Abstract
Over the last decade there has been a considerable effort directed toward reformulating the standard approach taken to preclinically model stroke and stroke recovery. The principal objective of this undertaking has been to improve the success with which preclinical findings can be translated. Although several advancements have already been introduced, one potentially critical feature that appears to have been overlooked is psychological stress. Stroke is well recognized to produce high levels of stress in patients, and ongoing exposure to stress is recognized to deleteriously interfere with recovery. The presence of high levels of stress (distress) in stroke patients is also relevant because nearly all clinically deployed neurorestorative interventions occur against this background. Somewhat perplexingly, however, we could find no preclinical stroke studies concerned with investigating the efficacy of putative neurorestorative compounds that did so in the presence of stress. The following article will make the case that failure to recognize or compensate for the effects of ongoing stress in standard preclinical experimental models of recovery is likely to result in overestimation of the effectiveness of pharmacological or behavioral neurorestorative interventions.
Keywords: animal models, brain ischemia, regeneration and recovery, rehabilitation, stress, stroke
Introduction
Stroke is a major cause of death and long-term disability globally, and represents a major claim on health-care budgets.1 Although the precise financial burden imposed by stroke is difficult to quantify, the first-year costs of first-ever strokes have been estimated at more than $2 billion per year in Australia,2 $53.9 billion USD in the United States,3 and over €38 billion per year across the European Union.4 Of this total cost, rehabilitative services (inpatient and outpatient) account for the largest component.2 Despite the considerable effort invested in existing rehabilitative approaches, research has shown that the majority of stroke patients who exhibit significant disability at three months, continue to do so at 12 months.5 This challenging situation has driven an intense research effort directed toward evaluating promising therapeutic interventions using experimental, animal-based, preclinical models of stroke. Many of the most promising strategies identified using preclinical models have not translated well into clinical practice. In the broadest of terms, this has led to a massive overhaul of the ‘standard' model of preclinical research with efforts now directed at redefining testing protocols so that they embrace the complexity and heterogeneity associated with human stroke. One critical element of human recovery, however, that has largely been overlooked in the current discourse relating to the improvement of preclinical models is stress. Although psychological stress has long been recognized to be a major risk factor for stroke,6, 7, 8, 9, 10, 11, 12, 13, 14 it is also a major, and effectively ubiquitous aspect of stroke recovery.15, 16, 17 Importantly, because of its ubiquity, almost all clinical interventions directed at improving recovery occur against a background of stress. In contrast, we could find no preclinical studies that have evaluated the efficacy of potential neurorestorative interventions in the presence of stress. There are, however, several preclinical studies that have clearly demonstrated that stress by itself impairs recovery. Given this situation, it would appear that serious consideration should be given to incorporating stress as a standard background feature in preclinical stroke models that are concerned with evaluating the potential effectiveness of interventions designed to enhance recovery from stroke.
Stress, what are we really talking about?
Mammals, like most other species, have evolved to deal with a raft of unexpected environmental challenges, with some being more mundane and others life threatening. Hans Selye18 was the first to explicitly equate the word ‘stress' with the idea that the body engages in a nonselective response to any serious challenge. Selye18 further proposed that this response served to reorientate almost all of the animals' cognitive and physiologic systems to deal with the impending challenge.19 This theoretical foundation has now been elaborated to differentiate between the event that causes a response in the body, and the body's actual response to it. Specifically, it was accepted that a stressor is any stimulus (real or imagined) that substantially threatens the homeostatic balance that exists within the body, whereas by extension, the stress response (often just shortened to stress) is the reaction of the body aimed at reestablishing homeostatic balance.19, 20 Over recent decades, the conceptualization of stress has been somewhat elaborated, particularly in regard to what the defining features of events are that provoke a stress response. There is now a wide consensus that events be considered stressors only if they are perceived as threatening, and are to some degree uncontrollable and/or unpredictable in nature.21, 22 The magnitude of the stress response is determined by the intensity of these three combined factors and the biologic history of the organism.22
Although the definition of a stressor as an event that is perceived to be threatening, unpredictable and/or uncontrollable is useful theoretically, it is also helpful to place this definition within the context of life events. In this regard, for humans, actual or imminent unemployment, poverty, medical illness, and in particular, loss of function due to illness, injury, disability, or discord in intimate personal relationships, are all frequently experienced stressors.23
In the context of stroke recovery, there are numerous stressors present for most patients even if they are provided with the highest standard of rehabilitative care. For instance, patients frequently experience difficulties with movement, communication, and cognition.15 Owing to these limitations, which are extremely rapid in their onset poststroke, a recovering patient often has difficulty in performing what previously were routine tasks, such as navigating within the home environment, preparing food, and executing other basic household activities.24 Further, routine tasks performed outside the home can often, at least initially, require third party assistance, and returning to work in the short or even the medium term is often not feasible. Collectively, each of these stressors can culminate in a significant reduction in the quality of life for affected patients, and give rise to a range of negative emotional states including anxiety, fear, worry, and anger.15, 24 In clinical research, these negative emotional states are often captured under the umbrella term of distress.25
The presence of stress during recovery from stroke in clinical trials
Numerous studies have consistently reported the presence of high levels of distress in patients recovering from stroke.15, 17 Hilari et al16 found that approximately two-thirds of all stroke patients report high levels of psychological distress early after stroke, and further, that these levels show little reduction across the following 6 months. Apart from a reduction in the patient's perceived quality of life, the presence of high levels of distress is also associated with poorer physical functional outcomes.26, 27, 28, 29, 30, 31 For instance, West et al32 found that persistent psychological distress in the first 6 months after stroke predicted a lower Barthel index score, a measurement of competence in performance of activities of daily living, at 1 year.
