Abstract
Delayed cerebral ischemia (DCI) is at presentation a diagnosis per exclusionem, and can only be confirmed with follow-up imaging. For treatment of DCI a diagnostic tool is needed. We performed a systematic review to evaluate the value of CT perfusion (CTP) in the prediction and diagnosis of DCI. We searched PubMed, Embase, and Cochrane databases to identify studies on the relationship between CTP and DCI. Eleven studies totaling 570 patients were included. On admission, cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time-to-peak (TTP) did not differ between patients who did and did not develop DCI. In the DCI time-window (4 to 14 days after subarachnoid hemorrhage (SAH)), DCI was associated with a decreased CBF (pooled mean difference −11.9 mL/100 g per minute (95% confidence interval (CI): −15.2 to −8.6)) and an increased MTT (pooled mean difference 1.5 seconds (0.9–2.2)). Cerebral blood volume did not differ and TTP was rarely reported. Perfusion thresholds reported in studies were comparable, although the corresponding test characteristics were moderate and differed between studies. We conclude that CTP can be used in the diagnosis but not in the prediction of DCI. A need exists to standardize the method for measuring perfusion with CTP after SAH, and optimize and validate perfusion thresholds.
Keywords: CT perfusion, delayed cerebral ischemia, meta-analysis, subarachnoid hemorrhage, systematic review
Introduction
Delayed cerebral ischemia (DCI) is a serious complication after aneurysmal subarachnoid hemorrhage (SAH), occurring mostly 4 to 14 days after the hemorrhage.1 Clinical deterioration due to DCI can present as focal neurologic impairment, a decrease in the level of consciousness or both.2 Symptoms of DCI can reverse or progress to cerebral infarction, which increases the risk of poor functional outcome.3, 4 The diagnosis of DCI can be difficult, as clinical deterioration can also have other etiologies, such as hydrocephalus, rebleeding, metabolic disturbances, or infections. For guiding treatment of DCI, a diagnostic tool in the acute setting of clinical deterioration is needed, but currently, the diagnosis can only be confirmed by the identification of ischemic lesions on follow-up imaging. Previously, vasospasm on angiography or increased flow velocities on transcranial Doppler were used to diagnose DCI. However, vasospasm in the large vessels only correlates moderately with DCI. Approximately a third of patients with vasospasm develops DCI, and a third of patients with DCI has no vasospasm.4, 5, 6 Therefore, it is important to have a diagnostic tool that investigates brain ischemia instead of vasospasm to diagnose DCI. CT perfusion (CTP) is frequently used for this purpose at the time of clinical deterioration. In addition, CTP on admission is sometimes used to determine cerebral perfusion and assess the risk of DCI during hospitalization.7, 8 The clinical value of CTP in the prediction and diagnosis of DCI still remains unclear because of the different CTP methods used and because no single accepted and validated threshold exists. The aim of this review is to investigate the value of CTP in the prediction and diagnosis of DCI.
Materials and Methods
Search Strategy
We searched the PubMed, Embase, and Cochrane databases with the following combination of variables: SAH, CT perfusion, DCI, and cerebral infarction (for search syntax see Supplementary Data 1; last search 14 October, 2013). Inclusion criteria were as follows: (1)⩾10 patients with aneurysmal SAH, defined as the presence of subarachnoid blood as shown by CT or lumbar puncture and the presence of an aneurysm on conventional CT or magnetic resonance angiography; (2) manuscript reporting original data on the relationship between DCI and perfusion as measured by CTP; (3) perfusion measured quantitatively, semi-quantitatively (comparing quantitative perfusion in (a part of) one hemisphere to the other), or visually (comparing the affected hemisphere to the contralateral side in a qualitative manner); (4) definition for DCI includes clinical symptoms; and (5) CTP scan performed <72 hours after SAH for the prediction of DCI or in the time-window of DCI (4 to 14 days after SAH) for diagnosing DCI. Both prospective and retrospective studies were included. Exclusion criteria were as follows: (1) manuscripts written in another language than English, French, or German; (2) conference abstracts; and (3) manuscripts in which definition of DCI was completely or partially dependent on CTP measurements. If several manuscripts used the same or overlapping patient populations, we included only the report with the largest population or with the most relevant information for our review. References of included articles were searched for other eligible articles.
Two reviewing authors (CHPC and EW) independently screened titles and abstracts and extracted data on study design, methodology, study population, CT perfusion technique, and outcome. In case of disagreement two other authors (ICvdS and MDIV) reviewed the title, abstract, or article and the disagreement was resolved by consensus between the four reviewers. We collected mean values of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), and time-to-peak (TTP) for patients with and without DCI. In addition, we collected data on thresholds of perfusion values to differentiate between DCI and non-DCI and on the diagnostic accuracy of CTP in detecting DCI. In case an article did not provide the exact data needed for the review analysis, the corresponding author was contacted by e-mail to ask for additional information. If the corresponding author did not respond to our request, a second email was sent after 4 weeks.
As many different terms are used for DCI in the literature, we considered the following terms as DCI: DCI, clinical deterioration from DCI, symptomatic vasospasm, delayed ischemic deterioration, delayed neurologic deterioration (after exclusion of non-ischemic causes of deterioration). The following terms were considered as non-DCI for the control groups: non-DCI, non-DCI-related deterioration, clinically stable, asymptomatic vasospasm, no (symptomatic) vasospasm, no delayed ischemic deterioration, no delayed neurologic deterioration.
