Abstract
Hypothalamic glucose detection participates in maintaining glycemic balance, food intake, and thermogenesis. Although hypothalamic neurons are the executive cells involved in these responses, there is increasing evidence that astrocytes participate in glucose sensing (GS); however, it is unknown whether astroglial networking is required for glucose sensitivity. Astroglial connexins 30 and 43 (Cx30 and Cx43) form hexameric channels, which are apposed in gap junctions, allowing for the intercellular transfer of small molecules such as glucose throughout the astroglial networks. Here, we hypothesized that hypothalamic glucose sensitivity requires these connexins. First, we showed that both Cxs are enriched in the rat hypothalamus, with highly concentrated Cx43 expression around blood vessels of the mediobasal hypothalamus (MBH). Both fasting and high glycemic levels rapidly altered the protein levels of MBH astroglial connexins, suggesting cross talk within the MBH between glycemic status and the connexins' ability to dispatch glucose. Finally, the inhibition of MBH Cx43 (by transient RNA interference) attenuated hypothalamic glucose sensitivity in rats, which was demonstrated by a pronounced decreased insulin secretion in response to a brain glucose challenge. These results illustrate that astroglial connexins contribute to hypothalamic GS.
Keywords: astrocyte, connexin 43, connexin 30, glucose sensing, hypothalamus
Introduction
The hypothalamus has a pivotal role in energy homeostasis. In response to energy-related stimuli such as hormones and nutrients, the hypothalamus modulates multiple neuroendocrine responses as well as food intake.1 Among these energy-related clues, glucose constitutes an important signal. Hypothalamic detection of increased blood glucose level induces a set of rapid physiologic responses, including reduced hepatic glucose production,2 increased insulin secretion,3 and increased energy expenditure through thermogenesis.4 It has been suggested that glucose-sensing (GS) neurons that modulate their electrical activity in response to changes in extracellular glucose levels5 have a critical role in these physiologic responses. Glucose-sensing neurons are mostly located within specific hypothalamic nuclei. The most studied hypothalamic nuclei are the arcuate (ARC) and ventromedial nuclei (VMN), which together constitute the mediobasal hypothalamus (MBH).1 Moreover, altered hypothalamic GS is an early defect observed in some animal models of insulin resistance and type 2 diabetes,6, 7, 8 lending support to the idea that this sensing is essential to maintain energy homeostasis.
Astrocytes have been identified as partners of GS neurons in hypothalamic glucose detection. For instance, increasing brain glucose levels by a carotid injection of glucose (thus avoiding the effects of peripheral glucose detection) activates some neurons and astrocytes within the ARC, as revealed by increased c-fos proto-oncogene expression.9 This ARC activation and the ability of increased hypothalamic glucose to induce insulin secretion were both reversed by a preliminary injection of the xenobiotic amino acid methionine sulfoximine, a glutamine synthetase blocker that alters glial metabolism.9 In addition, inhibition of the hypothalamic astroglial-specific glucose transporter GLUT1 is associated with a decrease in lowered hepatic glucose production.10 Moreover, the inhibition of the low affinity GLUT2 in astrocytes was shown to alter insulin and food intake in response to increased central glucose levels.11, 12 Thus, these data support the idea that even though interstitial glucose diffusion occurs,13 glucose taken up by hypothalamic astrocytes participates in the detection of the increase in blood glucose levels and in the generation of appropriate responses.
These hypothalamic astrocytes, as well as tanycytes lining the third ventricle, express key factors involved in sensing and processing nutritional signals.14, 15 These cells also have high plastic properties depending on their metabolic and hormonal environment16 but are often considered as single cells. However, several lines of evidence demonstrate that astrocytes are organized into coordinated networks because of a high expression level of gap junction proteins.17 This networking provides a conduit for the exchange of numerous active molecules, including glucose,18 and is controlled by multiple factors.17, 19 Astroglial network exchanges comprise gap junction plaques formed by numerous channels, which are made up of connexins 43 and 30 (Cxs). These proteins form hemi-channels embedded in the plasma membrane, establishing an intercellular pathway by facing each other at the interface of two adjacent astrocytes.17
Recently, it has been demonstrated by ex vivo recordings on acute hippocampal slices that astroglial gap junctions are essential for providing energetic fuel to sustain neuronal activity.20 This study revealed that glucose or lactate perfused into a single astrocyte spreads within the astroglial network through Cxs, thereby reaching fuel-demanding neurons.20 The connectivity of this astroglial network might be crucial to provide rapid information for hypothalamic glucose detection of increased blood glucose levels. Glucose-sensing neurons are not specifically located in the vicinity of blood vessels, and the inhibition of astroglial glucose uptake has been shown to alter hypothalamic glucose detection.11, 21 These data suggest that glucose dispersion throughout the astroglial network contributes either directly or indirectly to glucose detection by these neurons.
We hypothesized that Cxs expressed in astrocytes have a role in vivo in hypothalamic glucose detection. We first examined their hypothalamic distribution and determined whether energy status affects the expression of MBH Cxs. Finally, we studied the effects of inhibiting transient MBH Cx43 expression on hypothalamic glucose-induced insulin secretion in rats.
Materials and Methods
Animals
Experiments were approved by the Animal Use and Care Advisory Committee of the University of Burgundy (according to the European Communities Council Directive (2010/63/UE). Nine-week-old adult male Wistar rats (250–275 g; Charles River Laboratories, Lyon, France) were housed under standard animal care conditions with ad libitum access to water and food (A04, Safe, Augy, France) and were individually caged after cranial surgery.