High levels of stress have also consistently been associated with the emergence of mood-related psychopathology after stroke, especially depression (see Table 1 for further details). It is important to recognize, however, that at the present time, it is not known whether the relationship between distress and mood-related psychopathology in the context of stroke is causal. What appears to be certain is that the emergence of mood-related psychopathology is not only linked to the presence of stress but also to a confluence of other important mediating factors including the following: the individual's coping skills;33, 34 personality;33, 35 social support;33, 34 and socioeconomic status.36, 37 Complicating the relationship between stress and mood is a now substantial body of evidence indicating that mood alterations after stroke can be caused by functional destruction or serious disturbance of serotonergic and noradrenergic fiber pathways.38 Further, several recent studies have indicated that disturbances in inflammatory signaling, that may occur as a result of stroke, can elicit significant changes in mood state39 in a manner that is apparently independent of any of the aforementioned psychosocial factors. In summary, although the interaction between stress and poorer outcomes poststroke appears to be remarkably consistent, it remains to be specifically determined how stress-signaling pathways interact with other physiologic and psychological alterations induced by stroke to alter mood state.
Table 1. Studies analyzing the relationship between distress and functional outcome in stroke recovery in humans.
| Author | Year | No. of Participants | Setting | Baseline (time poststroke) | Follow-up (time poststroke) | Distress measure | Outcome measure | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Herrmann et al26 | 1998 | 436 (baseline) 150 (3 months) 136 (12 months) | Regional stroke centre, Toronto, Canada | On admission to hospital | 3 and 12 months | MADRS, SDS | Functional Independence Measure, Oxford Handicap Scale. | Depression symptoms correlated with functional outcome at 3 and 12 months |
| House et al27 | 2001 | 448 | Hospital patients, UK | 1 month | 12 and 24 months | Present state examination, GHQ-28 | BI, Frenchay Activities Index, MMSE | Mood symptoms were associated with 12- and 24-month mortality |
| Kotila et al28 | 2003 | 311 | Population-based stroke register, Finland | 3 months | 12 months | BDI | RS, BI | Depression at 3 months predicted poorer functional outcome at 12 months, depressed patients at 3 months were more likely to be in institutional care between 3 and 12 months later |
| Morris et al29 | 1992 | 49 | NA | 2 months | 14 months | NA | BI | Clinical depression was associated with impaired recovery of functional status and cognitive performance at 14 months |
| Pohjasvaara et al30 | 2001 | 390 | Helsinki Stroke Ageing Memory (SAM) Study cohort | 3 months | 15 months | BDI (n=390), DSM III-R (n=256)a | RS, BI | Depression at 3 months correlated with poorer functional outcome at 15 months |
| Van de Weg et al31 | 1999 | 85 | 3 rehabilitation centers, Amsterdam | 3–6 weeks | 6 months | GDS, DSM III-R | FIM, RAP | Stroke patients with depression had significantly lower functional scores at both time points |
| West et al32 | 2010 | 544 | National Health Service Hospital Trusts, West Yorkshire, UK | 2–6 weeks | 9, 13, 26, & 52 weeks | GHQ-28 | BI | Strong correlation between persistent psychological symptoms in first 6 months and poorer physical functioning outcomes |
BDI, Beck's depression inventory; BI, Barthel Index; DSM III-R, American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders revised third edition; FIM, Functional Independence Measure; GDS, Geriatric Depression Scale; GHQ-28, General Health Questionnaire, MADRS, Montgomery Asberg Depression Rating Scale; MMSE, Mini-Mental State Examination; RAP, Rehabilitation Activities Profile; RS, Rankin Scale; SDS, Zung Self-Rating Depression Scale.
Functional Independence Measure and handicap were assessed by the Oxford Handicap Scale.
Two hundred and fifty-six of the initial 390 participants completed a comprehensive psychiatric evaluation including DSM III-R.
The consequences of stress in the recovery phase after stroke in animal models
A search of the Medline, Cochrane and Psychinfo databases uncovered just nine relevant studies that have examined the effects of stress on stroke recovery in an animal model (see Table 2).40, 41, 42, 43, 44, 45, 46, 47, 48 Although there was little consistency in the absolute time that the animals were exposed to stressful situations, the majority of studies employed a restraint stress paradigm for either 20 minutes daily after induction of stroke or 60 minutes daily commencing seven days before stroke and continuing up to 3 weeks after. Despite this, the majority of studies reported that exposure to persistently stressful situations resulted in significant impairment of recovery; effects that included increased infarct size, and increased motor impairments (see Table 2).42, 45, 46, 47, 48 A representative study by Kirkland et al44 demonstrated that chronic restraint stress in rats reduced motor recovery and increased infarct size after focal motor cortex lesion. Similarly, Jin et al43 demonstrated that rats subjected to restraint stress after a photothrombotic stroke in the sensorimotor cortex displayed increased infarct volume and apoptotic cell death in addition to reduced functional recovery compared with rats that underwent the stroke procedure but were not subjected to restraint stress.