The quality of the included articles was determined with the QUADAS-tool (quality assessment of diagnostic accuracy studies), with a maximum score of 12 points.9 The questions were adapted for the specific characteristics of the current review (Supplementary Data 2). A study with a QUADAS score of 0 to 4 was considered a low-quality study, 5 to 8 an intermediate-quality study, and 9 to 12 a high-quality study.
Analyses
First, we compared means of quantitative and semi-quantitative values of CTP parameters on admission (<72 hours after ictus) between patients who did and did not develop DCI during the clinical course to investigate the predictive value of CBF, CBV, MTT, and TTP. The differences in perfusion parameters between patients with and without DCI with 95% confidence intervals (CIs) were pooled in forest plots. We pooled studies using both fixed effects and random effects models. Because a random effects model assumes that individual studies are estimating different effects, we used a random effects model for our conclusions. For the quantitative differences, we performed a sensitivity analysis, in which only high-quality studies (QUADAS score 9 to 12) were included. In addition, we collected thresholds to differentiate between DCI and non-DCI patients with their test characteristics. Subsequently, we performed similar analyses on the diagnostic value of CTP in the time-window that DCI develops (4 to 14 days after ictus).
Results
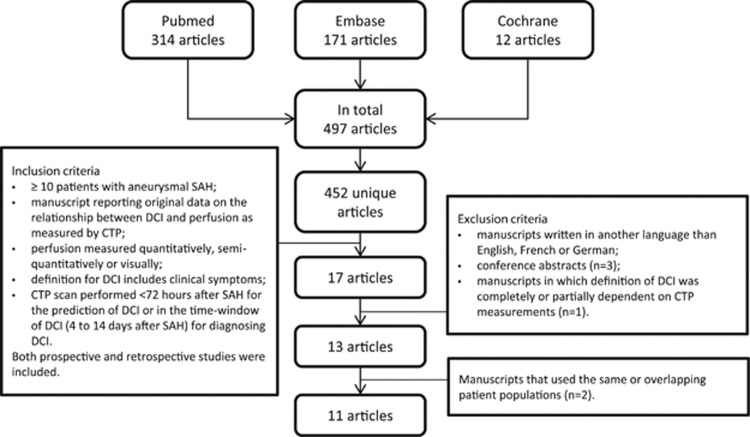
The search strategy yielded 497 articles, of which 11 were included with a total of 570 patients (Figure 1). Additional data on definitions and perfusion values were obtained from eight (of 10 requested) studies.6, 7, 8, 10, 11, 12, 13, 14 Characteristics of the included studies and the definitions used for DCI and control patients are shown in Table 1. Median number of patients per study was 46 (range 19 to 96). Six studies (55%) fulfilled the criteria for high-quality studies.
Figure 1.
Flow chart search strategy. CTP, CT perfusion; DCI, delayed cerebral ischemia; SAH, subarachnoid hemorrhage.
Table 1. Study characteristics.
| Study | Patients included in analyses (n) | Patient characteristics | QUADAS score | Subgroups in studies and outcome definitions (if applicable) |
|---|---|---|---|---|
| Chai12 | 85 | Non-selected SAH patients | 6 | Symptomatic vasospasm: Arterial vasospasm (DSA/CTA/TCD)+neurologic deficit Asymptomatic vasospasma: Only arterial segments demonstrating vasospasm (DSA/CTA/TCD), while patients do not have neurologicdeficit No vasospasma: no arterial vasospasm (DSA/CTA/TCD); with or without neurologic deficit due to other causes |
| Dankbaar6 | 85 | Non-selected SAH patients | 11 | DCI: Clinical deterioration (↓ GCS of ⩾2 points lasting >2 hours or a new focal deficit), excluding rebleeding, hydrocephalus, edema, postoperative swelling, infection, and metabolic disturbance as the cause Non-DCI: Clinically stable |
| Hickmann14 | 29 | Non-selected SAH patients | 8 | Delayed neurologic deterioration: An increase in the NIHSS score by ⩾1 point or a decrease in the GCS score ⩾1 point, which could not be accounted for by other factors such as bleeding, seizure, infection, edema, electrolyte- or acid-base disturbances No delayed neurologic deterioration |
| Kanazawa15 | 19 | Non-selected SAH patients | 5 | Symptomatic vasospasm: neurologic deficits due to vasospasm (by DSA) No symptomatic vasospasm |
| Killeen16 | 57 | Non-selected SAH patients | 9 | DCI: New neurologic deficit distinct from baseline deficit caused by a SAH or surgical intervention, excluding rebleeding, hemorrhage, hydrocephalus, infection, metabolic disturbance, and seizure as the cause and/or a new infarction on CT/MRI after day 4 Non-DCI: Patients with none of the criteria above |
| Lagares7 | 39 | Non-selected SAH patients | 8 | DCI: Clinical deterioration attributable to vasospasm, excluding rebleeding, hydrocephalus or electrolyte abnormalities as the cause, or a new infarct on CT related to vasospasm (not visible on admission or immediate posttreatment scan), or both Non-DCI: Patients without clinical deterioration or patients with clinical deterioration owing to other causes and without a new infarct on CT |
| Lanterna13 | 41 | Non-selected SAH patients | 11 | DCI: A deterioration (↓ GCS of ⩾2 points or a new focal deficit), excluding rebleeding, hydrocephalus, and metabolic disturbances as the cause, with or without an infarction on the CT scan Non-DCI: Clinical deterioration owing to other causes or clinically stable |
