factor involved in early BBB disruption after stroke.67
(3) Depleting leukocytes can decrease the BBB disruption and the rate of HT within the first 24 hours of ischemic stroke. When neutrophils are depleted with vinblastine or anti-neutrophil antibody, endothelial disruption and the rate of HT are decreased in rodent stroke.68, 69 Likewise, inhibiting neutrophils with a CD11b/CD18 antagonist decreases the degree of BBB disruption and tPA-related HT after MCAO in rats.70 In contrast, when neutrophils are increased via lipopolysaccharide there is an increase in early BBB disruption in rodent stroke.71
Neutrophils have been shown to be an important source of MMP-9 within the first 24 hours of ischemic stroke. In a rat MCAO stroke model, blocking neutrophil infiltration reduced MMP-9 in the brain.72 In particular, two forms of MMP-9 that were present in neutrophils (a 95-kDa form, and MMP-9 dimers) were only present when neutrophil infiltration was not blocked.72 In humans with ischemic stroke, MMP-9-positive neutrophil infiltration has also been associated with BBB breakdown, basal lamina type IV collagen degradation, and HT.73 Thus, neutrophils appear to be an important source of early MMP-9 in ischemic stroke.
(4) The mechanism by which blood-derived MMP disrupts the BBB and leads to HT is not clear. Plasma MMP-9 may open the BBB from the luminal side of the vessel by acting directly on tight junction proteins (TJPs), or may be taken up into endothelial cells and act on the basal lamina. Alternatively, neutrophils that enter into brain could release MMP that acts directly on TJPs and/or basal lamina.73 Matrix metalloproteinase actions on the basal lamina (e.g., collagen IV, fibronectin, and laminin) could disrupt the endothelial–pericyte–astrocyte complex and facilitate BBB injury and promote HT.41 The role of TJPs in early HT is unclear since one study found intact TJPs at 24 hours after stroke74 whereas a human study found increased blood levels of TJPs to be predictive of HT.75
Early Hemorrhagic Transformation: Role of Other Leukocyte Factors
Though leukocyte-derived MMPs appear to be one mediator of early HT, additional leukocyte-derived molecules have been implicated.76 We examined mRNA expression in blood within 3 hours of ischemic stroke onset in humans before administration of tPA. Twenty-nine genes were differentially expressed in patients that developed HT. Six genes predicted HT in a second cohort with 80% sensitivity and 70% specificity.76 These genes included amphiregulin, membrane-associated ring finger (C3HC4) 7, SMAD family member 4 (SMAD4), inositol polyphosphate-5-phosphatase (INPP5D), multiple coagulation factor deficiency 2 (MCFD2), and vascular endothelial growth inhibitor (Figure 2).
Amphiregulin enhances neutrophil migration across epithelial cell layers by altering E-cadherin tight junctions, promotes IL-8 release, and increases MMP-9 and vascular endothelial growth factor (VEGF) through MAP kinase pathways.76 Thus, it may also promote HT. Multiple coagulation factor deficiency 2 was decreased in those who developed HT. It regulates transport of coagulation factors V and VIII from endoplasmic reticulum to the Golgi apparatus, and mutations of the gene result in a bleeding disorder.76 Thus, low MCFD2 levels might predispose to HT.
SMAD family member 4 was also elevated in blood leukocytes by 3 hours in those who later developed HT.76 It is a target of transforming growth factor β (TGFβ) and regulates N-cadherin expression in endothelial cells to stabilize the BBB. Mutations of SMAD4 cause hereditary hemorrhagic telangiectasia, and SMAD4-TGFβ signaling is associated with extracellular matrix remodeling and formation of aortic dissections and aneurysms.76 Two SMAD4 target genes were also elevated in our study: interleukin-1 receptor-associated kinase 3 (IRAK3) and INPP5D. The IRAK3 is involved in toll-like receptor signaling, mainly in monocytes, and INPP5D regulates proliferation and programming of myeloid cells. Our data suggest that TGFβ signaling via SMAD4 and IRAK3/INPP5D in leukocytes within 3 hours of ischemic stroke may be associated with increased risk of HT after ischemic stroke (Figure 2).76 In contrast, TGFβ/SMAD2 signaling in a subset of monocytes that migrate into brain 1 to 7 days after stroke reduce the BBB disruption and the rate of HT (see section below titled Delayed Hemorrhagic Transformation: Role of Neuroinflammation).77
Early Hemorrhagic Transformation: Role of Brain Metalloproteinases
Though MMP from blood has a prominent role in early BBB disruption and HT, brain-derived proteases also contribute. The role of brain-derived proteases and brain-derived molecules develops over time after stroke, becoming a major contributor to BBB disruption and HT by 24 hours. This is discussed in greater detail in the section Delayed Hemorrhagic Transformation below. In early BBB disruption, brain MMP-2 and caveolin-1 have been implicated.78, 79
Matrix metalloproteinase-2 has a key role in early BBB opening after ischemic stroke.80 Brain MMP-2 is mostly derived from astrocytes,81 endothelial cells,82 and potentially leukocytes. It is increased in the brain within 1 to 3 hours of stroke onset in rats, mice, and primates and remains elevated for days.57, 78, 79 Activators of MMP-2 including furin, thrombin, xanthine oxidase, and MT1-MMP are also increased early after stroke, providing further support to an increase in brain MMP-2 in cerebral ischemia.40, 83 The rise in MMP-2 correlates with early opening of the BBB and degradation of the TJPs claudin-5 and occludin in rodent MCAO models.78, 80, 84, 85 In addition, direct injection of MMP-2 into rodent brain disrupts the BBB and results in hemorrhage, further supporting a role of MMP-2 in early BBB disruption and HT in stroke.84
Though MMP-2 is associated with early BBB disruption after stroke, its role in HT and effect on stroke outcome remains less clear. In primates, brain levels of MMP-2 were not significantly increased in animals with HT compared with those without.86 In a 2-hour MCAO mouse model, an MMP-2 knockout failed to reduce the infarct size.87 Though the MMP inhibitor (BB-1101) blocked MMP-2 increase in brain, a reduction in cerebral edema and BBB permeability was present only at 24 hours and not at 48 hours.80 In contrast, a recent study of mice within 1 to 1.5 hours of MCAO found that MMP-2-deficient mice had a reduced rate of HT, smaller hemorrhage volume, and improved neurologic function compared with wild type.79 The reasons for these differing results require further study to better understand the role of MMP-2 in acute stroke and early HT.
The membrane protein caveolin-1 (cav-1) has recently been shown to be involved in the early BBB disruption in ischemic stroke. Cav-1 may affect the BBB by inhibition of MMP activity and production of ROS.88, 89 Indeed, infarct size is larger in cav-1 knockout mice.88 After brain ischemia, cav-1 is decreased and correlates with an increase in brain MMP-2 and BBB permeability.89 In cultured endothelial cells, inhibition of cav-1 with siRNA alters the distribution of the TJP claudin-5 in response to oxygen glucose deprivation.78 Furthermore, in a 2-hour MCAO rat model microvessels have reduced occludin and redistribution of claudin-5. The authors suggest that after stroke cav-1 mediates changes in claudin-5, whereas MMP-2 mediates degradation of occludin. Thus, both MMP-2 and cav-1 may contribute to early BBB disruption after ischemic stroke.
Delayed hemorrhagic transformation
There is a persistent disruption of the BBB beginning by 1 day (18 to 24 hours depending on severity of ischemia) after ischemic stroke that can last for several weeks.90, 91 This delayed and prolonged BBB opening is mediated predominantly by brain-derived MMPs (MMP-9, MMP-2, and MMP-3), other brain proteases (plasmin, endogenous tPA, urokinase, and cathepsins),41 neuroinflammation, as well as vascular remodeling and neovascularization. We propose that HT that occurs beyond 18 to 24 hours after ischemic stroke (delayed HT) occurs mostly due to the processes involved in delayed BBB opening after stroke (Figure 3). Indeed, early BBB disruption assessed at 2.5 to 3 hours after stroke does not predict delayed HT at 3 to 7 days.92, 93
Delayed Hemorrhagic Transformation: Role of Brain-Derived Metalloproteinases
At 18 to 24 hours after stroke, brain cells become an increasingly more important source of MMP-9 and MMP-3.81, 86 Indeed, when neutrophils are depleted in a rat stroke model brain levels of MMP-9 or MMP-2 measured at 24 hours remain relatively unchanged compared with controls.86 This suggests that the major source of MMP-9 and MMP-2 in the brain at 24 hours is not neutrophils, but brain. This finding is supported by Maier et al65 who showed that the primary source of MMP-9 in the brain at 24 and 72 hours is not neutrophils but brain cells (Figure 2). In addition, other proteases derived from brain cells also contribute to delayed HT, including MMP-10, MMP-13, MMP-14, TNF-α converting enzyme, plasmins (tPA and urokinase), and cathepsins.32, 34, 38, 41, 56, 81
Several brain cells including astrocytes, neurons, microglia, and endothelium can express MMP-9 and have been found to be MMP-9 immunoreactive after stroke. The expression of MMP-9 may vary by severity of ischemia and time from stroke onset. Early after stroke (first 24 hours) MMP-9 is predominantly localized to endothelial cells.94 However, as a stroke evolves beyond 24 hours to several days, MMP-9 is predominantly identified in brain cells including neurons, astrocytes,94 and microglia.81 This supports the notion that leukocytes, including neutrophils, contribute to MMP-9 early in stroke, whereas brain cells are the predominant source of MMP-9 later in stroke. Indeed, brain levels of MMP-9 correlate with delayed opening at 24 to 48 hours,80 whereas plasma levels of MMP-9 at 3 hours are predictive of HT.60, 61, 62
Matrix metalloproteinase-9 degrades TJPs (claudin-5, occludin, and ZO-1), and basal lamina proteins (fibronectin, lamin, and collagen).85 After ischemic stroke, MMP-9 promotes BBB permeability, cerebral edema, and HT in animals and humans.61, 85, 95 In mice deficient in MMP-9 or treated early with an MMP-9 inhibitor, there is a reduction in infarct size and degree of tight junction degradation.55, 96, 97 Mechanisms for MMP-9 activation after ischemia include (1) ROS,40 (2) TNF, IL-1, and other cytokines that activate MMP-3 which converts proMMP-9 into active MMP-9,40 (3) actions of high-mobility-group-box-1 (HMGB1) on TLR4 receptors that then induce MMP-9,98 or (4) NFκB induction of MMP-9 after ischemia.