Table 2. Studies evaluating the effect of stress on stroke recovery in animals.
| Author/Year | Species | No. of animals | Stroke model | Stressor | When initiated | Frequency | Estimated Cohen's da for stroke versus stroke+stress | Duration (minutes) | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Faraji et al40 | Rat | 27 | Endothelin-1 (ET-1) induced mini stroke- hippocampus | Restraintb | 4-5 days poststroke | Daily for 15 days | Memory test d=1.73 | 60 | Stress and corticosterone reduced the severity of memory impairment and anatomic pathology produced by hippocampal mini stroke. |
| Faraji et al41 | Rat | 50 | ET-1 induced mini stroke in the hippocampus | Restraint | 4-5 days poststroke | Daily for 21 days | d=Insufficient details provided | 60 | Repeated restraint stress enhanced spatial cognition recovery in rats after focal stroke in the hippocampus. |
| Faraji et al42 | Rat | 22 | ET-1 induced mini stroke in the hippocampus | Restraint | 2-3 days post stroke | Daily for 21 days | Reaching d=4.30 | 60 | Focal stroke in the ventrolateral striatum, and restraint stress acted synergistically to impair motor but not spatial performance in rats. |
| Jin et al43 | Rat | 60 | Photothrombotic ischemic injury in the sensorimotor cortex | Restraint | 1 day poststroke | Daily for 5 days | Beam walking d=3.31 | 120 | Stress after stroke led to increased infarct volume, increased apoptotic cell death, and poorer functional recovery. |
| Kirkland et al44 | Rat | 78 | Devascularization lesion of the motor cortex | Restraint | Prestress group-15 days prestroke, Post stress gp- 1 day poststroke | Daily for 15 days | Reaching d=1.87 | 20 | Chronic restraint stress before or after stroke, impaired motor recovery and compensation, poststroke stress increased infarct size and edema in rats. |
| Kirkland et al45 | Rat | 32 | Devascularization lesion of the motor cortex | Restraint | 1 day poststroke | Daily for 15 days | Reaching d=1.59 | 20 | Chronic stress and corticosterone treatment impaired motor skill recovery and promoted compensatory movement strategies in a task-specific manner. |
| Merrett et al46 | Rat | 29 | Devascularization lesion of the motor cortex | Restraint | 7 days prestroke | Daily for 28 days | Reaching d=1.29 | 20 | Stress reduced motor function recovery after stroke in rats, with aged rats displaying greater functional impairment. |
| Wang et al89 | Rat | 30 | Middle cerebral artery occlusion (MCAO) | Chronic mild stress (CMS)- food and water deprivation, 45° cage tilt, overnight illumination, soiled cage, swimming in 4°C water, foot shock, tail pinch, paired caging. | Poststroke time not specified | Day and night for 18 days | Cell survival in dentate gyrus d=11.38 | Variable | CMS induced depressive behaviors in rats subjected to stroke and this was accompanied by reduced neurogenesis and altered neurogenic fate by increasing the differentiation of neural progenitor cells to glial lineage cells. |
| Zucchi et al48 | Rat | 59 | Devascularization lesion of the motor cortex | Restraint/variable (restraint, overcrowding, forced swimming, foot shock, saline injection, shaking, fox odor, social isolation). | 7 days pre stroke | Daily for 21 days | Reaching d=0.62 | 20 | Predictable restraint stress led to more pronounced motor impairments than unpredictable variable stress in skilled motor tasks before and after stroke. |
| Zucchi et al47 | Rat | 22 | Devascularization lesion of the motor cortex | Restraint | 7 days pre stroke | Daily for 28 days | Reaching d=1.10 | 20 | Stress induced greater impairment and diminished recovery of motor function, which was related to increased glucocorticoid receptor activation in a rat model. |
Where data (means and s.d.) necessary for the calculation of Cohen's d were not explicitly reported in text, they were estimated from the figures provided in the manuscript.
Restraint involved animals being placed in a transparent Plexiglass tube (5 to 7.5 cm diameter).
