Abstract
The authors test the hypothesis that vocal fold morphology and biomechanical properties covary with species-specific vocal function. They investigate mule deer (Odocoileus hemionus) vocal folds, building on, and extending data on a related cervid, the Rocky Mountain elk (Cervus elaphus nelsoni). The mule deer, in contrast to the elk, is a species with relatively little vocal activity in adult animals. Mule deer and elk vocal folds show the typical three components of the mammalian vocal fold (epithelium, lamina propria and thyroarytenoid muscle). The vocal fold epithelium and the lamina propria were investigated in two sets of tensile tests. First, creep rupture tests demonstrated that ultimate stress in mule deer lamina propria is of the same magnitude as in elk. Second, cyclic loading tests revealed similar elastic moduli for the vocal fold epithelium in mule deer and elk. The elastic modulus of the lamina propria is also similar between the two species in the low-strain region, but differs at strains larger than 0.3. Sex differences in the stress–strain response, which have been reported for elk and human vocal folds, were not found for mule deer vocal folds. The laminae propriae in mule deer and elk vocal folds are comparatively large. In general, a thick and uniformly stiff lamina propria does not self-oscillate well, even when high subglottic pressure is applied. If the less stiff vocal fold seen in elk is associated with a differentiated lamina propria it would allow the vocal fold to vibrate at high tension and high subglottic pressure. The results of this study support the hypothesis that viscoelastic properties of vocal folds varies with function and vocal behavior.
Keywords: larynx, vocal ligament, stress–strain response, cervidae, bioacoustics, mammals, source-filter theory, Young's modulus
INTRODUCTION
The role that vocal communication plays in mammalian social living varies between species, and each species’ vocal repertoire possesses unique spectral and temporal characteristics (Tembrock, 1996). Ultimate and proximate factors that have shaped vocal systems and acoustic signals may have also affected the structures that produce them (Bradbury and Vehrencamp, 1998).
Mammalian vocal folds protect the lower respiratory system from foreign bodies, regulate airflow from and to the lungs (Bartlett et al., 1973), and help regulate intrathoracal pressure by controlling the entrance to the lower respiratory tract (Negus, 1924). Vocal folds also function as an aerodynamic, myoelastic sound source (Van den Berg, 1958; Titze, 2006) because tissue oscillations are self-sustained by an airstream passing between the vocal folds. This is their most mechanically demanding function (Titze, 1994). Coordinated by laryngeal muscles, vocal folds are adducted into prephonatory position. During phonation, muscle activity determines only vocal fold length (and thereby tension) and adduction, but not the vibration pattern. The oscillation itself is a passive mechanism, and the vocal output is largely depending on the viscoelastic properties of the vocal fold tissue (Van den Berg, 1958; Titze, 1988). An important feature of the mammalian vocal fold is its multilayered structure (Hirano, 1974), consisting of an epithelium, a lamina propria and a muscle (Fig. 1A; Hahn et al., 2006a,b; Hammond et al., 1997).
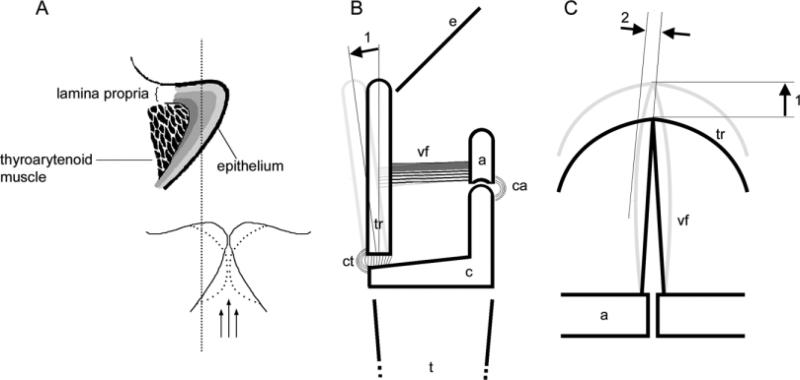
Fig. 1.
A: Schematic of a frontal section of a vocal fold, indicating the layered structure with three main layers: epithelium, lamina propria, and the thyroarytenoid muscle. The thickness and the microstructure of the lamina propria are species-specific. In adult humans a superficial, intermediate, and deep layer of the lamina propria can be differentiated. Bottom figure in A: Another schematic of a frontal section of two vocal folds. The dotted outlines indicate the change of shape during oscillation. Note that the complete vocal fold is not always oscillating but only a few layers of a certain depth, called cover. The vertical dotted line indicates the boundary between the nonoscillating body of the vocal fold (to the left) and the oscillating cover (to the right). The three arrows indicate the air flow coming from the lungs. B: Schematic of the lateral view of a larynx indicating how the cricothyroid muscle pulls the thyroid cartilage, thereby elongating the vocal fold. C: Schematic of top view of a larynx. Force acts at least in two directions on the vocal folds, 1) elongation along the longitudinal axis, 2) bending due to oscillation amplitude. a, arytenoid cartilage; c, cricoid cartilage; ca, cricoarytenoid muscle; ct, cricothyroid muscle; e, epiglottis; t, trachea; tr, thyroid cartilage; vf, vocal fold.