Matrix metalloproteinase-2 has previously been discussed for its role in early HT as it is increased in brain within the first hours of stroke. However, MMP-2 in the brain remains persistently elevated for days. Thus, it likely contributes to both early and delayed HT.
Matrix metalloproteinase-3 is produced by pericytes99 and endothelial cells100 after ischemic stroke. It acts on proMMP-9 to produce active MMP-9 and thus may promote HT.40 Indeed, MMP-3 has been associated with HT particularly when tPA is used. In postmortem human stroke brain MMP-3 is increased.101 In mice brain MMP-3 mRNA and protein begins to rise within 24 hours of stroke onset.100 The increase in MMP-3 may occur as a result of stroke-related inflammation or through induction by tPA. In mice, cerebral inflammation induced by lipopolysaccharide results in a breakdown of the BBB, an effect that is reduced in MMP-3 knockouts.102 The MMP-3 activity is also promoted by tPA binding LRP and inducing the transcription factor NFκB.103 In a mouse MCAO model, a knockout of MMP-3 reduced the rate of HT at 24 hours after tPA treatment.100 In addition, a broad spectrum of MMP inhibitor (GM6001) did not further reduce the rate of HT in this MMP-3 knockout, suggesting that MMP-3 is an important metalloproteinase in HT.
Delayed Hemorrhagic Transformation: Role of Neuroinflammation
Inflammation after stroke is of increased interest as a mechanism of secondary brain damage and potentially a role in tissue repair. The role of inflammation in HT is an evolving area of study. Microglia are activated after stroke and have been shown to be an important source of MMP-9 that promotes BBB disruption and HT.81 Peripheral monocyte interactions with endothelial cells induce MMP-9 expression,104 and monocytes then enter brain and become macrophages also produce MMP-9.81 Given monocyte infiltration into brain peaks over several days after stroke, they may contribute to delayed HT, though this needs further study.
Although monocytes may increase the MMP-9 after stroke, whether they promote HT remains unclear. In humans, augmentation of peripheral monocytes with granulocyte-macrophage colony-stimulating factor did not increase the rate of tPA-related HT.105 Indeed certain subsets of monocytes may be beneficial after stroke. One study has shown a subset of peripheral monocytes can prevent delayed HT after ischemic stroke.77 Within 24 hours of stroke onset, a subset of inflammatory monocytes (Ly6chi, C-C chemokine receptor type 2 (CCR2+), CX3CR1+) infiltrate the infarct border via a CCR2-dependent pathway and differentiate into macrophages (Figure 2).77 When these monocytes were depleted in a mouse stroke model, the rate of HT at both day 3 and day 7 increased.77 In addition, when CCR2 was blocked there was an increase in delayed HT and worsening of neurologic function. Bleeding occurred around thin-walled, dilated neovessels in the infarct border zone and was accompanied by a decrease in TGFβ1, collagen 4, and Smad2. Injection of TGFβ1 into the infarct border reduced HT in monocyte-depleted mice.77 These data suggest that a subset of peripheral monocytes recruited early via CCR2 into brain promote delayed repair of the neurovascular unit and prevent delayed HT77 (Figure 2).
In contrast, other studies have shown that inflammatory monocytes and the CCR2 pathway have harmful effects on the brain.106 In mice deficient in CCR2, 30 minutes of MCAO results in reduced infarct size, decreased BBB permeability, and decreased infiltration of monocytes and neutrophils.107 In humans, increased levels of the CD14highCD16− monocyte subtype (corresponds to mouse Ly6chigh CCR2+) are associated with worse outcome and early clinical deterioration, whereas increased levels of CD14highCD16+ are associated with reduced mortality.108 Thus, certain monocyte subsets may contribute to BBB disruption/repair and HT after ischemic stroke, though additional study is needed.
Delayed Hemorrhagic Transformation: Role of Vascular Remodeling
Vascular remodeling and angiogenesis are an important component of recovery after stroke; however, they may also promote HT. For new vessels to be incorporated into existing vasculature, a number of growth factors, MMPs, and other molecules form new vessels and the neurovascular unit. During this vascular remodeling, vessels are leakier and prone to HT.90
Vascular endothelial growth factor has an important role in vascular remodeling and angiogenesis. In rodent stroke, a reduction in VEGF through suppression of hypoxia inducible factor-1α results in a reduction in HT.109 Likewise, inhibition of VEGF signaling reduces tPA-related HT.110 In contrast, when VEGF is administered to a rodent within 1 to 24 hours of stroke the rate and severity of HT is increased.111 Furthermore, VEGF administered 1 hour after stroke increases the BBB disruption, the rate of HT, and the size of infarction.112 However, when VEGF is administered 3 to 21 after stroke, angiogenesis is enhanced with improved neurologic recovery, reduced BBB permeability, increased pericyte coverage of brain capillaries, and improved cerebral blood flow.112, 113 Thus, VEGF has a biphasic role in stroke, with early VEGF promoting BBB disruption and HT, and later VEGF promoting BBB integrity and vascular function.
Matrix metalloproteinases also contribute to vascular remodeling and neovascularization.40 Early and transient inhibition of MMPs after stroke reduces brain tissue injury, promotes new vessel formation at 3 weeks, and increases pericyte and endothelial expression of TJPs (ZO-1, occludin, and claudin-5).99 In contrast, delayed treatment with MMP inhibitors 7 days after stroke suppresses neovascular remodeling, increases brain injury, and impairs functional recovery at 14 days.94
Angiopoietins might also be involved in delayed HT. In rodent stroke, alteration of angiopoietin-1 in the ischemic core mediates BBB disruption whereas angiopoietin-2 regulates neovascularization.114 In stroke patients, increased blood levels of angiopoietin-1 are associated with tPA-related hemorrhage.115
High-mobility-group-box-1 is also involved in neurovascular repair after ischemic stroke. It is a damage-associated molecular-pattern molecule that is released by perivascular astrocytes after ischemic stroke. It acts on the HMGB1 receptor on endothelial progenitor cells (EPCs) to promote peri-infarct angiogenesis (Figure 2).116 The effect of HMGB1 on EPC has been shown to improve long-term behavioral outcome after stroke, though any effects on HT have not been studied.
Delayed Hemorrhagic Transformation: Role of Reactive Oxygen Species
Reactive oxygen species have an important role in vascular remodeling and angiogenesis,117 and thus likely contribute to delayed HT. They have regulatory effects on many angiogenic factors including VEGF, hypoxia inducible factor-1α signaling, MMP activation, NFκB activity, protein kinase C activity, ERK1/2 activity, and p38 MAP kinase activity.118 They can act as signaling molecules to regulate cell growth, differentiation, and angiogenesis.119 When endothelial cells are exposed to ROS, angiogenic patterns are induced including cell proliferation, migration, and tube formation.117 ROS also promote EPC mobilization, differentiation, and homing and thus contribute to vascular remodeling and angiogenesis.119
NADPH oxidase (NOX) is an important source of superoxide and ROS. NOX4-induced ROS has been shown to promote endothelial cell migration and proliferation as well as promote blood flow recovery after ischemia.117 When NOX1 is knocked out in a mouse, angiogenic activity is impaired.120 Likewise, in the NOX2 mouse knockout or when NOX2 is inhibited, ischemia-induced neovascularization is impaired.119 Though ROS are known to have an important role in ischemic angiogenesis, additional study is required to more specifically determine the role of ROS in delayed HT after ischemic stroke.
