Abstract
Desmoid tumors (DTs) are benign tumors that exhibit fibroblastic proliferation, which arises from fascial or musculoaponeurotic structures. The aim of this study was to investigate the characteristics and outcomes of patients with resectable DTs. A total of 21 patients were included and their clinicopathological characteristics were retrospectively analyzed. The 21 patients (16 females and 5 males) were identified through reviewing the patient charts at our institute. The tumor was located in the lower extremities in 7 cases, in the upper extremities in 4 cases, in the abdominal region in 9 cases and in the neck region in 1 case. Patients who had been initially treated by surgical excision were included in the study. Of these 21 patients, a positive surgical margin (SM) was reported in 11 patients, 7 of whom received postoperative radiotherapy (RT). Ten patients had a negative SM and 6 received RT. A total of 5 patients (46%) in the positive SM group and 4 (40%) in the negative SM group had documented disease relapse (P>0.05). The median relapse-free survival (RFS) was 20.5 months for the patients treated by surgery alone and 50 months for those treated with surgery followed by adjuvant RT (P>0.05). Age, gender, SM and adjuvant RT were not identified as predictors of recurrence. No predictive factors appeared to indicate local DT recurrence following surgery.
Keywords: desmoid disease, radiotherapy, surgery
Introduction
Desmoid tumors (DTs) are benign tumors that exhibit fibroblastic proliferation, which arises from fascial or musculoaponeurotic structures. This type of tumor is rare and accounts for 0.003% of all neoplasms (1,2). This connective tissue hyperplasia infiltrates locally and may result in debilitating pain. This type of tumor does not usually metastasize, although it tends to recur locally following surgical excision (3–5). DTs are poorly circumscribed and grow along tissue planes with a peculiar, infiltrative-like pattern toward the mesenchymal tissues; however, they do not spare the connective support of the viscera, glands or teguments. These pathological characteristics emphasize the high incidence of local recurrences, even in patients with a pathologically confirmed negative surgical margin (SM) (6). DTs may be sporadic, or associated with familial adenomatous polyposis (FAP) and Gardner’s syndrome. Approximately 2% of all DTs are associated with FAP, with these patients exhibiting a 1,000-fold increased risk of developing this type of tumor compared to the general population.
Surgical excision with clear margins is currrently the mainstay of treatment. Radiotherapy (RT) may be administered in cases where there is a locally positive SM, or when repeat surgery may prove to be difficult. Extensive surgery, with or without RT, may achieve a control rate of >60% (7–10). Systemic agents used for the treatment of DTs include doxorubicin-based combinations, methotrexate/vinblastine, tyrosine kinase inhibitors, tamoxifen, non-steroidal anti-inflammatory drugs, such as indomethacin and sulindac, and colchicine. Since this is a rare type of tumor, there is currently no established or evidence-based approach for the treatment of these neoplasms in the available literature.
The aim of this study was to determine the clinicopathological characteristics, treatment details and clinical course of patients with DTs.
Materials and methods
Patient characteristics
We retrospectively examined the charts of 21 patients who had been diagnosed with DT between January, 2005 and December, 2010. The patients were evaluated by a pathologist, a radiologist, an orthopedic surgeon, a radiation oncologist and a medical oncologist at our institute for definitive treatment and all the patients had histologically confirmed DTs. In addition, a colonoscopy was performed to confirm or exclude polyposis coli syndrome. The clinical records were reviewed and information regarding age, gender, tumor site, surgical details, pathological margin status, adjuvant therapy and treatment of recurrences was collected. The patients received 50–60 gray (Gy) RT.
This retrospective study was approved by the Institutional Review Board of the Institute of Oncology, University of Istanbul.