Vocal fold oscillation behavior has been studied not only in humans but also in excised larynx experiments in various mammalian species (e.g., Brown et al., 2003; Alipour and Jaiswal, 2009). Vocal fold vibrations interrupt a passing airstream. Their oscillation rate determines the fundamental frequency of the sound. It is important to note that only the superficial layers of the vocal fold (called the cover), rather than the complete vocal fold, are set into vibration. The soft and pliable cover (Fig. 1A) can respond to the airstream because it is easily deformed and because it is very close to the aerodynamic driving forces. The cover consists of epithelium and some part of the lamina propria. Its effective thickness in vibration depends on vocal fold deformation and subglottic pressure. The postural deformation of the vocal fold (longitudinally and perpendicularly; Fig. 1B,C) is determined by the viscoelastic properties of the tissue and activation of laryngeal muscles.
The mammalian larynx shows enormous morphological variability (e.g., Negus, 1949; Schneider, 1964; Harrison, 1995) which affects airflow, vocal fold oscillation, and vocal output. Our goal in the current study is to test the hypothesis that two closely related species with very different vocal repertoires possess vocal folds with different bio-mechanical properties. Here, we investigate the biomechanical characteristics of the vocal folds of the mule deer (Odocoileus hemionus), a cervid species with very little vocal activity. This project builds on and extends the results of studies on Rocky Mountain elk (Cervus elaphus nelsoni; Riede and Titze, 2008). The mule deer/elk comparison provides an opportunity to explore laryngeal morphology variations associated with large spe cies differences in the rate of emission and the type of vocalizations among sympatric cervids.
Vocalizations of mule deer differ from those of elk with respect to the calling rate, amplitude, and fundamental frequency range. The calling rate in all age and sex classes of elk increases in the fall (Boywer and Kitchen, 1987). The bugle call produced by male and female elk can carry several hundred meters. The average fundamental frequency of male elk bugle calls ranges between 500 and 2,500 Hz although several other call types with lower fundamental frequencies have been described (Struhsaker, 1968; Feighny et al., 2006).
Most of the sounds described for mule deer are uttered at low rates, are low in amplitude, and are communicated at close range. No amplitude data exist for adult mule deer. Except for their distress calls, vocalizations of mule deer can be heard only over short distances (<50 m). These include different forms of contact calls, snorts, and grunts expressed during social and dominance interactions, in addition to soft buzzing sounds uttered by courting males as they tend individual females (Cowan and Geist, 1961; Geist, 1981; Kiley, 1972; Richardson et al., 1983). Juvenile distress calls, on the other hand, may be heard from distances exceeding a few hundred meters in favorable environmental conditions and their fundamental frequency (F0) frequently extends above 1 kHz. Distress calls have been described most often for juveniles but can be emitted by adults when an animal is attacked by a predator or restrained by humans (Lingle et al., 2007a,b; Richardson et al., 1983).
In the present study, we conducted histological sections of mule deer vocal folds, and we performed tensile tests to quantify viscoelastic properties. We tested whether the elastic modulus of a mule deer vocal fold differs from that of an elk vocal fold, previously reported in Riede and Titze (2008). Confirmation of this hypothesis would support the notion that morphology varies with function, whereas rejection of the hypothesis would suggest that viscoelastic properties of vocal fold tissue remained conservative, and other factors must explain the functional difference.
METHODS
Larynges were retrieved from hunter-harvested mule deer submitted to the Colorado Division of Wildlife's chronic wasting disease surveillance program during the 2007 hunting season. Tissue was collected within 48 h after death and was maintained at 48C from the time of death until the experiment which was performed within 2 h after the collection. Age was estimated by tooth wear. Elk vocal folds were collected and tested under the same conditions as mule deer vocal folds described previously in Riede and Titze (2008).
Two male and two female larynges were fixed in 10% buffered formalin for 4 weeks. After fixation, larynges were cut in half in the mid-sagittal plane, and the vocal folds were carefully isolated. Mid-membraneous frontal sections of the vocal folds (i.e., a frontal section through the vocal fold about halfway between arytenoid and thyroid cartilage, 5 μm thick) were stained with hematoxylin-eosin, Masson's Trichrome (for collagen fiber stain) and Elastica-Van Gieson (for elastic fiber stain).