Delayed Hemorrhagic Transformation: The Role of Src Family Kinases
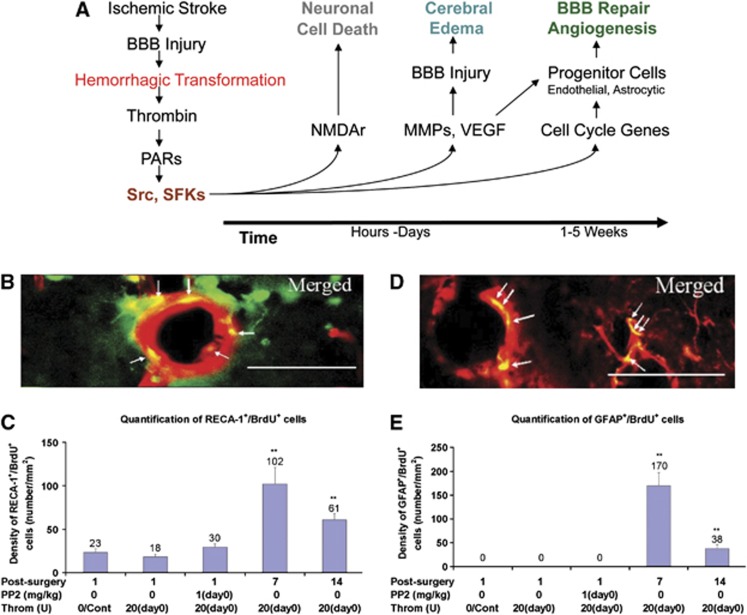
After HT of ischemic stroke, blood products such as thrombin are released into the brain and promote further damage of ischemic tissue. To study this injury, thrombin was injected into the ventricle of adult rats.121 This resulted in vascular leakage of Evans Blue at 24 hours and increased brain water content at 24 hours. We postulated that thrombin acted on protease activated receptors, which activated Src family kinase (SFK) family members to phosphorylate and activate NMDA receptors to kill neurons, and to phosphorylate MMPs, VEGF, and other factors to open the BBB (Figure 5A). Indeed, the SFK inhibitor PP2 prevented BBB breakdown and brain edema produced by ventricular thrombin.121 Since this BBB breakdown and brain edema resolved by 14 days after thrombin, we further postulated that SFK phosphorylation of cell-cycle genes would lead to BBB repair. Indeed, new endothelial cells and new perivascular astrocytes are born at 7 days and 14 days after intraventricular thrombin (Figures 5B to 5E).121 Furthermore, administration of PP2 (an SFK inhibitor) from days 2 to 6 after intraventricular thrombin prevented the repair in the BBB as manifested by persisting Evans Blue leakage and persisting brain edema.121 Thus, Src kinase family members mediate acute BBB injury produced by thrombin, but also mediate chronic BBB repair.121 Additional study of thrombin and blood products that induced BBB disruption and tissue injury after stroke may identify novel targets to reduce the effects of HT.
Figure 5.
(A) Hemorrhagic transformation (HT) activates src and src family kinases (SFKs) via thrombin induction of protease-activated receptors (PARs). Within the first hours after stroke SFKs activate N-methyl D-aspartate receptors (NMDAr) and induce neuronal cell death. Within the first few days of hemorrhage, SFKs activate matrix metalloproteinases (MMPs), vascular endothelial growth factor (VEGF), and other molecules involved in blood–brain barrier (BBB) injury and cerebral edema. Beginning around 1 week after hemorrhage, SFKs activate cell-cycle genes in progenitor cells to form new brain endothelial cells and astrocytes that are involved in BBB repair and angiogenesis. (B) HT produces thrombin that induces the birth of new cerebral endothelial cells at day 7 and day 14 in rat hippocampus (green=BrdU (bromodeoxyuridine, a marker of cell proliferation); red=RECA-1 (rat endothelial cell antigen-1, endothelial cell marker); yellow=merged). (C) There is an increase in new cerebral endothelial cells (BrdU+ RECA-1+) in the hippocampus (LMol layer) on days 7 and 14 after thrombin injection. **P<0.01 (Cont=control; PP2=non-specific src family kinase inhibitor; Throm=thrombin; (D) HT produces thrombin that induces the birth of new perivascular astrocytes at day 7 and day 14 in rat hippocampus (green=BrdU; red=GFAP (green fluorescence protein, astrocyte marker); yellow=merged). (E) There is an increase in new perivascular astrocytes (BrdU+ GFAP+) in the hippocampus (LMol layer) on days 7 and 14 after thrombin injection. **P<0.01 (figures from Liu et al).121
Treatment of hemorrhagic transformation
Several therapies have been evaluated for the prevention and treatment of HT. Though many compounds have been shown to decrease HT in animals,122 to date none have successfully been translated to humans. Targets that have been evaluated include reducing ROS, inhibiting MMPs, and modulating targets that affect BBB permeability.
Early administration of MMP inhibitors reduces BBB permeability and the rate of HT. MMP-9 and MMP-3 inhibitors decrease HT produced by tPA.123 Batimastat (BB-94) is a board spectrum MMP inhibitor that reduces tPA-related HT in rats. Minocycline inhibits MMP-9 and microglia and reduces tPA-related HT in rats.124 Ongoing studies are evaluating the role of minocycline to reduce HT in stroke patients.125 Though early inhibition of MMP-9 can reduce HT in animals, delayed or persisting MMP inhibition is known to enhance brain injury and worsen stroke outcome.94
Several therapies have targeted ROS to reduce HT. Though initial studies of the spin-trap-agent NXY-059 showed promise to reduce HT in animals,51 it failed to reduce HT in patients with stroke.53 Another spin trap agent, N-t-Butyl-Phenylnitrone (PBN), also reduced tPA-related HT in rodent stroke.52 However, when used in a rabbit clot embolic model, PBN worsened HT.126 Edaravone is a free radical scavenger that can reduce HT in rat stroke.127 However, in patients with cardioembolic stroke edaravone was found to increase the rate of HT.128 Isoflurane may increase ROS by inhibiting superoxide dismutase and catalase. It has been found to increase HT in rats with focal ischemia.129 Hydrogen gas may reduce oxidative stress in the brain and has been found to reduce hyperglycemia-enhanced HT in a rat stroke model.130
Other agents that also attenuate tPA-related HT include activated protein C, PDGFRα antagonists, cilostazol (a phosphodiesterase inhibitor),131 melatonin, fasudil (rho kinase inhibitor), fingolimod (sphingosine 1-phosphate receptor agonist),132 poly ADP ribose polymerase (PARP) inhibitors,133 sulfonylurea receptor 1 (Sur1) inhibitors (glyburide),134 17-beta estradiol, FK506 (tacrolimus),135 VEGF inhibition,110 and hyperbaric oxygen.136 In contrast, erythropoietin exacerbates tPA-related HT.137
The reasons that several promising therapies in animals have failed to translate to successful therapies for HT in human stroke remain unclear. It highlights that animal studies may not predict pharmacological effectiveness in human HT and supports the use of several different animal models to evaluate treatments to prevent HT.138 We suggest consideration be given to the different mechanisms and subtypes of HT. Though a therapy may decrease tPA-related HT, this does not necessarily indicate a benefit in HT related to reperfusion without tPA. Thus, experimental studies designed to treat all types of HT might include tPA-related HT and reperfusion induced HT without tPA. Furthermore, HT that occurs early may require a different therapeutic approach than delayed HT. Many of the small animal studies to date have focused on early HT. A therapy that can show effectiveness both in models of early HT and in models of delayed HT may be more likely to translate to the clinic.51, 138 The spontaneously hypertensive rat (SHR) may be a good model of delayed HT, as a 0.5-hour MCAO has been found to produce HT beyond 24 hours in 75% of animals and >50% of the HTs occur after 48 hours139 (Figure 1B). Another model of delayed HT is an MCAO in a mouse with depleted monocytes or selective CCR2 inhibition, as this has been found to have increased HT on days 3 and 7 after ischemic stroke.77
Clinical factors associated with hemorrhagic transformation
A number of clinical factors have been associated with HT in patients with stroke. Stroke severity and infarct size is the single factor that best correlates with HT.1 Other factors include increasing age, baseline systolic blood pressure, hypertension, serum glucose, and antiplatelet use. A meta-analysis of 55 human studies reported factors associated with tPA-related HT including older age, greater stroke severity, higher glucose, atrial fibrillation, congestive heart failure, renal impairment, previous antiplatelet agents, leukoaraiosis, and a visible acute cerebral ischemic lesion on pretreatment brain imaging.50 These findings are consistent with animal stroke models that have found increasing age, hypertension, and blood glucose predispose to HT.140, 141, 142
Clinical Factors: Age, Hypertension, Hyperglycemia, and Hemorrhagic Transformation
Increasing age has frequently been associated with an increased risk of HT in patients with stroke.8, 26, 50, 143, 144, 145, 146, 147, 148 The mechanisms by which age may contribute to HT are unclear. Age may enhance the production of ROS which promotes BBB disruption. An increase in BBB permeability has been observed with age.149 Age may also be linked to changes in the cerebral vasculature that promote HT such as a reduction in collateral circulation or increase in arterial disease.150 Though age is associated with an increased risk of HT, treatment with tPA has been shown to improve outcomes in stroke patients >80 years of age.151
Hypertension is also associated with an increased risk of HT in stroke patients.8, 144, 145, 146, 152, 153 Acute elevations in blood pressure are presumed to directly affect BBB permeability and increase HT. In a rabbit clot model acute hypertension promotes tPA-related HT,154 whereas decreasing prestroke blood pressure in rats reduces HT.155 In patients increased blood pressure after tPA treatment is associated with HT.152 The risk of HT increases with each 10 mm Hg rise in systolic blood pressure from 140 to 180 mm Hg.153 Furthermore, reducing blood pressure to below 185/110 mm Hg before administration of tPA is part of the treatment guidelines to reduce the risk of HT.156 The effects of chronic hypertension on cerebral circulation may also increase the risk of HT. Chronic hypertension alters the vasculature, increasing vascular resistance, reducing vascular compliance, and impairing collateral circulation.157 Indeed, in the SHR infarcts tend to be larger, with increased BBB disruption and increased rates of HT compared with normotensive Wistar rats.52, 139 Hypertension may also enhance formation of ROS, activation of MMPs, and inflammation and thus potentially promote BBB disruption and HT.158 An interaction between age and hypertension may also exist. In a SHR stroke model, the rate of HT was the same in young rats compared with aged rats. However, in normotensive rats age did increase the rate of HT.140
Increased serum glucose is associated with an increase risk of HT in stroke patients. In transient MCAO rat models, acute hyperglycemia increases BBB disruption and the rate of HT.141, 142 Hyperglycemia also increases HT in a cat stroke model.159 How hyperglycemia enhances HT remains unclear. It may promote ROS production, MMP activity, and proinflammatory cytokine expression.141, 159 Elevated glucose may also enhance the severity of ischemic injury through effects on the vasculature, and thus contribute to HT.160 Whether lowering glucose in patients with acute stroke can reduce the rate of HT remains unclear, though insight may be provided by an ongoing clinical trial.161
Clinical Factors: The Coagulation System and Hemorrhagic Transformation
The coagulation system also contributes to HT. Use of anticoagulants, antiplatelets, and thrombolytics, as well as an elevation in clotting times, reduction in platelets, and abnormalities in clotting factors have been associated with an increased risk of HT. Altered hemostasis may increase the probability of HT when the BBB is disrupted and may worsen HT by transforming smaller petechial hemorrhages into a larger parenchymal hemorrhage.
