Abstract
White matter sparing after traumatic brain injury (TBI) is an important predictor of survival and outcome. Blood vessels and axons are intimately associated anatomically and developmentally. Neural input is required for appropriate vascular patterning, and vascular signaling is important for neuron development and axon growth. Owing to this codependence between endothelial cells and axons during development and the contribution of endothelial progenitor cells (EPCs) in ischemic injury, we hypothesized that EPCs are important in axonal survival after TBI. We examined the effects of allogenic-cultured EPCs on white matter protection and microvascular maintenance after midline fluid percussion injury in adult Sprague–Dawley rats. We used two in vitro models of injury, mechanical stretch and oxygen–glucose deprivation (OGD), to examine the effects of EPCs on the mechanical and ischemic components of brain trauma, respectively. Our results indicate that EPCs improve the white matter integrity and decrease capillary breakdown after injury. Cultured cortical neurons exposed to OGD had less axon degeneration when treated with EPC-conditioned media, whereas no effect was seen in axons injured by mechanical stretch. The results indicate that EPCs are important for the protection of the white matter after trauma and represent a potential avenue for therapy.
Keywords: endothelial progenitor cell, fluid percussion injury, ischemia, traumatic brain injury, vasculature, white matter injury
Introduction
Treatment of traumatic brain injury (TBI) continues to be a complex clinical issue. Specifically, injury to the white matter has been shown to be a strong predictor of survival and long-term outcome after TBI.1 Hemodynamic changes after trauma resulting in compromised cerebral blood flow include vasoconstriction due to increased intracranial pressure as well as vasospasm and microthrombi.2, 3 We have demonstrated diffuse microvascular changes in the brain using a rodent model of TBI associated with expression of hypoxia-induced factor 1α.4 However, the contribution of microvascular compromise on the maintenance of axons from posttraumatic degeneration is not known.
The vascular system and the nervous system are codependent during development. Axons provide signaling cues for arterial growth whereas vasculature provides signaling cues during axon guidance. Blood vessels also provide structural support during axon growth and development while simultaneously providing oxygen transport and metabolic waste removal.5 Given the role of vascular cells in guiding axon development and in homeostasis, endothelial progenitor cells (EPCs) may also be important in mediating local angiogenesis in disease states.6, 7 Increased levels of circulating EPCs correlate to decreased neuronal cell death and decreased infarct area after stroke.8 Evidence also suggest a contribution to improved behavioral outcomes after brain injury.6, 9 Given the importance of white matter in the outcome after TBI, we sought to clarify the relationship between endothelial progenitor cell-mediated protection of axons after modeled TBI.
We used a well-established in vivo model of midline fluid percussion injury (FPI) and two in vitro models of axonal injury to evaluate the effects of EPC-mediated therapy on microvascular outcome and axonal sparing. The response of cultured cortical neurons to EPC treatment was evaluated using a stretch injury model and oxygen–glucose deprivation (OGD) to isolate the mechanical and ischemic components of TBI in vitro, respectively.
Materials and Methods
All animal procedures were performed in accordance with guidelines established by the Animal Care Committee at St Michael's Hospital in accordance with the standards set by the Canadian Council on Animal Care. Animal protocols for this study were approved by the Animal Care Committee before the commencement of experiments. The study was designed in accordance with the ARRIVE (Animal Research: Reporting and In Vivo Experiments) guidelines.
In Vivo Experiments
The midline FPI model was performed on adult male Sprague–Dawley rats (350 to 400 g) as previously described resulting in a reproducible injury to the corpus callosum.10 Rats were housed in pairs in a dedicated animal facility under a 12-hour light/dark cycle. Water and rat chow were provided ad libitum. In brief, rats were anaesthetized with 2% isoflurane in laboratory-grade air. A thermal heating blanket was used to maintain body temperature at 37°C. A 2 mm diameter extradural craniotomy was performed along the midline suture, midway between the bregma and lambda. A polypropylene tube with an inner diameter 1.5 mm was fixed to the opening with cyanoacrylate adhesive and dental acrylic, filled with 0.9% isotonic saline and attached to the FPI device. Rats were subjected to a moderate extradural FPI with the weighted pendulum set to an angle of 25° resulting in a fluid pressure pulse of 2.5 atmospheres with 15-millisecond duration. Bone wax was used to seal the hole in the skull and the scalp was sutured before recovery in a temperature-controlled chamber. Sham rats underwent identical surgical procedures with the omission of the fluid percussion application (n=6 rats per injury group for in vivo studies). Rats were randomly assigned to receive FPI, FPI with EPC treatment, or sham surgical treatment.
Twenty-four hours after injury, rats were reanesthetized with 2% isoflurane gas. Body temperature was maintained on a heating pad. Tail vein injections consisted of either 500 μL vehicle consisting of Hanks' Balanced Salt Solution or with 4–5 × 106 EPCs in 500 μL Hanks' Balanced Salt Solution. Animals were survived for 3 days after injection. In a subset of animals (n=4 rats subject to FPI, n=3 sham surgery rats), EPCs were fluorescently labeled using PKH26, a lipophilic fluorescent cell dye, (Sigma-Aldrich, Oakville, Ontario, Canada) and were injected into the tail vein and survived up to 5 days post injury.
Immunohistochemistry
Rats were anesthetized with ketamine/xylazine and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. Immunohistochemistry for β-amyloid precursor protein expression in the corpus callosum was performed in paraffin-embedded tissues. Serial coronal brain sections were cut at a thickness of 10 μm on a microtome. Immunofluorescent detection of PKH26 and rat endothelial cell antigen-1 (RECA-1) labeling was performed in frozen coronal brain and lung sections at a thickness of 20 μm sectioned on a cryostat.
Histologic brain sections were deparaffinized, and antigen retrieval was performed by pretreatment with heated citrate buffer, pH 6.0 for 15 minutes. Primary anti-β-APP antibodies (1:200; Invitrogen, Burlington, ON, Canada), were incubated in 10% normal horse serum blocking solution overnight at 4°C. Secondary incubation was also performed in 10% normal horse serum blocking solution for 2 hours at room temperature using horse–anti-mouse Biotin (1:200; Vector, Burlington, ON, Canada). Signal detection consisted of a VIP substrate kit (Vector) used according to the manufacturer's protocol.
In frozen brain and lung sections, extracted tissues were postfixed overnight in 4% paraformaldehyde, then cryoprotected in 30% sucrose solution at 4°C for 2 days. Tissues were then flash frozen in Cryomatrix (ThermoScientific, Kalamazoo, MI, USA) and stored at −80°C. For immunofluorescent labeling, sections were pretreated for 1 hour in 0.1 mol/L phosphate-buffered saline (PBS) containing 10% normal goat serum and 0.1% Triton X. Endothelial cells in the brain and lung tissues were labeled with mouse anti-RECA-1 (Santa Cruz, Santa Cruz, CA, USA) at a dilution of 1:100 overnight at 4°C. Secondary antibody incubation using Alexa 488 goat–anti-mouse at a dilution of 1:200 (Invitrogen) was performed in 10% normal goat serum blocking solution for 1 hour at room temperature. Three washes were made in 0.1 mol/L PBS between each step. Negative controls were run simultaneously with the omission of primary antisera.