To affect F0 in vivo, the vocal fold tissue is longitudinally stretched between thyroid and arytenoid cartilage (Hollien, 1960), almost like a string, (Fig. 1C). According to the string model, F0 is determined by:
| (1) |
where L is the string (or vocal fold) length, σ is the stress (force per unit area) applied to the string (or vocal fold), and ρ is the tissue density (1.02 g/cm3).
Stress–Strain Measurements
The force-elongation data were obtained by a) a stepwise loading procedure and subsequent creep and b) a 1 Hz sinusoidal cyclic loading to the tissue by an automated electromechanical system, recording force and distance.
a. Step-wise load increase
In two males and two females, the lamina propria was tested. The epithelium and the thyroarytenoid muscle were removed. Great care was taken to separate epithelium and lamina propria to ensure that the complete lamina propria stayed attached to the arytenoid and the thyroid cartilage. The arytenoid cartilage was fixed with a suture on a 50-cm-high steel rack with the lamina propria and the remaining thyroid cartilage freely hanging vertically. A 2 g cup was attached to the thyroid cartilage end piece. Defined weights were added to the cup. A ruler (serving as reference distance) was positioned next to the tissue. Thirty seconds after placing a new weight in the cup, a picture of the tissue and the reference distance was taken (Sony DSC-S85 camera, 2,272 × 1,704 pixel resolution). Lamina propria length was measured for each increase in load with reference to the reference distance using Scion Image software (version 4.0.3.2., available at www.scioncorp.com). Lamina propria was stressed until irreversible damage occurred to estimate ultimate stress. Irreversible damage occurred when the tissue did not experience full stress recovery after stretching. The mass of the lamina propria was determined after the experiment. During the experiment, the lamina propria was kept moist by covering it with wet cellulose tissue, which was repeatedly sprayed with warm (388C) saline solution.
b. 1 Hz sinusoidal cyclic loading
Ideally the vocal fold would be tested in its complete length. However, the servo-control lever system allows excursions up to 15 mm. Male mule deer older than 3 years have vocal folds larger than 25 mm and would be strained by less than 60% at maximum lever arm excursion. Manual extension of a vocal fold in situ in the larynx allows maximum strain of up to 70%. We, therefore, tested two sets of larynges, one with a vocal fold of a reduced length (in males and females older than 3 years) and a second with a vocal fold of complete length (hereafter ‘‘complete,’’ in females of all ages and males up to the age of 3 years).
In the first set of larynges, the length of the epithelium and the lamina propria of six males and six females were reduced (hereafter “partial”). A 2–cm-long piece of vocal fold remained attached to a small portion of the arytenoid cartilage. The thyroarytenoid muscle was removed whereas the lamina propria and epithelium remained intact. The lamina propria was tested whereas the epithelium was carefully separated, left unstressed, attached to the arytenoid cartilage and kept wet. After testing the lamina propria, it was removed and the epithelium was tested. Although great care was taken to make sure that the epithelium was not damaged, the epithelium suffered transverse ruptures during the preparation in two male and two female larynges. These were discarded from the data set and four additional larynges were dissected to maintain six male and six female epithelium samples. A suture connected the arytenoid cartilage to the lever arm of the servo-control lever system. A clamp held the ventral end of the lamina propria or the epithelium in a fixed position. The mounted tissue measured about 1 cm in length without any slack in the suture. Slippage was minimized by wrapping the clamped part of the ventral end with dry cellulose.
Using a second set of larynges, the full length of the lamina propria from five males and six females were tested. The thyroarytenoid muscle and the epithelium were carefully removed to ensure that the lamina propria remained undamaged. The lamina propria was tested whereas still attached to a small portion of the arytenoid cartilage and a small portion of the thyroid cartilage. One suture connected the arytenoid cartilage to the lever arm of the servo-control lever system and a second connected the thyroid cartilage to a fixation point below the lever arm.
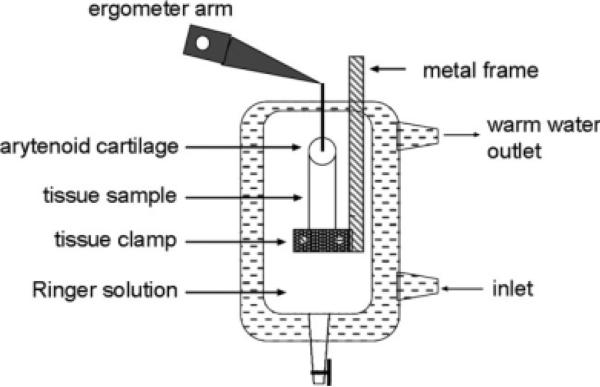
In both sets of larynges, the tissue was vertically mounted in a water-surrounded chamber containing saline solution (Ringer solution) maintained at 388C (Fig. 2). The exact length between cartilage and fixation point in the clamp was measured with a caliper (±0.1 mm accuracy).