| Rijsdijk10 | 27 | Non-selected SAH patients | 11 | Clinical deterioration from DCI: clinical deterioration (↓ in consciousness or new focal deficit) lasting ⩾2 hours, excluding rebleeding, hydrocephalus, infections, and metabolic disturbances as the cause, with or without hypodensity on CT Non-DCI-related deteriorationb: A decrease in GCS of ⩾2 points from hydrocephalus or other medical causes (metabolic disturbances, infection) Clinically stableb |
| Sanelli11 | 96 | Non-selected SAH patients | 9 | DCI: Clinical deterioration (↓ GCS of ⩾2 points or focal neurologic impairment), that was not apparent immediately after aneurysm occlusion and was not attributed to other causes by clinical assessment, CT or MR imaging, and laboratory studies Non-DCI: Clinical deterioration owing to other causes or clinically stable |
| Sviri17 | 46 | Patients with cerebral vasospasm after aSAH | 8 | Delayed ischemic deterioration: worsening of neurologic condition (↓ GCS of ⩾2 points or a new hemiplegia or hemiparesis or↓muscle scale of ⩾2 points in a previously hemiparetic patient) lasting >30 minutes, excluding rebleeding, hydrocephalus, postoperative complications and systemic complications as the cause No delayed ischemic deterioration |
| Van der Schaaf8 | 46 | Non-selected SAH patients | 11 | DCI: persisting clinical deterioration (new focal deficit and/or decrease in consciousness), excluding rebleeding, hydrocephalus, infections, and metabolic disturbances as the cause, with or without hypodensity on follow-up CT Non-DCI: clinical deterioration owing to other causes or clinically stable |
aSAH, aneurysmal subarachnoid hemorrhage; CTA, CT angiography; DCI, delayed cerebral ischemia; DSA, digital subtraction angiography; GCS, Glasgow Coma Scale; MRI, magnetic resonance imaging; n, number; NIHSS, National Institutes of Health Stroke Scale; QUADAS: quality assessment of diagnostic accuracy studies;9 SAH, subarachnoid hemorrhage; TCD: transcranial Doppler.
In the analyses, the subgroups of asymptomatic vasospasm and no vasospasm were pooled (as non-DCI).
In the analyses, the subgroups of non-DCI-related deterioration and clinically stable patients were pooled (as non-DCI).
Both for predicting and diagnosing DCI, quantitative, semi-quantitative, visual assessments, or a combination of methods had been used. Eight of the nine studies using quantitative assessments reported mean perfusion values6, 7, 8, 10, 11, 12, 15, 16 and one gave the lowest values in predefined regions of interest (ROIs).17 In six studies, all ROIs were used in the analyses,7, 10, 12, 15, 16, 17 whereas two other studies only used the ROIs with lowest CBF or CBV and highest MTT or TTP.6, 8 Of these last two studies, we obtained additional information on the mean values of all ROIs together and included these values in the quantitative analyses. One of the nine studies used only the affected ROIs in patients with a perfusion deficit and all ROIs in patients without a perfusion deficit.11 Perfusion thresholds were determined in two studies7, 8 for predicting DCI and in three studies for diagnosing DCI.6, 11, 17
CT Perfusion and Prediction of Delayed Cerebral Ischemia
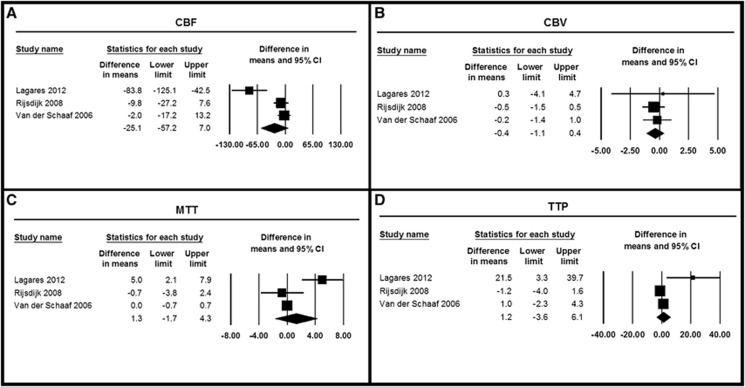
Four studies with 153 patients investigated the relationship between perfusion on admission and later development of DCI.7, 8, 10, 13 Three studies determined CBF, CBV, MTT, and TTP quantitatively (Table 2, Figure 2).7, 8, 10 Cerebral blood flow, CBV, MTT, and TTP on admission did not differ between patients who later developed DCI and those who did not develop DCI. One study with 46 patients not only reported quantitative data but also semi-quantitative data (Table 3).8 Although quantitative measurements on admission did not reveal differences between DCI and non-DCI patients in this study, semi-quantitative assessments showed a significantly decreased CBF and CBV and increased MTT and TTP in DCI patients compared with non-DCI patients (Table 3). Another study with 41 patients evaluated perfusion visually on the different color maps and found that DCI was significantly related to hypoperfusion on CTP, without specification of perfusion parameters.13
Table 2. Quantitative perfusion values on admission in patients who did and did not develop DCI.