Bone Marrow-Derived Endothelial Progenitor Cell Cultures
Endothelial progenitor cells were cultured from bone marrow mononuclear cells as previously described by others.11 Briefly, the femur and tibia were removed from male Sprague–Dawley rats (∼180 g) and all surrounding connective tissue and muscle were dissected. The proximal and distal epiphyses of the femur and tibia were cut, and Hanks' Balanced Salt Solution was used to flush out the bone marrow. The cell slurry was collected and centrifuged at 400 g for 5 minutes. The pellet was resuspended in endothelial growth media-2 (Lonza, Walkersville, MD, USA) and transferred into T25 or T75 flasks. Cells were placed in an incubator maintained at 37°C with 5% CO2. After 24 hours of initial plating, the media was replaced with fresh EGM2. Adherent cells were grown for 8 to 9 days resulting in a confluent monolayer of cells. Subsequent media changes were made every 3 to 4 days. Cells were removed from the flasks by trypsinization using 0.25% trypsin–EDTA solution. Cell numbers were quantified with a hemocytometer and collected for characterization, injection, or for coculture with cortical neurons. Fluorescent PKH26 labeling of cultured EPCs was performed before cell use at this time in a subset of cultures, as per manufacturer's instructions (Sigma).
Conditioned media was collected from EPCs grown for 9 days as follows: original growth factor supplemented endothelial growth media-2 media was removed, and the cells were washed three times in PBS, then incubated for 1 hour in growth factor-depleted basal media (EBM-2; Lonza) at 37 °C. The media was then removed and fresh EBM-2 was added. This media was collected 24 hours later and used as conditioned media either fresh or from frozen stock.
Rat Fibroblast Cultures
Fibroblasts were derived from rat skin and maintained in Dulbecco's modified Eagle's medium (Invitrogen) with 10% FBS, and 1% penicillin/streptomycin. Fibroblasts were plated into 75-mm flasks and passaged every 5 to 7 days. Cells from passage 5 to 7 were used in the experiments described. Cells were trypsinized using 0.25% trypsin–EDTA. A total of 50,000 cells were added to at least three wells in each culture condition. Fibroblast-conditioned media was obtained in the same fashion as EPC-conditioned media, with the exception that FBS was omitted from Dulbecco's modified Eagle's medium.
Cortical Neuron Cultures
Cortical neurons were dissected from E17 rat embryos as previously described.12 Briefly, cortices were isolated from the brains of E17 rat embryos and grown on poly-D-lysine/laminin-coated 4-well chamber slides or 6-well silastic membrane plates. Cells were grown in neurobasal media supplemented with 2% B27, 1% streptomycin/penicillin and 0.25% L-glutamine (all Gibco, Burlington, ON, Canada). Cells were grown for 5 days before OGD, stretch injury on deformable silastic membranes, or control treatment. All treatments on cortical neurons were performed at sublethal doses. Neurons were cocultured with EPCs (50,000 EPCs per well to 180,000 cortical neurons) or treated with EPC-conditioned media (25% conditioned media:75% cortical media). Oxygen–glucose deprivation treatment consisted of 1 hour of preincubation in aglycemic extracellular solution containing 140 mmol/L NaCl, 5.4 mmol/L KCl, 1.3 mmol/L CaCl, 10 mmol/L HEPES, and 1 mmol/L MgCl13 in 20% O2, followed by 90-minute incubation at 4% O2 in deoxygenated extracellular solution. Deoxygenated extracellular solution was then replaced with cortical media and EPCs or 25% conditioned media. Stretch injury was performed using a Cell Injury Controller II (Custom Design and Fabrication, Virginia Commonwealth University, Richmond, VA, USA). A transient pulse with a duration of 50 milliseconds and pressure magnitudes between 2.5 to 3 pounds per square inch (p.s.i.) resulted in a sublethal level of neuronal injury as previously characterized.14
Immunocytochemistry And Cellular Labeling
At 8 days in vitro, cortical neuron cultures were fixed and immunolabeled as previously described.12 Briefly, cells were fixed in 4% paraformaldehyde, permeabilized in 0.2% Igepal (Sigma) and blocked for 45 minutes in 0.5% BSA and 6% normal goat serum in PBS. Primary antibodies used were mouse anti-α-tubulin (New England Biolabs, Boston, MA USA), rabbit anti-CD133 (Abcam, Cambridge, MassachusettsMA, USA) for identification of axons and EPCs, respectively. Incubation was performed overnight at 4°C at a dilution of 1:500 for anti-α-tubulin and 1:200 for anti-CD133. For EPC characterization, rabbit anti-VEGFR2 antibody (Biorbyt, San Francisco, USA) was used at a dilution of 1:200 and rabbit anti-CD34 (Abbiotec, San Diego, CA, USA) was used at a dilution of 1:200. Secondary incubation was performed with Alexa 555 goat–anti-rabbit (Invitrogen) or Alexa 488 goat–anti-mouse antibodies (Invitrogen) for 1 hour at room temperature at a dilution of 1:500. Rabbit polyclonal anti-CD133 (1:200) and mouse monoclonal anti-OX-42 (Sigma-Aldrich, 1:500) expression in EPC cultures was used to determine the relative ratio of CD133-expressing EPCs to OX-42-expressing macrophages in EPC cultures. Nuclear Hoechst counterstaining was performed at 1:3,000 for 3 minutes at room temperature. Cells were washed in three changes of PBS between incubation steps and coverslipped with Permafluor (Thermo Scientific). Negative controls were run simultaneously with the omission of primary antisera.
The acetylated LDL assay was performed as per manufacturer's instructions (Invitrogen). For the tube-formation assays, EPCs were grown for 8 days in a T25 flask, resuspended, and plated (10,000 cells per well) in Matrigel (BD Biosciences, Mississauga, ON, Canada)-coated 24-well plates. Wells were imaged every 2 hours over a 6-hour period to assess for microtubule-forming capacity.
Image Analysis and Statistical Methods
Endothelial progenitor cell cultures labeled for CD133 and OX-42 were quantified from three separate culture experiments. Three random locations were chosen on each cultured slide and captured at × 10 magnification. The total number of CD133- and OX-42-expressing cells was quantified for each sampling field. Individual cells were identified by colocalization with the Hoechst nuclear stain.