Metabolic Conditions
Anesthesia was performed via an intraperitoneal pentobarbital injection (50 mg/kg, Ceva, Velaine en Haye, France). Anesthetized rats were injected subcutaneously with saline (controls) or a glucose solution (4 g/kg) that induced hyperglycemia for 3 hours. Only rats showing glycemia above 10 mmol/L were used for the study. Another group of animals were fasted for 24 hours (food removed at the beginning of the light phase) and compared with fed rats. At the end of the experiment, the animals were euthanized; the brains were quickly removed; and the MBH or areas of interest (cortex, thalamus, hippocampus, cerebellum, hindbrain) were dissected, snap frozen, and stored at −80 °C until western blot analysis.
Stereotaxic Surgery and Small Interfering RNA Injection
Cranial surgery was performed under isoflurane anesthesia (2% to 2.5%, Abbott, Rungis, France) at a flow rate of 1 L/minute oxygen. Once fully anesthetized, rats received a subcutaneous injection of buprenorphine (0.03 mg/kg, Axience, Pantin, France). The skin on the top of the skull was disinfected and lidocaine injected (10 mg/kg, Ceva, Libourne, France). A bilateral cannula guide (10 mm length, 26-gauge; Plastic-One, Roanoke, VA, USA) was inserted into the arcuate nucleus (coordinates: −3.1 mm posterior to the bregma,±0.4 mm lateral of midline, and −9.0 mm below the skull surface). The cannula guide was adhered to the skull with dental cement. At the end of the surgery, saline was injected subcutaneously to limit dehydration. After 5 days of recovery (monitored through daily food intake and weight gain), small interfering RNAs directed against connexin 43 (siCx43) were injected through the cannula guide in the arcuate nucleus using a transferring agent as described previously.22 Briefly, siCx43 consisted of double-stranded 21-nucleotide siRNAs (800 pmol) that had previously been demonstrated to knock down rat Cx43 expression in vitro23 (siRNA sense strand: 5′-GCUGGUUACUGGUGACAGAUdTdT-3′).
Intracarotid Glucose Injection-Induced Insulin Secretion
We have previously established an in vivo test to stimulate the hypothalamo-pancreatic axis.3, 22, 24 This test consists of injecting a carotid load of glucose toward the brain at a concentration (9 mg/kg), which does not affect peripheral blood glucose levels but triggers insulin secretion through pancreatic vagal nerve activation. During the test, blood samples were collected at the tail vein before (t=0 minutes) and 1, 3, 5, and 10 minutes after the load to monitor glycemia and plasma insulin levels.
Immunohistochemistry
Anesthetized animals were perfused transcardially with heparinized PBS (Eurobio, Courtaboeuf, France, and 50 U/mL heparin, Sanofi Aventis, Paris, France). Brains were quickly removed, frozen in −25°C isopentane and stored at −80°C until use. After embedding the brain in Tissue-Tek at −20°C (Sakura, Villeneuve d'Ascq, France), the hypothalamus was cut into 20-μm thick coronal sections using a cryostat. Slices were mounted on glass slides (SuperFrost Plus, ThermoFisher, Illkirch, France) and dried. Fixation was performed with 2% paraformaldehyde for 20 minutes at 4°C (Sigma, Saint-Quentin Fallavier, France) and blocked with 2% gelatin and 0.2% Triton X-100 (VWR, Fontenay-sous-Bois, France) in PBS for 1 hour at room temperature. Sections were incubated overnight at 4 °C with primary antibodies as follows: mouse anti-Cx43 (1/2,000, BD Biosciences, Le Pont de claix, France), rabbit anti-Cx30 (1/500, Zymed, Life technologies, Saint Aubin, France) or rabbit anti-von Willebrand Factor (1/400, Sigma). After washing, sections were incubated with the appropriate secondary antibodies: Alexa Fluor 488 (goat anti-mouse) or 555 (goat anti-rabbit) (Invitrogen, Life Technologies, Saint Aubin, France). For Cx43-von Willebrand Factor double immunostaining, incubations were made in series. Sections were observed using an apotome fluorescence microscope (Axio Imager 2, Zeiss, Le Pecq, France). The labeling specificity was determined with the omission of primary antibodies.
Western Blot
Frozen samples from rat brain were homogenized in lysis buffer (150 mmol/L NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 50 mmol/L Tris, pH8, 10 μL per μg of tissue), with the Tissuelyser apparatus, at 20 Hz, 2 × 2 minutes (Qiagen, Courtaboeuf, France). The protein concentration of homogenates from brain samples was determined with the DC Protein Assay kit (Bio-Rad, Marnes-la-Coquette, France). A total of 20 μg of protein was loaded onto premade 12% polyacrylamide gels (Bio-Rad). After migration, transfer (0.2 μm PVDF membrane, Bio-Rad) and blocking of nonspecific sites, an anti-Cx43 mouse monoclonal antibody (1/2,000, BD Biosciences) or a Cx30 rabbit polyclonal antibody (1/500, Zymed) diluted in 1% nonfat milk in TBST was incubated overnight at 4°C. After washing, the membrane was incubated for 1 hour with a donkey anti-rabbit secondary or sheep anti-mouse HRP-linked secondary antibody (1/10,000, in TBST, GE Healthcare, Orsay, France). An anti-glyceraldehyde 3-phosphate dehydrogenase mouse monoclonal antibody (1/6,000, Ambion, Life technologies, Saint Aubin, France) was used for relative quantification, following the same steps (sheep anti-mouse HRP-linked secondary antibody). Bands were visualized by chemiluminescence with the addition of western C reagent (Bio-Rad). The stability of glyceraldehyde 3-phosphate dehydrogenase protein levels was determined by staining total protein (MemCode Reversible Protein Stain kit, Pierce, Rockford, IL,USA) and showed no variation in our conditions (data not shown). Membranes were scanned using the ChemiDoc XRS+ system and the ImageLab software (Bio-Rad) for the detection and quantification of the band density.