Significance of stress in preclinical experimental models of stroke
Perhaps the single greatest frustration faced by those working toward developing improved rehabilitative approaches has been the relatively low rates of successful research translation. This issue relates most specifically to pharmacological interventions that have demonstrated therapeutic potential in the acute phase poststroke during preclinical animal-based evaluation but have faulted during translation.49, 50, 51, 52, 53, 54, 55, 56 The fact that so many pharmacological interventions have proceeded down this same pathway has resulted in widespread reevaluation of the general approach taken to preclinical modeling. Indeed, there is now a consensus that animal modeling of stroke, and of recovery from stroke needs to be altered to take into account the heterogeneity of human stroke, the variation between human and animal brain structures and functions, and differences in the therapeutic time window between animals and humans, to optimize its ability to preclinically assess interventions.57, 58, 59, 60, 61 More recent theoretical approaches have highlighted the need to consider factors such as age and gender55 as well as issues related to other clinical conditions that may be present in human stroke patients such as hypertension, diabetes, heart disease,62 and use of concomitant medications,63 in animal stroke models. The Stroke Therapy Academic Industry Roundtable (STAIR) recommendations, developed in 1999 in response to the failure of translation of preclinical findings and updated in 2009, provide guidelines developed to improve the quality of preclinical studies and provide standards for the development of neurorestorative interventions that would ensure that demonstrated preclinical benefits would not be lost in translation to the clinical setting.63 In addition to improvements to animal-based modeling, these guidelines also include recommendations to ensure good scientific inquiry such as randomization and masked assessment of outcome, and the inclusion of biomarker endpoints common to both the animal model and human patients, to assess modification of the therapeutic target.63 Clearly, many of the proposed modifications have the potential to vastly improve the precision with which the clinical condition is modeled, and should enhance the generalizability of findings. One issue, however, with these current efforts to improve stroke models, is that they have not given extensive consideration to the importance that stress has on recovery.
Explicitly recognizing the fact that most individuals affected by stroke experience significant levels of stress throughout the recovery process is critical, as almost all interventions designed to enhance recovery are being evaluated against a background of stress. In stark contrast, we could find no animal-based studies that were focused on evaluating the efficacy of neurorestorative interventions in stroke models that did so against a background of stress. However, a recent study evaluating the effect of chronic mild stress on the effectiveness of Copaxone in mice subjected to a crush injury to the optic nerve, found that stress eliminated the neuroprotective effect of Copaxone observed in non-stressed mice.64 Owing to the negative effects of stress on recovery, it seems probable that evaluating neurorestorative interventions in its absence is likely to result in significant overestimation of treatment efficacy.
Statistical implications of ‘building in' stress to animal models of stroke recovery
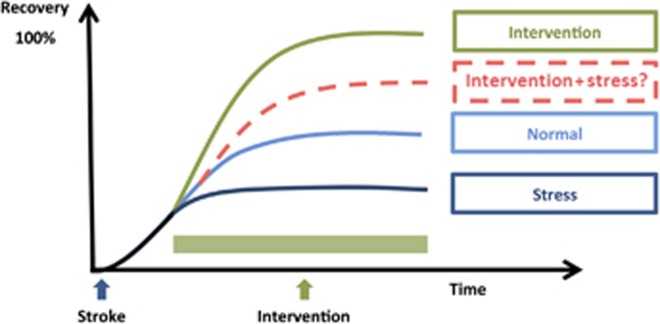
Consideration of the typical effect sizes for deleterious effects of stress gives further indication of the potential problems created by not factoring stress into standard models of recovery. For instance Jin et al43, in a representative study, demonstrated that stress significantly increased infarct volume, with an estimated effect size d=3.67 and impaired motor performance, with an estimated effect size of d=3.31. In comparison, the effect size for a number of neuroprotective strategies is found to be considerably more modest. For instance, Sphingosine-1-phosphate receptor agonist FTY720 (Fingolimod) has been documented to decrease infract volume with an effect size of d=1.3.65 Unfortunately, there is not a sufficiently consistent literature investigating the effects of stress on stroke recovery to undertake a systematic meta-analysis of effect sizes. Nevertheless, on the basis of what has already been shown we could reasonably speculate that many, if not all, of the preclinical interventions so far investigated, if re-evaluated in the presence of ongoing stress, would exhibit considerably more modest effects on recovery (see Figure 1 for a schematic representation of this concept).
Figure 1.
Schematic representation of the potential impact of stress on stroke recovery interventions. The figure illustrates four recovery trajectories: ‘normal' recovery in which animals receive neither exposure to stress nor any neurorestorative intervention; ‘intervention' in which the animal receives only a neurorestorative intervention; ‘stress' in which the animal is exposed to ongoing stress during the recovery period; and ‘intervention+stress' in which the neurorestorative intervention is conducted against a background of ongoing stress. The trajectory for the ‘normal' group is based on numerous reports on the recovery of motor function in rodents.85, 86 Typically, motor performance is significantly degraded by stroke and shows marked improvement across the first 7 to 14 days prior to achieving a relatively stable level, which is substantially below the animals' prestroke level of functioning. The trajectory for the ‘intervention' group is illustrative of the level of improvement that has been documented in animals treated with drugs such as γ-aminobutyric acid antagonists;87 Sphingosine-1-phosphate (S1P) receptor agonists;65 and environmental enrichment.88 The trajectory for the stress group is indicative of the average level of motor impairment described in Refs 42,43,44,45,46,47,48,89. The ‘intervention+stress' group is the only group for which empirical data have not been published. We propose, however, given the broadly negative impact of stress on recovery processes that it is likely to substantially reduce the effectiveness of standard neurorestorative interventions.