Immunohistochemical analysis of RECA-1 labeling included quantification of the diameter, length, and number of RECA-1-positive objects captured within a sampling field in the corpus callosum (Figure 3D). Sections were sampled from regions ∼3 mm rostral to the injury epicenter at bregma −1.5 mm. Two sampling fields per region from a total of n=6 rats per treatment group were captured on a × 20 objective using a Nikon Eclipse 90i microscope. Capillaries were identified as RECA-1-positive objects with diameters of less than 10 μm. The longitudinal length of capillary fragments was quantified for each sampling field and summated as a representative total summated capillary length for a given sampling field. In instances where a bifurcation was preset, a separate length measurement was made for each bifurcation.
Analysis of β-APP labeling included counting the number of stained swellings, as well as the area of each swelling found in two bilateral regions of the corpus callosum at bregma −1.5 mm. Images were captured on a Nikon Eclipse microscope on a × 20 objective and quantified using NIS Elements software. Quantitative analysis on RECA-1 and β-APP labeled sections was performed in a masked manner.
Cortical neuron data were averaged from five separate experiments. Each OGD experiment was performed in triplicate. Images captured at × 20 were used for survival counts. Live and dead cells were identified by diffuse or condensed nuclear morphology, respectively. A one-way analysis of variance was used to compare the number of live and dead cells between treatment groups followed by the Student–Newman–Keuls post hoc test where cell numbers were significantly different between treatment groups. Axon degeneration was evaluated by counting the number of axons that exhibited evidence of at least two breaks in the α-tubulin labeling along the axon. Breakdown was counted only on axons that could be traced to its axon hillock. Statistical tests for RECA-1, β-APP, and axonal degeneration quantification were performed using a one-way analysis of variance and a post hoc Student–Newman–Keuls method to examine differences owing to treatment between injury groups. Statistical significance was assumed at a value of P⩽0.05. Data are presented as mean values±s.e. measurements.
Results
Bone Marrow Mononuclear Cells Express Endothelial Progenitor Cell Markers when Cultured in Endothelial Growth Media-2
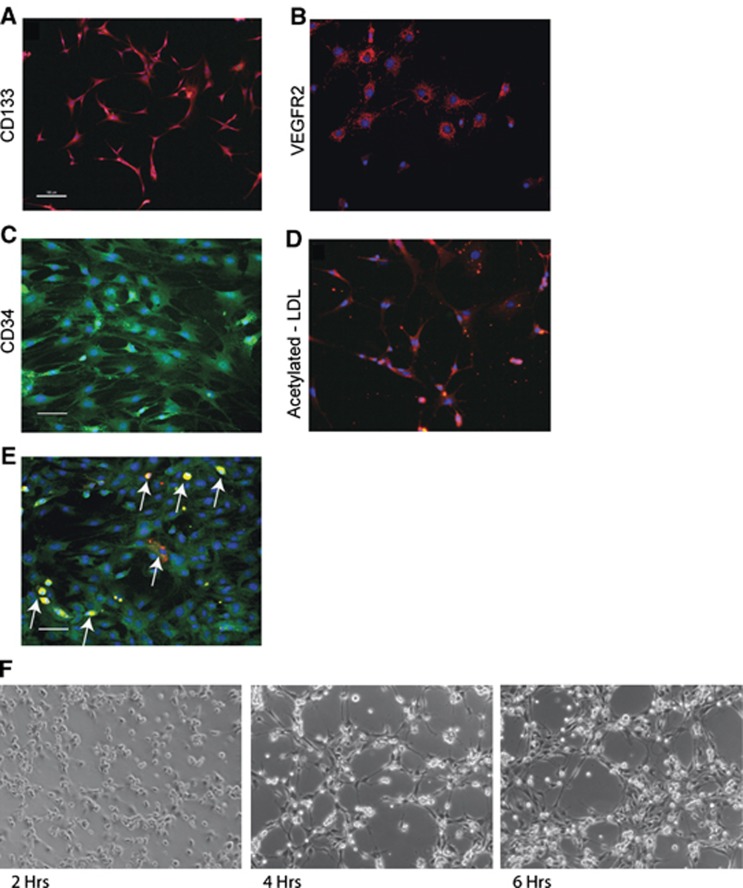
Although the precise definition of EPCs has not been conclusively established,15 we verified the cell surface expression, metabolic capacities, and functional activity of our cells and found them to be consistent with previous reports of EPCs cultured from bone marrow. We characterized the bone marrow mononuclear cells 8 days after plating in endothelial-optimized growth media. Morphology was initially spindle shaped at low-cell density and progressed to previously described cobblestone morphology upon increasing cell proliferation and higher-cell density when grown to 80% to 90% confluency. The cells were positive for cell surface markers consistent with an EPC phenotype. These included CD133 (Figure 1A), VEGFR2 (Figure 1B), and CD34 (Figure 1C).16 Metabolic activity consistent with an endothelial lineage was confirmed through uptake of acetylated LDL (Figure 1D). Double-label immunocytochemistry for OX-42 (red) and CD133 (green) expression in EPC cultures indicated 7.4±1.2% cells expressed the macrophage marker (Figure 1E). Bone marrow-derived EPC cultures formed rudimentary capillary networks in a Matrigel assay (Figure 1F) thus further confirming their functional capacity. As a control cell type for in vitro studies, we also examined the response of rat subcutaneous fibroblasts to the acetylated LDL assay and found no evidence of LDL metabolism (data not shown), which indicated some metabolic properties unique to EPCs in comparison with fibroblasts.
Figure 1.
Cells cultured from rat bone marrow express endothelial progenitor cells (EPC) markers. (A) Harvested EPCs were cultured for 8 days and were positive for CD133, (B) VEGFR2, and (C) CD34. (D) Endothelial progenitor cells were evaluated for metabolic activity and were observed to metabolize acetylated low-density lipoprotein (LDL), consistent with an endothelial cell lineage. (E) Double-label immunocytochemistry for OX-42 (red) and CD133 (green) indicated that 7.4±1.2 % were positive for the macrophage marker. Arrows indicate OX-42-positive cells. Note: OX-42-positive cells were also strongly immunofluorescent at 488 nm excitation. (F) Endothelial progenitor cells plated on a Matrigel substrate demonstrated the ability to form rudimentary capillary networks over a 6-hour observation period. (Scale bar, 50 μm).
