Abstract
Oral NaCl produces a greater natriuresis and diuresis than the intravenous infusion of the same amount of NaCl. Gastrin is the major gastrointestinal hormone taken up by renal proximal tubule (RPT) cells. We hypothesized that renal gastrin and dopamine receptors interact to synergistically increase sodium excretion, an impaired interaction of which may be involved in the pathogenesis of hypertension. In Wistar-Kyoto (WKY) rats, infusion of gastrin induced natriuresis and diuresis, which was abrogated in the presence of a gastrin (CCKBR; CI-988) or D1-like receptor antagonist (SCH23390). Similarly, the natriuretic and diuretic effects of fenoldopam, a D1-like receptor agonist, were blocked by SCH23390, as well as by CI-988. However, the natriuretic effects of gastrin and fenoldopam were not observed in spontaneously hypertensive rats (SHRs). The gastrin/D1-like receptor interaction was also confirmed in RPT cells. In RPT cells from WKY but not SHRs, stimulation of either D1-like or gastrin receptor inhibited Na+-K+-ATPase activity, an effect that was blocked in the presence of SCH23390 or CI-988. In RPT cells from WKY and SHRs, CCKBR and D1 receptor (D1R) co-immunoprecipitated, which was increased after stimulation of either D1R or CCKBR in RPT cells from WKY rats; stimulation of one receptor increased RPT cell membrane expression of the other receptor, effects that were not observed in SHRs. These data suggest that there is a synergism between CCKBR and D1-like receptors to increase sodium excretion. An aberrant interaction between the renal CCKBR and D1-like receptors (e.g., D1R) may play a role in the pathogenesis of hypertension.
Keywords: Gastrin receptor; D1 dopamine receptor; kidney, hypertension; renal proximal tubule
Introduction
Hypertension with its complications is currently a paramount problem imperiling human health which is caused by the combined effects of environmental and genetic factors1. There is general agreement that salt (NaCl) intake is one such environmental factor2, 3 and epidemiological studies have shown a positive correlation between sodium intake and blood pressure4. Thus, limiting salt intake and promoting sodium excretion are effective in the treatment of sodium-induced hypertension, keeping in mind that there may be a J-shaped relationship between sodium intake and mortality5. Indeed, in some individuals, blood pressure may actually increase with a low sodium intake6.
The kidney, especially the renal proximal tubule (RPT), which is responsible for about 60% of total renal sodium reabsorption, is critical in the regulation of sodium balance7–9. We and others have reported that renal dopamine, via D1-like (comprised of D1R and D5R subtypes) and D2-like receptors (comprised of D2R, D3R, and D4R subtypes), plays an important role in preventing volume expansion by increasing sodium excretion secondary to a decrease in renal sodium reabsorption, in several nephron segments, including the RPT8–10. At least 50% of basal sodium excretion in moderately volume expanded states is mediated by the paracrine action of renal dopamine exerted on D1 receptors (D1Rs)9,10. Dopamine, via D1R, inhibits the activity of Na+-K+-ATPase in the basolateral membrane and Na+/H+ exchanger (NHE3) in the apical membrane of RPT cells8,10–14. A dysfunction of the D1R is involved in the pathogenesis of hypertension8–10.
Depending on the state of sodium balance, an oral NaCl load has been reported to produce stronger natriuresis and diuresis than an intravenous infusion of the same amount of NaCl, indicating the existence of a gastro-renal axis15–18. Several hormones secreted by the stomach and duodenum have been suggested to be the effectors of the gastro-renal axis16–22. An effector of the gastro-renal axis may be gastrin, produced by the G cells of stomach antrum and duodenum16,20,21. Food intake increases circulating gastrin levels16,20,21 10–20-fold more than those of cholecystokinin (CCK)22 and of all the gut hormones, gastrin is the one that is taken up the most by RPTs23. Moreover, the gastrin receptor, also called cholecystokinin B receptor (CCKBR), is expressed in the kidney, including glomerular mesangial cells and collecting duct and proximal convoluted tubule cells16,21,24,25. Additionally, stimulation of CCKBR promotes natriuresis21,24,25. However, as aforementioned, we and others have suggested that the renal dopaminergic system is important in the excretion of an oral or an intravenous sodium load8–10. Therefore, we tested the hypothesis that gastrin interacts with dopamine receptors in the kidney, to synergistically increase sodium excretion. We hypothesized, further, that this interaction is impaired in hypertension. To test these hypotheses, we studied the natriuretic effect of intrarenal arterial infusion of gastrin with or without co-infusion of a D1-like receptor agonist, fenoldopam, in the presence or absence of their respective antagonists, CI-988 (CCKBR antagonist)26 and SCH2339027,28, D1-like receptor antagonist, in the normotensive Wistar-Kyoto (WKY) rat and the spontaneously hypertensive rat (SHR). We also studied the colocalization and physical and functional interactions between CCKBR and D1R in rat RPT cells.
Materials and Methods
(see the online Data Supplement.)