Anticoagulants have been shown to increase the risk of HT, and an international normalized ratio of >1.7 is a contraindication to tPA use in patients as a result of this increased risk.156 In rats, anticoagulation increases the risk of HT in both tPA-treated and -untreated stroke.162 After stroke onset, early initiation of anticoagulants, heparin or enoxaparin increases the risk of HT.163 The activated partial thromboplastin time is associated with HT,156, 164 and genetic mutations in coagulation factors (factor XII and factor XIII) have been associated with HT.164, 165 A mutation in collagen type IV, an important component of the BBB basal lamina, promotes cerebral hemorrhage related to anticoagulants.166 In ischemic stroke, collagen type IV is reduced in the basal lamina within the first 1 to 24 hours, thus it may contribute to coagulation-related HT.12, 42
Antiplatelet agents also increase the risk of HT in patients.8, 50, 144, 167, 168, 169 Antiplatelet use before tPA treatment or antiplatelet use within the first 24 hours of tPA treatment increases the risk of tPA-related HT.8, 156 In addition, dual antiplatelet therapy also increased the risk of HT after stroke.167 Antiplatelet use has been included in some of the clinical HT risk scores (Table 1). A low platelet count is predictive of HT,143 and levels below <100 000/mm3 are a contraindication to tPA because of increased HT risk.156
Tissue plasminogen activator is a serine protease that degrades fibrin-based blood clots by generating plasmin from plasminogen. As previously discussed, tPA increases the risk of HT through several mechanisms including increased reperfusion, activation of MMPs, and receptor signaling through LRP and PDGFRα. Other thrombolytics such as streptokinase and tenecteplase also promote HT.25
Clinical Factors: Prediction of Hemorrhagic Transformation in Ischemic Stroke Patients
To improve the safety of tPA and potentially extend its therapeutic window, clinical factors, imaging parameters, and blood biomarkers have been evaluated as predictors to identify patients at risk for tPA-related HT.
Clinical scores to predict HT that have been developed include the Multicenter Stroke Survey,143 the Hemorrhage After Thrombolysis score,170 and the Safe Implementation of Thrombolysis in Stroke Symptomatic Intracerebral Hemorrhage score,144 the blood Sugar, Early infarct signs and hyperDense cerebral artery sign, Age, and NIHSS score,145 the Glucose Race Age Sex Pressure Stroke Severity score,146 the Stroke Prognostication using Age and NIHSS-100 index,147 and the iScore148 (Table 1). Though each score is able to identify patients at increased risk of HT, they do not achieve sufficient sensitivity or specificity to withhold tPA in otherwise tPA eligible patients.171 Thus, additional predictors to stratify risk of HT after ischemic stroke have been sought, including neuroimaging and blood biomarkers.
Several brain imaging approaches have been evaluated to predict HT after stroke (Table 1). In one study, magnetic resonance imaging (MRI) enhancement patterns were associated with HT.172 In another, the presence of gadolinium enhancement of cerebrospinal fluid termed Hyperintense Acute Reperfusion Marker was found to be an independent predictor of HT.173 Blood–brain barrier permeability assessed by perfusion CT is also predictive HT.174 Other MRI measures that predict HT include T2*-permeability MRI,175 apparent diffusion coefficient,176 and very low cerebral blood volume.177
Collateral circulation has also been associated with HT in patients with stroke. After vessel occlusion, leptomeningeal collaterals can maintain cerebral blood flow to distal brain tissue.150 In patients, the extent and rate of pial artery backfilling has been used as an indirect assessment of collateral circulation. Poor collaterals are associated with larger infarction and worse functional outcome at 3 months.178 Furthermore, poor collaterals are a predictor of HT in patients where recanalization occurs. In a study of 105 stroke patients, HT occurred more frequently in those with poor pial collaterals (25%, 8/32) compared with those with good pial collaterals (2.8%, 2/72) (P=0.0004).179 In a study of 222 endovascular-treated stroke patients, symptomatic HT was more common in patients that recanalized and had poor collaterals (30.2%) compared with patients that recanalized and had good collaterals (14.3%). This highlights that reperfusion of infarcted brain tissue is a critical factor in HT, and poor collaterals likely increase the size and severity of cerebral infarction and thus promote HT.
Blood biomarkers may have utility to identify ischemic stroke patients at risk for HT. Patients at increased risk for HT might have their tPA therapy modified or be administered a drug to prevent HT. Blood biomarkers that have been associated with HT in ischemic stroke include MMP-9,60, 61, 122 cellular fibronectin,62 fibrinogen,180 S100B,181 ferritin,182 activated protein C,183 thrombin activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1184 (Table 1). Other molecules that predict HT include: Vascular Adhesion Protein-1,185 a cell surface and circulating enzyme involved in recruitment of lymphocytes and neutrophils; serum levels of TJPs (CLDN5, OCLN, and CLDN5/ZO1 ratio);75 and PDGF-CC isoforms,37 which is activated by tPA and acts on astrocyte PDGFRα (Figure 2).
A few genetic factors have been associated with tPA-related HT. Single-nucleotide polymorphisms in α-2-macroglobulin, Factor XII164 and Factor XIII165 have each been associated with HT. Several RNA expressed in circulating leukocytes within 3 hours of ischemic stroke are also associated with HT and can predict tPA-related HT with >85% accuracy76 (Figure 2).
The above review summarizes HT after ischemic stroke in animals and humans. Though major progress has been made, this has yet to translate to the clinic and alter practice. Well-designed clinical trials are needed to advance the basic and clinical science of HT to clinical care. Ideally, agents evaluated in clinical trials will target a specific type of HT, or potentially be those therapies that demonstrate effectiveness in animal models of both early and delayed HT produced with and without tPA.
Acknowledgments
The authors thank the National Institutes of Health/NINDS and the American Heart Association for support that made this review possible. We apologize to colleagues whose excellent work has not been cited as a result of space constraints.
The authors declare no conflict of interest.