Statistical analysis
Data are presented as median and inter-quartile range (IQR) for events such as time to relapse after surgery. Age and follow-up time were expressed as median and range, respectively. The Chi-square and Mann-Whitney U tests were used for comparisons among the groups and the overall survival and progression-free survival (PFS) rates were calculated utilizing the Kaplan-Meier method. All analyses were performed using the SPSS statistical software, version 15.0 (SPSS Inc., Chicago, IL, USA). Two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
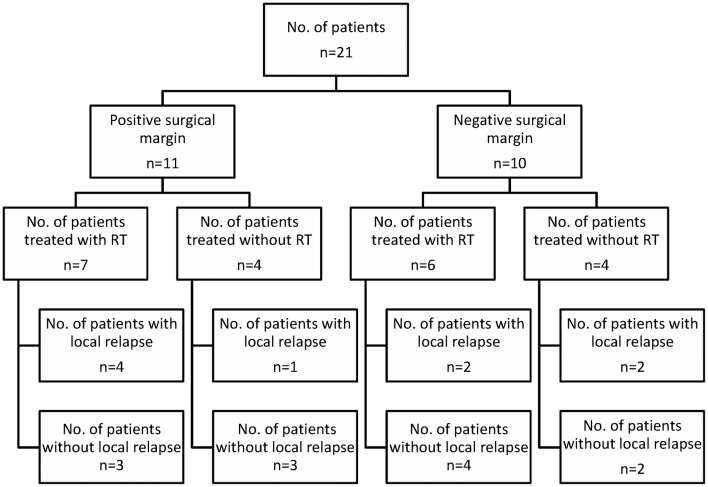
After examining patient records over a 5-year period, 21 patients with DTs [16 females (76.2%) and 5 males (23.8%)] were identified and included in this study. The median age of the subjects was 28 years (range, 12–52 years). The patient characteristics, treatment details and patterns of relapse are presented in Table I and Fig. 1. Seven tumors were located in the lower extremities (1 in the calf, 3 in the buttocks, 2 in the knees and 1 in the popliteal fossa). Four tumors were located in the upper extremities (2 in the shoulder, 1 in the upper arm and 1 in the forearm). Nine tumors were located in the abdomen and 1 in the neck. Additionally, 2 patients were diagnosed with Gardner’s syndrome and FAP (cases 5 and 14, respectively). The mean tumor size was 10.6 cm at presentation (range, 1.5–40 cm), 6.3 cm at the first relapse (range, 1.5–13 cm) and 2.6 cm at the second relapse (range, 1.3–4 cm).
Table I.
Clinical characteristics and treatment options of 21 patients with desmoid tumors.
| No. | Age (yrs)/gender | Primary site | Size (cm) | SM | Adjuvant treatment | Recurrence | Treatment for recurrence | Last follow-up | PFS | OS |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12/M | Popliteal fossa | 22×17×11 | + | RT | 2 | Surgery (R1–2), RT (R2) | NED | 91 | 91 |
| 2 | 12/F | Calf | 17×15×5 | + | None | 2 | Surgery (R1) + RT (R2) | NED | 4 | 16 |
| 3 | 14/F | Knee | 4×3×2.4 | + | RT | 3 | Surgery (R1–3), chemotherapy (R3, MTX) | NED | 18 | 53 |
| 4 | 20/F | Forearm | 8×4×3 | + | None | None | None | NED | 67 | 67 |
| 5 | 22/F | Abdomen | 13×8×8.5 | − | None | 4 | Surgery (R1–4), RT (R2) + chemotherapy (TAM, TAL) | Exitus | 1 | 59 |
| 6 | 23/M | Upper arm | 4.2×3.5×3 | − | RT | None | None | NED | 114 | 114 |
| 7 | 25/M | Knee | 1.5×0.2 | − | RT | 1 | Chemotherapy (R1, MTX) | NED | 17 | 24 |
| 8 | 26/F | Buttocks | 7×4×1.5 | − | RT | None | None | NED | 123 | 123 |
| 9 | 27/F | Shoulder | 9×3×2 | − | RT | 2 | Surgery (R1), chemotherapy (see results in text) | AWD | 153 | 170 |
| 10 | 27/F | Abdomen | 7.5×5×2.5 | + | None | None | None | NED | 53 | 53 |
| 11 | 28/F | Abdomen | 4×3.6×1.6 | − | RT | None | None | NED | 8 | 8 |
| 12 | 30/F | Buttocks | 12×8×7.5 | − | RT | None | None | NED | 23 | 23 |
| 13 | 32/M | Abdomen | 2.5×2×2 | + | RT | None | None | NED | 6 | 6 |
| 14 | 35/F | Abdomen | 13×10×5 | − | None | None | None | NED | 3 | 3 |
| 15 | 36/F | Neck | 3×2×1.5 | + | RT | 5 | Surgery (R1–5), RT (R1, R2) + chemotherapy (R3 EPI, R4 IMA, R5 TAM) | NED | 11 | 43 |