Results
1. In vivo study
1.1. Intrarenal infusion of gastrin induces natriuresis and diuresis in WKY rats but not SHRs
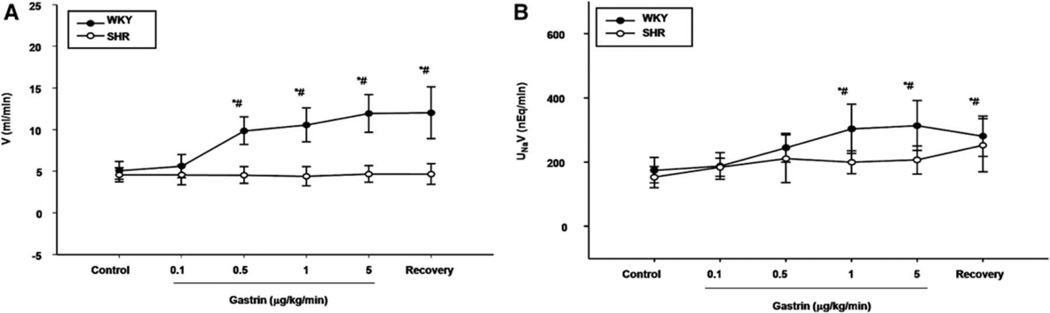
To determine the effect of renal CCKBRs on sodium excretion and urine flow, varying dosages of gastrin (0, 0.1, 0.5, 1.0, 5.0 µg/kg/min for 40 min in each period; n=6), were infused into the right suprarenal artery of WKY rats. In WKY rats, the intra-renal arterial infusion of the vehicle (normal saline) into the right kidney had no effect on urine flow (V) or absolute sodium excretion (UNaV) (Table S1), while gastrin increased V and UNaV; the increase in V was first observed at the dose of 0.5 µg/kg/min while the increase in UNaV was first observed at the dose of 1.0 µg/kg/min (Figures 1A and 1B, respectively). In contrast, gastrin had no effect on V and UNaV in SHRs (Figures 1A and 1B). The right intrarenal infusion of gastrin had no effect on blood pressure in WKY rat (Table S1) and SHR (not shown).
Figure 1. Effect of the renal infusion of gastrin on urine flow and sodium excretion in WKY and SHRs.
(A) Urine flow (V); (B) Absolute sodium excretion (UNaV). Varying dosages of gastrin (0.1–5.0 µg/kg/min) were infused into the right suprarenal artery of anesthetized rats. *P<0.05 vs. control, (repeated measures ANOVA, Holm-Sidak test). #P<0.05 vs. SHR, n= 6 (t-test).
To determine the specificity of the gastrin effect on urine flow and sodium excretion, a gastrin receptor (CCKBR) antagonist, CI-988, was used. CI-988, infused at 1.0 mg/kg/min, did not affect V and UNaV in WKY rats (Table S2); in the presence of CI-988, the diuretic and natriuretic effects of gastrin (1.0 µg/kg/min) were blocked (Table S3).
1.2. Stimulation of renal D1-like receptors induces natriuresis and diuresis in WKY rats
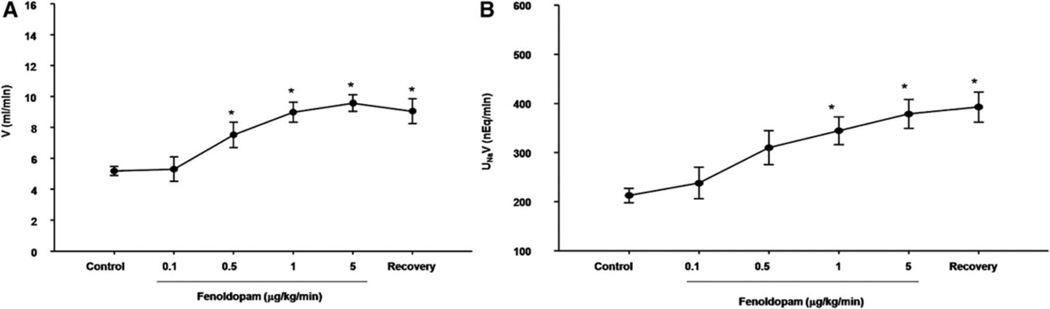
To determine the effect of D1-like receptors on natriuresis and diuresis, varying dosages of fenoldopam (0, 0.1, 0.5, 1.0, 5.0 µg/kg/min for 40 min in each period; n = 6), were infused into the right kidney (via the right suprarenal artery) in WKY rats. The intra-renal arterial infusion of fenoldopam increased V and UNaV; the increase in V was first observed at the dose of 0.5 µg/kg/min while the increase in UNaV was first observed at the dose of 1.0 µg/kg/min (Figures 2A and 2B). The impaired natriuretic and diuretic effects of dopamine and D1-like receptor agonists, including fenolodopam, in SHRs have been reported10,29, therefore, this experiment was not performed in the current study.
Figure 2. Effect of the renal infusion of a D1-like receptor agonist, fenoldopam, on urine flow and sodium excretion in WKY rats.
(A) Urine flow (V); (B) Absolute sodium excretion (UNaV). Varying dosages of the D1-like receptor agonist, fenoldopam (0.1–5.0 µg/kg/min), were infused into the right suprarenal artery of anesthetized rats. *P<0.05 vs. control, n= 4 (Repeated measures ANOVA, Holm-Sidak test).
A D1-like receptor antagonist, SCH23390, was used to determine the specificity of the D1-like receptors agonist (fenoldopam) on renal function. SCH23390, infused at the low dose of 0.4 µg/kg/min, did not affect V and UNaV in WKY rats (Table S4). In the presence of SCH23390, the natriuretic and diuretic effects of fenoldopam were blocked in WKY rats (Table S5).