References
- Terruso V, D'Amelio M, Di Benedetto N, Lupo I, Saia V, Famoso G, et al. Frequency and determinants for hemorrhagic transformation of cerebral infarction. Neuroepidemiology. 2009;33:261–265. doi: 10.1159/000229781. [DOI] [PubMed] [Google Scholar]
- Beslow LA, Smith SE, Vossough A, Licht DJ, Kasner SE, Favilla CG, et al. Hemorrhagic transformation of childhood arterial ischemic stroke. Stroke. 2011;42:941–946. doi: 10.1161/STROKEAHA.110.604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli M, Bastianello S, von Kummer R, del Zoppo GJ, Larrue V, Lesaffre E, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- Khatri P, Wechsler LR, Broderick JP. Intracranial hemorrhage associated with revascularization therapies. Stroke. 2007;38:431–440. doi: 10.1161/01.STR.0000254524.23708.c9. [DOI] [PubMed] [Google Scholar]
- Berger C, Fiorelli M, Steiner T, Schabitz WR, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic. Stroke. 2001;32:1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- Dzialowski I, Pexman JH, Barber PA, Demchuk AM, Buchan AM, Hill MD. Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian alteplase for stroke effectiveness study registry. Stroke. 2007;38:75–79. doi: 10.1161/01.STR.0000251644.76546.62. [DOI] [PubMed] [Google Scholar]
- Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- Larrue V, von Kummer RR, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- Park JH, Ko Y, Kim WJ, Jang MS, Yang MH, Han MK, et al. Is asymptomatic hemorrhagic transformation really innocuous. Neurology. 2012;78:421–426. doi: 10.1212/WNL.0b013e318245d22c. [DOI] [PubMed] [Google Scholar]
- Motto C, Ciccone A, Aritzu E, Boccardi E, De Grandi C, Piana A, et al. Hemorrhage after an acute ischemic stroke. MAST-I Collaborative Group. Stroke. 1999;30:761–764. doi: 10.1161/01.str.30.4.761. [DOI] [PubMed] [Google Scholar]
- Thomalla G, Sobesky J, Kohrmann M, Fiebach JB, Fiehler J, Zaro Weber O, et al. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38:313–318. doi: 10.1161/01.STR.0000254565.51807.22. [DOI] [PubMed] [Google Scholar]
- Hamann GF, del Zoppo GJ, von Kummer R. Hemorrhagic transformation of cerebral infarction—possible mechanisms. Thromb Haemost. 1999;82 (Suppl 1:92–94. [PubMed] [Google Scholar]
- Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Khatri P, et al. Mechanical reperfusion is associated with post-ischemic hemorrhage in rat brain. Exp Neurol. 2009;216:407–412. doi: 10.1016/j.expneurol.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Clark JF, Broderick JP, Pyne-Geithman GJ, Wagner KR, Ran R, et al. Reperfusion activates metalloproteinases that contribute to neurovascular injury. Exp Neurol. 2008;210:549–559. doi: 10.1016/j.expneurol.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado L, Castano C, Millan M, Aleu A, de la Ossa NP, Gomis M, et al. Hemorrhagic risk of emergent endovascular treatment plus stenting in patients with acute ischemic stroke J Stroke Cerebrovasc Disadvance online publication, 22 January 2013 (e-pub ahead of print). [DOI] [PubMed]
- Fagan SC, Nagaraja TN, Fenstermacher JD, Zheng J, Johnson M, Knight RA. Hemorrhagic transformation is related to the duration of occlusion and treatment with tissue plasminogen activator in a nonembolic stroke model. Neurol Res. 2003;25:377–382. doi: 10.1179/016164103101201526. [DOI] [PubMed] [Google Scholar]
- Copin JC, Gasche Y. Effect of the duration of middle cerebral artery occlusion on the risk of hemorrhagic transformation after tissue plasminogen activator injection in rats. Brain Res. 2008;1243:161–166. doi: 10.1016/j.brainres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Garcia-Yebenes I, Sobrado M, Zarruk JG, Castellanos M, Perez de la Ossa N, Davalos A, et al. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452. [DOI] [PubMed] [Google Scholar]
- Molina CA, Montaner J, Abilleira S, Ibarra B, Romero F, Arenillas JF, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke. 2001;32:1079–1084. doi: 10.1161/01.str.32.5.1079. [DOI] [PubMed] [Google Scholar]
- Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42:2235–2239. doi: 10.1161/STROKEAHA.110.604603. [DOI] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Paciaroni M, Agnelli G, Corea F, Ageno W, Alberti A, Lanari A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. 2008;39:2249–2256. doi: 10.1161/STROKEAHA.107.510321. [DOI] [PubMed] [Google Scholar]
- Seidel G, Cangur H, Albers T, Burgemeister A, Meyer-Wiethe K. Sonographic evaluation of hemorrhagic transformation and arterial recanalization in acute hemispheric ischemic stroke. Stroke. 2009;40:119–123. doi: 10.1161/STROKEAHA.108.516799. [DOI] [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Jaillard A, Cornu C, Durieux A, Moulin T, Boutitie F, Lees KR, et al. Hemorrhagic transformation in acute ischemic stroke. The MAST-E study. MAST-E Group. Stroke. 1999;30:1326–1332. doi: 10.1161/01.str.30.7.1326. [DOI] [PubMed] [Google Scholar]
- Group TNt-PSS Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. The NINDS t-PA Stroke Study Group. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- Fraser PA. The role of free radical generation in increasing cerebrovascular permeability. Free Radic Biol Med. 2011;51:967–977. doi: 10.1016/j.freeradbiomed.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM, et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35:2726–2730. doi: 10.1161/01.STR.0000143219.16695.af. [DOI] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood-brain barrier breakdown following thrombolysis. Exp Neurol. 2006;200:38–49. doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagai N, Umemura K. Novel situations of endothelial injury in stroke—mechanisms of stroke and strategy of drug development: intracranial bleeding associated with the treatment of ischemic stroke: thrombolytic treatment of ischemia-affected endothelial cells with tissue-type plasminogen activator. J Pharmacol Sci. 2011;116:25–29. doi: 10.1254/jphs.10r27fm. [DOI] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Gonzalez R, Blanco M, Rodriguez-Yanez M, Moldes O, Castillo J, Sobrino T. Platelet derived growth factor-CC isoform is associated with hemorrhagic transformation in ischemic stroke patients treated with tissue plasminogen activator. Atherosclerosis. 2013;226:165–171. doi: 10.1016/j.atherosclerosis.2012.10.072. [DOI] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado E, Ortega L, Hernandez-Guillamon M, Penalba A, Fernandez-Cadenas I, Rosell A, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukoc Biol. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Shuaib A. Critical role of microvasculature basal lamina in ischemic brain injury. Prog Neurobiol. 2007;83:140–148. doi: 10.1016/j.pneurobio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Liebetrau M, Martens H, Burggraf D, Kloss CU, Bultemeier G, et al. Microvascular basal lamina injury after experimental focal cerebral ischemia and reperfusion in the rat. J Cereb Blood Flow Metab. 2002;22:526–533. doi: 10.1097/00004647-200205000-00004. [DOI] [PubMed] [Google Scholar]
- Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- Klohs J, Steinbrink J, Bourayou R, Mueller S, Cordell R, Licha K, et al. Near-infrared fluorescence imaging with fluorescently labeled albumin: a novel method for non-invasive optical imaging of blood-brain barrier impairment after focal cerebral ischemia in mice. J Neurosci Methods. 2009;180:126–132. doi: 10.1016/j.jneumeth.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- Tang XN, Zheng Z, Giffard RG, Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol. 2011;70:606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PJ, Morrow JD, Ning M, Koroshetz W, Lo EH, Terry E, et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: the Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke. 2008;39:100–104. doi: 10.1161/STROKEAHA.107.488189. [DOI] [PubMed] [Google Scholar]
- Bas DF, Topcuoglu MA, Gursoy-Ozdemir Y, Saatci I, Bodur E, Dalkara T. Plasma 3-nitrotyrosine estimates the reperfusion-induced cerebrovascular stress, whereas matrix metalloproteinases mainly reflect plasma activity: a study in patients treated with thrombolysis or endovascular recanalization. J Neurochem. 2012;123 (Suppl 2:138–147. doi: 10.1111/j.1471-4159.2012.07952.x. [DOI] [PubMed] [Google Scholar]
- Whiteley WN, Slot KB, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke. 2012;43:2904–2909. doi: 10.1161/STROKEAHA.112.665331. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Song D, Wei J, Purdy R, Zivin JA. Effects of the spin trap agent disodium- [tert-butylimino)methyl]benzene-1,3-disulfonate N-oxide (generic NXY-059) on intracerebral hemorrhage in a rabbit Large clot embolic stroke model: combination studies with tissue plasminogen activator. Stroke. 2002;33:1665–1670. doi: 10.1161/01.str.0000017145.22806.aa. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2000;20:452–457. doi: 10.1097/00004647-200003000-00002. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, et al. NXY-059 for the treatment of acute ischemic stroke. N Engl J Med. 2007;357:562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Chen H, Garcia JH. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- Stamova B, Xu H, Jickling G, Bushnell C, Tian Y, Ander BP, et al. Gene expression profiling of blood for the prediction of ischemic stroke. Stroke. 2010;41:2171–2177. doi: 10.1161/STROKEAHA.110.588335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Xu H, Du X, Lit L, Walker W, Lu A, et al. Gene expression in blood changes rapidly in neutrophils and monocytes after ischemic stroke in humans: a microarray study. J Cereb Blood Flow Metab. 2006;26:1089–1102. doi: 10.1038/sj.jcbfm.9600264. [DOI] [PubMed] [Google Scholar]
- Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, et al. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- Castellanos M, Sobrino T, Millan M, Garcia M, Arenillas J, Nombela F, et al. Serum cellular fibronectin and matrix metalloproteinase-9 as screening biomarkers for the prediction of parenchymal hematoma after thrombolytic therapy in acute ischemic stroke: a multicenter confirmatory study. Stroke. 2007;38:1855–1859. doi: 10.1161/STROKEAHA.106.481556. [DOI] [PubMed] [Google Scholar]
- Demir R, Ulvi H, Ozel L, Ozdemir G, Guzelcik M, Aygul R. Relationship between plasma metalloproteinase-9 levels and volume and severity of infarct in patients with acute ischemic stroke. Acta Neurol Belg. 2012;112:351–356. doi: 10.1007/s13760-012-0067-4. [DOI] [PubMed] [Google Scholar]
- Lucivero V, Prontera M, Mezzapesa DM, Petruzzellis M, Sancilio M, Tinelli A, et al. Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol Sci. 2007;28:165–170. doi: 10.1007/s10072-007-0814-0. [DOI] [PubMed] [Google Scholar]
- Maier CM, Hsieh L, Yu F, Bracci P, Chan PH. Matrix metalloproteinase-9 and myeloperoxidase expression: quantitative analysis by antigen immunohistochemistry in a model of transient focal cerebral ischemia. Stroke. 2004;35:1169–1174. doi: 10.1161/01.STR.0000125861.55804.f2. [DOI] [PubMed] [Google Scholar]
- Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- Wang G, Guo Q, Hossain M, Fazio V, Zeynalov E, Janigro D, et al. Bone marrow-derived cells are the major source of MMP-9 contributing to blood-brain barrier dysfunction and infarct formation after ischemic stroke in mice. Brain Res. 2009;1294:183–192. doi: 10.1016/j.brainres.2009.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier S, Ouk T, Petrault O, Caron J, Bordet R. Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia. Br J Pharmacol. 2009;156:673–679. doi: 10.1111/j.1476-5381.2009.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrault O, Ouk T, Gautier S, Laprais M, Gele P, Bastide M, et al. Pharmacological neutropenia prevents endothelial dysfunction but not smooth muscle functions impairment induced by middle cerebral artery occlusion. Br J Pharmacol. 2005;144:1051–1058. doi: 10.1038/sj.bjp.0706124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Zhang RL, Lu M, Krams M, Chopp M. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34:1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Krueger M, Hartig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS ONE. 2013;8:e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierski R, Michalak S, Wencel-Warot A, Nowinski WL. Serum tight-junction proteins predict hemorrhagic transformation in ischemic stroke patients. Neurology. 2012;79:1677–1685. doi: 10.1212/WNL.0b013e31826e9a83. [DOI] [PubMed] [Google Scholar]
- Jickling GC, Ander BP, Stamova B, Zhan X, Liu D, Bsc LR, et al. RNA in blood is altered prior to hemorrhagic transformation in ischemic stroke. Ann Neurol. 2013;74:232–240. doi: 10.1002/ana.23883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71:743–752. doi: 10.1002/ana.23529. [DOI] [PubMed] [Google Scholar]
- Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suofu Y, Clark JF, Broderick JP, Kurosawa Y, Wagner KR, Lu A. Matrix metalloproteinase-2 or -9 deletions protect against hemorrhagic transformation during early stage of cerebral ischemia and reperfusion. Neuroscience. 2012;212:180–189. doi: 10.1016/j.neuroscience.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, Estrada EY, Dencoff JE. Matrix metalloproteinases and TIMPs are associated with blood-brain barrier opening after reperfusion in rat brain. Stroke. 1998;29:2189–2195. doi: 10.1161/01.str.29.10.2189. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Frankowski H, Gu YH, Osada T, Kanazawa M, Milner R, et al. Microglial cell activation is a source of metalloproteinase generation during hemorrhagic transformation. J Cereb Blood Flow Metab. 2012;32:919–932. doi: 10.1038/jcbfm.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter B, Rodemer C, Grudzenski S, Couraud PO, Weksler B, Romero IA, et al. Temporal profile of matrix metalloproteinases and their inhibitors in a human endothelial cell culture model of cerebral ischemia. Cerebrovasc Dis. 2013;35:514–520. doi: 10.1159/000350731. [DOI] [PubMed] [Google Scholar]
- Liu W, Rosenberg GA, Shi H, Furuichi T, Timmins GS, Cunningham LA, et al. Xanthine oxidase activates pro-matrix metalloproteinase-2 in cultured rat vascular smooth muscle cells through non-free radical mechanisms. Arch Biochem Biophys. 2004;426:11–17. doi: 10.1016/j.abb.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Kornfeld M, Estrada E, Kelley RO, Liotta LA, Stetler-Stevenson WG. TIMP-2 reduces proteolytic opening of blood-brain barrier by type IV collagenase. Brain Res. 1992;576:203–207. doi: 10.1016/0006-8993(92)90681-x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Harris AK, Ergul A, Kozak A, Machado LS, Johnson MH, Fagan SC. Effect of neutrophil depletion on gelatinase expression, edema formation and hemorrhagic transformation after focal ischemic stroke. BMC Neurosci. 2005;6:49. doi: 10.1186/1471-2202-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi M, Sumii T, Fini ME, Itohara S, Lo EH. Matrix metalloproteinase 2 gene knockout has no effect on acute brain injury after focal ischemia. Neuroreport. 2001;12:3003–3007. doi: 10.1097/00001756-200109170-00050. [DOI] [PubMed] [Google Scholar]
- Gu Y, Dee CM, Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front Biosci (Schol Ed) 2011;3:1216–1231. doi: 10.2741/222. [DOI] [PubMed] [Google Scholar]
- Gu Y, Zheng G, Xu M, Li Y, Chen X, Zhu W, et al. Caveolin-1 regulates nitric oxide-mediated matrix metalloproteinases activity and blood-brain barrier permeability in focal cerebral ischemia and reperfusion injury. J Neurochem. 2012;120:147–156. doi: 10.1111/j.1471-4159.2011.07542.x. [DOI] [PubMed] [Google Scholar]
- Durukan A, Marinkovic I, Strbian D, Pitkonen M, Pedrono E, Soinne L, et al. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res. 2009;1280:158–165. doi: 10.1016/j.brainres.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, et al. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153:175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin T, Kastrup A, de Crespigny A, Ringer TM, Sun GH, Yenari MA, et al. MRI of subacute hemorrhagic transformation in the rat suture occlusion model. Neuroreport. 2001;12:309–311. doi: 10.1097/00001756-200102120-00025. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Brinker G, Uhlenkuken U, Pillekamp F, Hossmann KA, Hoehn M. Prediction of hemorrhagic transformation after thrombolytic therapy of clot embolism: an MRI investigation in rat brain. Stroke. 2002;33:1392–1398. doi: 10.1161/01.str.0000014619.59851.65. [DOI] [PubMed] [Google Scholar]
- Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22:E4. doi: 10.3171/foc.2007.22.5.5. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, et al. High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41:2077–2082. doi: 10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang L, Ji X, Yang B, Zhang Y, Fu XD. Hepatitis B Viral RNA Directly Mediates Down-regulation of the Tumor Suppressor MicroRNA miR-15a/miR-16-1 in Hepatocytes. J Biol Chem. 2013;288:18484–18493. doi: 10.1074/jbc.M113.458158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Nagai N, Umemura K, Collen D, Lijnen HR. Stromelysin-1 (MMP-3) is critical for intracranial bleeding after t-PA treatment of stroke in mice. J Thromb Haemost. 2007;5:1732–1739. doi: 10.1111/j.1538-7836.2007.02628.x. [DOI] [PubMed] [Google Scholar]
- Cuadrado E, Rosell A, Penalba A, Slevin M, Alvarez-Sabin J, Ortega-Aznar A, et al. Vascular MMP-9/TIMP-2 and neuronal MMP-10 up-regulation in human brain after stroke: a combined laser microdissection and protein array study. J Proteome Res. 2009;8:3191–3197. doi: 10.1021/pr801012x. [DOI] [PubMed] [Google Scholar]
- Gurney KJ, Estrada EY, Rosenberg GA. Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis. 2006;23:87–96. doi: 10.1016/j.nbd.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nagai N, Yamakawa K, Kawakami J, Lijnen HR, Umemura K. Tissue-type plasminogen activator (t-PA) induces stromelysin-1 (MMP-3) in endothelial cells through activation of lipoprotein receptor-related protein. Blood. 2009;114:3352–3358. doi: 10.1182/blood-2009-02-203919. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Osanai T, Nishizaki F, Sukekawa T, Izumiyama K, Sagara S, et al. Matrix metalloprotein-9 activation under cell-to-cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor-alpha. Heart Vessels. 2012;27:624–633. doi: 10.1007/s00380-011-0214-5. [DOI] [PubMed] [Google Scholar]