Endothelial Progenitor Cells Decrease the Number and Size of β-Amyloid Precursor Protein Swellings in the Corpus Callosum after Midline Fluid Percussion Injury
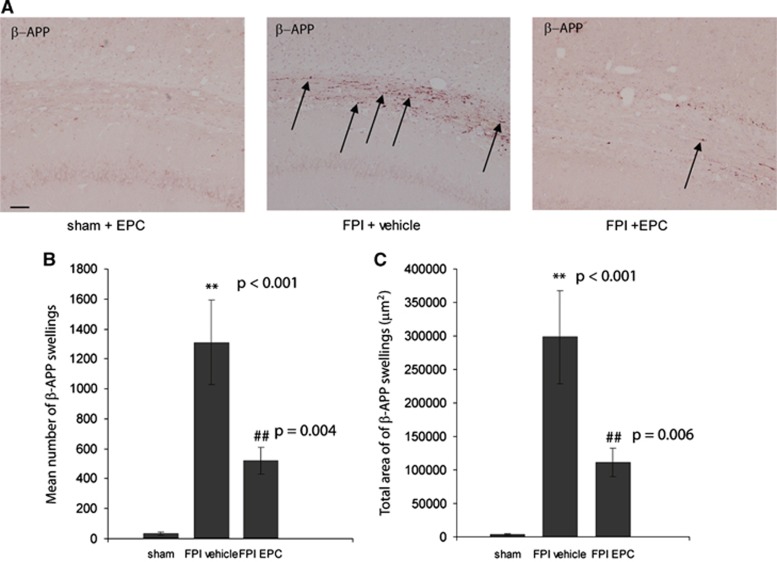
Accumulation of β-APP in brain regions is found in a number of central nervous system diseases, including ischemic and TBI.17, 18 Axonal pathology after TBI is characterized by impaired axonal transport resulting in β-APP accumulation, axonal swelling, and ultimately axonal disconnection.19 We examined coronal sections of the corpus callosum for evidence of β-APP accumulation after FPI and treatment. A significant difference was found between treatment groups (P<0.001). There was little β-APP staining in the corpus callosum in sham animals injected with either Hanks' Balanced Salt Solution or EPCs (35.5±11.5 objects; Figures 2A and 2B). There was a significant increase in the number of β-APP deposits in FPI+vehicle rats relative to sham rats (1,309.8±281.1 objects; P<0.001). EPC treatment resulted in a significant reduction in the number of quantified β-APP deposits relative to FPI+vehicle rats (520.8±9.13; P=0.004). Although there was still a noticeable difference in the number of β -APP deposits quantified between sham and FPI+EPC-treated rats, values were just below the threshold for statistical difference (P=0.06). Quantifying the density of β-APP deposits yielded similar findings (Figure 2C). There was a significant difference between treatment groups (P<0.001) with 3509.5±923.0 μm2 β-APP coverage in sham, 298,221.8±69,648.7 μm2 coverage in FPI+vehicle and 111,086.0±21,237.70 μm2 β-APP coverage in FPI+EPC-treated rats. Post hoc analysis indicated that values between FPI+EPC and FPI+vehicle rats were significantly different (P=0.006). This data provide supportive evidence that injected EPCs decreased axonal pathology in vivo.
Figure 2.
Endothelial progenitor cells (EPCs) reduce β-amyloid precursor protein (β-APP) accumulation in the corpus callosum after fluid percussion injury (FPI). (A) β-amyloid precursor protein labeling in 10 mm coronal sections of the corpus callosum 3 days after treatment. Endothelial progenitor cells treatment resulted in a reduction in both the (B) number and (C) overall density of β-APP deposits in the corpus callosum. **P values were significantly different relative to sham values. ##P values were significantly different relative to FPI+vehicle. (Scale bar, 200 μm) n=6 per treatment group.
Endothelial Progenitor Cells reduce Capillary Breakdown after Midline Fluid Percussion Injury
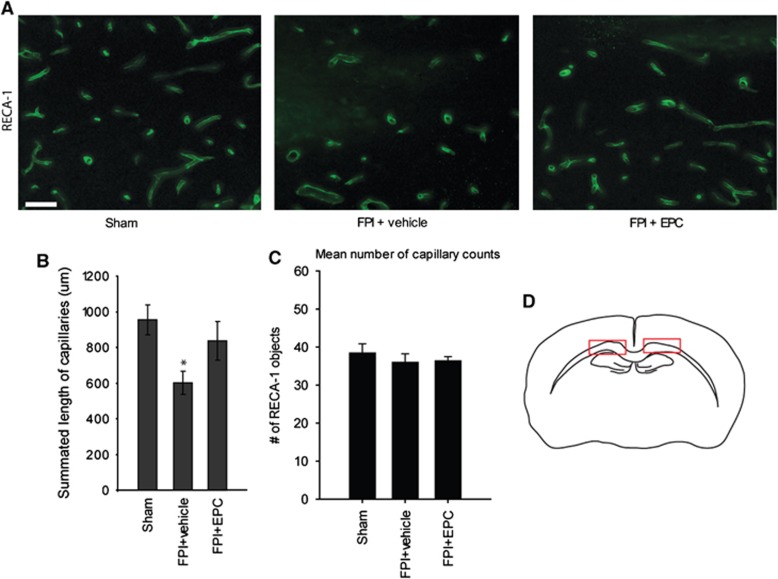
In addition to white matter outcome after FPI, we examined whether EPC treatment improved microvascular outcome in the corpus callosum. There was a significant difference in the mean total capillary lengths in the corpus callosum between treatment groups (P=0.035). Mean totaled capillary lengths were 955.4±82.6 μm in sham, 601.2±65.8 μm in FPI+vehicle and 837.8±108.8 μm in FPI+EPC-treated rats (Figures 3A and 3B). Post hoc analysis indicated a significant reduction in mean total capillary length in FPI+vehicle-treated rats relative to sham rats (P=0.03). There was a strong trend toward improved mean total capillary length relative to vehicle-treated rats, although values were not significantly different (P=0.075) in post hoc analysis. Endothelial progenitor cell treatment resulted in improved microvascular lengths of individually measured fragments; 27.5±1.7 μm in sham, 22.2±2.6 μm in FPI+vehicle, and 30.2±3.8 μm in FPI+EPC-treated rats. However, values were not significantly different by 1-way analysis of variance (P=0.167). In order to confirm that capillary length quantification was not due to sampling bias of longitudinal fragments, we quantified the total number of longitudinal capillary fragments within the sampling regions. There was no significant difference in the mean number of RECA-1 objects counted in the sampling fields between injury groups. Mean object number values were 38.4±2.4 objects in sham, 36.0±objects in FPI+vehicle, and 36.4±1.1 objects in FPI+EPC-treated rats (P=0.91; Figure 3C).
Figure 3.
Endothelial progenitor cells improve microvascular outcome after traumatic brain injury. (A) Representative images of rat endothelial cell antigen-1 (RECA-1)-labeled sections of the corpus callosum in sham, FPI+vehicle, and FPI+EPC-treated rats. (B) FPI+vehicle-treated rats had a significant reduction in mean capillary length relative to sham (*P=0.03). Although EPC treatment indicated a strong trend toward improvement relative to FPI+vehicle-treated rats, values were not statistically significant (P=0.075). (C) The total number of longitudinal capillaries in quantified sections were not significantly different thus validating the lack of bias in length quantification. (D) Red boxes indicate the fields used for RECA-1 quantification in the corpus callosum. (Scale bar, 50 μm) n=6 per treatment group.
