Abstract
In this study, we measured ADA and DPP-IV enzymatic activity and sCD26 concentration in 150 pleural effusion (PE) samples and tested for correlations between these and other cellular and biochemical measures. We found that DPP-IV in particular might improve the specificity (but not the sensitivity) of the ADA test for diagnosis of pulmonary tuberculosis, since half of the false ADA positive results in non-tuberculous PE were also DPP-IV positive. A percentage of patients with malignant PE were sCD26 or DPP-IV positive; however, some patients with benign PE also tested positive. As a pattern associated with DPP-IV (but not the CD26 protein) was observed in PE, we searched for a finding that might increase the value of these biomarkers for diagnosis of malignancy. The observed pattern was related to the presence of leukocytes, as indicated by correlations with the cell count, and to a band of 180 kDa, detected by immunoblotting.
The soluble form of CD26 (sCD26), ascribed to the DPP-4 gene and originating from shedding of the transmembrane protein, is found in many biological fluids. The physiological role of sCD26 and its relation, if any, to CD26 functions remain poorly understood1. Dipeptidyl peptidase IV (DPP-IV), a serine protease belonging to type II transmembrane glycoproteins (EC 3.4.14.5), is expressed on the surface of epithelial cells of diverse tissues, on endothelial cells of blood vessels and on some immune cells such as T lymphocytes, B lymphocytes and NK cells. By cleaving dipeptides from the N-terminal end of peptides and polypeptides with proline or alanine in the second position, DPP-IV controls the activity of many bioactive molecules, including cytokines and chemokines, incretins and gastrointestinal hormones, vasoactive peptides and neuropeptides2,3,4. DPP-IV is a multifunctional regulatory biomolecule, and in addition to its enzymatic activity, it interacts with many plasma membrane proteins, such as ADA and the chemokine receptor CXCR4, and with extracellular matrix components such as collagen. Thus, DPP-IV is involved in diverse biological processes apart from protein degradation in the gut, especially immune functions and inflammation2,4, and it has also become a novel therapeutic target for inhibitors that extend endogenously produced insulin half-life in diabetics1.
Pleural effusion (the pathological accumulation of fluid in the pleural cavity that surrounds the lung) may appear as the result of different benign abnormalities, which are often caused by tuberculosis (TB), or as the result of malignant disease5. Tuberculous pleural effusion (TPE), which is considered a form of extrapulmonary TB, remained a diagnostic challenge for clinicians until the identification of molecular biomarkers that yielded a rapid and accurate diagnosis of tuberculous pleuritis6,7. Among the best-established techniques, determination of adenosine deaminase (ADA) activity and the concentration of cytokine IFN-gamma in the pleural effusion are included in the supplemental diagnostic index for pleural tuberculosis8,9,10. However, none of the available tests for TPE are wholly accurate10,11, and any biomarker that can increase their reliability in indicating whether anti-tuberculosis therapy should be resumed or discontinued will be valuable.
Interest has recently been shown in determining sCD26 concentration and DPP-IV acativity in PE, for two reasons. First, the plasma membrane CD26 was previously known as ADA complexing or binding protein (ADA-CP or ADA-BP)1,12, although the ADA of most diagnostic importance in biological fluids (including TPE) is ADA2, the isoenzyme that is produced by monocytes and that predominates in the sera of normal individuals. The other isoenzyme, ADA1, is expressed by most cells and can be found separately or connected via a dimer of soluble ADA-CP (sCD26). Extracellular ADA is probably involved in the control of adenosine-mediated signalling through purinergic receptors, at least in leukocytes13,14.
The second reason for studying sCD26/DPP-IV is that tuberculous infections generate Th1-like immune responses, such as IFN-gamma secretion. Membrane-bound expression of soluble CD26 correlated with Th1-like responses including cytokine production15,16,17.
Previous studies have shown that measurement of DPP-IV activity18 and sCD26 concentration19,20 slightly improved the already high sensitivity and diagnostic efficiency of the ADA test for tuberculous pleurisy. However, as shown in some diseases1, and even in healthy donor serum samples, enzymatic activity and enzyme concentrations are not closely correlated. We therefore determined the sCD26 concentration and DPP-IV and ADA activity in the same samples to investigate this hypothesis in relation to PE. We included PE associated with malignant pathologies (mainly lung cancer)20 in the study, partly to widen the cohort of the study and also because many recent studies have demonstrated an important role for DPPIV/CD26 (both altered cell surface CD26 expression as well as changes in serum DPP-IV activity) in the initial steps of malignant transformation, in progression of the tumour and in the metastatic process2,3,4,21,22,23,24. Therefore, any information regarding the role of these molecules in PE will be important for developing diagnostic tests or treatments.
Results
Basic demographic parameters of ADA and DPP-IV activity and sCD26 concentration in PE samples from patients with benign and malignant pathologies
The ADA and DPP-IV enzymatic activities (expressed in IU L−1) and soluble CD26 concentration (expressed in μg L−1) were determined in PE samples from 138, 147 and 159 patients, respectively. Gender and age factors were considered in a basic demographic study as they affect DPP-IV enzymatic activity and sCD26 concentration in serum4 and also because TPE is generally diagnosed in younger patients and MPE in older patients.
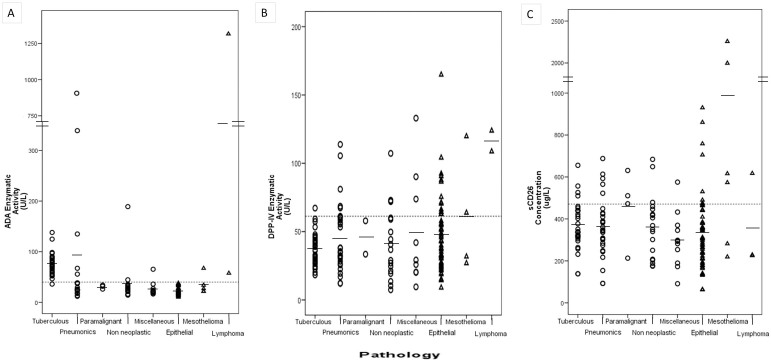
The mean ADA activity of the BPE group was 63.7 ± 106.1 UL−1 (Tables 2 and 3, and Figure 1). The mean value for the TPE group was 75.3 ± 21.4 UL−1, range 11.9–905.9. The high SD associated with the mean value for the BPE group was due to cases of parapneumonic PE, some associated with high ADA values. The values were much higher than in other BPE and MPE groups (except for one lymphoma sample), as expected (Fig. 1). Almost half of the TPE patients were less than 36 years old, in contrast to the other groups (Table 2). However, although with a few cases, the ADA activity was higher in older than in young individuals only in the parapneumonic group (Table 2). Further studies are required to confirm the trend towards higher levels of activity in women.