- Ringelstein EB, Thijs V, Norrving B, Chamorro A, Aichner F, Grond M, et al. Granulocyte colony-stimulating factor in patients with acute ischemic stroke: results of the AX200 for ischemic stroke trial. Stroke. 2013;44:2681–2687. doi: 10.1161/STROKEAHA.113.001531. [DOI] [PubMed] [Google Scholar]
- Mildner A, Mack M, Schmidt H, Bruck W, Djukic M, Zabel MD, et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain. 2009;132:2487–2500. doi: 10.1093/brain/awp144. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- Urra X, Villamor N, Amaro S, Gomez-Choco M, Obach V, Oleaga L, et al. Monocyte subtypes predict clinical course and prognosis in human stroke. J Cereb Blood Flow Metab. 2009;29:994–1002. doi: 10.1038/jcbfm.2009.25. [DOI] [PubMed] [Google Scholar]
- Chen C, Ostrowski RP, Zhou C, Tang J, Zhang JH. Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res. 2010;88:2046–2055. doi: 10.1002/jnr.22361. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Igarashi H, Kawamura K, Takahashi T, Kakita A, Takahashi H, et al. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31:1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumiya T, Yokota C, Kuge Y, Minematsu K. Aggravation of hemorrhagic transformation by early intraarterial infusion of low-dose vascular endothelial growth factor after transient focal cerebral ischemia in rats. Brain Res. 2005;1049:95–103. doi: 10.1016/j.brainres.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechariah A, Elali A, Doeppner TR, Jin F, Hasan MR, Helfrich I, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. 2013;44:1690–1697. doi: 10.1161/STROKEAHA.111.000240. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Tsang W, Soltanian-Zadeh H, Morris D, Zhang R, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–392. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Sobrino T, Millan M, Castellanos M, Blanco M, Brea D, Dorado L, et al. Association of growth factors with arterial recanalization and clinical outcome in patients with ischemic stroke treated with tPA. J Thromb Haemost. 2010;8:1567–1574. doi: 10.1111/j.1538-7836.2010.03897.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci USA. 2012;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bir SC, Kolluru GK, Fang K, Kevil CG. Redox balance dynamically regulates vascular growth and remodeling. Semin Cell Dev Biol. 2012;23:745–757. doi: 10.1016/j.semcdb.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, et al. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS ONE. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DZ, Ander BP, Xu H, Shen Y, Kaur P, Deng W, et al. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann Neurol. 2010;67:526–533. doi: 10.1002/ana.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Soliman S, Guan W, Saler M, Fagan SC. Vascular protection to increase the safety of tissue plasminogen activator for stroke. Curr Pharm Des. 2012;18:3677–3684. doi: 10.2174/138161212802002779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA. Hemorrhagic transformation following ischemic stroke: significance, causes, and relationship to therapy and treatment. Curr Neurol Neurosci Rep. 2002;2:38–43. doi: 10.1007/s11910-002-0051-0. [DOI] [PubMed] [Google Scholar]
- Fan X, Lo EH, Wang X. Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats. Stroke. 2013;44:745–752. doi: 10.1161/STROKEAHA.111.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer JA, Hess DC, Ergul A, Waller JL, Machado LS, Portik-Dobos V, et al. Matrix metalloproteinase-9 in an exploratory trial of intravenous minocycline for acute ischemic stroke. Stroke. 2011;42:2633–2635. doi: 10.1161/STROKEAHA.111.618215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. Pharmacological effects of the spin trap agents N-t-butyl-phenylnitrone (PBN) and 2,2,6, 6-tetramethylpiperidine-N-oxyl (TEMPO) in a rabbit thromboembolic stroke model: combination studies with the thrombolytic tissue plasminogen activator. Stroke. 2001;32:147–153. doi: 10.1161/01.str.32.1.147. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kamiya T, Deguchi K, Inaba T, Zhang H, Shang J, et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29:715–725. doi: 10.1038/jcbfm.2008.164. [DOI] [PubMed] [Google Scholar]
- Mishina M, Komaba Y, Kobayashi S, Kominami S, Fukuchi T, Mizunari T, et al. Administration of free radical scavenger edaravone associated with higher frequency of hemorrhagic transformation in patients with cardiogenic embolism. Neurol Med Chir (Tokyo) 2008;48:292–297. doi: 10.2176/nmc.48.292. [DOI] [PubMed] [Google Scholar]
- Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, et al. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42:1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Manaenko A, Zhan Y, Liu WW, Ostrowki RP, Tang J, et al. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169:402–414. doi: 10.1016/j.neuroscience.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase Y, Okamoto Y, Fujita Y, Kitamura A, Nakabayashi H, Ito H, et al. Cilostazol, a phosphodiesterase inhibitor, prevents no-reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol. 2012;233:523–533. doi: 10.1016/j.expneurol.2011.11.038. [DOI] [PubMed] [Google Scholar]
- Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH, et al. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke. 2013;44:505–511. doi: 10.1161/STROKEAHA.112.679043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad M, Beray-Berthat V, Coqueran B, Palmier B, Szabo C, Plotkine M, et al. Reduction of hemorrhagic transformation by PJ34, a poly(ADP-ribose)polymerase inhibitor, after permanent focal cerebral ischemia in mice. Eur J Pharmacol. 2008;588:52–57. doi: 10.1016/j.ejphar.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, et al. Does inhibiting Sur1 complement rt-PA in cerebral ischemia. Ann NY Acad Sci. 2012;1268:95–107. doi: 10.1111/j.1749-6632.2012.06705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda M, Furuichi Y, Noto T, Matsuoka N, Mutoh S, Yoneda Y. Tacrolimus (FK506) suppresses rt-PA-induced hemorrhagic transformation in a rat thrombotic ischemia stroke model. Brain Res. 2009;1254:99–108. doi: 10.1016/j.brainres.2008.11.080. [DOI] [PubMed] [Google Scholar]
- Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, et al. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41:2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan SC, Lapchak PA, Liebeskind DS, Ishrat T, Ergul A. Recommendations for preclinical research in hemorrhagic transformation. Transl Stroke Res. 2013;4:322–327. doi: 10.1007/s12975-012-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henning EC, Latour LL, Hallenbeck JM, Warach S. Reperfusion-associated hemorrhagic transformation in SHR rats: evidence of symptomatic parenchymal hematoma. Stroke. 2008;39:3405–3410. doi: 10.1161/STROKEAHA.108.520304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden PD. Hemorrhagic Transformation during thrombolytic therapy and reperfusion: effects of age, blood pressure, and matrix metalloproteinases. J Stroke Cerebrovasc Dis. 2013;22:532–538. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Elgebaly MM, Ogbi S, Li W, Mezzetti EM, Prakash R, Johnson MH, et al. Neurovascular injury in acute hyperglycemia and diabetes: A comparative analysis in experimental stroke. Transl Stroke Res. 2011;2:391–398. doi: 10.1007/s12975-011-0083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Jiang X, Yang Y, Xi G. Hemorrhagic transformation induced by acute hyperglycemia in a rat model of transient focal ischemia. Acta Neurochir Suppl. 2011;111:49–54. doi: 10.1007/978-3-7091-0693-8_9. [DOI] [PubMed] [Google Scholar]
- Cucchiara B, Tanne D, Levine SR, Demchuk AM, Kasner S. A risk score to predict intracranial hemorrhage after recombinant tissue plasminogen activator for acute ischemic stroke. J Stroke Cerebrovasc Dis. 2008;17:331–333. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. 2012;43:1524–1531. doi: 10.1161/STROKEAHA.111.644815. [DOI] [PubMed] [Google Scholar]
- Strbian D, Engelter S, Michel P, Meretoja A, Sekoranja L, Ahlhelm FJ, et al. Symptomatic intracranial hemorrhage after stroke thrombolysis: the SEDAN score. Ann Neurol. 2012;71:634–641. doi: 10.1002/ana.23546. [DOI] [PubMed] [Google Scholar]
- Menon BK, Saver JL, Prabhakaran S, Reeves M, Liang L, Olson DM, et al. Risk score for intracranial hemorrhage in patients with acute ischemic stroke treated with intravenous tissue-type plasminogen activator. Stroke. 2012;43:2293–2299. doi: 10.1161/STROKEAHA.112.660415. [DOI] [PubMed] [Google Scholar]
- Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH stroke scale: SPAN-100. Neurology. 2013;80:21–28. doi: 10.1212/WNL.0b013e31827b1ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saposnik G, Fang J, Kapral MK, Tu JV, Mamdani M, Austin P, et al. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. Stroke. 2012;43:1315–1322. doi: 10.1161/STROKEAHA.111.646265. [DOI] [PubMed] [Google Scholar]