Biochemical Analysis
Plasma glucose concentrations were measured using the glucose analyzer Performa AccuChek (Roche, Meylan, France). Plasma insulin concentrations were determined using an ultrasensitive ELISA test (AlpCo, Eurobio, France).
Statistical Analysis
Statistical analyses were performed with GraphPad Prism 4.0 for Windows (GraphPad Software, La Jolla, CA, USA). Data are presented as the mean±sem. After testing normality, Student's unpaired or paired t-tests, Mann–Whitney tests or one-way or two-way analysis of variance followed by a Bonferroni post hoc test were used to identify significant differences between groups as described in the figure legends. A P⩽0.05 indicates statistical significance.
Results
Glial Proteins Cx43 and Cx30 are Highly Expressed in the Mediobasal Hypothalamus
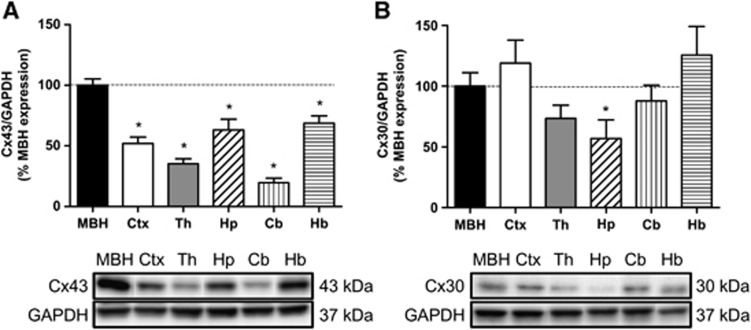
We first quantified astroglial Cx43 and Cx30 protein levels in different brain areas, including the MBH, cortex, thalamus, hippocampus, cerebellum, and hindbrain, using western blotting (Figure 1A). The relative MBH Cx43 protein levels from the highest to the lowest (MBH taken as the reference, 100%, P⩽0.05 for each value, n=4) were as follows: hindbrain (68.7±6.2%)⩾hippocampus (63.2±8.8%)⩾parietal cortex (52.0±5.2%)⩾thalamus (35.3±4.2%)⩾cerebellum (19.7±3.7%). Cx30 protein levels of the same samples were detected in equal amounts throughout the brain areas investigated except, to a lesser degree, in the hippocampus (56.9±15.4%, relative to MBH levels P⩽0.05, n=8).
Figure 1.
Cx43 and Cx30 protein levels in various brain areas. (A,B). Relative protein quantification (upper panel) and representative western blots (lower panel) for Cx43 (n=4) and Cx30 (n=8) in rat mediobasal hypothalamus (MBH), cortex (Ctx), thalamus (Th), hippocampus (Hp), cerebellum (Cb), and hindbrain (Hb). The results are expressed as a percentage of relative MBH protein levels for Cx43 or Cx30 taken as references. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) signal (density ratio of Cx/GAPDH) was used as the control for protein loading. Comparison for each brain region relative to MBH was performed using a Student unpaired t-test for Cx30 and a Mann–Whitney test for Cx43, *P⩽0.05.
Cx43 and Cx30 are Distributed Differently in the Hypothalamus
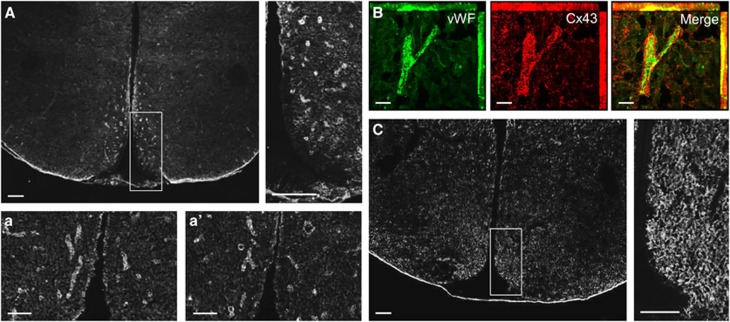
To get further insights on the astroglial distribution of proteins Cx43 and Cx30 throughout the hypothalamus, immunohistochemical studies were performed on brain slices containing the hypothalamus. Cx30 and Cx43 proteins exhibited a punctate staining throughout the hypothalamus (Figures 2A and 2C). Nevertheless, the VMN and the ARC of the MBH displayed a high concentration of Cx43-labeled structures, especially on the edge of the medial part of the third ventricle (Figure 2A), whereas Cx30 staining appeared more uniformly distributed throughout the hypothalamus (Figure 2C). The parenchyma exhibited a high density of Cx43 immunoreactivity that appeared to delineate capillary-like structures, particularly in the ARC and VMN (Figure 2A, insets a and a′). Double immunostaining against the von Willebrand Factor, a marker of endothelial cells, revealed that this specific pattern of Cx43 immunoreactivity was located around capillaries (Figure 2B). This observation was specific to Cx43 because Cx30 immunolabeling displayed a uniform punctate staining that was higher in the ARC (Figure 2C, inset) and completely absent from the median eminence and the edge of the third ventricle (Figure 2C).
Figure 2.
Hypothalamic Cx43 and Cx30 distribution. (A) Cx43 immunostaining in the hypothalamus and observations at higher magnification of the mediobasal area. Scale bar, 200 μm. (a, a′) Cx43 immunostaining in the mediobasal hypothalamus. Scale bar, 50 μm. (B) Double immunohistochemistry representing endothelial staining with von Willebrand Factor (in green, left panel), Cx43 staining (in red, central panel), and merged images (right panel). Scale bar, 20 μm. (C) Cx30 immunostaining in the hypothalamus and observations at higher magnification of the mediobasal area. Scale bar, 200 μm.