In Vivo Endothelial Progenitor Cell Tracking
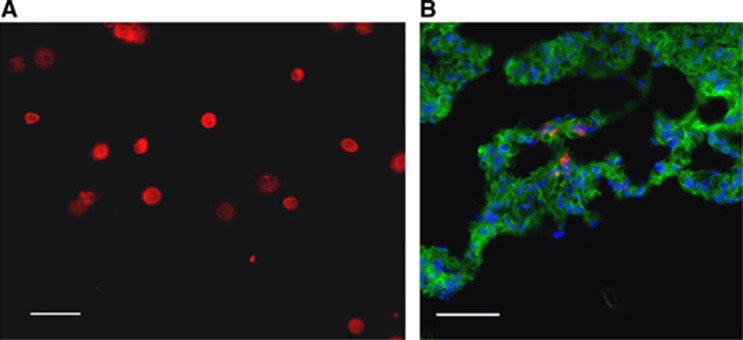
PKH26 labeling was applied to EPCs to track their in vivo localization at 4 days post injection (5 days post injury) or in sham surgery rats. Before injection, we assessed the longevity of the lipophilic fluorescent dye and found the fluorescent signal to be highly visible up to 60 days in vitro (Figure 4A), well beyond the in vivo assessment time point. We examined the cortex of injured rats 4 days after labeled EPC injection to determine whether cultured EPCs were detectable at the injury site. Despite evidence of numerous PKH-labeled EPCs in the lungs (Figure 4B), we were unable to consistently detect quantifiable PKH-labeled cells in the injured brain or in the brains of sham rats.
Figure 4.
PKH26-labeled endothelial progenitor cells (EPCs) were detected in the lung tissue. (A) Cultured EPCs were fluorescently labeled with a lipophilic dye, PKH26. Dye longevity was assessed up to 60 days in culture. (B) PKH26-labeled EPCs were detected in the lungs at 4 days post injection. (Scale bar, 50 μm).
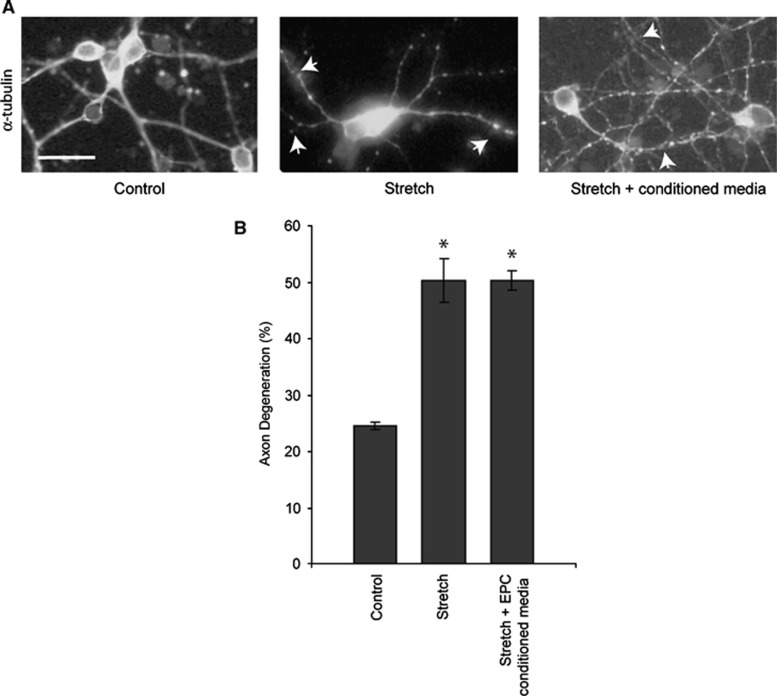
Endothelial Progenitor Cell-Conditioned Media does not Rescue Axons from Degenerating after Stretch Injury
Two major components of brain injury are the primary mechanical shearing of tissues and subsequent ischemia. We used a sublethal stretch injury model14 to isolate the mechanical injury component on cortical neuron cultures to determine if EPC-conditioned media was sufficient to rescue axon degeneration. There was evidence of basal axonal degeneration as evidenced by α-tubulin beading and breakdown in control neuron cultures (35.8±7.9% axonal degeneration; Figure 5A). However, neuron cultures subject to sublethal stretch demonstrated increased axonal injury (59.6±8.1% P=0.018) relative to controls (Figure 5B). Treatment of stretch-injured cultures with EPC-conditioned media had no effect on axonal degeneration relative to stretch-injured cultures (53.3±3.3% degeneration; P=0.28). Coculturing of EPCs with stretch-injured neurons resulted in no protective effects on axons as the majority of axonal projections from injured neurons were absent (data not shown).
Figure 5.
Endothelial progenitor cells (EPC)-conditioned media does not rescue axon degeneration after stretch injury. (A) Cortical neurons labeled for α-tubulin in control and stretch conditions. Arrowheads indicate α-tubulin breakdown and beading indicative of axonal degeneration. (B) Stretch injury resulted in a significant increase in axonal degeneration relative to control cultures (*P=0.018). Treatment with EPC-conditioned media did not result in axonal protection; n=5 independent stretch experiments performed in triplicate. (Scale bar, 50 μm).