| 16 | 36/F | Abdomen | 2.5×1.6×1 | − | None | 1 | Surgery | NED | 62 | 62 |
| 17 | 36/F | Buttocks | 11×10×8 | + | RT | None | None | NED | 108 | 113 |
| 18 | 36/F | Abdomen | 6.2×3.3 | + | None | None | None | NED | 37 | 37 |
| 19 | 38/F | Abdomen | 16×15×11 | − | None | None | None | NED | 2 | 2 |
| 20 | 41/M | Abdomen | 19×13×6.5 | + | RT | None | None | NED | 50 | 50 |
| 21 | 52/F | Shoulder | 40×10×10 | + | RT | 2 | Surgery (R1–2), RT (R2) | NED | 108 | 219 |
F, female; M, male; yrs, years; SM, surgical margin; RT, radiotherapy; PFS, progression-free survival; OS, overall survival; R1–5, recurrence treatment; MTX, methotrexate; EPI, epirubicin; IMA, imatinib; TAM, tamoxifen; TAL, thalidomide; NED, no evidence of disease; AWD, alive with disease.
Figure 1.
Treatment details and patterns of relapse.
Follow-up
The median follow-up time was 53 months (range, 2–219 months). None of the patients developed distant metastasis. All the patients were initially treated by surgical excision and 13 patients also received RT. Five of the 11 patients (45%) with a positive SM and 4 of the 10 patients (40%) with a negative SM experienced a tumor relapse. The local recurrence rate was not significantly different between patients with positive and those with negative SM. Two patients had only 1 local recurrence and 7 patients had >1 recurrence. Of the 21 patients, 9 (42.8%) suffered a local relapse [3 of the 8 patients (38%) who received RT and 6 of the 13 patients (46%) who did not receive RT] (P>0.05). In addition, 2 of the 6 patients with a negative SM who were treated with postoperative RT also developed a local recurrence. The median PFS was 20.5 months (IQR: 2.25–63.5) in patients treated by surgical excision alone and 50 months (IQR: 14–111) in those who had surgery followed by RT (P>0.05).
Treatment
The patients received different systemic drugs, such as adriamycin, epirubicin, methotrexate, tamoxifen and thalidomide. In case 9, the DT transformed into a fibrosarcoma at the primary site. The patient subsequently developed lung metastasis during pregnancy and was treated with 3 cycles of single-agent adriamycin during pregnancy, followed by adriamycin and ifosfamide after pregnancy. The patient with Gardner’s syndrome underwent repetitive surgeries, RT and systemic treatment and eventually died from cranial hemorrhage, unrelated to her disease. None of the evaluated parameters, including age, gender, tumor site, tumor size or tumor border were correlated with the risk of local recurrence.
Discussion
The primary treatment for DTs is surgical resection with a wide SM. However, complete resection of the tumor with negative microscopic margins is often constrained by anatomical boundaries. The role of RT in the treatment of DTs has not been clearly defined. In the literature, a positive SM was shown to be a negative predictive factor for relapse-free survival (RFS). Systemic agents like imatinib and methotrexate are often used for patients for whom local therapy is not suitable.