1.3. Blockade of one receptor (D1–like or gastrin [CCKBR] receptor) abolishes the natriuresis and diuresis due to gastrin or fenoldopam, respectively, in WKY rats
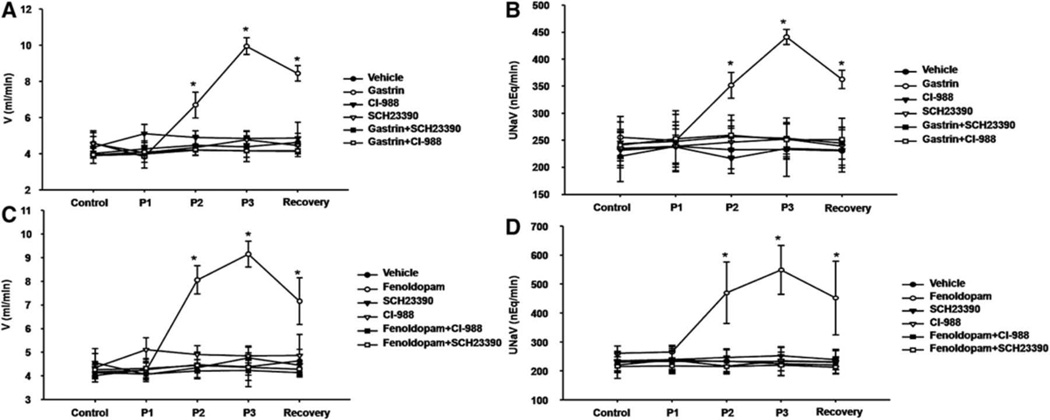
To determine if there is an interaction between D1-like and gastrin receptors, we investigated the diuretic and natriuretic effects of gastrin in the presence of the D1-like receptor antagonist, SCH 23390, and the diuretic and natriuretic effects of fenoldopam in the presence of the CCKBR antagonist, CI-988. In the presence of D1-like receptor antagonist, SCH23390 (0.4 µg/kg/min), the diuretic and natriuretic effects of gastrin were blocked (Figures 3A and 3B). Similarly, in the presence of gastrin receptor (CCKBR) antagonist, CI-988, the diuretic and natriuretic effects of fenoldopam (1.0 µg/kg/min) were also blocked (Figures 3C and 3D).
Figure 3. Interaction between gastrin and D1-like receptors on urine flow and sodium excretion in WKY rats.
A and B: The gastrin-mediated diuresis and natriuresis were blocked by a D1-like receptor antagonist (SCH23390) in WKY rats. Effect of gastrin (1.0 µg/kg/min; n=5), gastrin receptor antagonist CI-988 (1.0 mg/kg/min; n=5), D1-like receptor antagonist SCH23390 (0.4 µg/kg/min; n=5), or in combination (gastrin+SCH23390, n=5; gastrin+CI-988, n=6) on urine flow (V) (A) and absolute sodium excretion (UNaV) (B). During the control period, only the vehicle (saline) was infused. During period 1 (P1), the vehicle (saline) was infused in the gastrin group; CI-988 was infused in vehicle+CI-988, gastrin+CI-988; SCH23390 was infused in the vehicle+SCH23390 and gastrin+SCH23390 groups. During periods 2–3 (P2, P3), gastrin, instead of vehicle, was infused in the gastrin+SCH23390 and gastrin+CI-988 groups. In the recovery period, only the vehicle (saline) was infused in all the groups. Data are expressed as mean±SEM. *P<0.05 vs. other groups (Factorial ANOVA, Holm-Sidak test).
C and D: The fenoldopam-mediated diuresis and natriuresis were blocked by gastrin receptor antagonist (CI-988) in WKY rats. Effect of the D1-like receptor agonist fenoldopam (1.0 µg/kg/min; n=5), D1-like receptor antagonist SCH23390 (0.4 µg/kg/min; n=5), or in combination (fenoldopam+CI-988, n= 6; fenoldopam+SCH23390, n=5) on urine flow (V) (C) and absolute sodium excretion (UNaV) (D). During the control period, only the vehicle (saline) was infused. During period 1 (P1), the vehicle (saline) was infused in the fenoldopam group; CI-988 was infused in the vehicle+CI-988 and fenoldopam+CI-988; SCH23390 was infused in vehicle+SCH23390 and fenoldopam+SCH23390 groups. During periods 2–3 (P2, P3), fenoldopam, instead of vehicle, was infused in the fenoldopam+SCH23390 and fenoldopam+CI-988 groups. In the recovery period, only the vehicle (saline) was infused in all the groups. Data are expressed as mean±SEM. *P<0.05 vs. other groups (Factorial ANOVA, Holm-Sidak test).
2. In vitro study
2.1. The inhibitory effect of gastrin or fenoldopam on Na+-K+-ATPase activity is impaired in RPT cells from SHRs
The effect of gastrin on the activity of Na+-K+-ATPase, the primary active sodium transporter located at the basolateral membrane, was studied in RPT cells. We found that gastrin inhibited Na+-K+-ATPase activity in WKY RPT cells in a concentration (10−11–10−8 M) - and time-dependent manner (Figures S1A and S1B). In contrast, the inhibitory effect of gastrin (10−9 M) on Na+-K+-ATPase activity was not observed in SHR RPT cells (Figure S1C).
The receptor specificity of the inhibitory effect of gastrin on Na+-K+-ATPase activity was also studied. CI-988 (10−5 M/15 min), by itself, had no effect on the basal Na+-K+-ATPase activity, but in the presence of CI-988, the inhibitory effect of gastrin (10−9 M/15 min) on Na+-K+-ATPase activity was blocked (basal = 0.44±0.05; gastrin = 0.31±0.05 (P<0.05 vs. others); CI-988 = 0.38±0.03; gastrin+CI-988 = 0.38±0.03 µmol•Pi/mg•protein/min).