| Article | Timing of CTP | Subgroup | Mean CBF (mL/100 g per minute) | Mean CBV (mL/100 g) | Mean MTT (s) | Mean TTP (s) |
|---|---|---|---|---|---|---|
| Lagares7 | <24 hours after ictus | DCI (n=19) | 75.0±45.1 | 9.1±9.1 | 8.8±6.5 | 39.1±41.1 |
| Rijsdijk10 | Non-DCI (n=20) | 158.8±80.7 | 8.8±4.2 | 3.8±1.3 | 17.6±5.2 | |
| <72 hours after ictus | DCI (n=12) | 54.3±20.7 | 4.1±1.1 | 4.8±1.0 | 22.7±2.8 | |
| Non-DCI (n=15)a | 64.1±24.5 | 4.6±1.4 | 5.5±5.3 | 23.9±4.3 | ||
| Van der Schaaf8 | <72 hours after ictus | DCI (n=16) | 66.9±24.1 | 4.7±2.0 | 4.3±1.2 | 21.7±4.3 |
| Non-DCI (n=30) | 68.9±25.6 | 4.9±2.0 | 4.3±1.1 | 20.7±6.0 |
CBF, cerebral blood flow; CBV, cerebral blood volume; CTP, CT perfusion; DCI, delayed cerebral ischemia; MTT, mean transit time; TTP, time-to-peak.
This subgroup contains both clinically stable patients as patients with non-DCI-related deterioration.
Figure 2.
Differences in means of perfusion parameters on admission in patients who did and did not develop delayed cerebral ischemia (DCI). Differences in cerebral blood flow (CBF) (A), cerebral blood volume (CBV) (B), mean transit time (MTT) (C), and time-to-peak (TTP) (D) between patients who did and did not develop DCI. CI, confidence interval.
Table 3. Semi-quantitative perfusion valuesa in patients with DCI versus non-DCI.
| CTP time | Study | CTP performance | Subgroup | Median CBF ratio (range) | Median CBV ratio (range) | Median MTT difference (s) (range) | Median TTP difference (s) (range) |
|---|---|---|---|---|---|---|---|
| Admission | Van der Schaaf8 | <72 hours after ictus | DCI (n=16) | 0.61 (0.29–0.93) | 0.71 (0.46–0.88) | 1.35 (0.4–4.0) | 1.35 (0.3–3.5) |
| Non-DCI (n=30) | 0.81 (0.56–0.93) | 0.85 (0.47–0.98) | 0.70 (0.1–2.1) | 0.5 (0.1–2.8) | |||
| Clinical deterioration | Dankbaar6 | Clinical deterioration or 1 week after ictus (asymptomatic patients) | DCI (n=50) | 0.67 (0.00–0.86) | 0.74 (0.02–0.92) | 1.91 (0.68–57.7) | 1.34 (0.39–23.2) |
| Non-DCI (n=35) | 0.78 (0.27–0.91) | 0.82 (0.24–0.91) | 0.96 (0.35–3.2) | 0.70 (0.16–2.1) | |||
| Hickmann14 | Day 10 after ictus (delayed neurologic deterioration was mostly documented on day 10) | DCI (n=11) | 0.95 (0.79–0.98) | 0.94 (0.86–0.98) | NR | NRb | |
| Non-DCI (n=18) | 0.93 (0.73–0.99) | 0.94 (0.75–0.99) | NR | NRb |
CBF, cerebral blood flow; CBV, cerebral blood volume; CTP, CT perfusion; DCI, delayed cerebral ischemia; MTT, mean transit time; NR, not reported; s, seconds; TTP, time-to-peak.
Affected hemisphere compared with healthy hemisphere. Lowest CBF and CBV ratios and largest MTT and TTP differences were used.
Hickmann14 determined a TTP ratio instead of a TTP difference.
Sensitivity Analyses for Predicting Delayed Cerebral Ischemia
In the sensitivity analyses with only high-quality studies (n=2),8, 10 the pooled mean differences between DCI and non-DCI patients were for CBF −5.4 mL/100 g per minute (95% CI: −16.8 to 6.1), for CBV −0.4 mL/100 g (95% CI: −1.1 to 0.4), for MTT 0.0 seconds (95% CI: −0.7 to 0.6), and for TTP −0.3 seconds (95% CI: −2.4 to 1.9).
Thresholds and Test Characteristics for Predicting Delayed Cerebral Ischemia
Two studies with 85 patients in total investigated perfusion thresholds on admission for predicting DCI (Table 5).7, 8 The first study7 used a quantitative diagnostic threshold (mean MTT of 5.9 seconds) obtained in a previous study6 and found a sensitivity of 47% and a specificity of 100%. The second study8 derived optimal semi-quantitative thresholds by seeking the best tradeoff between highest possible sensitivities and specificities of the threshold values. Optimal thresholds were as follows: a CBF ratio of 0.72 (sensitivity 75%, specificity 93%); a CBV ratio of 0.78 (sensitivity 75%, specificity 70%); a MTT difference of 0.87 seconds (sensitivity 75%, specificity 70%); and a TTP difference of 1.0 seconds (sensitivity 75%, specificity 90%).