DTs have a high rate of recurrence, even following complete surgical removal, and the contribution of a positive SM to local recurrence rates has not yet been determined. The recurrence rates of patients with resection and a negative SM were reported to be 16–39% (10–12). In addition, previous studies indicated that the risk of recurrence is independent of the margin status (13,14). In one of the largest series, 203 patients who underwent surgery for either primary or recurrent DT over a 35-year period were evaluated and the margins were found to be microscopically positive in 57 of the participants and negative in the remaining 146 (15). That study also demonstrated that patients with a positive margin had a 5-year RFS rate of 79% and a 10-year RFS rate of 74%, whereas those with a negative margin had a 5-year RFS rate of 82% and a 10-year RFS rate of 77% (P=0.5). In our series, the recurrence rate for patients with and without a positive SM were 45 and 40%, respectively (P>0.05).
RT has often been used for patients with a positive SM in oncology practice. However, the role of RT for DTs with a positive SM has not been established. Previous studies reported that RT alone (50–60 Gy) or RT combined with surgery in patients with incomplete resection achieved long-term disease control in ∼70–80% of DT patients (12,16,17). Spear et al (18) demonstrated that the 5-year local control rate of patients treated with a combination of surgery and RT (n=41) was 72%. Another study on 52 patients who were treated with RT in conjunction with gross total resection of the tumor, the 5- and 10-year relapse rates were 18 and 23%, respectively (16). The local control rate in patients treated with a combination of surgery and RT was 54% in our series. Even if RT was considered to be an option for the non-surgical definitive therapy of DTs, there would be an increased risk of potential late side effects, including secondary malignancies, particularly in younger patients, and RT-related fibrosis. Out of the 14 patients (7%) treated with RT in our series, 1 patient developed a fibrosarcoma.
Currently, repetitive surgery is considered the treatment of choice; however, certain patients may have an unpredictable clinical course. Therefore, a period of conservative management (systemic therapy or monitoring and assessing tumor progression) may be considered, particularly if resection may entail major morbidity (3,19). In a previous retrospective study, 83 patients underwent a ‘wait and see’ policy, whereas 59 patients were administered systemic therapy (20). The 5-year PFS in that study was 49.9% for the ‘wait and see’ group and 58.6% for the group treated with hormonal therapy or chemo-therapy (P=0.32). A multivariate analysis identified no clinical variables that may be considered independent predictors of PFS. However, all the patients in our series received treatment.
There are no evidence-based or widely accepted guidelines for the management of unresectable DTs. Systemic therapy is increasingly being integrated into a multidisciplinary approach for selected patients with unresectable or intra-abdominal DTs for which local therapy options may lead to unacceptable morbidity. However, our results were insufficient for interpreting the role of systemic agents in the management of such tumors. We may only suggest that systemic agents may be a part of the treatment approach.
In conclusion, DTs may be challenging due to their variable biological behavior and local morbidity, although their metastatic potential is low. Surgery remains the standard treatment option for resectable tumors. The effect of a positive SM, RT and systemic agents on recurrence have not yet been fully determined. With a better understanding of the molecular and genetic basis of this disease, targeted therapies with minimal toxicity profiles may become a more attractive treatment option in the future.