Fasting Decreases and Hyperglycemia Increases Cx43 in the Mediobasal Hypothalamus
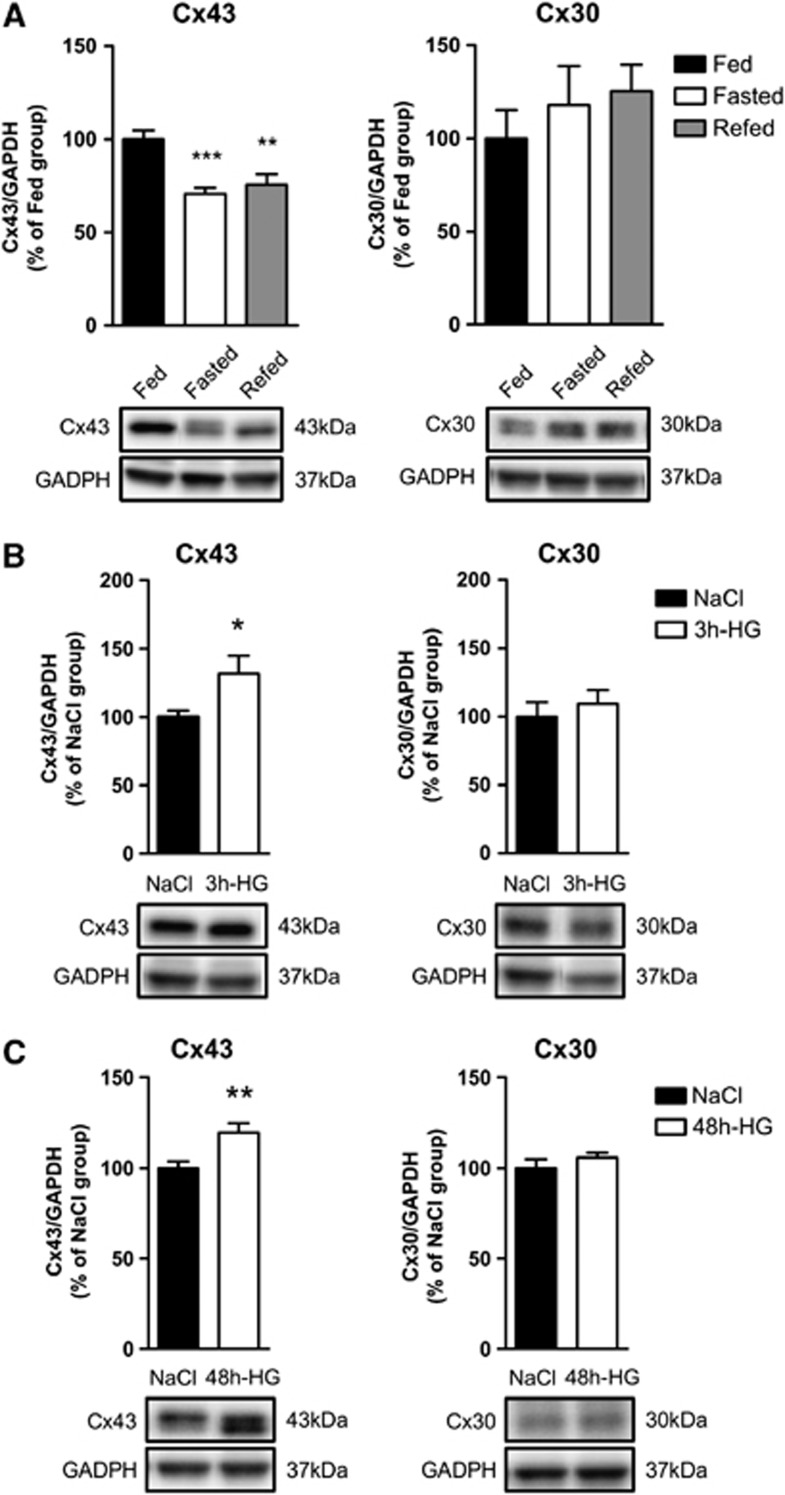
We next examined whether the expression levels of both Cx43 and Cx30 were affected by changes in the metabolic status. We tested the effect of 24-hour fasting and 4-hour refeeding after overnight fasting. Although Cx30 expression levels were not affected by these conditions, MBH Cx43 protein levels significantly decreased (−29.4±3.4%, n=10) during fasting and remained low (−24.4±5.6%, n=10) after refeeding when compared with fed control rats (Figure 3A). In a second set of experiments, Cx43 and Cx30 protein levels were measured after an acute subcutaneous glucose injection inducing 3 hours of hyperglycemia, and a protracted hyperglycemia induced by 48 hours of intravenous glucose perfusion that mimics a prediabetic state.25 Short-term hyperglycemia (18.0±3.1 mmol/L at 3 hours, n=7) led to a 31.8±13.0% increase in Cx43 protein levels. Cx30 protein levels were not affected (Figure 3B). After 48 hours of hyperglycemia (15.1±0.7 mmol/L), the Cx43 protein levels were also increased (+19.5±5.3%, n=15, P⩽0.01) whereas Cx30 protein levels remained unchanged (Figure 3C). These results highlight that only Cx43 protein levels are rapidly affected by nutritional status and glycemic levels.
Figure 3.
Changes in metabolic status and blood glucose concentration alter mediobasal hypothalamus (MBH) Cx43 and Cx30 protein levels. Relative protein level quantification (upper panel) and representative western blots (lower panel) of MBH Cx43 (left panel) and Cx30 (right panel) in panel A fed (black bar, n=8), 24-hour fasted (white bar, n=10) and 4-hour refed rats (gray bars, n=10), (B) in saline-injected (black bar, n=7) and 3-hour hyperglycemic rats (white bar, n=7; glycemia: 18.0±3.1 mmol/L), and (C) in saline-infused (black bars, n=11 for Cx43, n=5 for Cx30) versus 48-h hyperglycemic rats (white bars, n=15 for Cx43, n=8 for Cx30; glycemia: 15.1±0.7 mmol/L). The results are expressed as percentages of the respective control group after values have been normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) signal density (loading control). (A) One-way analysis of variance and (B,C) unpaired Student's t-test have been performed compared with respective control group, *P⩽0.05, **P⩽0.01 and ***P⩽0.001.