Table 1. Demographic data for the population under study.
| Aetiology of PE | No. of Patients | No. of Men/Women | Age, years Mean (Range) | |
|---|---|---|---|---|
| BPE | 93 | 64/29 | 55 (20–96) | |
| Tuberculosis | 30 | 20/10 | 44 (20–96) | |
| Parapneumonic | 29 | 22/7 | 55 (26–89) | |
| Paramalignant | 4 | 2/2 | 61 (40–81) | |
| Non neoplastic of unknown origin | 18 | 13/5 | 65 (37–87) | |
| Miscellaneous | 12 | 7/5 | 62 (24–94) | |
| After surgery | 3 | 2/1 | 64 (53–74) | |
| Chylothorax | 3 | 2/1 | 73 (54–94) | |
| Secondary to collagen vascular diseases | 3 | 1/2 | 47 (37–59) | |
| Secondary to drug reaction | 1 | 1/0 | 24 | |
| Dressler's Syndrome | 1 | 1/0 | 80 | |
| After trauma | 1 | 0/1 | 83 | |
| MPE | 67 | 42/25 | 67 (20–91) | |
| Epithelial origin neoplasias | 58 | 34/24 | 68 (34–91) | |
| NSCLC | 42 | 30/12 | 68 (37–91) | |
| Breast cancer | 6 | 0/6 | 63 (34–75) | |
| SCLC | 3 | 3/0 | 72 (57–81) | |
| Ovarian cancer | 2 | 0/2 | 82 (79–85) | |
| Thymic epithelial neoplasm | 2 | 0/2 | 69 (65–73) | |
| Gastric cancer | 2 | 0/2 | 73 (60–83) | |
| Cholangiocarcinoma | 1 | 1/0 | 61 | |
| Mesothelioma | 6 | 5/1 | 67 (57–87) | |
| Lymphoma | 3 | 3/0 | 55 (20–79) |
Abbreviations: MPE, malignant pleural effusion; BPE, benign pleural effusion.
Table 2. ADA and DPP-IV activity and sCD26 concentration in PE according to gender groups in the study population (mean ± SD).
| All | Men | Women | ||
|---|---|---|---|---|
| ADA activity (UL−1) | Total | 56.2 ± 135.9 (138) | 55.1 ± 140.1 (91) | 58.3 ± 129.0 (47) |
| BPE | 63.7 ± 106.1 (81) | 50.5 ± 48.2 (57) | 91.1 ± 171.8 (24) | |
| +TPE | 75.3 ± 21.4 (30)† | 69.5 ± 15.1 (20) | 86.9 ± 27.7 (10) | |
| +pneumonic* | 90.4 ± 200.1 (21)‡ | 53.6 ± 78.8 (17) | 246.7 ± 439.9 (4) | |
| +paramalignant | 30.7 ± 4.1 (3) | 29.0 ± 4.0 (2) | 34.2 ± 0 (1) | |
| +non neoplastic | 37.1 ± 42.8 (15) | 25.8 ± 9.4 (11) | 53.9 ± 66.6 (4) | |
| +miscellaneous | 26.5 ± 14.0 (12) | 22.8 ± 6.2 (7) | 32.8 ± 22.2 (5) | |
| MPE | 46.1 ± 168.5 (57)∧ | 62.0 ± 215.3 (34) | 21.1 ± 6.1 (23) | |
| +epithelial | 22.7 ± 7.2 (50)∥ | 24.0± 7.8 (28) | 21.1 ± 6.2 (22) | |
| +mesothelioma | 34.4 ± 19.1 (5) | 37.6 ± 20.4 (4) | 21.5 ± 0 (1) | |
| +lymphoma | 686.8 ± 889.8 (2) | 686.8 ± 889.8 (2) | 0 | |
| DPP-IV activity (UL−1) | Total | 45.4 ± 27.19 (140) | 47.9 ± 28.4 (89) | 42.0 ± 25.8 (51) |
| BPE | 41.9 ± 24.0 (80) | 42.9 ± 23.1 (53) | 41.0 ± 27.1 (27) | |
| +TPE | 36.8 ± 12.1 (30) | 37.3 ± 12.9 (20) | 35.8 ± 10.9 (10) | |
| +pneumonic* | 45.3 ± 25.5 (29) | 48.2 ± 26.4 (22) | 36.1 ± 21.6 (7) | |
| +paramalignant | 45.7 ± 17.3 (2) | 33.5 ± 0 (1) | 57.9 ± 0 (1) | |
| +non neoplastic | 40.9 ± 26.7 (10) | 39.6 ± 30.8 (5) | 45.8 ± 35.9 (5) | |
| +miscellaneous | 49.4 ± 41.2 (9) | 47.2 ± 34.4 (5) | 52.1 ± 54.1 (4) | |
| MPE | 50.5 ± 30.8 (60) | 55.4 ± 33.6 (36) | 43.1 ± 24.9 (24) | |
| +epithelial | 47.3 ± 27.7 (54)§ | 50.0 ± 29.5 (31)§ | 43.6 ± 25.3 (23) | |
| +mesothelioma | 60.5 ± 37.0 (4) | 70.1 ± 46.8 (3) | 31.7 ± 0 (1) | |
| +lymphoma | 116.3 ± 10.7 (2) | 116.3 ± 10.7 (2) | 0 | |
| sCD26 concentration (μg L−1) | Total | 337.5 ± 253.1 (159) | 388.5 ± 286.4 (106) | 355.2 ± 165.8 (53) |
| BPE | 360.8 ± 136.1 (92) | 356.1 ± 128.3 (64) | 371.8 ± 154.1 (28) | |
| +TPE | 367.1 ± 113.4 (30) | 357.5 ± 107.7 (20) | 386.4 ± 127.8 (10) | |
| +pneumonic* | 363.1 ± 143.3 (29) | 391.2 ± 119.1 (22) | 274.5 ± 184.7 (7) | |
| +paramalignant | 456.7 ± 176.1 (4) | 422.0 ± 295.6 (2) | 491.4 ± 27.2 (2) | |
| +non neoplastic | 362.5 ± 151.5 (18) | 336.6 ± 142.9 (13) | 429.8 ± 168.4 (5) | |
| +miscellaneous | 300.4 ± 132.2 (11) | 258.9 ± 115.6 (7) | 373.1 ± 143.2 (4) | |
| MPE | 392.8 ± 352.4 (67) | 426.3 ± 421.9 (42) | 336.6 ± 179.4 (25) | |
| +epithelial | 332.8 ± 173.1 (58) | 328.6 ± 168.6 (34) | 338.9 ± 182.9 (24) | |
| +mesothelioma | 990.6 ± 897.6 (6)# | 1132.4 ± 925.5 (5) | 281.8 ± 0 (1) | |
| +lymphoma | 357.0 ± 225.3 (3) | 357.0 ± 225.3 (3) | 0 |
In parentheses (n) number of samples.
*, para and metapneumonic. Interesting statistically significant differences indicated: For ADA;
†U-Mann p < 0.001 between TPE and other groups, except pneumonic PE;
‡U-Mann p < 0.001 between pneumonic PE and other groups except TPE;
∧, T-student p < 0.001 between BPE and MPE;
∥, Kruskal-Wallis ANOVA p < 0.001 for MPE groups. For DPP-IV;
§, U-Mann p = 0.022 (total) and p = 0.029 (men) between MPE epithelial and lymphoma. For CD26;
#, Kruskal-Wallis p < 0.001 for mesotheliomas compared with the other groups.
Table 3. ADA and DPP-IV activity and sCD26 concentration in PE according to age groups in the study population (mean ± SD).