Recovery processes that are potentially sensitive to the effects of stress
Although there are numerous studies indicating that infrequent, brief exposures to mildly stressful situations can produce highly enhancing effects on learning,66, 67 memory,67, 68, 69 executive function,70, 71 and immunocompetence,72, 73, 74 exposure to chronic stress exerts almost uniformly negative effects on recovery. This is relatively unsurprising given that chronic stress has been shown to impair a vast number of processes critical to tissue regeneration. Specifically, it has been shown that stress, principally through the actions of cortisol and norepinephrine, impairs angiogenesis; neurogenesis; cytokine expression; immune cell maturation; immune cell trafficking; chemoattractant production; glucose metabolism; neurotransmission; and apoptosis. Each of these impacts has been extensively described in a variety of excellent reviews.75, 76, 77, 78, 79, 80, 81, 82
Recent research has indicated that the ability of chronic stress to alter tissue regeneration within the central nervous system after stroke may extend far beyond many of the traditionally recognized areas of interference. For instance, in a recent in vivo investigation of the effects of chronic stress on astrocytes, Tynan et al83 identified that exposure to chronic stress appeared to induce profound structural atrophy of a key cytoskeletal protein within astrocytes known as glial fibrillary acid protein. This finding is of particular interest, as it has previously been shown that genetic disruption of astrocyte-specific cytoskeletal proteins (glial fibrillary acid protein and vimentin) is associated with significantly larger infarct volumes. The full extent of how stress alters glial function, and how these changes could impact upon recovery processes is yet to be fully explored; research findings in this area, however, are rapidly accumulating.84
How much stress is already inherent in our animal models of recovery?
Although the theoretical case for why ongoing stress exposure should be included as a standard feature of animal models is relatively clear, some additional information must first be acquired before any practical adjustments are made. Foremost amongst this information is the extent to which stress is already a part of existing animal models. Certainly, it is the case that stress is not being explicitly built-in to preclinical experimental models of stroke recovery interested in evaluating neurorestorative approaches. This does not mean, however, that the animals are not experiencing some level of stress. Perhaps, the most direct way to obtain this information is by measuring standard indices considered to be influenced by stress, such as the levels of circulating corticosterone, corticosterone-binding globulin, glucocorticoid receptor expression and/or occupancy, adrenal weight, thymus weight, and body weight. Alternatively, with an appropriate experimental design, stress-linked behaviors could also be used to assess levels of stress experienced by the recovering animal. Explicitly quantifying the level of stress present in the recovering animal (and there may be no increase under normal circumstances) will provide clarity about the extent to which ongoing stress exposure needs to be built into existing experimental models so that they more accurately reflect what is seen clinically.
Conclusion
Within the field, there is now widespread recognition of the fact that existing models of stroke recovery can be improved to more accurately reflect the diverse experiences that are normal to human recovery. The benefits of improving existing models are numerous, but most importantly this endeavor has the potential to allow for higher levels of confidence to be placed in those interventions identified as extremely promising. This in turn creates a stronger platform from which more extensive academic engagement and higher levels of commercial investment can be obtained. Alterations already made to our existing models have steadily improved their realism and accuracy. Clearly, however, there is still room for improvement. Although there are many points of difference between the recovery environments of animals and humans, we suggest that one of the most obvious next steps to improve translational success is to ‘build-in' exposure to ongoing stress to directly mimic the clinical reality of the recovery process. Indeed, assuming that stress is low in current models, an argument could be made to add ongoing stress exposure by default to both conventional intervention and control groups, and not include conventional unhandled home cage controls at all. In moving down this pathway, a further practical issue to consider is the likely negative impact that stress will have on therapeutic effect sizes. Accordingly, if this approach is adopted, it is likely that studies evaluating a potential therapeutic against a background of stress, will need to substantially increase sample size to retain the ability to identify differences if they do indeed exist. Undertaking these modifications certainly has the potential to make the execution of recovery models, which are already time consuming, even more challenging, and expensive. In doing so, however, animal models of stroke recovery could take another substantial step toward realistically capturing the processes that determine the trajectory of human recovery from stroke.
Acknowledgments
FRW acknowledges project grant support from the Australian National Health and Medical Research Council. MN acknowledges the support from the University of Newcastle's Brawn Fellowship, the Hunter Medical Research Institute (HMRI), and the Stan. A. Olsson Foundation for Science and Culture.
The authors declare no conflict of interest.