Astroglial Cx43 Transient Inhibition in the Mediobasal Hypothalamus is Associated with Alterations in Hypothalamic Glucose Sensing
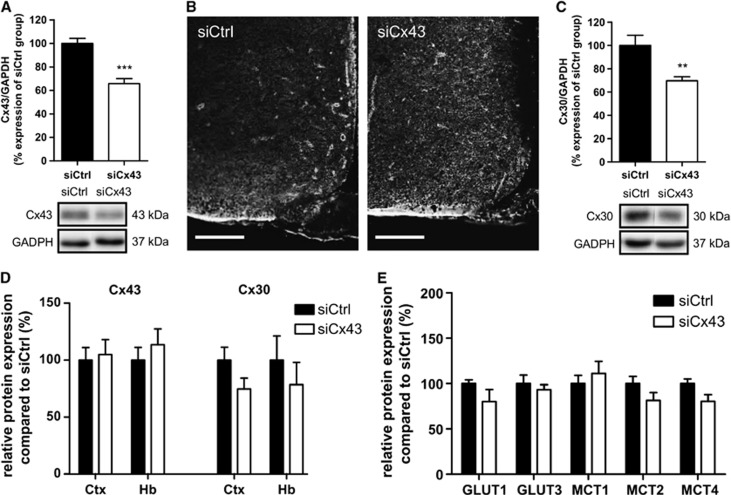
To determine whether Cx43 is involved in hypothalamic GS, we transiently inhibited MBH Cx43 expression using RNA interference. Hypothalamic glucose sensitivity was assessed 72 hours post injection when animals fully recovered from siRNA injection (Supplementary Figure S1). At this time, Cx43 protein levels were significantly decreased by 34.2±4.1% (n=13; Figure 4A). Immunohistochemical studies confirmed that immunolabeling for Cx43 in the MBH in siCx43-injected animals was less pronounced than in siCtrl-injected or normal animals (Figure 4B). The reduction in Cx43 protein levels was specific to the MBH as evidenced by the lack of any noticeable difference in Cx43 immunolabeling in the parietal cortex and hindbrain between siCx43- or siCtrl-injected animals (Figure 4D). Surprisingly, despite the absence of homology with the Cx30 RNA sequence, the siRNA directed against Cx43 caused a similar reduction in MBH Cx30 levels (−30.3±3.4%, n=6) with no effect on other areas (Figures 4C and 4D). Other proteins of interest implicated in metabolic sensing (GLUT1 and 3, MCT1, 2 and 4) were investigated to rule out nonspecific modifications that could result in modulating central glucose sensitivity. There were no alterations in GLUT1 and 3 and MCT1, 2 and 4 protein levels detected in siCx43-treated animals (Figure 4E).
Figure 4.
Transient mediobasal hypothalamus (MBH) Cx43 inhibition decreases protein levels of astroglial connexins Cx43 and Cx30, without altering main glucose and lactate transporters expression. (A) Relative protein quantification for MBH Cx43 (upper panel, siCtrl: n=16; siCx43: n=13) and representative western blots (lower panel). (B) Cx43 immunostaining in the MBH of siCx43 and siCtrl-injected rats after 72 hours. Scale bar, 200 μm. (C) Relative protein quantification for MBH Cx30 (upper panel, siCtrl: n=10; siCx43, n=11) and representative western blots (lower panel). (D) Relative Cx43 and Cx30 proteins quantification in the parietal cortex (Ctx) and hindbrain (Hb), compared with siCtrl (siCx43 and siCtrl: n=6). (E) Relative proteins quantification in MBH of 72 hours siCx43-treated rats, of astroglial transporters GLUT1, MCT1 (siCtrl, n=13; siCx43, n=11) and MCT4 (siCx43 and siCtrl: n=6); and neuronal transporters GLUT3 (siCx43 and siCtrl: n=6) and MCT2 (siCtrl: n=13; siCx43: n=12). The results are expressed as a percentage of siCtrl protein levels after the values were normalized to GAPDH signal density (loading control). Unpaired t-tests (or Mann–Whitney tests for the MCT1 western blot) have been performed compared with siCtrl group, **P⩽0.01, ***P⩽0.001.
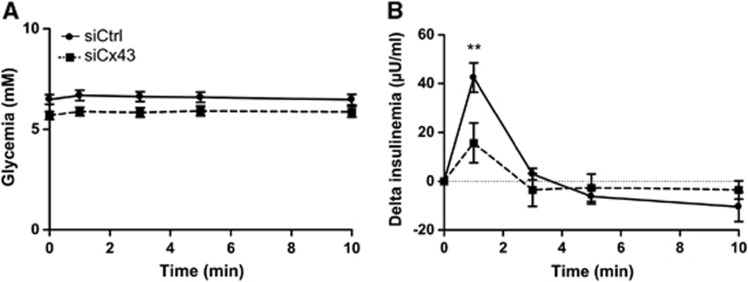
We next evaluated the impact of siCx43 inhibition on hypothalamic GS. We quantified the insulin secretion in response to an intracarotid glucose load toward the brain that does not alter peripheral blood glucose levels (and therefore beta cell stimulation by glucose).3 Neither basal glycemia nor basal insulinemia were modified in siRNA-treated rats (Supplementary Figure S2). Both siCtrl- and siCx43-injected animals displayed stable glycemia during the test (Figure 5A). siCtrl-injected rats exhibited a characteristic increase in insulin secretion 1 minute after the glucose load (+40.9±6.2 μU/mL, n=10) (Figure 5B). In contrast, siCx43 treatment resulted in a 61.6% inhibition in that peak (+15.7±8.1 μU/mL, n=7), demonstrating that lowering MBH Cxs protein levels diminished brain glucose detection.