| ≤35 years old | 36–55 years old | ≥56 years old | ||
|---|---|---|---|---|
| ADA activity (UL−1) | Total | 131.3 ± 288.4 (19) | 43.9 ± 57.4 (32) | 45.1 ± 98.4 (86) |
| BPE | 68.0 ± 28.9 (17) | 50.2 ± 65.2 (24) | 70.1 ± 142.6 (39) | |
| +TPE | 78.4 ± 19.1 (14) | 63.6 ± 15.6 (7) | 79.5 ± 27.1 (9) | |
| +pneumonic* | 18.7 ± 7.6 (2) | 73.7 ± 130.1 (6) | 109.1 ± 241.5 (13) | |
| +paramalignant | 0 | 29.0 ± 4.0 (2) | 34.2 ± 0 (1) | |
| +non neoplastic | 0 | 26.1 ± 12.6 (5) | 42.6 ± 51.8 (10) | |
| +miscellaneous | 21.1 ± 0 (1) | 32.0 ± 22.5 (4) | 23.7 ± 7.0 (6) | |
| MPE | 669.5 ± 914.4 (2) | 25.1 ± 8.7 (8) | 24.3 ± 10.6 (47) | |
| +epithelial | 22.9 ± 0 (1) | 25.1 ± 8.7 (8) | 22.2 ± 7.0 (41) | |
| +mesothelioma | 0 | 0 | 34.4 ± 19.1 (5) | |
| +lymphoma | 1316.0 ± 0 (1) | 0 | 57.7 ± 0 (1) | |
| DPP-IV activity (UL−1) | Total | 43.7 ± 20.9 (21)‡ | 51.1 ± 26.2 (35)‡ | 43.6 ± 28.8 (91) |
| BPE | 41.0 ± 15.2 (19) | 47.1 ± 25.2 (26) | 39.2 ± 26.3 (42) | |
| +TPE | 39.8 ± 10.4 (14) | 38.3 ± 17.7 (7) | 31.1 ± 8.0 (9) | |
| +pneumonic* | 33.0 ± 5.0 (4) | 50.8 ± 26.1 (10) | 44.9 ± 28.2 (15) | |
| +paramalignant | 0 | 57.9 ± 0 (1) | 33.5 ± 0 (1) | |
| +non neoplastic | 0 | 57.2 ± 40.7 (6) | 32.0 ± 19.1 (11) | |
| +miscellaneous | 90.1 ± 0 (1) | 23.0 ± 4.1 (2) | 51.3 ± 45.8 (6) | |
| MPE | 69.6 ± 55.3 (2)∧ | 62.8 ± 26.8 (9)∧ | 47.4 ± 30.5 (49)∧ | |
| +epithelial | 30.5 ± 0 (1) | 62.8 ± 26.8 (9) | 44.5 ± 27.4 (44) | |
| +mesothelioma | 0 | 0 | 60.5 ± 42.7 (4) | |
| +lymphoma | 108.7 ± 0 (1) | 0 | 123.8 ± 0 (1) | |
| sCD26 concentration (μg L−1) | Total | 389.1 ± 128.6 (21) | 412.3 ± 148.2 (37)† | 357.4 ± 295.1 (101)† |
| BPE | 379.6 ± 123.5 (19) | 392.2 ± 158.3 (28)§ | 322.5 ± 136.3 (45)§ | |
| +TPE | 379.3 ± 112.3 (14) | 353.1 ± 60.6 (7) | 359.1 ± 151.2 (9) | |
| +pneumonic* | 367.3 ± 189.0 (4) | 377.4 ± 149.8 (10) | 352.3 ± 136.8 (15) | |
| +paramalignant | 0 | 491.4 ± 27.2 (2) | 422.0 ± 295.6 (2) | |
| +non neoplastic | 0 | 500.5 ± 134.0 (6) | 293.5 ± 108.2 (12) | |
| +miscellaneous | 432.9 ± 0 (1) | 414.4 ± 144.0 (3) | 232.6 ± 86.4 (7) | |
| MPE | 479.3 ± 195.0 (2) | 420.1 ± 205.7 (9) | 385.4 ± 376.4 (56) | |
| +epithelial | 341.4 ± 0 (1) | 420.1 ± 205.7 (9) | 316.3 ± 165.6 (48) | |
| +mesothelioma | 0 | 0 | 990.6 ± 897.6 (6) | |
| +lymphoma | 617.2 ±0 (1) | 0 | 226.9 ± 2.4 (2) |
In parentheses (n) number of samples. Significant differences between age groups: For DPP-IV;
‡U-Mann p = 0.007;
∧p = 0.013 and p = 0.003 respectively. For sCD26;
†p < 0.001 and
§p = 0.007.
Figure 1.
Levels of (A) ADA enzymatic activity, (B) DPP-IV enzymatic activity and (C) sCD26 concentration in pleural effusion (PE) samples from patients diagnosed with the following types of PE, from left to right: benign PE (circles), including tuberculous, parapneumonic, paramalignant, non neoplasic and miscellaneous PE; malignant PE (triangles), including epithelial, mesothelioma and lymphoma PE.
The DPP-IV activity was lowest in the TPE group (mean 36.8 ± 12.1 UL−1, range 38.3–127.6: Table 2 and 3, and Figure 1), for 41.8 ± 24.0 UL−1 in total BPE, but only mesotheliomas (60.5 ± 37.0) and lymphomas (116.3 ± 10.7) MPE groups were significantly different (Table 2). Although not statistically significant, there was a trend towards lower DPP-IV activity in the older patients (see BPE and MPE means, Table 2).
The commercial ELISA (Bender MedSystems) used in the present study was the same as used in other studies, and therefore comparison of the serum sCD26 levels is easier1. The mean concentrations of sCD26 measured in BPE and MPE were 360.8 ± 136.1 μg L−1 (range 92.31–687.92 μg L−1) and 392.8 ± 352.4 μg L−1 (range 62.6 – 2 256.54 μg L−1) respectively (Table 1 and 2). The results were similar to those obtained for carcinomatous pleurisy in a study using the same kit18 and to those obtained with a different kit20. However, the present study included a larger number of patients, and the mean concentration of sCD26 in TPE was not only similar to that of BPE but also to those of total MPE and MPE groups (Tables 2 and 3). High values of sCD26 were only observed in paramalignant PEs in the BPE group and particularly mesotheliomas and one lymphoma in MPE. Taking age into account, we found that the concentrations of sCD26 were significant lower in older (≥56) than in younger patients (Table 3).
No differences were found for the other epidemiological factors studied, such as smoking habit, cytology and neoplasic history.
Diagnostic value of determining ADA and DPP-IV activity and sCD26 concentration in samples of benign and malignant PE
For TPE diagnosis with the ADA activity test, a cut-off value of 40 UL−1 was chosen as this is commonly used in the clinical setting. For DPP-IV and sCD26, ROC curves that best distinguished TPE and non-neoplasic BPE groups from the other groups were constructed. The cut-off value of 61 UL−1 obtained for the DPP-IV activity was adjusted to 60. For the sCD26 levels, the cut-off obtained was not useful and therefore the mean value plus the standard deviation, a cut-off of 470 μg L−1, was used.
Thus, only 1 of 30 cases of TPE (in a middle-aged patient) was found to be negative for ADA (value below the cut-off). The ADA levels were lower in this age group than in the other groups (Table 3). The main problem associated with the use of ADA activity to diagnose TPE was that another 8 of 51 non-tuberculous BPE (5/21 parapneumonic PE patients) were also positive, as well as three (3/57) MPE (Table 4), i.e. this biomarker shows poorer specificity (89.8%) than sensitivity (96.7%) in clinical diagnosis.
Table 4. Frequency of positive results for ADA and DPP-IV enzymatic activity, and sCD26 levels in PE according to the groups under study.
| ADA>40 U L−1 | DPP-IV>60 U L−1 | sCD26>470 μg L−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Total n | +n | % | Total n | +n | % | Total n | +n | % |
| BPE | 81 | 36 | 44.4 | 80 | 15 | 18.8 | 92 | 14 | 15.2 |
| +TPE | 30 | 29 | 96.7 | 30 | 1 | 3.3 | 30 | 4 | 13.3 |
| +pneumonic | 21 | 5 | 23.8 | 29 | 8 | 27.6 | 29 | 5 | 17.2 |
| +paramalignant | 3 | 0 | 0 | 2 | 0 | 0 | 4 | 1 | 25.0 |
| +non neoplastic | 15 | 2 | 13.3 | 10 | 3 | 30.0 | 18 | 3 | 16.7 |
| +miscellaneous | 12 | 1 | 8.3 | 9 | 3 | 33.3 | 11 | 1 | 9.1 |
| MPE | 57 | 3 | 5.3 | 60 | 18 | 30.0 | 67 | 13 | 19.4 |
| +epithelial | 50 | 0 | 0 | 54 | 14 | 25.9 | 58 | 8 | 13.8 |
| +mesothelioma | 5 | 1 | 20.0 | 5 | 3 | 60.0 | 6 | 4 | 66.7 |
| +lymphoma | 2 | 2 | 100.0 | 2 | 2 | 100.0 | 3 | 1 | 33.3 |
Positive results for ADA, sCD26 and DPP-IV activity (+) represent all cases above the cut-off values of 40 and (60) U L−1 and 470 μg L−1, respectively. Frequencies shown in shaded type indicate significant differences from the TPE group, as revealed by Fischer's exact tests done with contingency tables.