References
- Begg SJ, Vos T, Barker B, Stanley L, Lopez AD. Burden of disease and injury in Australia in the new millennium: measuring health loss from diseases, injuries and risk factors. Med J Aust. 2008;188:36–40. doi: 10.5694/j.1326-5377.2008.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Cadilhac DA, Carter R, Thrift AG, Dewey HM. Estimating the long-term costs of ischemic and hemorrhagic stroke for Australia: new evidence derived from the North East Melbourne Stroke Incidence Study (NEMESIS) Stroke. 2009;40:915–921. doi: 10.1161/STROKEAHA.108.526905. [DOI] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener H, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathologica. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989-1990. Stroke. 2002;33:1034–1040. doi: 10.1161/01.str.0000012515.66889.24. [DOI] [PubMed] [Google Scholar]
- Henderson KM, Clark CJ, Lewis TT, Aggarwal NT, Beck T, Guo H, et al. Psychosocial distress and stroke risk in older adults. Stroke. 2013;44:367–372. doi: 10.1161/STROKEAHA.112.679159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen P, Rosengren A, Tsipogianni A, Wilhelmsen L. Risk factors for stroke in middle-aged men in Göteborg, Sweden. Stroke. 1990;21:223–229. doi: 10.1161/01.str.21.2.223. [DOI] [PubMed] [Google Scholar]
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Surtees P, Wainwright N, Luben R, Wareham N, Bingham S, Khaw K-T. Psychological distress, major depressive disorder, and risk of stroke. Neurology. 2008;70:788–794. doi: 10.1212/01.wnl.0000304109.18563.81. [DOI] [PubMed] [Google Scholar]
- Caso J, Lizasoain I, Lorenzo P, Moro M, Leza J. The role of tumor necrosis factor-alpha in stress-induced worsening of cerebral ischemia in rats. Neuroscience. 2006;142:59–69. doi: 10.1016/j.neuroscience.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Caso JR, Moro MA, Lorenzo P, Lizasoain I, Leza JC. Involvement of IL-1β in acute stress-induced worsening of cerebral ischaemia in rats. Eur Neuropsychopharmacol. 2007;17:600–607. doi: 10.1016/j.euroneuro.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Leza JC, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in subacute stress-induced neuroinflammation and in the worsening of experimental stroke. Stroke. 2008;39:1314–1320. doi: 10.1161/STROKEAHA.107.498212. [DOI] [PubMed] [Google Scholar]
- Guiraud V, Amor MB, Mas J-L, Touzé E. Triggers of ischemic stroke: a systematic review. Stroke. 2010;41:2669–2677. doi: 10.1161/STROKEAHA.110.597443. [DOI] [PubMed] [Google Scholar]
- Madrigal J, Caso J, De Cristobal J, Cardenas A, Leza J, Lizasoain I, et al. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 2003;979:137–145. doi: 10.1016/s0006-8993(03)02892-0. [DOI] [PubMed] [Google Scholar]
- Brunborg B. Stressful situations the first six months after a stroke. Illness, Crisis, Loss. 2009;17:39–50. [Google Scholar]
- Hilari K, Northcott S, Roy P, Marshall J, Wiggins RD, Chataway J, et al. Psychological distress after stroke and aphasia: the first six months. Clin Rehab. 2010;24:181–190. doi: 10.1177/0269215509346090. [DOI] [PubMed] [Google Scholar]
- Lyon BL. Psychological stress and coping: framework for poststroke psychosocial care. Top Stroke Rehabil. 2002;9:1–15. doi: 10.1310/YA8Q-EQK9-00EF-EUG7. [DOI] [PubMed] [Google Scholar]
- Selye H. McGraw Hill: New York, USA; 1956. The stress of life. [Google Scholar]
- Day TA. Defining stress as a prelude to mapping its neurocircuitry: no help from allostasis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1195–1200. doi: 10.1016/j.pnpbp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Walker F, Nalivaiko E, Day TA.Stress an inflammation: an emerging storyIn: Garg ML, Vood LG (eds). Nutrition & Physical Activity in Inflammatory Diseases Cab International; 2013 [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, et al. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 2011;35:1291–1301. doi: 10.1016/j.neubiorev.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Day TA, Walker FR. More appraisal please: a commentary on Pfaff et al.(2007) “Relations between mechanisms of CNS arousal and mechanisms of stress”. Stress. 2007;10:311–313. doi: 10.1080/10253890701638204. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- de Haan RJ, Limburg M, Van der Meulen JHP, Jacobs HM, Aaronson NK. Quality of life after stroke: impact of stroke type and lesion location. Stroke. 1995;26:402–408. doi: 10.1161/01.str.26.3.402. [DOI] [PubMed] [Google Scholar]
- Ridner SH. Psychological distress: concept analysis. J Adv Nurs. 2004;45:536–545. doi: 10.1046/j.1365-2648.2003.02938.x. [DOI] [PubMed] [Google Scholar]
- Herrmann N, Black S, Lawrence J, Szekely C, Szalai J. The Sunnybrook Stroke Study: a prospective study of depressive symptoms and functional outcome. Stroke. 1998;29:618–624. doi: 10.1161/01.str.29.3.618. [DOI] [PubMed] [Google Scholar]
- House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32:696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- Kotila M, Numminen H, Waltimo O, Kaste M. Post-stroke depression and functional recovery in a population-based stroke register. The Finnstroke study. Eur J Neurol. 2003;6:309–312. doi: 10.1046/j.1468-1331.1999.630309.x. [DOI] [PubMed] [Google Scholar]