Figure 5.
Transient downregulation of mediobasal hypothalamus (MBH) Cx43 inhibits hypothalamic glucose sensing in vivo. (A) Peripheral blood glucose levels after 9 mg/kg intracarotid glucose injection in siCtrl (full black line; n=10) and siCx43 rats (dotted black line; n=7). (B) Delta plasma insulin levels (values compared with time 0 minutes before injection) after 9 mg/kg intracarotid glucose injection in siCtrl and siCx43 rats. Statistical analysis was performed using a repeated measures two-way analysis of variance followed by a Bonferroni post hoc test ** P⩽0.01.
Discussion
Over the last few years, there has been a significant improvement in the knowledge of cellular and molecular mechanisms involved in hypothalamic glucose sensitivity. We and others have identified astrocytes as being important factors in hypothalamic GS.2, 9, 10, 11 Likewise, astroglial gap junctions formed by Cx43 and Cx30 are necessary for glucose transfer through astroglial networks, as recently demonstrated in the hippocampus.20 Thus, we hypothesized that Cxs-dependent astroglial networks have a role in the hypothalamic glucose detection. Here, we show that (1) the MBH is highly enriched in Cx43; (2) changes in blood glucose levels modulate Cx43 protein levels; and importantly, (3) decreased MBH Cxs expression inhibits the increase of brain glucose-induced insulin secretion.
A previous analysis of Cxs-rich structures in the brain reported high levels of Cxs protein in the hypothalamus.26 Here, we show that in hypothalamic areas, strong immunostaining for Cx43 was restricted to the MBH. This labeling exhibited a pattern, confirmed by co-staining of endothelial cells, that suggested that astroglial endfeet are enriched with Cx43, as previously described.20, 26, 27 Endfeet are specialized astroglial processes apposed to the brain capillaries. They are part of the blood–brain barrier and constitute a highly regulated diffusion barrier between vasculature and neuronal tissue.28, 29 Mathematical simulations based on their in vitro glucose transport properties reported that astrocytes have moderate glucose uptake capacity compared with neurons, suggesting a higher glucose transfer via the intersticium.13 Moreover, intraventricular injection of horseradish peroxidase diffused into extracellular fluid and stopped only at endothelial tight junctions.30 Whether these general properties apply to the specific hypothalamic glucose-sensing area remains to be demonstrated. Recently, the diffusion dynamics of molecules within astrocytes, including a fluorescent glucose analog, have been investigated in the visual cortex. Diffusion was slower at the endfeet than at the ends of other processes, resulting in subcellular compartments of the glucose analog.29 The authors of the study suggest that glucose retention in these microcompartments could be achieved through the existence of special retention mechanisms, thus providing astrocytes a temporal window to distribute glucose to appropriate locations.29 A similar hypothesis for the regulation of dispatching glucose and related molecules toward hypothalamic GS neurons appears highly attractive. In addition, rich Cx43 immunostaining was exhibited at the border of the third ventricle, where tanycytes have been described.31 Interestingly, these cells have been recently reported in vitro to sense increased glucose levels using a Cx43-dependent mechanism.15, 32 These data are in agreement with our hypothesis that hypothalamic Cxs have a role in glucose sensitivity. Interestingly, Cx30 immunostaining was present throughout the hypothalamus, except along the border of the third ventricle or the median eminence. Cx30 labeling was concentrated in the ARC, without delimiting blood vessel-like shapes, which was in contrast to the observed Cx43 immunostaining. In contrast, in the hippocampus, Cx43 and Cx30 were reported to have a similar expression pattern at the contact between endfeet.20 Consequently, the differential and specific distribution of Cxs in the MBH suggests that distinct roles coexist for these proteins.
Changes in metabolic status have been shown to alter MBH glucose sensitivity.8, 10, 33, 34 Here, we examined whether Cxs protein levels were differently affected by changes in blood glucose levels. We found a positive correlation between blood glucose concentration and MBH Cx43 protein levels. A fasting-induced decrease in blood glucose levels attenuates MBH Cx43 expression, whereas short- (3 hours) or long-term (48 hours) hyperglycemia increases MBH Cx43 expression. Nevertheless, it is noteworthy that by opposition to the 3-hour hyperglycemia associated with increased Cx43 protein levels, mild hyperglycemia obtained after 4-hour refeeding was not sufficient to increase Cx43 protein levels. Blood glucose levels after 4 hours of refeeding did not exceed 7.5±0.2 mmol/L (n=10), suggesting that a substantial increase in glucose levels is necessary to alter Cx43 expression. Here, the short half-life of Cx43 (only a few hours) underlies rapid Cx43 protein level changes, which allow degradation and/or de novo synthesis as soon as stimulations occur.35 These alterations suggest that astroglial networks are capable of fast adaptation to address new metabolic conditions. However, our study focused only on protein levels and further investigations will be needed to evaluate the activity of these channels under various physiologic conditions and pathophysiological stressors because their properties depend not only on protein content but also on numerous posttranslational modifications.35 For instance, Cx43 undergoes several phosphorylation events during its life cycle, which can also affect Cx43 properties by either decreasing or increasing gap junction communication (assembly and channel stability).35 Interestingly, brain oxidative or nitrosative stress observed in streptozotocin-induced, long-term hyperglycemic and diabetic rats is associated with a reduction of gap-junctional communication.36 This reduction was not necessarily associated with decreased astroglial Cx30 and Cx43 levels, implying that other mechanisms of regulation occurred (as oxidative modifications). Moreover, only certain brain regions present this correlation between Cxs protein levels and gap junction communication,36 indicating the presence of some specificity related to the area studied. Furthermore, Cx43 was predominantly phosphorylated in control and diabetic samples, suggesting that reduced gap-junctional activity was not due to overall dephosphorylation.36 Although streptozotocin-diabetic rats present decreased hypothalamic glucose detection,10 changes in MBH Cxs levels or gap-junctional communication remain to be studied. Forty-eight hours of hyperglycemia, which mimics early nervous defects associated with a prediabetic state,25 strongly extends the insulin secretion duration in response to a cerebral glucose load.34 This hyperresponse is associated with increased Cx43 protein levels, suggesting that Cx43 protein levels in the MBH participate in the modulation of gap-junctional communication to respond to high glycemic levels after a few hours. Thus, it would be interesting to determine whether impaired hypothalamic glucose sensitivity during prolonged hyperglycemia is associated with decreased MBH gap-junctional communication and/or Cxs protein expression level, as well as the temporal sequence of these events.