- Dankbaar JW, Hom J, Schneider T, Cheng SC, Lau BC, van der Schaaf I, et al. Age- and anatomy-related values of blood-brain barrier permeability measured by perfusion-CT in non-stroke patients. J Neuroradiol. 2009;36:219–227. doi: 10.1016/j.neurad.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–921. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- Group ISTc. Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher K, Christensen S, Parsons M, De Silva DA, Ebinger M, Levi C, et al. Postthrombolysis blood pressure elevation is associated with hemorrhagic transformation. Stroke. 2010;41:72–77. doi: 10.1161/STROKEAHA.109.563767. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, et al. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR) Stroke. 2009;40:2442–2449. doi: 10.1161/STROKEAHA.109.548602. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Bowes MP, Lyden PD, Zivin JA. Acute hypertension promotes hemorrhagic transformation in a rabbit embolic stroke model: effect of labetalol. Exp Neurol. 1998;150:153–158. doi: 10.1006/exnr.1997.6756. [DOI] [PubMed] [Google Scholar]
- Tejima E, Katayama Y, Suzuki Y, Kano T, Lo EH. Hemorrhagic transformation after fibrinolysis with tissue plasminogen activator: evaluation of role of hypertension with rat thromboembolic stroke model. Stroke. 2001;32:1336–1340. doi: 10.1161/01.str.32.6.1336. [DOI] [PubMed] [Google Scholar]
- Jauch EC, Saver JL, Adams HP, Jr., Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- Tuttle JL, Sanders BM, Burkhart HM, Fath SW, Kerr KA, Watson WC, et al. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation. 2002;9:343–351. doi: 10.1038/sj.mn.7800151. [DOI] [PubMed] [Google Scholar]
- Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol Heart Circ Physiol. 2013;304:H1598–H1614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Holm P, DeVoe G, Schmitt G, Wagner KR, et al. Hemorrhagic infarct conversion in experimental stroke. Ann Emerg Med. 1992;21:120–126. doi: 10.1016/s0196-0644(05)80144-1. [DOI] [PubMed] [Google Scholar]
- Kawai N, Keep RF, Betz AL. Hyperglycemia and the vascular effects of cerebral ischemia. Stroke. 1997;28:149–154. doi: 10.1161/01.str.28.1.149. [DOI] [PubMed] [Google Scholar]
- Bruno A, Durkalski VL, Hall CE, Juneja R, Barsan WG, Janis S, et al. The Stroke Hyperglycemia Insulin Network Effort (SHINE) trial protocol: a randomized, blinded, efficacy trial of standard versus intensive hyperglycemia management in acute stroke. Int J Stroke. 2013. [DOI] [PMC free article] [PubMed]
- Pfeilschifter W, Spitzer D, Czech-Zechmeister B, Steinmetz H, Foerch C. Increased risk of hemorrhagic transformation in ischemic stroke occurring during warfarin anticoagulation: an experimental study in mice. Stroke. 2011;42:1116–1121. doi: 10.1161/STROKEAHA.110.604652. [DOI] [PubMed] [Google Scholar]
- Hallevi H, Albright KC, Martin-Schild S, Barreto AD, Savitz SI, Escobar MA, et al. Anticoagulation after cardioembolic stroke: to bridge or not to bridge. Arch Neurol. 2008;65:1169–1173. doi: 10.1001/archneur.65.9.noc70105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio-Espinola A, Fernandez-Cadenas I, Giralt D, Quiroga A, Gutierrez-Agullo M, Quintana M, et al. A predictive clinical-genetic model of tissue plasminogen activator response in acute ischemic stroke. Ann Neurol. 2012;72:716–729. doi: 10.1002/ana.23664. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Conejero R, Fernandez-Cadenas I, Iniesta JA, Marti-Fabregas J, Obach V, Alvarez-Sabin J, et al. Role of fibrinogen levels and factor XIII V34L polymorphism in thrombolytic therapy in stroke patients. Stroke. 2006;37:2288–2293. doi: 10.1161/01.STR.0000236636.39235.4f. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–1496. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- Hankey GJ. Dual antiplatelet therapy in acute transient ischemic attack and minor stroke. N Engl J Med. 2013;369:82–83. doi: 10.1056/NEJMe1305127. [DOI] [PubMed] [Google Scholar]
- Palacio S, Hart RG, Pearce LA, Anderson DC, Sharma M, Birnbaum LA, et al. Effect of addition of clopidogrel to aspirin on stroke incidence: meta-analysis of randomized trials Int J Strokeadvance online publication, 22 May 2013 (e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Zhou P, Wang Z, Yuan X, Zhou C, Liu L, Wan X, et al. Mixed lineage leukemia 5 (MLL5) protein regulates cell cycle progression and E2F1-responsive gene expression via association with host cell factor-1 (HCF-1) J Biol Chem. 2013;288:17532–17543. doi: 10.1074/jbc.M112.439729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M, Safdar A, Mehdiratta M, Kumar S, Schlaug G, Caplan L, et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. 2008;71:1417–1423. doi: 10.1212/01.wnl.0000330297.58334.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SF, Chen SC, Lin HJ, Chen YW, Tseng MC, Chen CH. Comparison of risk-scoring systems in predicting symptomatic intracerebral hemorrhage after intravenous thrombolysis. Stroke. 2013;44:1561–1566. doi: 10.1161/STROKEAHA.111.000651. [DOI] [PubMed] [Google Scholar]
- Vo KD, Santiago F, Lin W, Hsu CY, Lee Y, Lee J-M. MR imaging enhancement patterns as predictors of hemorrhagic transformation in acute ischemic stroke. AJNR Am J Neuroradiol. 2003;24:674–679. [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, Latour L, Saver JL, Alger JR, Starkman S, Duckwiler G, et al. Thrombolytic toxicity: blood brain barrier disruption in human ischemic stroke. Cerebrovasc Dis. 2008;25:338–343. doi: 10.1159/000118379. [DOI] [PubMed] [Google Scholar]
- Aviv RI, d'Esterre CD, Murphy BD, Hopyan JJ, Buck B, Mallia G, et al. Hemorrhagic transformation of ischemic stroke: prediction with CT perfusion. Radiology. 2009;250:867–877. doi: 10.1148/radiol.2503080257. [DOI] [PubMed] [Google Scholar]
- Bang OY, Buck BH, Saver JL, Alger JR, Yoon SR, Starkman S, et al. Prediction of hemorrhagic transformation after recanalization therapy using T2*-permeability magnetic resonance imaging. Ann Neurol. 2007;62:170–176. doi: 10.1002/ana.21174. [DOI] [PubMed] [Google Scholar]
- Tong DC, Adami A, Moseley ME, Marks MP. Relationship between apparent diffusion coefficient and subsequent hemorrhagic transformation following acute ischemic stroke. Stroke. 2000;31:2378–2384. doi: 10.1161/01.str.31.10.2378. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Christensen S, Parsons MW, Churilov L, Desmond PM, Barber PA, et al. Advanced imaging improves prediction of hemorrhage after stroke thrombolysis. Ann Neurol. 2013;73:510–519. doi: 10.1002/ana.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–2238. doi: 10.1093/brain/awp155. [DOI] [PubMed] [Google Scholar]
- Christoforidis GA, Karakasis C, Mohammad Y, Caragine LP, Yang M, Slivka AP. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: the role of pial collateral formation. AJNR Am J Neuroradiol. 2009;30:165–170. doi: 10.3174/ajnr.A1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillas P, Derex L, Philippeau F, Nighoghossian N, Honnorat J, Hanss M, et al. Early fibrinogen degradation coagulopathy is predictive of parenchymal hematomas in cerebral rt-PA thrombolysis: a study of 157 cases. Stroke. 2004;35:1323–1328. doi: 10.1161/01.STR.0000126040.99024.cf. [DOI] [PubMed] [Google Scholar]
- Foerch C, Wunderlich MT, Dvorak F, Humpich M, Kahles T, Goertler M, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–2495. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- Choi KH, Park MS, Kim JT, Nam TS, Choi SM, Kim BC, et al. The serum ferritin level is an important predictor of hemorrhagic transformation in acute ischaemic stroke. Eur J Neurol. 2012;19:570–577. doi: 10.1111/j.1468-1331.2011.03564.x. [DOI] [PubMed] [Google Scholar]
- Mendioroz M, Fernandez-Cadenas I, Alvarez-Sabin J, Rosell A, Quiroga D, Cuadrado E, et al. Endogenous activated protein C predicts hemorrhagic transformation and mortality after tissue plasminogen activator treatment in stroke patients. Cerebrovasc Dis. 2009;28:143–150. doi: 10.1159/000225907. [DOI] [PubMed] [Google Scholar]
- Ribo M, Montaner J, Molina CA, Arenillas JF, Santamarina E, Quintana M, et al. Admission fibrinolytic profile is associated with symptomatic hemorrhagic transformation in stroke patients treated with tissue plasminogen activator. Stroke. 2004;35:2123–2127. doi: 10.1161/01.STR.0000137608.73660.4c. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Garcia-Bonilla L, Sole M, Sosti V, Pares M, Campos M, et al. Plasma VAP-1/SSAO activity predicts intracranial hemorrhages and adverse neurological outcome after tissue plasminogen activator treatment in stroke. Stroke. 2010;41:1528–1535. doi: 10.1161/STROKEAHA.110.584623. [DOI] [PubMed] [Google Scholar]
- Tong DC, Adami A, Moseley ME, Marks MP. Prediction of hemorrhagic transformation following acute stroke: role of diffusion- and perfusion-weighted magnetic resonance imaging. Arch Neurol. 2001;58:587–593. doi: 10.1001/archneur.58.4.587. [DOI] [PubMed] [Google Scholar]