- Morris P, Raphael B, Robinson RG. Clinical depression is associated with impaired recovery from stroke. Med J Aust. 1992;157:239. doi: 10.5694/j.1326-5377.1992.tb137126.x. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Vataja R, Leppävuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome poststroke. Eur J Neurol. 2001;8:315–319. doi: 10.1046/j.1468-1331.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- Van de Weg F, Kuik D, Lankhorst G. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehab. 1999;13:268–272. doi: 10.1191/026921599672495022. [DOI] [PubMed] [Google Scholar]
- West R, Hill K, Hewison J, Knapp P, House A. Psychological disorders after stroke are an important influence on functional outcomes a prospective cohort study. Stroke. 2010;41:1723–1727. doi: 10.1161/STROKEAHA.110.583351. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH. Personality, coping, and family resources in stress resistance: a longitudinal analysis. J Pers Soc Psychol. 1986;51:389. doi: 10.1037//0022-3514.51.2.389. [DOI] [PubMed] [Google Scholar]
- King RB, Shade-Zeldow Y, Carlson CE, Feldman JL, Philip M. Adaptation to stroke: a longitudinal study of depressive symptoms, physical health, and coping process. Top Stroke Rehabil. 2002;9:46–66. doi: 10.1310/KDTA-WELC-T2WR-X51W. [DOI] [PubMed] [Google Scholar]
- Aben I, Denollet J, Lousberg R, Verhey F, Wojciechowski F, Honig A. Personality and vulnerability to depression in stroke patients: a 1-year prospective follow-up study. Stroke. 2002;33:2391–2395. doi: 10.1161/01.str.0000029826.41672.2e. [DOI] [PubMed] [Google Scholar]
- Koster A, Bosma H, Kempen GI, Penninx BW, Beekman AT, Deeg DJ, et al. Socioeconomic differences in incident depression in older adults: the role of psychosocial factors, physical health status, and behavioral factors. J Psychosom Res. 2006;61:619–627. doi: 10.1016/j.jpsychores.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Lazzarino AI, Hamer M, Stamatakis E, Steptoe A. Low socioeconomic status and psychological distress as synergistic predictors of mortality from stroke and coronary heart disease. Psychosom Med. 2013;75:311–316. doi: 10.1097/PSY.0b013e3182898e6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R, Bloom F. Pharmacological treatment following experimental cerebral infarction: implications for understanding psychological symptoms of human stroke. Biol Psychiatry. 1977;12:669. [PubMed] [Google Scholar]
- Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11:984–991. doi: 10.1038/sj.mp.4001879. [DOI] [PubMed] [Google Scholar]
- Faraji J, Lehmann H, Metz GA, Sutherland RJ. Stress and corticosterone enhance cognitive recovery from hippocampal stroke in rats. Neurosci Lett. 2009;462:248–252. doi: 10.1016/j.neulet.2009.06.096. [DOI] [PubMed] [Google Scholar]
- Faraji J, Metz GA, Sutherland RJ. Stress after hippocampal stroke enhances spatial performance in rats. Physiol Behav. 2011;102:389–399. doi: 10.1016/j.physbeh.2010.11.032. [DOI] [PubMed] [Google Scholar]
- Faraji J, Sutherland RJ, Metz GA. Stress precipitates functional deficits following striatal silent stroke: a synergistic effect. Exp Neurol. 2011;232:251–260. doi: 10.1016/j.expneurol.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Jin Z, Wu J, Oh S-Y, Kim K-W, Shin B-S. The effect of stress on stroke recovery in a photothrombotic stroke animal model. Brain Res. 2010;1363:191–197. doi: 10.1016/j.brainres.2010.09.081. [DOI] [PubMed] [Google Scholar]
- Kirkland SW, Coma AK, Colwell KL, Metz GA. Delayed recovery and exaggerated infarct size by post-lesion stress in a rat model of focal cerebral stroke. Brain Res. 2008;1201:151–160. doi: 10.1016/j.brainres.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland SW, Smith LK, Metz GA. Task-specific compensation and recovery following focal motor cortex lesion in stressed rats. J Integr Neurosci. 2012;11:33–59. doi: 10.1142/S0219635212500033. [DOI] [PubMed] [Google Scholar]
- Merrett DL, Kirkland SW, Metz GA. Synergistic effects of age and stress in a rodent model of stroke. Behav Brain Res. 2010;214:55–59. doi: 10.1016/j.bbr.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi FC, Matthies N-F, Badr N, Metz GA. Stress-induced glucocorticoid receptor activation determines functional recovery following ischemic stroke. Exp Transl Stroke Med. 2010;2:18. doi: 10.1186/2040-7378-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi FCR, Kirkland SW, Jadavji NM, van Waes LT, Klein A, Supina RD, et al. Predictable stress versus unpredictable stress: a comparison in a rodent model of stroke. Behav Brain Res. 2009;205:67–75. doi: 10.1016/j.bbr.2009.06.030. [DOI] [PubMed] [Google Scholar]
- Green A. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2009;153:S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RC, Lucke-Wold B, Lucke-Wold N, Elliott AS, Logsdon AF, Rosen CL, et al. Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. Int J Mol Sciences. 2013;14:1890–1917. doi: 10.3390/ijms14011890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DJ, Tymianski M. Translating promising preclinical neuroprotective therapies to human stroke trials. Expert Rev Cardiovasc Ther. 2011;9:433–449. doi: 10.1586/erc.11.34. [DOI] [PubMed] [Google Scholar]