Because only MBH Cx43 expression levels were affected by metabolic status, we aimed to determine whether inhibition of MBH Cx43 expression alters glucose sensitivity. MBH Cx43 protein levels were significantly decreased 72 hours after MBH siRNA injection, as confirmed by western blot and immunohistochemistry. When using Cx43-deficient mice, a compensatory upregulation of Cx30 has been reported.37 Surprisingly, we found that a decrease in siCx43-induced MBH Cx43 levels is associated with a decrease in Cx30 protein levels. The siRNA sequence we used does not match that of the Cx30 RNA sequence, suggesting that a decrease in Cx43 may indirectly affect Cx30 expression by a signaling pathway yet to be determined. A recent study by Ezan et al38 demonstrates that complete deletion of Cx43 and Cx30 in GFAP-positive cells in mice is associated with a partial loss of several proteins (astroglial water channel aquaporin-4 and β-dystroglycan, for instance), leading to a loss of integrity of the blood–brain barrier. Importantly, this study was performed on animals where Cxs were totally absent from birth, and some extreme compensation might have also occurred. Here, MBH Cx43 downregulation reached only −30% and lasted only a few days, which could limit compensatory mechanisms. In our study, levels of other major proteins involved in astrocyte metabolite transport were unaffected. Importantly, the lack of alterations in astroglial and neuronal GLUTs and MCTs upon treatment with Cx43 siRNA also suggests that the traffic of the main metabolites, such as glucose and lactate, were not affected through these other pathways.
Under basal conditions, astroglial MBH Cxs inhibition did not affect glycemia and insulinemia of siCx43-injected rats (Supplementary Figure S2). In contrast to siCtrl animals, siCx43 animals exhibited a strong decrease in increased insulin secretion in response to an intracarotid glucose injection toward the brain, suggesting that glucose detection was incomplete. In this experiment, even if gap-junction activity might be modified to compensate for decreased Cxs protein levels, it is not sufficient to ensure a complete sensing of increased hypothalamic glucose levels. Although it is not possible to infer this reduction to one or the other Cx because both Cx43 and Cx30 expression were inhibited in siCx43-treated animals, this result suggests that the MBH astroglial networks overall are necessary for GS.
With regard to the underlying molecular mechanisms by which Cxs are needed for hypothalamic glucose detection, tanycyte glucose detection has been shown in vitro to involve Cx43 hemichannel activity.32 To our knowledge, hypothalamic astroglial gap-junction channel or hemichannel activity has never been investigated ex vivo in brain slices. Retamal et al39 have demonstrated in astrocyte culture that decreased Cx43 expression is associated with a decrease in gap-junctional communication as well as an increase in hemichannel activity.39 Although hemichannel activity has been mostly linked to pathophysiological conditions, the existence of specific properties in the hypothalamus in physiologic conditions cannot be excluded, as recently reported in the hippocampus and neocortex.40
Finally, this study highlights the importance of the astroglial connexins to ensure proper hypothalamic GS and suggests that astroglial networks form a complex metabolic-sensing unit with GS neurons. Collectively, these in vivo data point to a major role of astrocytes in the hypothalamic glucose-sensing mechanism. Finally, the study of astroglial networks in brain fuel sensing in prediabetic and diabetic states could help researchers to better understand the etiology of these metabolic diseases.
Acknowledgments
CA conducted and designed in vivo, western blot, and immunohistochemistry experiments; performed data analyses; and wrote the manuscript. LC conducted and designed in vivo and western blot experiments and performed data analyses. SG performed biochemical analysis, western blot, and immunohistochemistry experiments. BC performed western blot experiments. CC and FB-A assisted with in vivo experiments. XF, CG and LP contributed to discussion, reviewed, and edited the manuscript. CL supervised the project, designed experiments, and participated in writing, reviewing, and editing of the manuscript. The authors are extremely grateful to Dr A Tabernero for having given us the siCx43 sequence, to Dr SC Collins for his invaluable assistance in reviewing the English of the manuscript and to A Lefranc from the animal facility of CSGA for excellent technical assistance.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by a grant from the Agence Nationale de la Recherche (ANR-11-BSV1-0007, ConnexSensing to CL), a VITAGORA's contribution and by a Burgundy Region FEDER-FABER grants (to CL and LP). CA was supported by the FABER Studentship.