According to these data, determination of DPP-IV activity and sCD26 concentration will not enhance the sensitivity of TPE detection since only 1 and 4 out of 30 patients were found to be positive, and the negative ADA case was also negative for both biomarkers. In fact, many more cases were found to positive for these markers, particularly DPP-IV, in the other BPE and MPE groups (Table 4). Fischer's exact test for the contingency tables showed that all groups except the paramalignant group were statistically significantly different from the TPE group for DPP-IV (p = 0.002 for TPE compared with the other groups), whereas the sCD26 levels only differed in mesothelioma and lymphoma PEs.
However, DPP-IV and sCD26 may enhance the specificity of the ADA test for TPE detection, i.e. detection of false TPE positive results. From the group of 11 false positives, 3/5 of ADA+ parapneumonic patients were also DPP-IV+ and a fourth was close to the cut-off (the other BPE cases were negative for DPP-IV) (Table 3) and the three MPE ADA+ cases were also positive for the other two measures. Calculation of the Net Reclassification Improvement (NRI) showed that of the 12 wrongly classified cases (11 false positives and one false negative: 8.7%), 6 false positives (4.3%) could be reclassified, with an NRI of 4.4% (95% CI 2.3–6.5% and p = 0.031, see Methods). Important for the patient, the ones remaining wrongly classified have not a malignant pathology.
We used exploratory NRI with more cut-off values per biomarker (continuous NRI), to develop a model that could improve diagnosis of other PE groups, but we did not find any values of clinical interest. However, an important number of the MPE samples were ADA negative and DPP-IV (18/60) and sCD26 (13/67) positive: these were mainly from patients with lymphomas and mesotheliomas, but also epithelial MPE (14/54 for DPP-IV compared with 0/50 for ADA) (Table 4). As 11/81 BPE were also ADA- DPP-IV+, these biomarkers are not useful for MPE diagnosis in their present form. However, we did not observe any relationship between these frequencies in epithelial MPE and any other pathological condition. Also, 11/40 cases of non-small cell lung cancer (NSCLC) were positive for DPP-IV, compared with 7/42 cases for sCD26; by contrast, more sCD26+ than DPP-IV+ cases were detected in the mesothelioma group (Table 3).
Correlations between DPP-IV activity, sCD26 concentration, standard biochemical parameters and cell counts, in samples of benign and malignant PE
The DPP-IV and sCD26 biomarkers did not perform in the same way in the different groups of patients (Table 4). Also, some data were lost due to technical problems with the measurement of enzymatic activity. Thus, we tried to obtain further information that could enhance the value of these biomarkers.
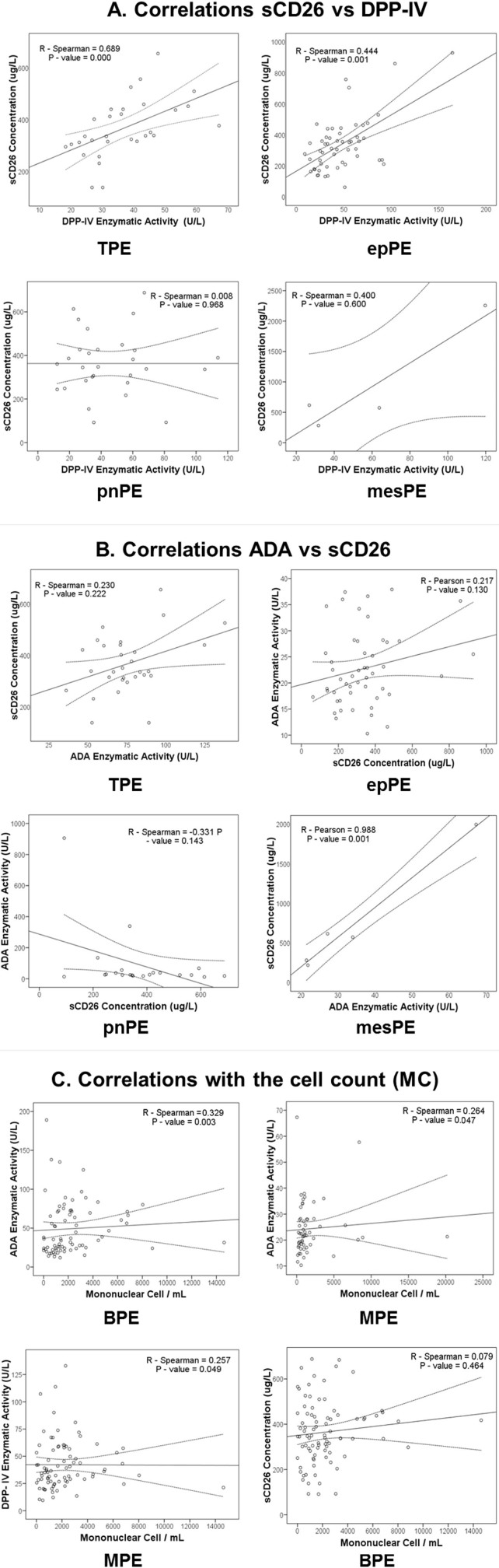
Since serum DPP-IV activity and sCD26 concentration are not always correlated1, the correlation values in PE were analysed for the different groups of patients. The DPP-IV activity and sCD26 values were correlated in both the BPE group (Spearman's R = 0.34 and p = 0.001) and MPE group (Spearman's R = 0.433 and p < 0.001) (Table 5 and Fig. 2). The DPP-IV activity and sCD26 values in the TPE sub-group and in the epithelial MPE sub-group (with higher enzymatic activity) were also correlated (Spearman R = 0.69, p < 0.001; and R = 0.44, p = 0.001, respectively). We conclude that the values of both biomarkers in PE are generally specific to the sCD26/DPP-IV protein. Interestingly, in the other sub-groups in which the correlations could be determined, mesotheliomas in MPE and parapneumonia and miscellaneous in BPE, the correlation was totally absent, except in non-neoplasic BPE (Table 5). An additional pattern related to that protein therefore appeared in these pathological conditions.