Previous studies29,30–32 have shown that the inhibitory effect of D1-like receptor agonists, on renal or RPT Na+-K+-ATPase activity is impaired in the SHR. We also studied the effect of fenoldopam on Na+-K+-ATPase activity in WKY RPT cells. We found that SCH23390 (10−6 M/15 min), by itself, had no effect on the basal Na+-K+-ATPase activity but blocked the inhibitory effect of fenoldopam (10−7 M/15 min) on Na+-K+-ATPase activity in WKY RPT cells (basal = 0.42±0.04; fenoldopam = 0.27±0.08 (P<0.05 vs. others); SCH23390 = 0.41±0.07; fenoldopam + SCH23390 = 0.37±0.01 µmol•Pi/mg•protein/min).
2.2. Gastrin (CCKBR) and D1-like receptors interact to inhibit Na+-K+-ATPase activity in WKY RPT cells
Gastrin receptor (CCKBR) and D1-like receptor interact in the inhibition of Na+-K+-ATPase activity in WKY RPT cells because in the presence of gastrin receptor (CCKBR) antagonist, CI-988 (10−5 M/15 min), the inhibitory effect of fenoldopam (10−7 M/15 min) on Na+-K+-ATPase activity was blocked (Figure S2A). Similarly, in the presence of a D1-like receptor antagonist, SCH23390 (10−6 M/15 min), the inhibitory effect of gastrin (10−9 M/15 min) on Na+-K+-ATPase activity was blocked (Figure S2B).
2.3. Augmented plasma membrane expression is involved in the synergistic interaction between D1–like and gastrin receptors in WKY RPT cells
To determine whether or not gastrin affects the cellular localization of D1R, the effect of short-term stimulation with gastrin on cell surface D1Rs was studied. Gastrin (10−9 M/15 min) caused a 44.5% increase in cell surface D1R expression (Figures S3A and S3B) in WKY RPT cells, but not in SHR RPT cells (WKY: control=24.28±6.92, gastrin=34.52±4.91 (P<0.05); SHRs: control=19.22±4.58, gastrin=21.98±5.40 DU [density units]; n=4/group; Figure S3C). Similarly, we also found that stimulation of D1-like receptors with fenoldopam increased gastrin receptor (CCKBR) expression in cellular membranes of WKY RPT cells (Figure S4A), but not in SHR RPT cells (Figure S4A). Cell surface D1R but not CCKBR expression was also lesser in SHRs than WKY rats. The ability of fenoldopam to increase cell surface D1R expression was blocked by both CCKBR and D1R antagonist which by themselves had no effect (Figure S4B).
2.4. Gastrin receptors (CCKBRs) co-localize with D1Rs in RPT cells
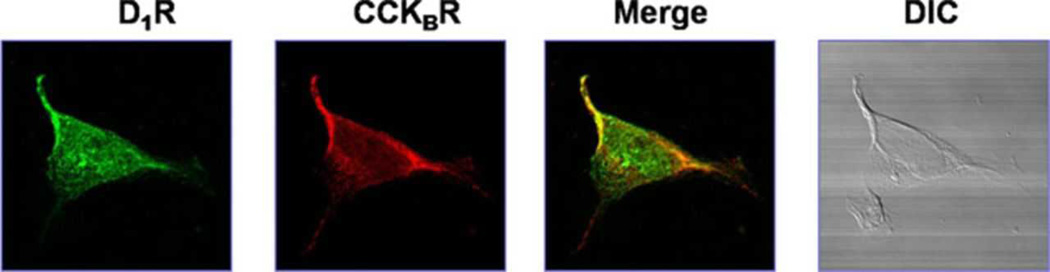
In order to affirm the potential for a direct or indirect interaction between gastrin (CCKBR) and D1Rs, we studied the co-localization of gastrin (CCKBR) and D1Rs in RPT cells from WKY rats. Immunofluorescence laser confocal microscopy showed that gastrin (CCKBR) and D1Rs were found throughout the cell, and with evidence of co-localization, especially at the plasma membrane (Figure 4). To show the specificity of the antibodies for CCKBR and D1Rs, those antibodies were pre-incubated with their corresponding immunizing peptides (1:10 w/w incubation for 12 hrs). The staining for TRITC-tagged CCKBR (red) and FITC-tagged D1R (green) were no longer visible when the CCKBR antibody was incubated with the CCKBR immunizing peptide and when the D1R antibody was incubated with the D1R immunizing peptide (Figure S5).
Figure 4. Co-localization of CCKBR and D1R in WKY RPT cells.
Co-localization appears as yellow after merging the images of FITC-tagged D1R (green) and TRITC-tagged CCKBR (red). No staining is seen without the antibodies (DIC). DIC: differential interference contrast.
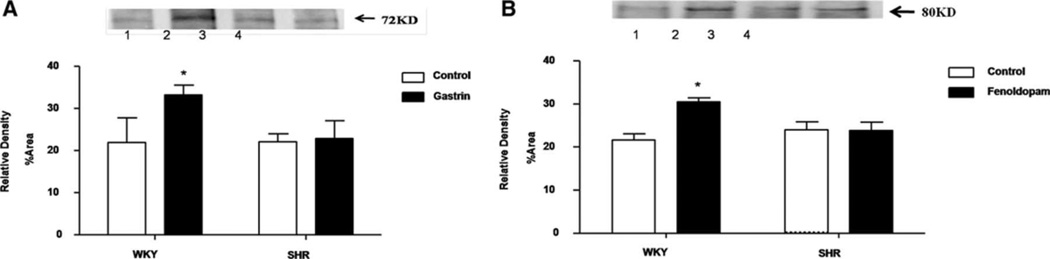
To determine whether or not there is a physical interaction between gastrin (CCKBR) and D1Rs, a co-immunoprecipitation study was performed; gastrin receptors were first immunoprecipitated with anti-gastrin (CCKBR) receptor antibodies and the co-immunoprecipitates were immunoblotted with anti-D1R antibodies. The CCKBR/D1R co-immunoprecipitation was increased with gastrin (10−9 M/15 min) treatment in WKY RPT cells, but not in SHR RPT cells; similar results were obtained after treatment with fenoldopam (Figures 5A and 5B).