Supplementary Material
References
- Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Lam TKT, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Leloup C, Magnan C, Benani A, Bonnet E, Alquier T, Offer G, et al. Mitochondrial reactive oxygen species are required for hypothalamic glucose sensing. Diabetes. 2006;55:2084–2090. doi: 10.2337/db06-0086. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T, Bray Ga. The effect of intrahypothalamic injections of glucose on sympathetic efferent firing rate. Brain Res Bull. 1987;18:591–595. doi: 10.1016/0361-9230(87)90128-6. [DOI] [PubMed] [Google Scholar]
- Fioramonti X, Contié S, Song Z, Routh VH, Lorsignol A, Pénicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide Y and pro-opio melanocortin networks. Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- Cruciani-Guglielmacci C, Vincent-Lamon M, Rouch C, Orosco M, Ktorza A, Magnan C. Early changes in insulin secretion and action induced by high-fat diet are related to a decreased sympathetic tone. Am J Physiol Endocrinol Metab. 2005;288:E148–E154. doi: 10.1152/ajpendo.00225.2004. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang C-Y, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Colombani A-L, Carneiro L, Benani A, Galinier A, Jaillard T, Duparc T, et al. Enhanced hypothalamic glucose sensing in obesity: alteration of redox signaling. Diabetes. 2009;58:2189–2197. doi: 10.2337/db09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillod-Maximin E, Lorsignol A, Alquier T, Pénicaud L. Acute intracarotid glucose injection towards the brain induces specific c-fos activation in hypothalamic nuclei: involvement of astrocytes in cerebral glucose-sensing in rats. J Neuroendocrinol. 2004;16:464–471. doi: 10.1111/j.1365-2826.2004.01185.x. [DOI] [PubMed] [Google Scholar]
- Chari M, Yang CS, Lam CKL, Lee K, Mighiu P, Kokorovic A, et al. Glucose transporter-1 in the hypothalamic glial cells mediates glucose sensing to regulate glucose production in vivo. Diabetes. 2011;60:1901–1906. doi: 10.2337/db11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup C, Orosco M, Serradas P, Nicolaïdis S, Pénicaud L. Specific inhibition of GLUT2 in arcuate nucleus by antisense oligonucleotides suppresses nervous control of insulin secretion. Brain Res Mol Brain Res. 1998;57:275–280. doi: 10.1016/s0169-328x(98)00097-7. [DOI] [PubMed] [Google Scholar]
- Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D, et al. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–995. doi: 10.2337/diabetes.55.04.06.db05-1386. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi C-X, Habegger KM, Chowen Ja, Stern J, Tschöp MH. A role for astrocytes in the central control of metabolism. Neuroendocrinology. 2011;93:143–149. doi: 10.1159/000324888. [DOI] [PubMed] [Google Scholar]
- Dale N. Purinergic signaling in hypothalamic tanycytes: potential roles in chemosensing. Semin Cell Dev Biol. 2011;22:237–244. doi: 10.1016/j.semcdb.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17:607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Harris AL. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Martinez AD, Retamal MA.Gap junction channels and hemichannels in the CNS: regulation by signaling molecules Neuropharmacology 2013. advance online publication, 7 March 2013;doi: 10.1016/j.neuropharm.2013.02.020(e-pub ahead of print). [DOI] [PubMed]
- Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, et al. Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocyte-dependent glucose sensors. J Clin Invest. 2005;115:3545–3553. doi: 10.1172/JCI26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro L, Allard C, Guissard C, Fioramonti X, Tourrel-Cuzin C, Bailbé D, et al. Importance of mitochondrial dynamin-related protein 1 in hypothalamic glucose sensitivity in rats. Antioxid Redox Signal. 2012;17:433–444. doi: 10.1089/ars.2011.4254. [DOI] [PubMed] [Google Scholar]
- Herrero-González S, Valle-Casuso JC, Sánchez-Alvarez R, Giaume C, Medina JM, Tabernero A. Connexin43 is involved in the effect of endothelin-1 on astrocyte proliferation and glucose uptake. Glia. 2009;57:222–233. doi: 10.1002/glia.20748. [DOI] [PubMed] [Google Scholar]
- Niijima A. The effect of glucose on the activity of the adrenal nerve and pancreatic branch of the vagus nerve in the rabbit. Neurosci Lett. 1975;1:159–162. doi: 10.1016/0304-3940(75)90032-4. [DOI] [PubMed] [Google Scholar]
- N'Guyen JM, Magnan C, Laury MC, Thibault C, Leveteau J, Gilbert M, et al. Involvement of the autonomic nervous system in the in vivo memory to glucose of pancreatic beta cell in rats. J Clin Invest. 1994;94:1456–1462. doi: 10.1172/JCI117483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23:9254–9262. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Nuriya M, Yasui M. Endfeet serve as diffusion-limited subcellular compartments in astrocytes. J Neurosci. 2013;33:3692–3698. doi: 10.1523/JNEUROSCI.3050-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana JA, Sáez PJ, Cortés-Campos C, Elizondo RJ, Shoji KF, Contreras-Duarte S, et al. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels. Glia. 2012;60:53–68. doi: 10.1002/glia.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti X, Song Z, Vazirani RP, Beuve A, Routh VH. Hypothalamic nitric oxide in hypoglycemia detection and counterregulation: a two-edged sword. Antioxid Redox Signal. 2011;14:505–517. doi: 10.1089/ars.2010.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard C, Carneiro L, Collins SC, Chrétien C, Grall S, Pénicaud L, et al. Alteration of hypothalamic glucose and lactate sensing in 48h hyperglycemic rats. Neurosci Lett. 2013;534:75–79. doi: 10.1016/j.neulet.2012.11.033. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA. Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications. J Neurosci Res. 2011;89:2052–2067. doi: 10.1002/jnr.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theis M, Jauch R, Zhuo L, Speidel D, Wallraff A, Döring B, et al. Accelerated hippocampal spreading depression and enhanced locomotory activity in mice with astrocyte-directed inactivation of connexin43. J Neurosci. 2003;23:766–776. doi: 10.1523/JNEUROSCI.23-03-00766.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezan P, André P, Cisternino S, Saubaméa B, Boulay A-C, Doutremer S, et al. Deletion of astroglial connexins weakens the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32:1457–1467. doi: 10.1038/jcbfm.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal MA, Froger N, Palacios-Prado N, Ezan P, Sáez PJ, Sáez JC, et al. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, et al. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.