Fig. 2.
The tissue sample was mounted by suturing the arytenoid cartilage to the ergometer arm. The second end of the tissue was either soft tissue or a piece of thyroid cartilage. The soft tissue was wrapped in dry cellulose paper and clamped. The thyroid cartilage was sutured to the metal frame that could also hold the clamp.
The force-elongation data were obtained by 1 Hz sinusoidal stretch and release of the vocal fold by means of a dual-mode servo-control lever system (Aurora Scientific Model 305B, Aurora, ON, Canada). Displacement of and force on the lever arm (resolution 1 μm and 0.3 mN) were recorded. Elongation was applied in a longitudinal direction (although with the current species this means dorso-ventral, in many species the vocal folds are not oriented perfectly dorso-ventrally), followed by a shortening to the original length. The present set of experiments was conducted with a system under displacement control.
A controlled sinusoidal displacement was applied to the lever arm so that the vocal fold was lengthened and shortened (loading–unloading condition) 15 times at a frequency of 1 Hz. The force and elongation signals were then transmitted via a 16-bit analog-to-digital acquisition board (Windaq Model DI722, DATAQ Instruments; Akron, OH) at 400 Hz sampling frequency to a PC.
A prestrain of 20% was applied to each specimen. Prestrain is the elongation (relative to total specimen length) imposed on the specimen before each lengthening-shortening test. Vocal fold length differed between in situ (intact larynx) and ex situ (vocal fold excised from the cartilage framework of the larynx), most likely due to suspension of the vocal fold in larynx morphology. A prestrain of 20% compensated for length changes due to isolating the tissue and was based on measurements of vocal fold length in 10 adult female and 10 adult male larynges before and after excising the vocal fold from the cartilage framework. The distance from insertion points at the thyroid cartilage and the arytenoid cartilage was 21.9% ± 5.6% shorter (mean ± std dev, N = 20) after isolating the vocal fold. In a subset of 20 larynges, the differences in length before and after excising the vocal fold between males and females were not significant (Mann-Whitney test; U = 27; P = 0.08; Nf = 10, Nm = 10). The 20% prestrain was applied for 1 min before experiments started. For comparison, a 5–10% prestrain was found in domestic dog (Canis familiaris) (Hunter et al., 2004) and 20% in elk (Riede and Titze, 2008).
Mass measurements were affected by hydration (tissue bath, see earlier) of the isolated tissue. For example, elk vocal fold epithelium and lamina propria increased in weight by 11.9% after 10 min in Ringer solution (Riede and Titze, 2008). Four mule deer lamina propria specimens increased in mass by 12.1% (N = 4; std dev: ±4.5%) after 10 min. We, therefore, corrected all mass measurements by 12% (in both experiments) because our mass measurements were taken after the experiment. We adapted density values from an earlier study on human vocal folds (Min et al., 1995) because Hunter et al. (2007) demonstrated that small variations in density have a negligible effect on stress calculations.
Data Analysis
Tensile strain (ε) was calculated as a specimen's length change (displacement at unconstrained end) divided by its original mounting length:
| (2) |
where l0 is the mounting length and Δl is the change in length of the specimen during stretching. Note that when strain is given as percentage in this article, the term on the right side of Eq. 2 is multiplied by 100.
Tensile stress (σ) is defined as the ratio between force (F in N) and cross-sectional area (A in m2) of the specimen. Assuming tissue incompressibility, uniform specimen cross-sectional area with roughly cylindrical geometry, and tissue isotropy allow for calculating the average cross-sectional area, the average cross-sectional area is then:
| (3) |
where m is the specimen mass and q is the tissue density. This equation considers the strain-dependent cross-sectional area A, which decreases as the specimen is elongated and increases as the specimen returns to its initial mounting length. Usually engineering or nominal stress is used (where the cross-sectional area, A0, is assumed constant). However, when the changes in cross-sectional area are significant, then stress must be calculated using the strain-dependent cross-sectional area called true stress. Considering the large strains applied to the tissue, we assumed that the true stress was more appropriate in this case. With the varying cross-sectional area, the tensile stress (σ in Pa) can be calculated as:
| (4) |
where F is the applied force.
The ratio of stress and strain tells us how much the material stretches for a given load. It is called variously “stiffness,” “elastic modulus,” or “Young's modulus of elasticity.”