Figure 5. Effect of gastrin or fenoldopam on the co-immunoprecipitation of CCKBR and D1R in RPT cells from WKY and SHRs.
The cells were incubated with gastrin (10−9 M, A) or fenoldopam (10−7 M, B) for 15 min. Thereafter, the samples were immunoprecipitated with CCKBR antibodies and immunoblotted with D1R antibodies (* P<0.05 vs. control; n=3–5; factorial ANOVA, Holm-Sidak test). One immunoblot (74 kDa) is depicted in the inset: (lane 1= vehicle-treated RPT cells from WKY rats, lane 2= gastrin-treated RPT cells from WKY rats, lane 3= vehicle-treated RPT cells from SHRs, and lane 4= gastrin-treated RPT cells from SHRs).
Discussion
As indicated earlier, depending on the state of sodium balance, an oral NaCl load produces a stronger diuresis and natriuresis than an intravenous infusion of the same amount of NaCl15,16. In addition to neural mechanisms, several gut hormones (e.g., CCK, uroguanylin) have been proposed to mediate the natriuresis of an oral NaCl load16–21. Although a high NaCl intake increases renal uroguanylin expression, it may not always increase circulating uroguanylin levels16,17,33,34. Guca2b−/− mice have an impaired natriuretic response to an acute oral NaCl load but blood pressure is only slightly increased and salt sensitivity is similar to that of Guca2b+/+ mice18. CCK is natriuretic16,19 but circulating CCK levels are not increased by an oral NaCl load35 and CCK is not taken up by renal tubules23.
Food intake increases circulating gastrin levels 10–20-fold more than those of CCK22, and, of all the gut hormones tested, gastrin is taken up the most by RPTs23, therefore, gastrin is a good candidate for the gastro-renal reflex. Moreover, the oral intake of NaCl, without food, increases circulating gastrin levels (Jose PA, et al, 2012, unpublished studies). The gastrin receptor, CCKBR, is expressed in specific nephron segments, including the proximal tubule16,21,24,25. Moreover, gastrin is important in the excretion of an acute oral sodium load because mice deleted of the gastrin (Gast) gene (Gast−/−) do not have a natriuresis after ingestion of food21 and develop salt-sensitive hypertension (Jose PA, et al. 2012, unpublished observations). Our current study confirms the diuretic and natriuretic effects of gastrin in WKY rats, and found that the natriuretic effect of gastrin is, in part, via the inhibition of Na+-K+-ATPase activity. However, in SHRs, the diuretic and natriuretic effects of gastrin are lost, as well as its inhibitory effect on Na+-K+-ATPase activity. These results may, at first glance, be taken as contradictory to our previous report that the intrarenal infusion of sulfated CCK octapeptide produced a similar natriuretic and diuretic effect in WKY and SHRs36. However, sulfated CCK octapeptide has a much greater affinity to CCKAR than CCKBR while gastrin has a much greater affinity to CCKBR than CCKAR20. Indeed, the natriuretic and diuretic effects of gastrin were blocked by the selective CCKBR antagonist, CI-98826,37. While CCKBR is expressed in the renal proximal tubule24,25, CCKAR is not38.
In addition to gastrin, dopamine, a catecholamine produced endogenously by the renal proximal tubule, plays an important role in the regulation of sodium excretion and blood pressure8–10. Several studies have shown that the natriuretic effect of dopamine is mainly exerted via D1-like receptors8,10,28,29,39. Consistent with previous reports,29,31,32, we found that renal D1-like receptor-mediated diuresis and natriuresis are impaired in SHRs. The impaired diuretic and natriuretic effects of D1-like receptors in the SHR29,31,32 is due, in part, to hyperphosphorylation and desensitization of the renal D1R because of increased constitutive activity of the G protein-coupled receptor kinase types 2 and 48–10,30,40–42.
Several other GPCRs and hormones/humoral factors negatively interact with the D1R, including aldosterone42 and α-adrenergic receptors43.AT1R27,44, insulin45, and renin46. While several GPCRs and other hormones/humoral factors have been reported to positively interact with the D1R in the regulation of sodium excretion, including atrial natriuretic peptide (ANP)/ANPA47, AT2R48, nitric oxide49, and prolactin50, the role of such interactions in hypertension has not been reported. Our present study finds that CCKBR is another GPCR that positively interacts with the D1R in the regulation sodium excretion. As indicated above, both gastrin and fenoldopam induce both diuresis and natriuresis in WKY rats. However, in the presence of a CCKBR antagonist, the fenoldopam-mediated diuresis and natriuresis are blocked and vice versa, i.e., a D1-like receptor antagonist blocks the diuretic and natriuretic effects of gastrin. However, whether or not Na+ balance and blood pressure regulation are the results of a net positive and negative interactions among D1R, other dopamine receptor, and other GPCRs remain to be determined.