Table 5. Correlations between ADA and DPP-IV activity and sCD26 concentration in PE according to the groups under study.
| n | R- Pearson | P- Pearson | R-Spearman | P-Spearman | ||
|---|---|---|---|---|---|---|
| BPE: TPE | sCD26 vs ADA | 30 | - | - | 0.230 | 0.222 |
| sCD26 vs DPP-IV | 30 | - | - | 0.689 | 0.000 | |
| ADA vs DPP-IV | 30 | 0.039 | 0.839 | - | - | |
| BPE: Pneumonic | sCD26 vs ADA | 21 | - | - | −0.331 | 0.143 |
| sCD26 vs DPP-IV | 29 | - | - | 0.008 | 0.968 | |
| ADA vs DPP-IV | 21 | - | - | 0.466 | 0.033 | |
| BPE: Miscellaneous | sCD26 vs ADA | 10 | - | - | 0.224 | 0.533 |
| sCD26 vs DPP-IV | 9 | - | - | 0.067 | 0.865 | |
| ADA vs DPP-IV | 8 | −0.209 | 0.619 | - | - | |
| BPE: Non neoplasic | sCD26 vs ADA | 15 | - | - | −0.196 | 0.483 |
| sCD26 vs DPP-IV | 17 | 0.796 | 0.000 | - | - | |
| ADA vs DPP-IV | 14 | - | - | −0.323 | 0.260 | |
| MPE: Epithelial | sCD26 vs ADA | 50 | 0.217 | 0.130 | - | - |
| sCD26 vs DPP-IV | 54 | - | - | 0.444 | 0.001 | |
| ADA vs DPP-IV | 48 | - | - | 0.115 | 0.437 | |
| MPE: mesothelioma | sCD26 vs ADA | 5 | 0.988 | 0.001 | - | - |
| sCD26 vs DPP-IV | 4 | - | - | 0.400 | 0.600 | |
| ADA vs DPP-IV | 3 | - | - | 0.500 | 0.667 |
n: Number of samples analysed, with statistically significant differences shown in bold type. Pearson's analysis was used for normally distributed samples, and Spearman's analyses was used for non-normally distributed samples.
Figure 2.
Correlations between (A) sCD26 concentration and DPP-IV activity, and (B) ADA activity and sCD26 concentration, in some types of PE. Spearman's correlation coefficient (R) and p values are shown on the graphs; (C) Correlation between these biomarkers and the MC counts in BPE or MPE groups.
A significant correlation between ADA and sCD26 was also detected in the MPE samples (R = 0.3, p = 0.023), probably due to the high coefficient (Pearson's) obtained for mesotheliomas, although no such correlation was observed in epithelial MPE samples (Table 5). No correlation between these parameters was found in BPE, as well (Table 5). A correlation between DPP-IV and ADA activity was observed in patients grouped as MPE or BPE (Pearson's test, data not shown), but when the different sub-types were analysed separately, a correlation (Spearman's test) was only found in patients with parapneumonic PE (Table 5). These findings confirm a biological relationship between these two proteins, although the type of relationship will depend on the aetiology of the PE.
We also compared our data with the standard values of biochemical parameters and cell counts. With respect to biochemical parameters, the most consistent finding was that LDH levels and total protein concentration were significantly correlated with sCD26, ADA and DPP-IV in MPE and BPE (with the following exceptions: LDH and sCD26 in BPE, and total protein concentration and DPP-IV or ADA in MPE (data not shown). ADA and DPP-IV (and sCD26 nearly significant) were negatively correlated with glucose and pH only in BPE (data not shown).
With respect to cell counts, ADA was correlated (Spearman test) with the number of MC in both BPE (R = 0.329; p = 0.003) and MPE (R = 0.264; p = 0.047)( Fig. 2C, upper dots), as well as with the number of PMN (Pearson R = 0.604; p < 0.001 in BPE, and R = 0.315; p = 0.018 in MPE), as expected. However, while DPP-IV activity was significantly correlated with the number of PMN (R = 0.351; p = 0.001) and almost correlated with the number of MC (R = 0.191; p = 0.077) in BPE, and it was correlated with the number of MC in MPE (R = 0.257; p = 0.049) (Fig. 2C, low left dot), sCD26 concentration was not correlated with the cell count (data not shown and Fig. 2C bottom right).
Pleural effusion sCD26 immunoblot
As mentioned before, in the mesothelioma group, unlike in epithelial MPE, we detected more sCD26+ cases than DPP-IV+ cases. Furthermore, in mesothelioma MPE and pneumonic and miscellaneous BPE, there was a total lack of correlation between CD26 and DPP-IV; finally, DPP-IV activity (but not sCD26 concentration) was significantly correlated with the number of leukocytes. Thus, we analysed samples from patients of different pathologies with disparate or uncorrelated values for sCD26 and DPP-IV by western blotting to try to obtain biochemical information to explain the above results.
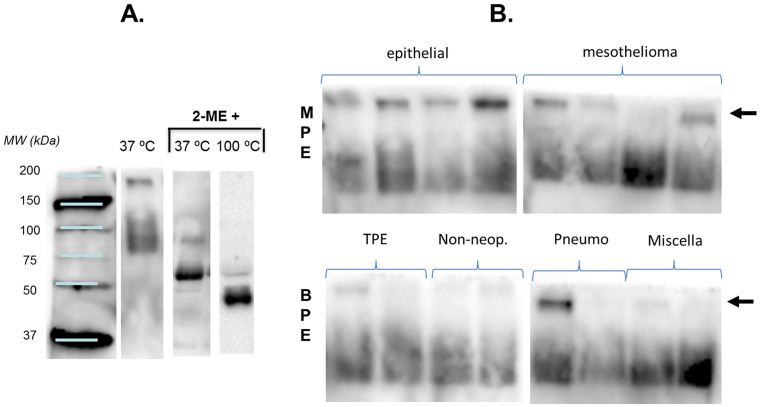
The TP1/16 anti-CD26 mAb was characterized in immunoblots of cell lysates under partially denaturing conditions so that it could be compared with other known anti-CD26 mAb, which only work under such conditions25,26. In serum, a region of three diffuse bands of between 125 and 135 kDa was observed (data not published), as in cell lysates, plus a band of around 180 kDa and an additional region of three diffuse bands of between 80 and 90 kDa. These MW are very similar to the monomer of 727 aa of sCD26 (Δ39-766 of PM CD26) with different degrees of glycosylation (80–90 kDa), plus the additional presence of one ADA molecule (around 42 kDa)-CD26 interaction (as already mentioned, CD26 was first identified as ADA binding or complexing protein)1,2,3,4,12,27.
In PE (Fig. 3A), under the same partially denaturing conditions, staining in the 125–135 kDa region was very slight or was absent in the samples analysed and the major bands were around 80–90 kDa. It can be deduced that the sCD26 in PE was mainly free of ADA (of the ADA-1 isoform). In addition, under denaturing conditions, the anti-CD26 mAb was able to stain bands of 60 kDa (37°C) or 45 kDa (100°C) (Fig. 3A), which may correspond to the same protein lacking a glycosylated epitope.
Figure 3.
(A) Western blot of PE sCD26 with anti-CD26 mAb TP1/16 according to the sample conditions used in SDS-PAGE.(B) The same western blot in samples from different pathologies showing the band of 180 kDa (arrows) mainly in MPE, which was absent or weaker in BPE samples. No clear trend was observed for the other bands of lower MW. Gel analyses were repeated 3–4 times.
The main finding (Fig. 3B) was that the 180 kDa band detected in serum under the partially denaturing conditions was present in most of the MPE samples and absent or fainter in most of the BPE samples. Because of the potential diagnostic value, we analysed the band by Mass Spectrometry (MS/MS) in triplicate experiments. We identified fibrinogen alpha, beta and gamma chains, and alpha-2 macroglobulin proteins, but not sCD26.
Discussion
We observed lower levels of DPP-IV enzymatic activity in TPE patients than in patients with other benign or malignant PE and similar levels of sCD26 in all types of patients. This contrasts with previous reports of significant increases in DPP-IV activity19 and sCD26 levels18,20 in TPE. Although the sCD26 levels we observed in TPE were similar to those reported by Wang et al20, these authors used a different type of control group (fewer patients and pathologies) than in the present study. We also found low levels of sCD26 in epithelial MPE (although not as low as in Wang's study); however, many patients had high levels of sCD26 in the MPE group. We did not find any epidemiological factor or anatomopathological data that discriminated epithelial MPE sub-groups according to sCD26 levels. In comparison with a study with fewer patients, Oshikawa and Sugiyama18 found that some TPE patients had very high sCD26 levels, but this was not repeated in our data.