References
- 1.Pikaar A, Nortier JW, Griffioen G, Vasen HF. Desmoid tumors in patients with familial adenomatous polyposis. Ned Tijdschr Geneeskd. 2001;146:1355–1359. (In Dutch). [PubMed] [Google Scholar]
- 2.Reitamo JJ, Hayry P, Nykyri E, Saxen E. The desmoid tumor. I. incidence, sex, age and anatomical distribution in the Finnish population. Am J Clin Pathol. 1982;77:665–673. doi: 10.1093/ajcp/77.6.665. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JJ, Boland PJ, Leung DH, Woodruff JM, Brennan MF. The enigma of desmoid tumors. Ann Surg. 1999;229:866–873. doi: 10.1097/00000658-199906000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wara WM, Phillips TL, Hill DR, et al. Desmoid tumors - treatment and prognosis. Radiology. 1977;124:225–226. doi: 10.1148/124.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Khorsand J, Karakousis CP. Desmoid tumors and their management. Am J Surg. 1985;149:215–218. doi: 10.1016/s0002-9610(85)80067-2. [DOI] [PubMed] [Google Scholar]
- 6.Posner MC, Shiu MH, Newsome JL, Hajdu SI, Gaynor JJ, Brennan MF. The desmoid tumor. Not a benign disease. Arch Surg. 1989;124:191–196. doi: 10.1001/archsurg.1989.01410020061010. [DOI] [PubMed] [Google Scholar]
- 7.Anthony T, Rodriguez-Bigas MA, Weber TK, Petrelli NJ. Desmoid tumors. J Am Coll Surg. 1996;182:369–377. [PubMed] [Google Scholar]
- 8.Lynch HT, Fitzgibbons R. Surgery, desmoid tumors, and familial adenomatous polyposis: case report and literature review. Am J Gastroenterol. 1996;91:2598–2601. [PubMed] [Google Scholar]
- 9.Merchant NB, Lewis JJ, Wodruff JM, Leung DH, Brennan MF. Extremity and trunk desmoid tumors: a multifactorial analysis outcome. Cancer. 1999;86:2045–2052. [PubMed] [Google Scholar]
- 10.Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA. Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Oncol. 1999;17:158–167. doi: 10.1200/JCO.1999.17.1.158. [DOI] [PubMed] [Google Scholar]
- 11.Meazza C, Bisogno G, Gronchi A, et al. Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer. 2010;116:233–240. doi: 10.1002/cncr.24679. [DOI] [PubMed] [Google Scholar]
- 12.Nuyttens JJ, Rust PF, Thomas CR, Turrisi AT., III Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors: a comparative review of 22 articles. Cancer. 2000;88:1517–1523. [PubMed] [Google Scholar]
- 13.Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol. 2007;25:1785–1791. doi: 10.1200/JCO.2006.10.5015. [DOI] [PubMed] [Google Scholar]
- 14.Reitamo JJ. The desmoid tumor. IV. Choice of treatment, results, and complications. Arch Surg. 1983;118:1318–1322. doi: 10.1001/archsurg.1983.01390110066014. [DOI] [PubMed] [Google Scholar]
- 15.Gronchi A, Casali PG, Mariani L, et al. Quality of surgery and outcome in extra-abdominal aggressive fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21:1390–1397. doi: 10.1200/JCO.2003.05.150. [DOI] [PubMed] [Google Scholar]
- 16.Ballo MT, Zagars GK, Pollack A. Radiation therapy in the management of desmoid tumors. Int J Radiat Oncol Biol Phys. 1998;42:1007–1014. doi: 10.1016/s0360-3016(98)00285-5. [DOI] [PubMed] [Google Scholar]
- 17.Micke O, Seegenschmiedt MH. Radiation therapy for aggressive fibromatosis (desmoid tumors): results of a national patterns of care study. Int J Radiat Oncol Biol Phys. 2005;61:882–891. doi: 10.1016/j.ijrobp.2004.07.705. [DOI] [PubMed] [Google Scholar]
- 18.Spear MA, Jennings LC, Mankin HJ, et al. Individualizing management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys. 1998;40:637–645. doi: 10.1016/s0360-3016(97)00845-6. [DOI] [PubMed] [Google Scholar]
- 19.Bonvalot S, Eldweny H, Haddad V, et al. Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol. 2008;34:462–468. doi: 10.1016/j.ejso.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol. 2009;16:2587–2593. doi: 10.1245/s10434-009-0586-2. [DOI] [PubMed] [Google Scholar]