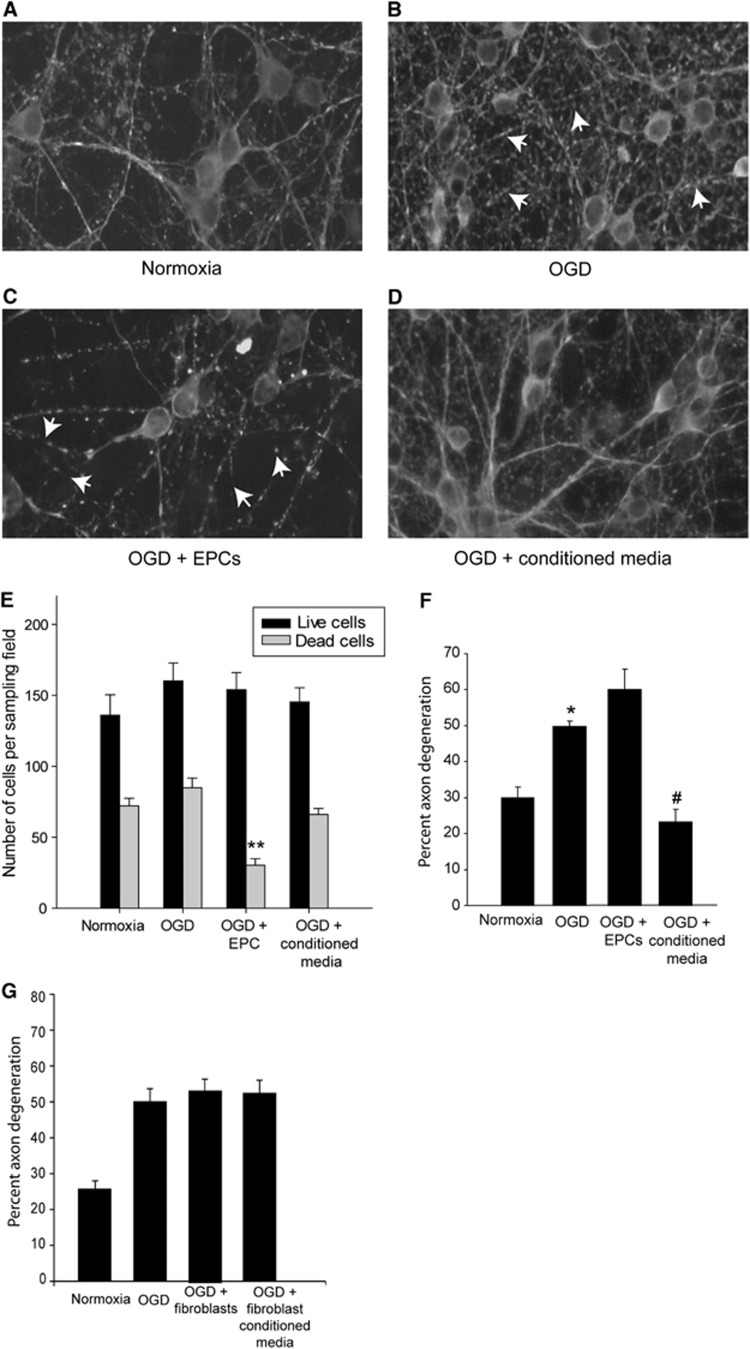
Endothelial Progenitor Cell-Conditioned Media Rescues Oxygen–Glucose Deprivation-Induced Axonal Degeneration
We used OGD in primary neuron cultures to model the ischemic component of TBI. After 5 days in vitro, the cells were subjected to a treatment of 90 minutes of OGD and confirmed as sublethal as no significant difference was found between the mean number of live neurons between each treatment group (Figure 6E). Mean number of live neurons counted for each treatment condition were 136.1±14.3 cells in normoxia, 160.1±12.8 cells in OGD, 154.0±12.1 cells in OGD+EPC, and 145.3±10.1 cells in OGD+conditioned media-treated cultures. We also examined the effect of EBM-2 basal media on neuronal survival after OGD and found no effects due to the basal media (166.6±19.4 cells, *data not shown in graph). Interestingly, we observed reduced neurite density (Figure 6C) as well as a significantly fewer dead or dying cells in OGD+EPC cocultures. There were significantly fewer dead cells present when EPCs were cocultured with neurons relative to other treatment groups (P<0.001 for all pair-wise comparisons). Sublethal OGD treatment resulted in a significant increase in axon degeneration (48.8±3.7%) relative to normoxia controls (29.9±3.1% P=0.01; Figures 6A, 6B, and 6F). Interestingly, cortical neurons subjected to OGD and subsequently cocultured with EPCs demonstrated an increase in axonal degeneration compared with OGD alone, although the values were not statistically significant (60.1±5.6 versus 48.8±3.7%, respectively; P=0.33; Figure 6F). Oxygen–glucose deprivation neuronal cultures treated with EPC-conditioned media had a significant decrease in the number of degenerating axons relative to OGD alone (23.2±3.5% P<0.01). In order to confirm that the protective effects were a result of the EPCs and not a result of nonspecific proliferating cell effects, fibroblast-conditioned media was added to a subset of cortical neuron cultures. No rescue of axon degeneration was found to occur in cortical neurons subjected to OGD and treated with fibroblast-conditioned media (50.32±3.74% versus 52.51±3.63%, respectively; Figure 6G). Taken together, these results indicate that signaling molecules released from EPCs are sufficient to decrease white matter degeneration. Furthermore, EPC-mediated protection is at least twofold, with improved microvascular outcome after injury, but also improved axon integrity via EPC-mediated signaling. These results are specific to EPCs, as conditioned media from fibroblast cultures did not result in a rescue of axon degeneration.
Figure 6.
Endothelial progenitor cells (EPC)-conditioned media rescues oxygen–glucose deprivation (OGD)-induced axon degeneration. Cortical neurons labeled for α-tubulin in panel A control, (B) OGD, (C) OGD+EPC-treated and (D) OGD+EPC-conditioned media-treated cultures. Arrowheads indicate α-tubulin breakdown. (E) OGD did not adversely affect the total number of live neurons in culture. However, coculturing with EPCs resulted in a significant reduction in the number of dead cells present in the culture (**P<0.001) relative to other treatment groups. (F) Sublethal OGD exposure resulted in significant axonal degeneration (*P=0.01) relative to normoxia controls. Coculturing of EPCs with OGD-injured neurons did not result in axonal protection. Addition of EPC-conditioned media to OGD cultures resulted in a significant reduction in axonal degeneration relative to OGD cultures (#P<0.01). (G) Coculturing of OGD neurons with fibroblasts or fibroblast-conditioned media did not result in axonal rescue; n=5 independent OGD experiments performed in triplicate.
Discussion
Endothelial progenitor cells have demonstrated therapeutic potential in a variety of vascular-related diseases.15 The high proliferative capacity of EPCs in culture, their relatively easy acquisition in adult peripheral blood, and suggested immune-privileged status20 makes their potential clinical use highly favorable. In this study, we demonstrate therapeutic benefit to cerebral white matter injury using allogenic-cultured EPCs.
In paradigms of cerebral injury, levels of endogenous circulating EPCs have been shown to correlate with improved outcome after stroke and TBI.8, 21 These findings are further supported by animal studies that have shown decreased cortical atrophy and cell death after ischemia with EPC treatment.6, 9, 22 We have previously demonstrated that cerebral microvasculature is susceptible after modeled lateral TBI resulting in the expression of signaling cascades associated with ischemia.4, 23 Endothelial progenitor cells have been shown to home into the injury site and contribute to the repair of damaged vasculature after ischemia.24, 25 Given the contribution of EPCs to neovascularization, the effect of EPCs on microvascular recovery after TBI is not entirely surprising. However, the role of EPCs in axonal protection and sparing has not been investigated and highlights an important concept in terms of the interaction between vascular components and neurobiology in disease states. During development, vascular remodeling takes place after the establishment of peripheral nerves, and in turn, requires neural input for proper patterning.26 Blood vessels also provide molecular cues to guide the development of neurons. For example, artemin, a member of the GDNF (glial cell-derived neurotrophic factor) family is expressed by vascular smooth muscle and also attracts developing sympathetic axons.27, 28 The codependence between vasculature and neurons during development also seems to share a similar relationship of interdependence during the acute posttraumatic period as evidenced by our findings. We demonstrate that EPCs are important mediators of axon survival after traumatic white matter injury in addition to their role in vascular protection or repair. The delay in treatment at 24 hours post injury also indicates a potentially clinically feasible window of opportunity for therapeutic intervention.