- Kahle MP, Bix GJ. Successfully climbing the “STAIRs”: surmounting failed translation of experimental ischemic stroke treatments. Stroke Res Treat. 2012;2012:374098. doi: 10.1155/2012/374098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RC, Dodson SC, Rosen CL, Huber JD. The science of cerebral ischemia and the quest for neuroprotection: navigating past failure to future success. J Neurosurg. 2013;118:1072–1085. doi: 10.3171/2012.11.JNS12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Sena E, Goehler J, Horn J, van der Worp B, Bath PMW, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39:929–934. doi: 10.1161/STROKEAHA.107.498725. [DOI] [PubMed] [Google Scholar]
- Braeuninger S, Kleinschnitz C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Transl Stroke Med. 2009;1:8. doi: 10.1186/2040-7378-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone DJ, Black SE, Hakim AM. Toward wisdom from failure lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33:2123–2136. doi: 10.1161/01.str.0000025518.34157.51. [DOI] [PubMed] [Google Scholar]
- Fisher M, Finklestein S, Furlan AJ, Goldstein LB, Gorelick PB, Kaste M, et al. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: current status and future perspectives. Int J Mol Sci. 2012;13:11753–11772. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod MR, O'Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35:1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG, Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies – the need for new approaches. Cerebrovasc Dis. 2004;17 (suppl 1:153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- Crossley NA, Sena E, Goehler J, Horn J, Van Der Worp B, Bath PM, et al. Empirical evidence of bias in the design of experimental stroke studies: a metaepidemiologic approach. Stroke. 2008;39:929–934. doi: 10.1161/STROKEAHA.107.498725. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov I, Walsh JT, Kipnis J. Chronic mild stress eliminates the neuroprotective effect of Copaxone after CNS injury. Brain, Behav Immun. 2013;31:177–182. doi: 10.1016/j.bbi.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang C, Tao W, Liu M. Systematic review and meta-analysis of the efficacy of Sphingosine-1-phosphate (S1P) receptor agonist FTY720 (Fingolimod) in animal models of stroke. Int J Neurosci. 2013;123:163–169. doi: 10.3109/00207454.2012.749255. [DOI] [PubMed] [Google Scholar]
- Smeets T, Wolf OT, Giesbrecht T, Sijstermans K, Telgen S, Joëls M. Stress selectively and lastingly promotes learning of context-related high arousing information. Psychoneuroendocrinology. 2009;34:1152–1161. doi: 10.1016/j.psyneuen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin N, Nair N. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27:401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work. Trends Cogn Sci. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Memory. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger D, Peters Razza R. Cortisol reactivity is positively related to executive function in preschool children attending head start. Child Dev. 2005;76:554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, Gunnar MR. The anterior attention network: associations with temperament and neuroendocrine activity in 6-year-old children. Dev Psychobiol. 2002;40:43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain, Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Acute stress enhances while chronic stress suppresses skin immunity: the role of stress hormones and leukocyte trafficking. Ann NY Acad Sci. 2000;917:876–893. doi: 10.1111/j.1749-6632.2000.tb05454.x. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Acute stress decreases inflammation at the site of infection: a role for nitric oxide. Physiol Behav. 2002;77:291–299. doi: 10.1016/s0031-9384(02)00861-2. [DOI] [PubMed] [Google Scholar]
- Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity*. Annu Rev Immunol. 2002;20:125–163. doi: 10.1146/annurev.immunol.20.082401.104914. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- McCowen KC, Malhotra A, Bistrian BR. Stress-Induced Hyperglycemia. Critical Care Clinics. 2001;17:107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Tajima K, Sasson R. Cell-specific regulation of apoptosis by glucocorticoids: implication to their anti-inflammatory action. Biochem Pharmacol. 2002;64:843–850. doi: 10.1016/s0006-2952(02)01147-4. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand J, Hellsten J, Tingström A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neurosci Lett. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Beynon SB, Hinwood M, Johnson SJ, Nilsson M, Woods JJ, et al. Chronic stress-induced disruption of the astrocyte network is driven by structural atrophy and not loss of astrocytes. Acta Neuropathol. 2013;126:75–91. doi: 10.1007/s00401-013-1102-0. [DOI] [PubMed] [Google Scholar]
- Walker F, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14:1262–1276. doi: 10.2174/13894501113149990208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer SC. Repairing the human brain after stroke: i. mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Mattsson B, Johansson BB, Eriksson PS. Enriched environment increases neural stem/progenitor cell proliferation and neurogenesis in the subventricular zone of stroke-lesioned adult rats. Stroke. 2005;36:1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- Wang S-h, Zhang Z-j, Guo Y-j, Teng G-j, Chen B-a. Hippocampal neurogenesis and behavioural studies on adult ischemic rat response to chronic mild stress. Behav Brain Res. 2008;189:9–16. doi: 10.1016/j.bbr.2007.11.028. [DOI] [PubMed] [Google Scholar]