We also sought to elucidate the underlying mechanism regulating the interaction between renal CCKBR and D1-like receptors, specifically, D1R. We found that CCKBR and D1R colocalize and physically interact, proved by the co-immunoprecipitation study. Stimulation of one receptor increases the cellular distribution of the receptor into the cell membrane, i.e., gastrin treatment increases D1-like receptor expression in the cell membrane; fenoldopam increases CCKBR in the cell membrane in WKY RPT cells. The RPT cell membrane targeting of D1R and CCKBR is physiologically relevant because blockade of D1-like receptors or CCKBR prevents the fenoldopam-mediated increase in RPT cell membrane D1R and the gastrin-mediated inhibition of Na+-K+-ATPase activity and vice versa; blockade of CCKBR blocks the inhibitory effect of fenoldopam on Na+-K+-ATPase activity. In SHR RPT cells, both the inhibitory effects of gastrin and fenoldopam on Na+-K+-ATPase activity are lost, and their synergism is no longer evident. Whether or not the synergistic inhibitory effect of CCKBR and D1R on sodium transport involves signaling mechanisms, in addition to direct protein/protein interaction, will be determined in the future.
In conclusion, we have demonstrated that gastrin, via CCKBR, interacts with D1-like receptors, specifically D1R in the kidney, synergistically increasing water and sodium excretions, effects that are not observed in SHRs. An impaired interaction among GPCRs, CCKBR and D1R, for instance, in the regulation of renal sodium excretion may be important in the pathogenesis of hypertension.
Perspective
The intrarenal infusion of gastrin or fenoldopam induces diuresis and natriuresis; CCKBR and D1-like receptors synergistically increase sodium excretion in WKY rats. However, in SHRs, both the diuresis and natriuresis mediated by CCKBR or D1-like receptors are no longer observed. Previous studies have shown that the D1R dysfunction in hypertensive states is due to the hyper-phosphorylation and uncoupling of D1R from its G protein-effector complex, caused by increased constitutive activity of G protein-coupled receptor kinases (GRKs) 2 and 48–10,30,40–42. Whether or not GRKs regulate CCKBR is not known. We hypothesize that GRKs impair CCKBR receptor function, including the interaction between CCKBR and D1R, in SHRs, which need to be confirmed in the future.
Supplementary Material
Novelty and Significance.
What Is New?
CCKBR and D1R synergistically increase sodium excretion in WKY rats. The natriuresis and diuresis mediated by CCKBR and its synergism with D1-like receptors to increase sodium excretion are not observed in SHRs.
What Is Relevant?
The impaired CCKBR and D1-like receptor-mediated natriuretic and diuretic effects in the SHR could be involved in the pathogenesis of hypertension.
Summary
CCKBR and D1-like receptors synergistically increase sodium excretion. Aberrant interaction between the renal CCKBR and D1-like receptors (e.g., D1R) may play a role in the pathogenesis of hypertension.
Acknowledgments
Sources of Funding
These studies were supported in part by grants from Natural Science Foundation Project of CQ CSTC (CSTC, 2009BA5044), the National Basic Research Program of China (973 Program, 2008CB517308, 2012CB517801, 2013CB531104), grants from the National Natural Science Foundation of China (30925018, 31130029, 81270338, 81070559), and grant from the US National Institutes of Health, DK039308.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Cowley AW, Jr, Nadeau JH, Baccarelli A, Berecek K, Fornage M, Gibbons GH, Harrison DG, Liang M, Nathanielsz PW, O'Connor DT, Ordovas J, Peng W, Soares MB, Szyf M, Tolunay HE, Wood KC, Zhao K, Galis ZS. Report of the National Heart, Lung, and Blood Institute Working Group on epigenetics and hypertension. Hypertension. 2012;59:899–905. doi: 10.1161/HYPERTENSIONAHA.111.190116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stamler J. The INTERSALT Study: background, methods, findings, and implications. Am J Clin Nutr. 1997;65:626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 3.Sanada H, Jones JE, Jose PA. Genetics of salt-sensitive hypertension. Curr Hypertens Rep. 2011;13:55–66. doi: 10.1007/s11906-010-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumler F. Dietary sodium intake and arterial blood pressure. J Ren Nutr. 2009;19:57–60. doi: 10.1053/j.jrn.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Dorresteijn JA, van der Graaf Y, Spiering W, Grobbee DE, Bots ML, Visseren FL Secondary Manifestations of Arterial Disease Study Group. Relation between blood pressure and vascular events and mortality in patients with manifest vascular disease: J-curve revisited. Hypertension. 2012;59:14–21. doi: 10.1161/HYPERTENSIONAHA.111.179143. [DOI] [PubMed] [Google Scholar]
- 6.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–1366. doi: 10.1161/HYPERTENSIONAHA.112.196071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman TM, Crowley SD. Kidney in hypertension: Guyton redux. Hypertension. 2008;51:811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 8.Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep. 2008;10:268–275. doi: 10.1007/s11906-008-0051-9. [DOI] [PubMed] [Google Scholar]