CT Perfusion and Diagnosis of Delayed Cerebral Ischemia
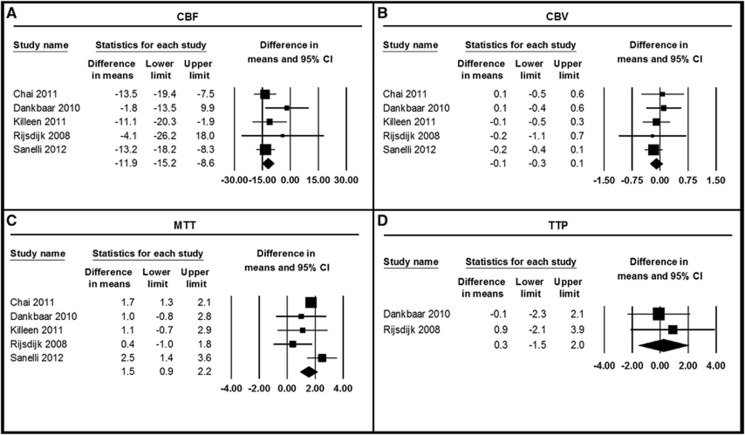
Eight studies with a total of 444 patients studied the diagnostic value of perfusion measured with CTP in the time-window of DCI.6, 10, 11, 12, 14, 15, 16, 17 From five studies, we obtained data on quantitative means of CBF, CBV, and MTT (Table 4, Figure 3).6, 10, 11, 12, 16 The pooled analysis showed that DCI patients had a significantly lower CBF (pooled mean difference −11.9 mL/100 g per minute (95% CI: −15.2 to −8.6)) and higher MTT (pooled mean difference 1.5 seconds (95% CI: 0.9–2.2)) compared with non-DCI patients. Cerebral blood volume was similar in both groups. Only two studies reported on TTP, which did not differ between DCI and non-DCI patients.6, 10
Table 4. Quantitative perfusion values in the time-window of DCI in patients with DCI versus non-DCI.
| Article | CTP performance | Subgroup | Mean CBF (mL/100 g/min) | Mean CBV (mL/100 g) | Mean MTT (s) | Mean TTP (s) |
|---|---|---|---|---|---|---|
| Chai12 | <14 days after ictus | DCI (n=22) | 23.1±9.3 | 1.9±0.8 | 5.5±0.8 | NR |
| Non-DCI (n=63)a | 36.6±13.0 | 1.8±1.3 | 3.8±0.8 | NR | ||
| Dankbaar6 | Clinical deterioration or 1 week after ictus (asymptomatic patients) | DCI (n=50) | 54.0±28.5 | 3.8±1.2 | 5.4±5.3 | 24.4±5.4 |
| Non-DCI (n=35) | 55.8±24.7 | 3.7±0.9 | 4.4±1.3 | 24.5±4.8 | ||
| Killeen16 | Days 6–8 after ictus | DCI (n=45) | 29.4±13.7 | 1.9±0.6 | 6.6±2.7 | NR |
| Non-DCI (n=12) | 40.5±16.9 | 2.0±0.4 | 5.5±3.2 | NR | ||
| Rijsdijk10 | Clinical deterioration (within 14 days) or 1 week after ictus (asymptomatic patients) | DCI (n=12) | 70.0±30.4 | 4.5±1.4 | 4.4±2.5 | 22.1±4.9 |
| Non-DCI (n=15)b | 74.1±28.1 | 4.7±1.0 | 4.0±1.0 | 21.2±3.1 | ||
| Sanelli11 | Clinical deterioration or days 6–8 after ictus (asymptomatic patients) | DCI (n=48) | 26.6±13.3 | 1.8±0.7 | 7.2±3.5 | NR |
| Non-DCI (n=48) | 39.8±11.3 | 2.0±0.5 | 4.7±1.4 | NR |
CBF, cerebral blood flow; CBV, cerebral blood volume; CTP, CT perfusion; DCI, delayed cerebral ischemia; MTT, mean transit time; NR, not reported; TTP, time-to-peak.
This subgroup contains both asymptomatic vasospasm as non-vasospasm patients.
This subgroup contains both clinically stable patients as patients with non-DCI-related deterioration.
Figure 3.
Differences in means of perfusion parameters in the time-window of delayed cerebral ischemia (DCI) in patients with and without DCI. Differences in cerebral blood flow (CBF) (A), cerebral blood volume (CBV) (B), mean transit time (MTT) (C), and time-to-peak (TTP) (D) between DCI and non-DCI patients. CI, confidence interval.
Two studies did not provide crude data on quantitative means of perfusion parameters that could be used in the meta-analysis.15, 17 Nonetheless, both studies reported similar findings as above: CBF was significantly lower and MTT higher in patients with DCI. Cerebral blood volume was reported in one study and was comparable in DCI and non-DCI patients.15 Time-to-peak was not reported in both studies.
Two studies reported semi-quantitative data,6, 14 including one study that also reported quantitative data.6 The first study did not find significant differences in CBF, CBV and TTP values between DCI and non-DCI patients in the time-window of DCI (Table 3).14 The second study found that although quantitative perfusion parameters were not different between DCI and non-DCI patients, semi-quantitative CBF and CBV values were significantly decreased and MTT and TTP significantly increased in DCI patients (Table 3).6
Sensitivity Analyses for Diagnosing Delayed Cerebral Ischemia
In the analyses with only high-quality studies (n=4),6, 10, 11, 16 the pooled mean differences between DCI and non-DCI patients were for CBF −10.6 mL/100 g per minute (95% CI: −15.4 to −5.7), for CBV −0.1 mL/100 g (95% CI: −0.3 to 0.1), for MTT 1.3 seconds (95% CI: 0.3–2.4), and for TTP 0.3 seconds (95% CI: −1.5 to 2.0).