The overall stress–strain response of viscoelastic tissue in general, and of vocal fold tissue, in particular, has been differentiated into a linear low-strain and a nonlinear high-strain region (Chan et al., 2007; Hunter and Titze, 2007; Riede and Titze, 2008). The low-strain region is modeled with a linear function (Eq. 5).
| (5) |
where a is the slope of the curve and b is the y-axis intercept. The high-strain region is best approximated with an exponential equation (Eq. 6)
| (6) |
where A and B are constants to be determined empirically.
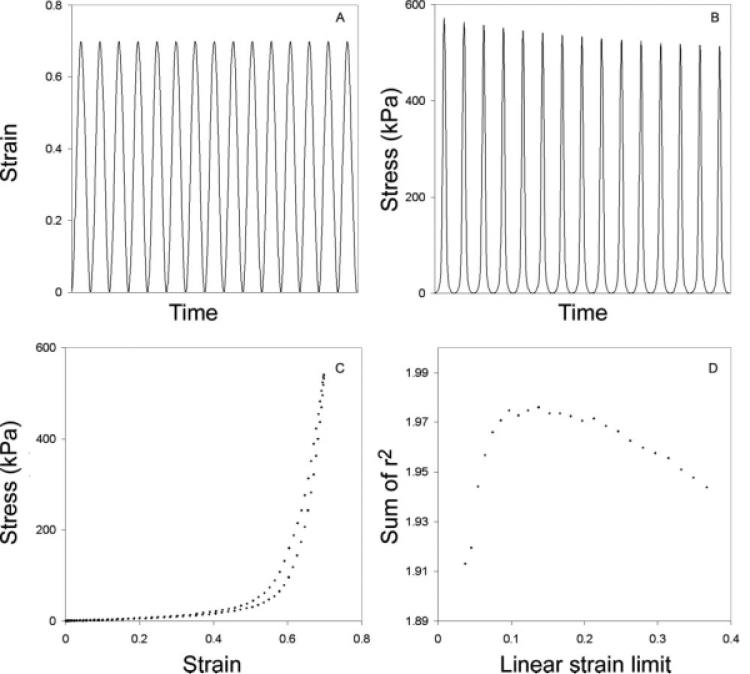
The amplitude of the cyclic strain application remains constant during 15 cycles (Fig. 3A) and the amplitude of the resulting stress response decreases (Fig. 3B). The combined stress– strain response results in a “banana-shaped” curve (Fig. 3C), with a loading part (upper curve) higher than the unloading part (lower curve). The constants in Eqs. 5 and 6 as well as the upper limit of the linear strain region (“linear strain limit,” ε1) are derived by fitting a linear and an exponential regression line, respectively, to the empirical data, whereas maximizing the sum of both regression coefficients. The maximization process is performed by conducting a step-wise movement of the linear strain limit, and performing a linear and exponential regression, respectively, on the two data sets at each step. The two resulting regression coefficients are added at each step. The maximum of the sum of both regression coefficients is considered the linear strain limit, ε1, in Eqs. 5, 6, and 7 (Fig. 3D).
Fig. 3.
A–C: Stress–strain response in time from a 1 Hz sinusoidal elongation of lamina propria. Note that the amplitude of strain remains constant (A) while stress (B) decreases over time. The decrease in stress is a result of tissue hysteresis, a phenomenon resulting from viscous properties of the tissue. Stress–strain relationship for a single cycle from the same data set is shown in (C). The upper part of the “banana-shaped” curve is the loading phase (stretching). The lower part is the unloading phase (relaxation). The difference between both curves is due to hysteresis of the tissue, i.e., lower stress in the tissue during the unloading phase. The low-strain region of the loading phase was fitted with a linear regression line, whereas the high-strain region was modeled with an exponential function. The limit of the linear region (Linear strain limit) determined by maximizing the sum of the two regression coefficients (sum of r2) is shown in (D). The maximum linear strain limit (ε1) in this example is approximately 0.14. Note, the tissue was prestrained by 20% and strain data have been corrected.
The range of Young's modulus was described by the first derivative of the exponential stress–strain function:
| (7) |
where A and B are constants.
Force-elongation data were obtained for the left and right vocal fold of each larynx. The linear and exponential coefficients for the left and right vocal fold were averaged for each larynx, so that only one data set per larynx was considered in further analyses.
Hysteresis was estimated as the difference between the area-under-the-curves of the loading and the unloading phases of the 1 Hz-sinusoidal stress–strain response. Hysteresis is frequency dependent because it involves the strain rate with respect to time. We provide here only one estimate for a 1 Hz loading-unloading regime. The stress–strain responses (loading phase and unloading phase separately) were fitted with an exponential curve according to Eq. 7. The area under the curve between ε1 and maximum applied strain was estimated by integrating both exponential equations. The difference in the area under the curve between loading and unloading stress–strain response was considered hysteresis and expressed as a percentage.