These results for sCD26 were confirmed by the similar data for the DPP-IV activity measured in parallel in our study. The DPP-IV activity levels were similar to those observed by Küpeli et al in TPE19. However, we found that the activity of this enzyme was higher in pneumonic BPE and MPE than in TPE. These differences may be due to the larger number of patients examined in our study.
We found that use of sCD26/DPP-IV as biomarkers did not improve the sensitivity of TPE diagnosis. However, measurement of DPP-IV may improve the specificity of TPE diagnosis because, when the ROC curve that best differentiated tuberculous plus non-neoplasic PE groups from the other groups was done, yielding a cut-off value of 60 UL−1, 6 out of 11 false positive TPE (ADA positive in other non-TPE groups) were also DPP-IV positive. Interestingly because of the lack of biomarkers for MPE, a relatively large number of MPE were ADA negative and sCD26 positive (in particular DPP-IV). However, there were no specific clinical data that might be associated to these cases, and some BPE cases would be classified as positive for this combination of the biomarkers.
Statistical analysis of the data on these biomarkers and ADA revealed that the sCD26 concentration and ADA activity were correlated in the mesothelioma MPE group but not in the other PE groups. This was probably because of changes in the presence of ADA2 isoform, which does not bind to sCD26 but is abundant in PE6,7,10 (we did not find the ADA1 isoform bound to sCD26 in the immunoblots). This suggests that analysis of ADA isoforms could help enhance the specificity of TPE diagnosis28. Similarly, DPP-IV and ADA activities were correlated in patients grouped as MPE and BPE, but not in patients grouped according to aetiology, except in patients with parapneumonic PE.
Analysis of biomarkers in relation to the cell count revealed a strong correlation between ADA and the number of leukocytes in both BPE and MPE groups, whereas sCD26 was not correlated with the cell count. Although ADA2 is derived from the monocyte fraction, the origin of sCD26/DPP-IV is more controversial. It has been suggested that serum sCD26 may originate from liver, endothelial capillary cells and immune cells (mainly T, cells)1. From our data, we conclude that only a minor fraction, if any, of sCD26 from PE (both MPE and BPE) originated from immune cells. In the lung, apart from capillaries, DPP IV has also been demonstrated in the submucosal serous glands and the alveolar cells4. However, the significant correlation between DPP-IV activity (but not sCD26) and leukocyte number (mainly MC) in both BPE and MPE groups indicates that a fraction of this activity is indeed derived from lymphocytes.
Serum DPP-IV enzymatic activity and sCD26 concentration are not closely correlated in patients of certain diseases and even in healthy donors, for at least three reasons1: i) some circulating proteins other than sCD26 display DPP-IV activity29; ii) hypersialylation (which is strongly enhanced in elderly individuals) of sCD26 can inhibit DPP-IV activity; and iii) the serum protein attractin may regulate the DPP-IV activity of CD2630,31,32 Interestingly, the performance of sCD26/DPP-IV as biomarkers was also different in PE samples. Although DPP-IV activity and sCD26 concentration were strongly correlated, confirming that most of the data above are specific for the sCD26/DPP-IV protein, the correlation was totally absent in some PE subgroups (mesotheliomas in MPE, and pneumonic and miscellaneous in BPE), indicating that one of the above-mentioned patterns probably occurred in these pathological conditions.
Like the anti-CD26 mAb used in the present study, in samples of serum the 1F7 anti-CD26 mAb detected by western blot a band of 180 kDa which was originally described as DPPT-L30,31, a protein originated in T cells with DPP-IV activity. The band has recently been identified as the protein attractin strongly bound to sCD2629,32. In this study, the TP1/16 anti-CD26 mAb identified a 180 kDa band, which was more abundant in those pathologies in which the correlations were absent, and in which DPP-IV was associated with mononuclear cells.
However, analysis of the band by MS revealed alpha, beta and gamma chains of fibrinogen and alpha-2 macroglobulin proteins, but not sCD26. These proteins have been found in PE33,34 and may mask sCD26-attractin. However, further study is warranted by previous findings that CD26/DPP-IV acts as receptor for plasminogen type 235,36 and that alpha-2-macroglobulin inactivates serine-proteases and also inhibits fibrinolysis via inhibition of plasmin37.
Methods
Patients
Patients (n = 159) admitted between April 2007 and December 2010 to the Unit of Interventional Bronchopleural Pathology, of the Pneumology Service of Complejo Hospitalario Universitario de Vigo (CHUVI) with a specific diagnosis for exudative PE were consecutively enrolled in this study.
All procedures followed the clinical-ethical practices of the Spanish Government; the protocol of the study was approved by the Galician Ethical Committee for Clinical Research (2007/179), and complied with the Helsinki Declaration, Oviedo Agreement, the Organic Law for Data Protection 15/1999 and Royal Decree 1720/2007. Informed consent was obtained from all subjects.
Sample diagnosis
As described in previous studies38,39, initial diagnostic thoracocentesis for biochemical, microbiological and cytological studies was performed in all patients. If a diagnosis was not obtained after this test in patients with exudative PE, a second thoracocentesis and a blind percutaneous pleural biopsy with an Abrams needle were carried out. In some patients in whom PE was still not identified, thoracoscopy was conducted. The following biochemical parameters and cell counts in pleural fluid were determined before any therapy was started: pH, protein concentration, glucose and LDH levels, and the number of leukocytes and percentage of mononuclear (MC) and polymorphonuclear (PMN) cells.
The aetiology of PE was based on accepted criteria, as described by the Spanish Society for Pneumology and Thoracic Surgery40 and the British Thoracic Society guidelines41. Benign PE (BPE) was divided into five groups: tuberculous, parapneumonic, miscellaneous, paramalignant and non-neoplasic of unknown origin, according to recently reported diagnositc measures39,42. Malignant PE (MPE) included PE in which malignant cells were demonstrated at cytological or histological examination or in a biopsy specimen. MPE was divided into three subgroups according to the aetiology of the PE: epithelial-origin neoplasia, mesothelioma and lymphoma. Patients who were not classified in any of these diagnostic groups were excluded from the study.
Sample collection
Approximately 10 mL of PE sample was obtained with a needle at the same time as thoracocentesis and pleural biopsy. Samples were centrifuged at 800 g for 15 minutes, and frozen in 0.5 aliquots at −20°C until the measurements were performed.
Enzyme assays
Abnormal values were obtained for haemolytic and turbid samples. These values were excluded from data analysis.
The ADA was determined by an automated test carried out in a Unicel DXC 600i Syncron (Beckman Coulter). The measurement was based on the colorimetric kinetics of ADA catalytic activity whereby adenosine is transformed to inosine and ammonia, and the ammonia then reacts with sodium hypochlorite and phenol at 37°C to form indophenol, which yields an intense blue colour. The indophenol was measured spectrophotometrically at 630 nm. The results are expressed in UL−1.
DPP-IV activity was measured in 96-well culture plates with Gly-Pro-p-nitroanilide (0.2 mM, Sigma) as substrate in reaction mixtures (100 μL) containing PF samples (10 μL) and 50 mM Tris-HCl, pH 8.015. After incubation of the plates for 15 min, substrate hydrolysis was monitored at a wavelength of 405 nm in a BioRad Model 680 microplate reader. The results were determined by comparison with a standard curve for p-nitroaniline (Sigma data sheet) and the activity is expressed as UL−1. All experiments were performed in duplicate, unless otherwise specified.
Determination of the sCD26 levels
The sCD26 concentration was measured with the sCD26 ELISA kit (Bender Medsystems; Vienna, Austria) according to the manufacturer's instructions1,13. Colorimetric quantification was performed with a microplate reader (model 550; Bio-Rad, USA) at 450/630 nm.