Thresholds and Test Characteristics for Diagnosing Delayed Cerebral Ischemia
Three studies including 227 patients investigated optimal quantitative thresholds for diagnosing DCI (Table 5).6, 11, 17 Two studies derived optimal thresholds by seeking the best tradeoff between highest possible sensitivities and specificities of the threshold values.6, 11 One study did not report a definition of their optimal thresholds.17 For CBF, the studies found optimal thresholds of 36.3 mL/100 g per minute (sensitivity 74%, specificity 63%)6 and 30.5 mL/100 g per minute (sensitivity 78%, specificity 70%),11 and a minimal CBF threshold of 25 mL/100 g per minute (sensitivity 73%, specificity 76%).17 The latter study investigated minimal CBF values per ROI (instead of mean values per ROI). Only one study determined an optimal CBV threshold (2.78 mL/100 g, sensitivity 52%, specificity 63%), and TTP threshold (25.2 seconds, sensitivity 54%, specificity 63%).6 Three studies determined optimal MTT thresholds ranging from 5.0 seconds (sensitivity 72%, specificity 70%),11 5.85 seconds (sensitivity 70%, specificity 77%),6 to a maximal MTT threshold of 6.5 seconds (sensitivity 71%, specificity 81%).17 In the last study, maximal MTT values per ROI (instead of mean values per ROI) were determined. One study determined both quantitative and semi-quantitative thresholds.6 The semi-quantitative thresholds were a CBF ratio of 0.77 (sensitivity 76%, specificity 63%), a CBV ratio of 0.80 (sensitivity 64%, specificity 63%), an MTT difference of 1.08 seconds (sensitivity 80%, specificity 63%), and a TTP difference of 0.99 seconds (sensitivity 70%, specificity 66%).
Table 5. Threshold values and test characteristics of CT perfusion parameters for the prediction and diagnosis of DCI.
| Quality | CTP parameter | Study | CTP threshold | AUC % (95% CI) | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | OR |
|---|---|---|---|---|---|---|---|---|---|
| Predicting DCI | CBF | Van der Schaaf8 | Least perfused flow territorya | 53 | |||||
| Van der Schaaf8 | Largest asymmetryb | 80 | |||||||
| Van der Schaaf8 | Ratio <0.72b | 75 (51–90) | 93 (79–98) | ||||||
| CBV | Van der Schaaf8 | Least perfused flow territorya | 56 | ||||||
| Van der Schaaf8 | Largest asymmetryb | 75 | |||||||
| Van der Schaaf8 | Ratio <0.78b | 75 (51–90) | 70 (52–83) | ||||||
| MTT | Lagares7 | Mean ⩾5.9 secondsa,c | 74 | 47 | 100 | 100 | 62 | ||
| Lagares7 | Any ROI with ⩾5.9 secondsa,c | 76 | 85 | 76 | 75 | 80 | 12 (2.5–57) | ||
| Van der Schaaf8 | Least perfused flow territorya | 57 | |||||||
| Van der Schaaf8 | Largest asymmetryb | 79 | |||||||
| Van der Schaaf8 | Difference >0.87 secondsb | 75 (51–90) | 70 (52–83) | ||||||
| TTP | Van der Schaaf8 | Least perfused flow territorya | 59 | ||||||
| Van der Schaaf8 | Largest asymmetryb | 81 | |||||||
| Van der Schaaf8 | Difference >1.0 secondsb | 75 (51–90) | 90 (74–97) | ||||||
| Diagnosing DCI | CBF | Dankbaar6 | <36.3 mL/100 g per minutea | 71 (60–82) | 74 (66–78) | 63 (54–69) | |||
| Dankbaar6 | Ratio <0.77b | 72 (61–83) | 76 (68–80) | 63 (54–69) | |||||
| Sanelli11 | a | 81 (77–85) | |||||||
| Sanelli11 | <30.5 mL/100 g per minutea | 78 | 70 | ||||||
| Sviri17 | minimal <25 mL/100 g per minutea | 73 | 76 | 73 | 78 | ||||
| CBV | Dankbaar6 | <2.78 mL/100 ga | 57 (45–70) | 52 (45–58) | 63 (54–69) | ||||
| Dankbaar6 | Ratio <0.80b | 64 (52–76) | 64 (57–69) | 63 (54–69) | |||||
| Sanelli11 | a | 61 (55–67) | |||||||
| MTT | Dankbaar6 | >5.85 secondsa | 76 (66–86) | 70 (62–74) | 77 (67–81) | ||||
| Dankbaar6 | Difference >1.08 secondsb | 78 (68–87) | 80 (72–83) | 63 (54–69) | |||||
| Sanelli11 | a | 78 (73–83) | |||||||
| Sanelli11 | >5.0 secondsa | 72 | 70 | ||||||
| Sviri17 | Maximal >6.5 secondsa | 71 | 81 | 78.5 | 74 | ||||
| TTP | Dankbaar6 | >25.2 secondsa | 60 (48–72) | 54 (47–60) | 63 (54–69) | ||||
| Dankbaar6 | Difference >0.99 secondsb | 75 (65–86) | 70 (62–74) | 66 (57–72) | |||||
| Qualitative | Hickmann14 | CTP defects on CBF mapd | 25 | 80.9 | |||||
| Hickmann14 | CTP defects on CBV mapd | 25 | 80.9 | ||||||
| Hickmann14 | CTP defects on TTP mapd | 25 | 84.6 | ||||||
| Killeen16 | CTP defectsd | 80 (68–92) | 67 (40–93) | 90 (82–96) | 47 (27–62) | 8.0 (1.96–32.6) | |||
| Sanelli11 | CTP defectsd | 81 (68–90) | 83 (70–91) | 83 (70–91) | 82 (69–90) |
AUC, area under the curve; CBF, cerebral blood flow; CBV, cerebral blood volume; CTP, CT perfusion; DCI, delayed cerebral ischemia; min, minutes; MTT, mean transit time; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value; sec, seconds; TTP, time-to-peak.