Viscoelastic properties of mule deer vocal folds were compared with elk. Species and sex differences were tested using unpaired t-tests.
RESULTS
Mule Deer Vocal Fold Histology
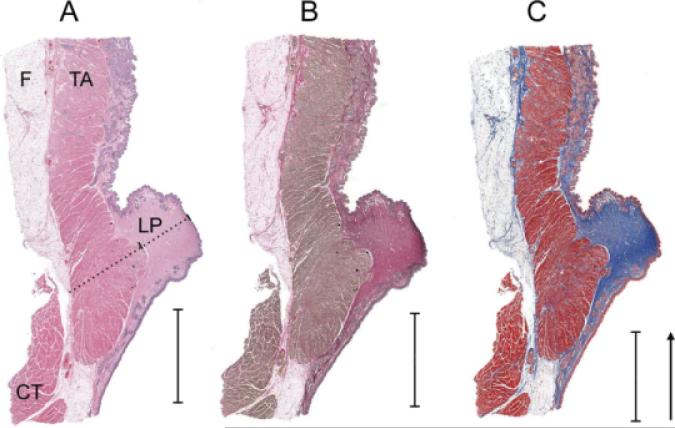
Epithelium thickness of the mule deer vocal folds ranged between 29 and 68 μm. The lamina propria ranged in cross-sectional area between 15 and 22 mm2. The largest medial-to-lateral depth (labeled “diameter”) of the lamina propria ranged between 3 and 3.7 mm and that of the thyroarytenoid muscles ranged between 4.4 and 4.8 mm. Thus, the lamina propria reached deep into the vocal fold (see Fig. 4) extending almost 42% of the distance between the epithelium and the thyroid cartilage (see dotted line in Fig. 4 between two short lines).
Fig. 4.
Three successive histological frontal sections of the mid-membraneous part of the vocal fold of a 4-year-old male mule deer. The arrow in part C indicates the direction of an expiratory air flow. A: hemalaun-eosin stain, B: Elastica-van-Gieson stain indicating elastic fibers in black stain, C: trichrome stain indicating collagen fibers in blue stain. The bars indicate a 5 mm distance. Along the dotted line in the hemalaun-eosin stain, thickness measurements of the epithelium, the lamina propria and the thyro-arytenoid muscle were taken; CT, cricothyroid muscle; F, fat layer that sits on the thyroid cartilage between cartilage and thyroarytenoid muscle; LP, lamina propria; TA, thyroarytenoid muscle.
Step-Wise Load Increase
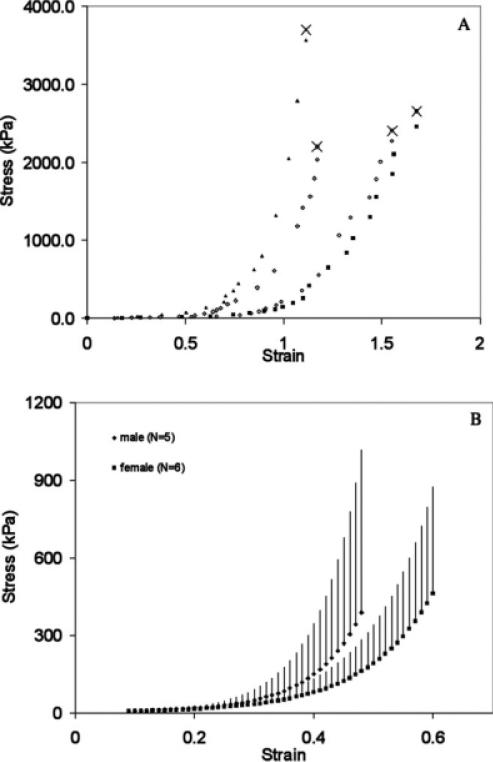
The maximum lamina propria lengthening before rupture ranged from 210% to 280% in eight samples from four larynges. Rupture occurred between 1.0 and 3.5 kg weight (10 to 35 N), corresponding to a stress of 2.0 to 3.7 MPa. It occurred near the anchoring point, either at the arytenoid (two specimens) or the thyroid insertion (five specimens). In one specimen, the suture was torn from the cartilage.
The loading of the tissue demonstrated an exponential stress–strain relationship (Fig. 5A). Beyond strain 0.8, data suggest some degree of yielding. We have attempted to exclude slippage of sutures in the cartilages or stretching of the sutures themselves and, therefore, assume that the shift around strain 0.8–1.0 is due to some internal material damage (Table 1). The stress–strain relationship was modeled exponentially for a strain range from 0 to 0.8. The average exponential function for stress in eight laminae propriae was:
| (8) |
Fig. 5.