Immunoblotting of soluble CD26
First, the amount of protein in the PE was quantified by the Bradford protein assay (Biorad, CA, USA). Samples containing 20 μg of protein were then subjected to 7.5% SDS-PAGE under non-totally reducing (samples with β-mercaptoethanol incubated at 37°C for 15 min) conditions and electroblotted onto PVDF membranes (Millipore, Bedford, MA, USA). Blots were treated with 5% skimmed milk blocking buffer (TBS 1% Tween-20) and were incubated overnight at 4°C. After three washes with TBS-Tween buffer, the membranes were incubated for 1 h at room temperature with anti-human CD26 mAb (clone TP1/16). The TP1/16 anti-CD26 antibody was previously characterized25 in immunoblots of cell lysates under partially denaturing conditions so that it could be compared with the better-known anti-CD26 1F7 mAb, both of which perform considerably better than other antibodies used in several immune techniques26. Goat anti-mouse IgG-HRP conjugated secondary antibody Fc-Specific (Sigma Aldrich) and Precision Plus Protein, Western C Standards (Bio Rad, USA) was used for protein detection before membranes were exposed a few minutes to ECL Prime Western Blotting Reagents substrate solution (GE, Amersham, UK) under appropriate dark room conditions. Results were analysed with ChemiDoc XRS (BioRad) software.
Protein identification by mass spectrometry
In parallel to the above-described immunoblot, a band of 180 kDa was excised from a replicate gel and digested with 12.5 ng/μL of trypsin (Roche Molecular Biochemicals) in 25 mM ammonium bicarbonate (pH 8.5) overnight at 37°C43. The supernatant was then collected and 1 μL was spotted onto a MALDI target plate and allowed to air-dry at RT. Then, 0.4 μL of α-cyano-4-hydroxy-cinnamic acid matrix (3 mg/mL) (Sigma) in 50% (v/v) ACN was added to the dried peptide digest spots and allowed to air-dry at RT. MALDI-TOF MS analysis (4800 Proteomics Analyzer MALDI-TOF/TOF mass spectrometer (Applied Biosystems) was performed by the Proteomics Service, University of Vigo. Mass spectra were calibrated with internal peptides from trypsin autodigestion. A database search was performed with MASCOT Daemon search engine 2.1 (Matrix Science) through the Global Protein Server version 3.6 (Applied Biosystems) against SwissProt 2012_07 (536789 sequences; 190518892 residues), as previously described43.
Statistical analysis
Statistical analysis was performed with the SPSS package (v16.0). Two tailed tests were carried out, and differences were considered significant at p < 0.05. Normal distributions and homogeneity of variances were verified by Kolmogorov-Smirnov and Mann-Whitney U tests respectively. Data from the three measures combined, alone, or grouped according to the pathological condition were not always normally distributed (shown when necessary).
The Mann-Whitney U test was used for analysis of two independent samples, and the Kruskal-Wallis ANOVA was used for more than two samples. Fischer's exact tests for contingency tables were used for analyses regarding positivity/negativity of the sCD26. Correlations between parameters were evaluated by parametric and non-parametric tests according to their distribution. Differences were considered significant at p < 0.05.
For ROC curve analysis of ADA, sCD26 and DPP-IV values, cut-offs points were generated with MedCalc, version 11.5.1 (MedCalc Software, Belgium). We selected the cut-off values with the highest value for the combined sensitivity and specificity for TPE detection as a proxy for the optimal cut-off value. For ADA, the cut-off obtained was close to 40 UL−1, i.e. the value used in the clinical setting. However, for sCD26, a cut-off corresponding to the mean value plus the standard deviation of the TPE group, performed better.
Net Reclassification Improvement (NRI) is an intuitive and easy to interpret measure for evaluation of potential added value of new diagnostic instruments or biomarkers in daily clinical practice. We chose this model44 to measure the biomarkers in combination with the ADA test for the diagnosis of TPE, using the same cut-off values obtained as explained above, i.e. category-based NRI. This enables the standard clinical values of specificity and sensitivity to be maintained45. We also calculated the NRI by testing a model with two or three cut-off values per variable (category-free or continuous-NRI) to determine whether the biomarkers could diagnose other non-TPE groups.
Author Contributions
O.J.C. and M.P.d.l.C. designed the study; N.S.O. and M.I.B.R. obtained the samples; M.I.B.R. recorded the clinical data; O.J.C. and N.S.O. performed the experiments; O.J.C., N.S.O. and M.P.d.l.C. wrote and revised the manuscript; F.J.R.B. organized the group and permitted the study.
Acknowledgments
This research was partially supported by grants PS09-00405 and Research Intensification activity from the Fondo de Investigación Sanitaria (FIS) of the Instituto de Salud Carlos III (Spain) and funding from Xunta de Galicia and FEDER (CN 2011/024). We are grateful to the patients who participated and made the study possible. We particularly thank A. Fernández, V. Leiro, C. Represas, and M. Núñez (Pneumology Department, CHUVI) for their collaboration in acquiring the samples.
References
- Cordero O. J., Salgado F. J. & Nogueira M. On the origin of serum CD26 and its altered concentration in cancer patients. Cancer Immunol. Immunother. 58, 1723–1747 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonacker E. & Van Noorden C. J. F. The multifunctional or moonlighting protein CD26/DPPIV. Eur. J. Cell. Biol. 82, 53–73 (2003). [DOI] [PubMed] [Google Scholar]
- Cordero O. J. et al. Potential of soluble CD26 as a serum marker for colorectal cancer detection. World J. Clin. Oncol. 2, 245–261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir A. M., Durinx C., Scharpé S. & De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 40, 209–294 (2003). [DOI] [PubMed] [Google Scholar]
- Jantz M. A. & Antony V. B. Pathophysiology of the pleura. Respiration. 75, 121–133 (2008). [DOI] [PubMed] [Google Scholar]
- Trajman A. et al. Pleural fluid ADA, IgA-ELISA and PCR sensitivities for the diagnosis of pleural tuberculosis. Scand. J. Clin. Lab. Invest. 67, 877–884 (2007). [DOI] [PubMed] [Google Scholar]
- Valdés L., Pose A., San José E. & Martínez-Vázquez J. M. Tuberculous pleural effusions. Eur. J. Intern. Med. 14, 77–88 (2003). [DOI] [PubMed] [Google Scholar]
- Baba K., Hoosen A. A., Langeland N. & Dyrhol-Riise A. M. Adenosine deaminase activity is a sensitive marker for the diagnosis of tuberculous pleuritis in patients with very low CD4 counts. PLoS One. 3, e2788 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon A. H. et al. Diagnostic tools in tuberculous pleurisy: a direct comparative study. Eur. Respir. J. 22, 589–591 (2003). [DOI] [PubMed] [Google Scholar]
- Krenke R. & Korczyński P. Use of pleural fluid levels of adenosine deaminase and interferon gamma in the diagnosis of tuberculous pleuritis. Curr. Opin. Pulm. Med. 16, 367–375 (2010). [DOI] [PubMed] [Google Scholar]