- 9.Harris RC. Abnormalities in renal dopamine signaling and hypertension: the role of GRK4. Curr Opin Nephrol Hypertens. 2012;21:61–65. doi: 10.1097/MNH.0b013e32834de2cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–H569. doi: 10.1152/ajpheart.01036.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibarra FR, Cheng SX, Agrén M, Svensson LB, Aizman O, Aperia A. Intracellular sodium modulates the state of protein kinase C phosphorylation of rat proximal tubule Na+,K+-ATPase. Acta Physiol Scand. 2002;175:165–171. doi: 10.1046/j.1365-201X.2002.00984.x. [DOI] [PubMed] [Google Scholar]
- 12.Pedrosa R, Gomes P, Soares-da-Silva P. Distinct signalling cascades downstream to Gsalpha coupled dopamine D1-like NHE3 inhibition in rat and opossum renal epithelial cells. Cell Physiol Biochem. 2004;14:91–100. doi: 10.1159/000076930. [DOI] [PubMed] [Google Scholar]
- 13.Bobulescu IA, Quiñones H, Gisler SM, Di Sole F, Hu MC, Shi M, Zhang J, Fuster DG, Wright N, Mumby M, Moe OW. Acute regulation of renal Na+/H+ exchanger NHE3 by dopamine: role of protein phosphatase 2A. Am J Physiol Renal Physiol. 2010;298:F1205–F1213. doi: 10.1152/ajprenal.00708.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salyer S, Lesousky N, Weinman EJ, Clark BJ, Lederer ED, Khundmiri SJ. Dopamine regulation of Na+-K+-ATPase requires the PDZ-2 domain of sodium hydrogen regulatory factor-1 (NHERF-1) in opossum kidney cells. Am J Physiol Cell Physiol. 2011;300:C425–C434. doi: 10.1152/ajpcell.00357.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey RM. Evidence for a splanchnic sodium input monitor regulating renal sodium excretion in man. Lack of dependence upon aldosterone. Circ Res. 1978;43:19–23. doi: 10.1161/01.res.43.1.19. [DOI] [PubMed] [Google Scholar]
- 16.Michell AR, Debnam ES, Unwin RJ. Regulation of renal function by the gastrointestinal tract: potential role of gut-derived peptides and hormones. Annu Rev Physiol. 2008;70:379–403. doi: 10.1146/annurev.physiol.69.040705.141330. [DOI] [PubMed] [Google Scholar]
- 17.Fukae H, Kinoshita H, Fujimoto S, Kita T, Nakazato M, Eto T. Changes in urinary levels and renal expression of uroguanylin on low or high salt diets in rats. Nephron. 2002;92:373–378. doi: 10.1159/000063311. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz J, Nieman M, Sabo J, et al. Uroguanylin knockout mice have increased blood pressure and impaired natriuretic response to enteral NaCl load. J Clin Invest. 2003;112:1244–1254. doi: 10.1172/JCI18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duggan KA, Hams G, MacDonald GJ. Modification of renal and tissue cation transport by cholecystokinin octapeptide in the rabbit. J Physiol. 1988;397:527–538. doi: 10.1113/jphysiol.1988.sp017017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wank SA. G protein-coupled receptors in gastrointestinal physiology. I. CCK receptors: an exemplary family. Am J Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- 21.Pisegna JR, Tarasova NI, Kopp JA, Asico LD, Jose P, Farnsworth DW, Michejda CJ, Wank SA. Postprandial changes in renal function are mediated by elevated serum gastrin acting at cholecystokinin type B receptors (CCKBR) in the kidney. Gastroenterology. 1996;64 151-151(1). [Google Scholar]
- 22.Rehfeld JF, Friis-Hansen L, Goetze JP, Hansen TV. The biology of cholecystokinin and gastrin peptides. Curr Top Med Chem. 2007;7:1154–1165. doi: 10.2174/156802607780960483. [DOI] [PubMed] [Google Scholar]
- 23.Melis M, Krenning EP, Bernard BF, de Visser M, Rolleman E, de Jong M. Renal uptake and retention of radiolabeled somatostatin, bombesin, neurotensin, minigastrin and CCK analogues: species and gender differences. Nucl Med Biol. 2007;34:633–641. doi: 10.1016/j.nucmedbio.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 24.de Weerth A, Jonas L, Schade R, Schöneberg T, Wolf G, Pace A, Kirchhoff F, Schulz M, Heinig T, Greten H, von Schrenck T. Gastrin/cholecystokinin type B receptors in the kidney: molecular, pharmacological, functional characterization, and localization. Eur J Clin Invest. 1998;28:592–601. doi: 10.1046/j.1365-2362.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- 25.von Schrenck T, Ahrens M, de Weerth A, Bobrowski C, Wolf G, Jonas L, Jocks T, Schulz M, Bläker M, Neumaier M, Stahl RA. CCKB/gastrin receptors mediate changes in sodium and potassium absorption in the isolated perfused rat kidney. Kidney Int. 2000;58:995–1003. doi: 10.1046/j.1523-1755.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- 26.Attoub S, Moizo L, Laigneau JP, Alchepo B, Lewin MJ, Bado A. YM022, a highly potent and selective CCKB antagonist inhibiting gastric acid secretion in the rat, the cat and isolated rabbit glands. Fundam Clin Pharmacol. 1998;12:256–262. doi: 10.1111/j.1472-8206.1998.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Scott L, Crambert S, Zelenin S, Eklöf AC, Di Ciano L, Ibarra F, Aperia A. Binding of losartan to angiotensin AT1 receptors increases dopamine D1 receptor activation. J Am Soc Nephrol. 2012;23:421–4298. doi: 10.1681/ASN.2011040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol. 1998;275:R986–R994. doi: 10.1152/ajpregu.1998.275.4.R986. [DOI] [PubMed] [Google Scholar]
- 29.Yao LP, Li XX, Yu PY, Xu J, Asico LD, Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensive rats. Hypertension. 1998;32:1049–1053. doi: 10.1161/01.hyp.32.6.1049. [DOI] [PubMed] [Google Scholar]
- 30.Yu P, Asico LD, Luo Y, Andrews P, Eisner GM, Hopfer U, Felder RA, Jose PA. D1 dopamine receptor hyperphosphorylation in renal proximal tubules in hypertension. Kidney Int. 2006;70:1072–1079. doi: 10.1038/sj.ki.5001708. [DOI] [PubMed] [Google Scholar]