Our results indicate EPC treatment potentially exerts a dual therapeutic effect on capillary fragmentation as well as axonal pathology in the corpus callosum in a midline FPI model. Whether improved axon integrity in vivo was a direct result of improved microvascular integrity is not clearly defined. Furthermore, as we only sampled from a single time point for in vivo assessments, it is not entirely clear whether improvement in microvascular outcome with EPC treatment was because of capillary protection or angiogenic repair. After injection of EPCs, we observed a marked decrease in the amount of β-APP accumulation in the corpus callosum of our injured rats, which also paralleled microvascular improvement suggesting a potential codependence. Our in vitro results, however, provide supportive evidence that indicate a role for EPCs in mediating axonal protection independent of microvascular effects.
The cell culture systems allowed us to isolate the injury on neurons and axons into ischemic and mechanical components. The cortical neuron cultures contained only postmitotic neurons, thus growing in isolation from surrounding glia and vasculature simplified the interactions of the neurovascular unit to effects between EPCs and neurons. Our in vitro data indicate that EPCs confer therapeutic effects on axons via paracrine trophic effects. In the cortical neuron cultures, conditioned media was sufficient to decrease axon degeneration after ischemic injury, indicating that EPC-derived factors were able to contribute to axonal protection. The coculturing of EPCs with injured neurons did not result in axonal protection. The observed lack of benefit in this particular instance could be due to insufficient time required for the adequate production of neurotrophic factors. The use of EPC-conditioned media has been shown to improve outcome in a model of hindlimb ischemia.29 However, modulation of the chemotactic signaling pathways involving CXCR4 expression in EPCs has been shown to affect the efficacy of EPC-mediated protection after cerebral ischemia11 suggesting that the localization of EPCs post insult have an integral role in neuroprotection. Whether EPC-conditioned media is sufficient for axonal protection after TBI in vivo requires further investigation.
Bone marrow mesenchymal stromal cells and EPCs are known to release cytokines and growth factors, which favor neuronal survival. These include but are not limited to vascular endothelial growth factor, stromal cell-derived factor 1α (SDF-1α), basic fibroblast growth factor, brain-derived neurotrophic factor.25, 30, 31 Our data indicate that EPCs can be exploited to provide survival cues to axons after ischemic but not mechanical injury. One reason for this difference in response may be related to the severity of injury incurred through mechanical trauma in relation to ischemic injury. Numerous seminal studies have demonstrated that calcium is a key component in initiating secondary axonal injury mechanisms after stretch trauma.32, 33, 34 Although the level of stretch applied to our neuron cultures was still below the threshold for cell death, the degree of injury to axons may have initiated irreparably large calcium influxes35 that initiated signaling cascades beyond the protective capacities of EPC-derived trophic factors. It is also possible that secreted factors derived from EPCs target different pathways in axons injured through mechanical means in a manner independent of signaling cascades involved in ischemic injury such as calcium release from intracellular stores. Further investigation is warranted to elucidate these mechanisms.
We were able to detect the presence of PKH26-labeled EPCs in the rat lungs at 4 days post injection, but very few were reliably detected in the injured brain. At the assessed time point in our study, there was little evidence to indicate that allogenic EPCs physically contribute to the repair or formation of new vasculature. This finding is consistent with a recent report in which magnetically labeled EPCs were tracked in a model of controlled cortical impact.36 Although allogenic EPCs were detected in the injured brain, the migration of EPCs was detected up to 24 hours post injury with subsequent diffusion of signal thereafter. Given our sampling time at 4 days post injection, the lack of EPCs in the brain does not rule out their involvement directly within the injured brain, rather it is likely that we missed the optimal detection period. Similarly, mesenchymal stem cells have also been shown to have very poor grafting abilities.37 Despite a lack of presence in the injured brain, the observed positive effects on white matter in vivo in conjunction with our in vitro data support the notion that posttraumatic EPC activity primarily involves localized release of trophic factors and cytokines.
We did not observe a beneficial effect when the conditioned media derived from fibroblasts of fibroblasts themselves were added to neuronal cultures subject to OGD. Fibroblasts are typically involved in wound healing processes through secretion of collagen and extracellular matrix proteins. Meningeal fibroblasts and glia form the extracellular matrix of a CNS scar, forming a growth-inhibitory barrier to injured axons.38 Fibroblasts secrete factors inhibitory to axon growth after injury including chondroitin sulfate proteoglycan39, 40 and do not lead to improved axon integrity after ischemic injury.
Our EPC cultures were derived from rat bone marrow. Although we did not preselect or isolate CD34+ cells, the majority of the cells (∼93%) in our cultures were of an EPC-like phenotype expressing CD133. There were, however, other mononuclear cell types in the culture system. We used double-label immunocytochemistry for OX-42, which recognizes CD11b/c expression, and CD133 to identify putative EPCs. We identified a subset of morphologically different cells as OX-42-positive macrophages. These cells were also immunofluorescent at the 488 nm wavelength indicating that the macrophages were also CD133 positive or exhibited autofluorescent properties at the excitation wavelength. Although these cells comprise a relatively small percentage of the total cell population, our data indicate a significant role in the clearance of dead or dying cellular material in culture. For example, in the EPC/neuronal cocultures, we observed a significant reduction in the number of dead cells in OGD-treated cultures. Similarly, in stretch trauma EPC/neuronal cocultures, we observed a dramatic loss in axons. In this regard, it seems plausible that the macrophage population was involved in clearance of damaged or degenerating neurite processes. Whether the macrophages had a significant role in the protection of white matter in our in vitro studies is not clear and is an area for further investigation.
Conclusions
In the present study, we demonstrate the application of allogenic-cultured EPCs as a potential therapeutic strategy to limit the progression of secondary axonal injury and improve microvascular outcome. We demonstrate that factors secreted by EPCs are sufficient to rescue axons from degeneration after ischemia. While these protective effects did not translate to mechanically injured axons, the overall translation in an in vivo model demonstrated reduced axon pathology in parallel with some evidence suggesting microvascular improvement. The targeting of the neurovascular unit using EPC therapy represents a potentially synergistic approach to improving outcome after trauma by targeting multiple facets of secondary injury mechanisms.
The authors declare no conflict of interest.
Footnotes
This study was funded by the Ministry of Economic Development and Innovation, Government of Ontario.