- Laniado-Laborin R. Adenosine deaminase in the diagnosis of tuberculous pleural effusion: is it really an ideal test? A word of caution. Chest 127, 417–418 (2005). [DOI] [PubMed] [Google Scholar]
- Ten Kate J., Dinjens W. N., Meera Khan P. & Bosman F. T. Adenosine deaminase complexing protein in cancer studies. Anticancer Res. 6, 983–988 (1986). [PubMed] [Google Scholar]
- Cordero O. J. et al. Cytokines regulate membrane adenosine deaminase on human activated lymphocytes. J. Leukoc. Biol. 70, 920–930 (2001). [PubMed] [Google Scholar]
- Herrera C. et al. Adenosine A2B receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol. Pharmacol. 59, 127–134 (2001). [PubMed] [Google Scholar]
- Cordero O. J., Salgado F. J., Viñuela J. E. & Nogueira M. Interleukin- 12 enhances CD26 expression and dipeptidyl peptidase IV function on human activated lymphocytes. Immunobiology 197, 522–533 (1997). [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D. et al. CD26 expression in leprosy and other granulomatous diseases correlates with the production of interferon-gamma. Lab. Invest. 73, 685–690 (1995). [PubMed] [Google Scholar]
- Willheim M. et al. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with T(h1) subsets. J. Allergy Clin. Immunol. 100, 348–355 (1997). [DOI] [PubMed] [Google Scholar]
- Oshikawa K. & Sugiyama Y. Elevated soluble CD26 levels in patients with tuberculous pleurisy. Int. J. Tuberc. Lung. Dis. 5, 868–872 (2001). [PubMed] [Google Scholar]
- Küpeli E., Karnak D., Elgün S., Argüder E. & Kayacan O. Concurrent measurement of adenosine deaminase and dipeptidyl peptidase IV activity in the diagnosis of tuberculous pleural effusion. Diag. Microbiol. Infect. Dis. 65, 365–371 (2009). [DOI] [PubMed] [Google Scholar]
- Wang H., Yue J., Yang J., Gao R. & Liu J. Clinical diagnostic utility of adenosine deaminase, interferon-g, interferon-g induced protein of 10 kDa, and dipeptidyl peptidase 4 levels in tuberculous pleural effusions. Heart Lung 41, 70–75 (2012). [DOI] [PubMed] [Google Scholar]
- Morrison M. E., Vijayasaradhi S., Engelstein D., Albino A. P. & Houghton A. N. A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J. Exp. Med. 177, 1135–1143 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havre P. A. et al. The role of CD26/dipeptidyl peptidase IV in cancer. Front. Biosci. 13, 1634–1645 (2008). [DOI] [PubMed] [Google Scholar]
- Kikkawa F. et al. Dipeptidyl peptidase IV in tumor progression. Biochim. Biophys. Acta 1751, 45–51 (2005). [DOI] [PubMed] [Google Scholar]
- Miyake Y. et al. Examination of CD26/DPPIV, p53, and PTEN expression in thyroid follicular adenoma. Diagn. Cytopathol. 40, 1047–1053 (2012). [DOI] [PubMed] [Google Scholar]
- Salgado F. J. et al. A role for interleukin-12 in the regulation of T cell plasma membrane compartmentation. J. Biol. Chem. 278, 24849–24857 (2003). [DOI] [PubMed] [Google Scholar]
- Salgado F. J. et al. Mechanisms of CD26/dipeptidyl peptidase IV cytokine-dependent regulation on human activated lymphocytes. Cytokine 12, 1136–1141 (2000). [DOI] [PubMed] [Google Scholar]
- Schrader W. P. & Stacy A. R. Immunoassay of the adenosine deaminase complexing proteins of human tissues and body fluids. J. Biol. Chem. 254, 11958–11963 (1979). [PubMed] [Google Scholar]
- Cordero O. J., Salgado F. J. & Nogueira M. Adenosine deaminase (ADA) isoenzymes ADA1 and ADA2 in biological fluids. Eur. Respir. J. 10, 2186–2187 (1997). [DOI] [PubMed] [Google Scholar]
- Sedo A., Stremenová J., Bušek P. & Duke-Cohan J. S. Dipeptidyl peptidase-IV and related molecules: markers of malignancy? Expert. Opin. Med. Diagn. 2, 1–13 (2008). [DOI] [PubMed] [Google Scholar]
- Duke-Cohan J. S., Morimoto C., Rocker J. A. & Schlossman S. F. Serum high molecular weight dipeptidyl peptidase IV (CD26) is similar to a novel antigen DPPT-L released from activated T cells. J. Immunol. 156, 1714–1721 (1996). [PubMed] [Google Scholar]
- Duke-Cohan J. S., Morimoto C., Rocker J. A. & Schlossman S. F. A novel form of dipeptidylpeptidase IV found in human serum. Isolation, characterization, and comparison with T lymphocyte membrane dipeptidyl peptidase IV (CD26). J. Biol. Chem. 270, 14107–14114 (1995). [DOI] [PubMed] [Google Scholar]
- Friedrich D. et al. Does human attractin have DP4 activity? Biol. Chem. 388, 155–162 (2007). [DOI] [PubMed] [Google Scholar]
- Alexandrakis M. G., Coulocheri S. A., Bouros D., Vlachonikolis I. G. & Eliopoulos G. D. Significance of alpha-2-macroglobulin, alpha-1-acid glycoprotein, and C-reactive protein in pleural effusion differentiation. Respiration 67, 30–35 (2000). [DOI] [PubMed] [Google Scholar]
- Wang Z. et al. Differential proteome profiling of pleural effusions from lung cancer and benign inflammatory disease patients. Biochim. Biophys. Acta 1824, 692–700 (2012). [DOI] [PubMed] [Google Scholar]
- Ghersi G. et al. The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res. 66, 4652–4661 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gronow M., Misra U. K., Gawdi G. & Pizzo S. V. Association of plasminogen with dipeptidyl peptidase IV and Na+/H+ exchanger isoform NHE3 regulates invasion of human 1-LN prostate tumor cells. J. Biol. Chem. 280, 27173–27178 (2005). [DOI] [PubMed] [Google Scholar]
- de Boer J. P. et al. Alpha-2-macroglobulin functions as an inhibitor of fibrinolytic, clotting, and neutrophilic proteinases in sepsis: studies using a baboon model. Infect. Immun. 61, 5035–5043 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botana-Rial M. et al. Validity of procalcitonin and c-reactive protein measurement when differentiating between benign and malignant pleural effusion. Clin. Lab. 57, 373–378 (2011). [PubMed] [Google Scholar]
- Sánchez-Otero N. et al. Calprotectin: a novel biomarker for the diagnosis of pleural effusion. Br. J. Cancer 107, 1876–1882 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena Garrido V. et al. Áreas de Técnicas y Trasplantes. SEPAR. Diagnóstico y tratamiento del derrame pleural. Sociedad Española de Neumología y Cirugía Torácica. Arch. Bronconeumol. 42, 349–372 (2006). [DOI] [PubMed] [Google Scholar]
- Roberts M. E., Neville E., Berrisford R. G., Antunes G. & Ali N. J. BTS Pleural Disease Guideline Group. Management of a malignant pleural effusion: British Thoracic Society pleural disease guideline 2010. Thorax 65, 32–40 (2010). [DOI] [PubMed] [Google Scholar]
- Heffner J. E. Diagnosis and management of malignant pleural effusion. Respirology 13, 5–20 (2008). [DOI] [PubMed] [Google Scholar]
- Rodríguez-Piñeiro A. M., Blanco-Prieto S., Sánchez-Otero N., Rodríguez-Berrocal F. J. & de la Cadena M. P. On the identification of biomarkers for non-small cell lung cancer in serum and pleural effusion. J. Proteomics 73, 1511–1522 (2010). [DOI] [PubMed] [Google Scholar]
- Pencina M. J., D'Agostino R. B. Sr, D'Agostino R. B. Jr & Vasan R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat. Med. 27, 157–172 (2008). [DOI] [PubMed] [Google Scholar]
- Richard E., Schmand B. A., Eikelenboom P. & Van Gool W. A. MRI and cerebrospinal fluid biomarkers for predicting progression to Alzheimer's disease in patients with mild cognitive impairment: a diagnostic accuracy study. BMJ Open 3, e002541, 10.1136/bmjopen-2012-002541 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]