- 31.Hussain T, Lokhandwala MF. Altered arachidonic acid metabolism contributes to the failure of dopamine to inhibit Na+,K+-ATPase in kidney of spontaneously hypertensive rats. Clin Exp Hypertens. 1996;18:963–974. doi: 10.3109/10641969609097911. [DOI] [PubMed] [Google Scholar]
- 32.Chen CJ, Lokhandwala MF. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens A. 1992;14:615–628. doi: 10.3109/10641969209036211. [DOI] [PubMed] [Google Scholar]
- 33.Potthast R, Ehler E, Scheving LA, Sindic A, Schlatter E, Kuhn M. High salt intake increases uroguanylin expression in mouse kidney. Endocrinology. 2001;142:3087–3097. doi: 10.1210/endo.142.7.8274. [DOI] [PubMed] [Google Scholar]
- 34.Kita T, Kitamura K, Sakata J, Eto T. Marked increase of guanylin secretion in response to salt loading in the rat small intestine. Am J Physiol. 1999;277:G960–G966. doi: 10.1152/ajpgi.1999.277.5.G960. [DOI] [PubMed] [Google Scholar]
- 35.Feinle C, Grundy D, Fried M. Modulation of gastric distension-induced sensations by small intestinal receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G51–G57. doi: 10.1152/ajpgi.2001.280.1.G51. [DOI] [PubMed] [Google Scholar]
- 36.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1-like and D2-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–R1078. doi: 10.1152/ajpregu.2001.281.4.R1071. [DOI] [PubMed] [Google Scholar]
- 37.Lindström E, Björkqvist M, Håkanson R. Pharmacological analysis of CCK2 receptor antagonists using isolated rat stomach ECL cells. Br J Pharmacol. 1999 May;127:530–536. doi: 10.1038/sj.bjp.0702538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto S, Shikata K, Miyasaka K, Okada S, Sasaki M, Kodera R, Hirota D, Kajitani N, Takatsuka T, Kataoka HU, Nishishita S, Sato C, Funakoshi A, Nishimori H, Uchida HA, Ogawa D, Makino H. Cholecystokinin plays a novel protective role in diabetic kidney through anti-inflammatory actions on macrophage. Anti-inflammatory effect of cholecystokinin. Diabetes. 2012;61:897–907. doi: 10.2337/db11-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connell DP, Ragsdale NV, Boyd DG, Felder RA, Carey RM. Differential human renal tubular responses to dopamine type 1 receptor stimulation are determined by blood pressure status. Hypertension. 1997;29:115–122. doi: 10.1161/01.hyp.29.1.115. [DOI] [PubMed] [Google Scholar]
- 40.Felder RA, Sanada H, Xu J, Yu PY, Wang Z, Watanabe H, Asico LD, Wang W, Zheng S, Yamaguchi I, Williams SM, Gainer J, Brown NJ, Hazen-Martin D, Wong LJ, Robillard JE, Carey RM, Eisner GM, Jose PA. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99:3872–3877. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe H, Xu J, Bengra C, Jose PA, Felder RA. Desensitization of human renal D1 dopamine receptors by G protein-coupled receptor kinase 4. Kidney Int. 2002;62:790–798. doi: 10.1046/j.1523-1755.2002.00525.x. [DOI] [PubMed] [Google Scholar]
- 42.Barrett RJ, Wright KF, Taylor DR, Proakis AG. Involvement of dopamine receptor subtypes in dopaminergic modulation of aldosterone secretion in rats. Life Sci. 1987;40:1499–1506. doi: 10.1016/0024-3205(87)90382-1. [DOI] [PubMed] [Google Scholar]
- 43.Li Z, Yu C, Han Y, Ren H, Shi W, Fu C, He D, Huang L, Yang C, Wang X, Zhou L, Asico LD, Zeng C, Jose PA. Inhibitory effect of D1-like and D3 dopamine receptors on norepinephrine-induced proliferation in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2008;294:H2761–H2768. doi: 10.1152/ajpheart.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest. 2008;118:2180–2189. doi: 10.1172/JCI33637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banday AA, Fazili FR, Lokhandwala MF. Insulin causes renal dopamine D1 receptor desensitization via GRK2-mediated receptor phosphorylation involving phosphatidylinositol 3-kinase and protein kinase C. Am J Physiol Renal Physiol. 2007;293:F877–F884. doi: 10.1152/ajprenal.00184.2007. [DOI] [PubMed] [Google Scholar]
- 46.Zhang MZ, Yao B, Fang X, Wang S, Smith JP, Harris RC. Intrarenal dopaminergic system regulates renin expression. Hypertension. 2009;53:564–570. doi: 10.1161/HYPERTENSIONAHA.108.127035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ortola FV, Seri I, Downes S, Brenner BM, Ballermann BJ. Dopamine1-receptor blockade inhibits ANP-induced phosphaturia and calciuria in rats. Am J Physiol. 1990;259:F138–F146. doi: 10.1152/ajprenal.1990.259.1.F138. [DOI] [PubMed] [Google Scholar]
- 48.Gildea JJ, Wang X, Shah N, Tran H, Spinosa M, Van Sciver R, Sasaki M, Yatabe J, Carey RM, Jose PA, Felder RA. Dopamine and angiotensin type 2 receptors cooperatively inhibit sodium transport in human renal proximal tubule cells. Hypertension. 2012;60:396–403. doi: 10.1161/HYPERTENSIONAHA.112.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Ting WL, Yang H, Wong PT. High doses of simvastatin upregulate dopamine D1 and D2receptor expression in the rat prefrontal cortex: possible involvement of endothelial nitric oxide synthase. Br J Pharmacol. 2005;144:933–939. doi: 10.1038/sj.bjp.0706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crambert S, Sjöberg A, Eklöf AC, Ibarra F, Holtbäck U. Prolactin and dopamine 1-like receptor interaction in renal proximal tubular cells. Am J Physiol Renal Physiol. 2010;299:F49–F54. doi: 10.1152/ajprenal.00582.2009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.