A: Stress–strain relationship for lamina propria from two female (filled symbols) and two male (open symbols) mule deer vocal folds derived in a creep rupture procedure. The crosses indicate the rupture point. B: Tensile stress–strain curves of lamina propria specimen (full-length tested) based on the loading part of cyclic loading (mean and standard deviation shown as error bars).
TABLE 1.
Constants A and B for fitting the exponential stress–strain relationship (according to Eq. 6) derived in a step-wise experiment, for the lamina propria
| Specimen | A | B | R 2 |
|---|---|---|---|
| Lamina propria, N = 4 | 1.8 ± 0.9 | 5.1 ± 0.9 | 0.992 ± 0.003 |
R2 is the regression coefficient and describes the quality of fit.
Stepwise loading experiments in elk vocal folds revealed similar yielding effects beyond a strain of about 1.0 (Riede and Titze, 2008). The stress at creep rupture in the vocal folds was not significantly different between both species [elk: N = 9, 2.2 ± 0.84 MPa, mean ± std dev (data from Riede and Titze, 2008); mule deer: N = 4, 2.6 ± 0.38 MPa, mean ± std dev; t-test, t = 20.96, P = 0.355].
1 Hz Sinusoidal Cyclic Loading
There was a significant difference in the linear strain limit between tests of the complete lamina propria (ranging from 8% to 9%) and tests of only a dorsal segment of the lamina propria (18–23%; Table 2). The slope of the linear model (constant a) and the A constant in the exponential model were significantly different when partial lamina propria or a complete one was tested; however, other parameters (b, B, hysteresis) did not differ significantly between the two data sets. None of the parameters (a, b, A, B, and ε1) differed significantly between males and females, neither for the lamina propria nor for the vocal fold epithelium (Tables 2 and 3). In summary, the average exponential function for lamina propria from five male mule deer vocal folds (complete lamina propria tested) were
| (9) |
and from six female mule deer vocal folds
| (10) |
TABLE 2.
Parameters of the linear model (Eq. 5) for curve-fitting the empirical stress–strain response of the lamina propria and vocal fold mucosa for strains between ε = 0 and linear strain limit (ε1) in %
| Specimen | a | b | R 2 | ε 1 |
|---|---|---|---|---|
| Mule deer | ||||
| Male | ||||
| Male MD, N = 6, partial lamina propria | 38.5 ± 8.6 | 0.04 ± 0.2 | 0.98 ± 0.01 | 16.3 ± 6.2 |
| Male MD, N = 5, complete lamina propria | 81.5 ± 16.1 | 0.3 ± 0.3 | 0.99 ± 0.002 | 8.1 ± 3.4 |
| Male MD, N = 6, epithelium | 64.5 ± 31.9 | −0.12 ± 0.5 | 0.98 ± 0.01 | 31.9 ± 13.2 |
| Female | ||||
| Female MD, N = 6, partial lamina propria | 37.2 ± 10.2 | −0.005 ± 0.2 | 0.98 ± 0.007 | 23.2 ± 8.6 |
| Female MD, N = 6, complete lamina propria | 70.0 ± 30.2 | 0.5 ± 0.9 | 0.99 ± 0.002 | 8.6 ± 1.3 |
| Female MD, N = 6, epithelium | 67.3 ± 19.9 | 0.03 ± 0.4 | 0.93 ± 0.002 | 26.6 ± 6.5 |
| Male × female | ||||
| Males × females, complete lamina propria (N1 = 6; N2 = 6) | t = −0.23, P = 0.82 | t = −0.38, P = 0.71 | t = 1.76, P = 0.11 | |
| Males × females, partial lamina propria (N1 = 5; N2 = 6) | t = −0.76, P = 0.46 | t = 0.49, P = 0.63 | t = 0.34, P = 0.36 | |
| Males × females, epithelium (N1 = 6; N2 = 6) | t = 0.17, P = 0.86 | t = 0.59, P = 0.56 | t = −0.88, P = 0.39 | |
| Partial × complete lamina propria, (N1 = 12; N2 = 11) (males and females pooled) | t = 4.96, P < 0.001 | t = 0.49, P = 0.63 | t = −4.89, P < 0.001 | |
| Elk (from Riede and Titze, 2008) | ||||
| Elk, partial lamina propria | 45.7 ± 16.3 | 0.11 ± 0.12 | 12.0 ± 2.71 | |
| Elk, epithelium | 77.8 ± 30.2 | 0.05 ± 0.5 | 22.5 ± 2.8 | |
| Elk × Mule deer | ||||
| Elk × mule deer, partial lamina propria (N1 = 12; N2 = 12) | t = 1.45, P = 0.08 | t = 1.35, P = 0.09 | t = −3.4, P = 0.002 | |
| Elk × mule deer, epithelium (N1 = 10; N2 = 12) | t = 1.0, P = 0.33 | t = 0.45, P = 0.65 | t = −1.99, P = 0.06 |
R2 is the regression coefficient and describes the quality of fit. In one set of tests the vocal fold length was reduced (partial), whereas in a second set the vocal fold length was not reduced (complete).