References
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Stein SC, Graham DI, Chen XH, Smith DH.Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury Neurosurgery 200454687–691.discussion 691. [DOI] [PubMed] [Google Scholar]
- Farahvar A, Gerber LM, Chiu YL, Carney N, Hartl R, Ghajar J. Increased mortality in patients with severe traumatic brain injury treated without intracranial pressure monitoring. J Neurosurg. 2012;117:729–734. doi: 10.3171/2012.7.JNS111816. [DOI] [PubMed] [Google Scholar]
- Park E, Bell JD, Siddiq IP, Baker AJ. An analysis of regional microvascular loss and recovery following two grades of fluid percussion trauma: a role for hypoxia-inducible factors in traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:575–584. doi: 10.1038/jcbfm.2008.151. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, Zhu W, Ye J, Liu J, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- Sobrino T, Hurtado O, Moro MA, Rodriguez-Yanez M, Castellanos M, Brea D, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- Ohta T, Kikuta K, Imamura H, Takagi Y, Nishimura M, Arakawa Y, et al. Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats Neurosurgery 200659679–686.discussion 679-686. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Phan N, Moulton RJ, Fehlings MG, Yucel Y, Zhao M, et al. Attenuation of the electrophysiological function of the corpus callosum after fluid percussion injury in the rat. J Neurotrauma. 2002;19:587–599. doi: 10.1089/089771502753754064. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen S, Zhang C, Zhang L, Xiao X, Das A, et al. Transfusion of CXCR4-primed endothelial progenitor cells reduces cerebral ischemic damage and promotes repair in db/db diabetic mice. PLoS ONE. 2012;7:e50105. doi: 10.1371/journal.pone.0050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AF, Ho DK, Zanassi P, Walsh GS, Kaplan DR, Miller FD. Evidence that DeltaNp73 promotes neuronal survival by p53-dependent and p53-independent mechanisms. J Neurosci. 2004;24:9174–9184. doi: 10.1523/JNEUROSCI.1588-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. J Neurochem. 2005;92:103–113. doi: 10.1111/j.1471-4159.2004.02841.x. [DOI] [PubMed] [Google Scholar]
- Bell JD, Park E, Ai J, Baker AJ. PICK1-mediated GluR2 endocytosis contributes to cellular injury after neuronal trauma. Cell Death Differ. 2009;16:1665–1680. doi: 10.1038/cdd.2009.106. [DOI] [PubMed] [Google Scholar]
- Resch T, Pircher A, Kahler CM, Pratschke J, Hilbe W. Endothelial progenitor cells: current issues on characterization and challenging clinical applications. Stem Cell Rev. 2012;8:926–939. doi: 10.1007/s12015-011-9332-9. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- Kalaria RN, Bhatti SU, Lust WD, Perry G. The amyloid precursor protein in ischemic brain injury and chronic hypoperfusion. Ann N Y Acad Sci. 1993;695:190–193. doi: 10.1111/j.1749-6632.1993.tb23050.x. [DOI] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246:35–43. doi: 10.1016/j.expneurol.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Ladhoff J, Fleischer B, Hara Y, Volk HD, Seifert M. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovasc Res. 2010;88:121–129. doi: 10.1093/cvr/cvq109. [DOI] [PubMed] [Google Scholar]
- Gong D, Hao M, Liu L, Liu C, Dong J, Cui Z, et al. Prognostic relevance of circulating endothelial progenitor cells for severe traumatic brain injury. Brain Inj. 2012;26:291–297. doi: 10.3109/02699052.2011.648710. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiq I, Park E, Liu E, Spratt SK, Surosky R, Lee G, et al. Treatment of traumatic brain injury using zinc-finger protein gene therapy targeting VEGF-A. J Neurotrauma. 2012;29:2647–2659. doi: 10.1089/neu.2012.2444. [DOI] [PubMed] [Google Scholar]
- Borlongan CV. Cell therapy for stroke: remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–S148. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells functional characterization. Trends Cardiovasc Med. 2004;14:318–322. doi: 10.1016/j.tcm.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, et al. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Honma Y, Araki T, Gianino S, Bruce A, Heuckeroth R, Johnson E, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron. 2002;35:267–282. doi: 10.1016/s0896-6273(02)00774-2. [DOI] [PubMed] [Google Scholar]
- He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35:2378–2384. doi: 10.1161/01.STR.0000141893.33677.5d. [DOI] [PubMed] [Google Scholar]
- Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, et al. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS ONE. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlacu A, Grigorescu G, Rosca AM, Preda MB, Simionescu M. Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev. 2013;22:643–653. doi: 10.1089/scd.2012.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XY, Chen ZZ, Cai YQ, Xu G, Shang JH, Kou SB, et al. Expression of cytokines in rat brain with focal cerebral ischemia after grafting with bone marrow stromal cells and endothelial progenitor cells. Cytotherapy. 2011;13:46–53. doi: 10.3109/14653249.2010.510505. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Kosanlavit R, McCreath BJ, Reid O, Graham DI. Freeze-fracture and cytochemical evidence for structural and functional alteration in the axolemma and myelin sheath of adult guinea pig optic nerve fibers after stretch injury. J Neurotrauma. 1999;16:273–284. doi: 10.1089/neu.1999.16.273. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, McCreath BJ, Graham DI, Gennarelli TA. Cytochemical evidence for redistribution of membrane pump calcium-ATPase and ecto-Ca-ATPase activity, and calcium influx in myelinated nerve fibres of the optic nerve after stretch injury. J Neurocytol. 1995;24:925–942. doi: 10.1007/BF01215643. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Stys PK, Lusardi T, Meaney D, Smith DH. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci. 2001;21:1923–1930. doi: 10.1523/JNEUROSCI.21-06-01923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A, Povlishock JT.All roads lead to disconnection?—Traumatic axonal injury revisited Acta Neurochir (Wien) 2006148181–193.discussion 193-184. [DOI] [PubMed] [Google Scholar]
- Chen X, Yin J, Wu X, Li R, Fang J, Chen R, et al. Effects of magnetically labeled exogenous endothelial progenitor cells on cerebral blood perfusion and microvasculature alterations after traumatic brain injury in rat model. Acta Radiol. 2013;54:313–323. doi: 10.1258/ar.2012.120605. [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Yamada KM, Yoneda M, Suzuki S, Kimata K. Chondroitin sulfate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J Biol Chem. 1986;261:13526–13535. [PubMed] [Google Scholar]
- Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem. 2011;119:176–188. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- Johansson S, Hedman K, Kjellen L, Christner J, Vaheri A, Hook M. Structure and interactions of proteoglycans in the extracellular matrix produced by cultured human fibroblasts. Biochem J. 1985;232:161–168. doi: 10.1042/bj2320161. [DOI] [PMC free article] [PubMed] [Google Scholar]