Quantitatively (absolute) measured.
Semi-quantitatively (relative) measured.
The MTT threshold of 5.9 seconds was a validation of the threshold by Dankbaar et al.6
Qualitatively (visual) measured.
Discussion
The results of this systematic review show that CTP on admission cannot be used reliably to predict DCI. However, at the time of clinical deterioration, patients with DCI had a significantly decreased CBF and an increased MTT compared with patients without DCI using quantitative assessments. For diagnosing DCI, quantitative thresholds for CBF ranged from 25 to 36.3 mL/100 g per minute (with sensitivity ranging from 73% to 78% and specificity ranging from 63% to 76%) and for MTT from 5.0 to 6.5 seconds (with sensitivity ranging from 70% to 72% and specificity ranging from 70% to 81%).
The present review differs from previous reviews. In one review, the diagnostic accuracy of CT angiography and CTP for cerebral vasospasm instead of DCI was studied.18 Although that meta-analysis included 10 studies, only three studies could be used for the statistical analysis because of inappropriate data (studies that did not report the actual data from a 2 × 2 table or enough data to reconstruct a 2 × 2 table were excluded from the statistical calculations). Another review on the role of clinical assessment, transcranial Doppler, CT angiography, and CTP in the detection and monitoring of vasospasm and DCI included a summary on the test characteristics of five studies on the relationship between CTP and DCI, without comparing means of perfusion parameter or pooling the data.19 In the present review, we included more studies and also compared quantitative and semi-quantitative means, thresholds and test characteristics, and performed pooled analyses.
Based on our results, CTP on admission is not useful to predict DCI in clinical practice. A recent study showed that the amount of blood on admission non-contrast CT in combination with an MTT increase on admission CTP correlated with the development of cerebral infarction due to DCI.20 This suggests that the combination of CTP and non-contrast CT might be a better predictor for DCI than CTP alone.
We showed that in the time-window of DCI (4 to 14 days after SAH) patients with DCI have a decreased CBF and an increased MTT compared with non-DCI patients. Therefore, CTP might be helpful in determining the cause of clinical deterioration in patients with SAH. When evaluating the diagnostic thresholds for CBF and MTT, these thresholds were reasonably comparable, but with varying sensitivities and specificities. As most of these test characteristics show moderate sensitivities and specificities, there is still no single threshold that can be used in the diagnostic process of DCI. The studies included in this review used different software packages and different methods of measuring perfusion. Therefore, our data emphasize the need to standardize the method and software for measuring perfusion with CTP in patients with SAH, so that more precise perfusion thresholds can be determined for diagnosing DCI.
As most patients with DCI have vasospasm in large cerebral arteries, these patients often have high-flow velocities on transcranial Doppler examination.21, 22 These increased flow velocities are in contrast with the observed decreased CBF on CTP in patients with DCI. This paradox can be explained by the measurement of blood flow in different vascular beds. Transcranial Doppler measures flow in the large proximal cerebral arteries whereas CTP measures blood flow at tissue level. The decreased CBF in patients with DCI supports alternative hypotheses for the pathogenesis of DCI such as microvascular spasm due to disturbed autoregulation, microthrombosis, and cortical spreading ischemia.23, 24, 25
Some limitations need to be addressed. First, many different terms and definitions for DCI were used in the included studies. Nevertheless, most definitions of these terms were comparable with the definition that was recently proposed by an international multidisciplinary research group.2 Therefore, despite differences in terminology, the similarity in definitions allowed us to pool data on DCI. Second, the studies included in this review used different methods for measuring perfusion. Some studies measured means in predefined ROIs, whereas another study measured the lowest values in predefined ROIs. In addition, most studies used quantitative measurements whereas some studies also used semi-quantitative values. Other studies also determined perfusion by visual assessment. A recently published review on definitions and thresholds for different states of ischemia in patients with acute ischemic stroke concluded, in line with our findings, that there is a considerable heterogeneity in definitions of outcome measures and perfusion analysis methods.26 In acute ischemic stroke, median threshold values varied up to fourfold. However, the range in thresholds found in our review for predicting or diagnosing DCI was considerably smaller. Third, the control patients differed between various studies. Some studies used SAH patients with clinical deterioration due to other causes as reference and some studies used clinically stable patients as reference group. In addition, in some studies, the reference group was not even described. Fourth, in some studies, the numbers of patients with and without DCI were comparable, which suggests that patients were selectively included in the study, because in clinical practice the patients without DCI outnumber those with DCI. This could have influenced the results of that specific study. Fifth, in most studies, positive predictive values and negative predictive values were not given.
Based on the current findings, CTP on admission cannot be used reliably to predict DCI. However, CTP might be helpful in determining the cause of clinical deterioration within the time-window of DCI, as patients with clinical deterioration due to DCI have a decreased CBF and an increased MTT compared with patients without DCI. Our data emphasize the need to standardize the method for measuring perfusion with CTP in patients with SAH, so that more precise perfusion thresholds can be determined for diagnosing DCI. In addition, future studies are necessary to validate thresholds and to investigate differences in perfusion thresholds for reversible and irreversible DCI.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Supplementary Material
References
- Van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–318. doi: 10.1016/S0140-6736(07)60153-6. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, Van Gijn J, Rinkel GJ, Wijdicks EF, Muizelaar JP, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:1545–1553. doi: 10.1038/jcbfm.2011.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–929. doi: 10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJ, Rinkel GJ. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–819. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbaar JW, de Rooij NK, Rijsdijk M, Velthuis BK, Frijns CJ, Rinkel GJ, et al. Diagnostic threshold values of cerebral perfusion easured with computed tomography for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41:1927–1932. doi: 10.1161/STROKEAHA.109.574392. [DOI] [PubMed] [Google Scholar]
- Lagares A, Cicuendez M, Ramos A, Salvador E, Alen JF, Kaen A, et al. Acute perfusion changes after spontaneous SAH: a perfusion CT study. Acta Neurochir (Wien) 2012;154:405–411. doi: 10.1007/s00701-011-1267-z. [DOI] [PubMed] [Google Scholar]
- van der Schaaf I, Wermer MJ, van der Graaf Y, Hoff RG, Rinkel GJ, Velthuis BK. CT after subarachnoid hemorrhage: relation of cerebral perfusion to delayed cerebral ischemia. Neurology. 2006;66:1533–1538. doi: 10.1212/01.wnl.0000216272.67895.d3. [DOI] [PubMed] [Google Scholar]
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJ, Rinkel GJ. Global and focal cerebral perfusion after aneurysmal subarachnoid hemorrhage in relation with delayed cerebral ischemia. Neuroradiology. 2008;50:813–820. doi: 10.1007/s00234-008-0416-4. [DOI] [PubMed] [Google Scholar]
- Sanelli PC, Anumula N, Johnson CE, Comunale JP, Tsiouris AJ, Riina H, et al. Evaluating CT perfusion using outcome measures of delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2013;34:292–298. doi: 10.3174/ajnr.A3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai WN, Sun XC, Lv FJ, Wan B, Jiang L. Clinical study of changes of cerebral microcirculation in cerebral vasospasm after SAH. Acta Neurochir Suppl. 2011;110 (Pt 1:225–228. doi: 10.1007/978-3-7091-0353-1_39. [DOI] [PubMed] [Google Scholar]
- Lanterna LA, Lunghi A, Martchenko S, Gritti P, Bonaldi G, Biroli F. Cerebral watershed hypoperfusion in subarachnoid hemorrhage: computed tomography perfusion analysis. J Neurosurg. 2011;114:961–968. doi: 10.3171/2010.8.JNS091766. [DOI] [PubMed] [Google Scholar]
- Hickmann AK, Langner S, Kirsch M, Baldauf J, Muller C, Khaw A, et al. The value of perfusion computed tomography in predicting clinically relevant vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2013;36:267–278. doi: 10.1007/s10143-012-0430-1. [DOI] [PubMed] [Google Scholar]
- Kanazawa R, Kato M, Ishikawa K, Eguchi T, Teramoto A. Convenience of the computed tomography perfusion method for cerebral vasospasm detection after subarachnoid hemorrhage. Surg Neurol. 2007;67:604–611. doi: 10.1016/j.surneu.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Killeen RP, Mushlin AI, Johnson CE, Comunale JP, Tsiouris AJ, Delaney H, et al. Comparison of CT perfusion and digital subtraction angiography in the evaluation of delayed cerebral ischemia. Acad Radiol. 2011;18:1094–1100. doi: 10.1016/j.acra.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sviri GE, Britz GW, Lewis DH, Newell DW, Zaaroor M, Cohen W. Dynamic perfusion computed tomography in the diagnosis of cerebral vasospasm. Neurosurgery. 2006;59:319–325. doi: 10.1227/01.NEU.0000222819.18834.33. [DOI] [PubMed] [Google Scholar]
- Greenberg ED, Gold R, Reichman M, John M, Ivanidze J, Edwards AM, et al. Diagnostic accuracy of CT angiography and CT perfusion for cerebral vasospasm: a meta-analysis. AJNR Am J Neuroradiol. 2010;31:1853–1860. doi: 10.3174/ajnr.A2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington CW, Zipfel GJ. Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care. 2011;15:312–317. doi: 10.1007/s12028-011-9594-8. [DOI] [PubMed] [Google Scholar]
- Etminan N, Beseoglu K, Heiroth HJ, Turowski B, Steiger HJ, Hanggi D. Early perfusion computerized tomography imaging as a radiographic surrogate for delayed cerebral ischemia and functional outcome after subarachnoid hemorrhage. Stroke. 2013;44:1260–1266. doi: 10.1161/STROKEAHA.111.675975. [DOI] [PubMed] [Google Scholar]
- Sloan MA, Haley EC, Jr., Kassell NF, Henry ML, Stewart SR, Beskin RR, et al. Sensitivity and specificity of transcranial Doppler ultrasonography in the diagnosis of vasospasm following subarachnoid hemorrhage. Neurology. 1989;39:1514–1518. doi: 10.1212/wnl.39.11.1514. [DOI] [PubMed] [Google Scholar]
- Burch CM, Wozniak MA, Sloan MA, Rothman MI, Rigamonti D, Permutt T, et al. Detection of intracranial internal carotid artery and middle cerebral artery vasospasm following subarachnoid hemorrhage. J Neuroimaging. 1996;6:8–15. doi: 10.1111/jon1996618. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129 (Pt 12:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Yundt KD, Grubb RL, Jr., Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18:419–424. doi: 10.1097/00004647-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Dani KA, Thomas RG, Chappell FM, Shuler K, MacLeod MJ, Muir KW, et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann Neurol. 2011;70:384–401. doi: 10.1002/ana.22500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.