TABLE 3.
Parameters of the exponential model (Eq. 6) for curve-fitting the empirical stress–strain response of the lamina propria and vocal fold epithelium for strains larger than ε1
| Specimen | A | B | R 2 | Young's modulus (kPa) | Hysteresis (%) |
|---|---|---|---|---|---|
| Mule deer | |||||
| Male | |||||
| Male, N = 6, partial lamina propria | 1.2 ± 0.4 | 8.6 ± 1.9 | 0.98 ± 0.002 | 44–690 | 16.6 ± 3.3 |
| Male, N = 5, complete lamina propria | 3.8 ± 1.6 | 8.2 ± 3.2 | 0.98 ± 0.01 | 60–1856 | 17.6 ± 5.7 |
| Male, N = 6, epithelium | 5.3 ± 2.2 | 4.2 ± 1.0 | 0.99 ± 0.003 | 82–181 | 14.9 ± 2.5 |
| Female | |||||
| Female, N = 6, partial lamina propria | 0.7 ± 0.5 | 9.7 ± 1.8 | 0.97 ± 0.01 | 63–854 | 21.3 ± 10.5 |
| Female, N = 6, complete lamina propria | 2.9 ± 0.8 | 7.9 ± 1.5 | 0.98 ± 0.006 | 43–1175 | 19.2 ± 5.3 |
| Female, N = 6, epithelium | 5.0 ± 2.2 | 4.6 ± 0.5 | 0.98 ± 0.01 | 76–228 | 14.6 ± 2.2 |
| Male × female | |||||
| Males × females, lamina propria (N1 = 6; N2 = 6) | t = −1.94, P = 0.08 | t = 0.97, P = 0.35 | t = 1.06, P = 0.31 | ||
| Males × females, complete lamina propria (N1 = 5; N2 = 6) | t = −1.24, P = 0.24 | t = −0.24, P = 0.82 | t = 0.48, P = 0.64 | ||
| Males × females, epithelium (N1 = 6; N2 = 6) | t = −0.19, P = 0.85 | t = 0.94, P = 0.36 | t = −0.26, P = 0.79 | ||
| Partial × complete lamina propria (N1 = 12; N2 = 11) (males and females pooled) | t = 5.89, P < 0.001 | t = −1.21, P = 0.21 | t = −0.16, P = 0.87 | ||
| Elk (from Riede and Titze, 2008) (males and females pooled) | |||||
| Elk, partial lamina propria | 3.2 ± 1.1 | 6.2 ± 0.4 | 41–436 | 14.1 ± 3.4 | |
| Elk, epithelium | 9.7 ± 3.6 | 3.9 ± 0.7 | 82–243 | 13.6 ± 2.3 | |
| Elk × mule deer (males and females pooled) | |||||
| Elk × mule deer, partial lamina propria (N1 = 12; N2 = 12) | t = 6.28, P < 0.0001 | t = −5.2, P < 0.0001 | t = −2.4, P = 0.025 | ||
| Elk × mule deer, epithelium (N1 = 10; N2 = 12) | t = 3.65, P = 0.001 | t = −1.28, P = 0.21 | t = −1.28, P = 0.21 |
Young's modulus was calculated for a range between ε1 and ε = 0.5. Hysteresis was calculated for a range between ε1 and maximum applied stress. In one set of tests the vocal fold length was reduced (partial), whereas in a second set the vocal fold length was not reduced (complete).
Differences between male and female tissue were not significant (Table 3, next chapter). However, they fell in the same direction as for elk, i.e., male vocal folds stiffer than female vocal folds (Fig. 5B).
During the unloading phase, energy loss relative to the loading phase was observed in all specimens (hysteresis, i.e., difference between upper and lower curve in Fig. 3C). Hysteresis in a 1 Hz cyclic tensile test ranged from 16% to 21% for male and female lamina propria and from 14% to 15% for male and female vocal fold epithelium (Table 3).
Species Difference
The constants a, b, A, B, ε1 and hysteresis were tested for differences between elk and mule deer (Tables 2 and 3, Fig. 6). Data for elk were used from an earlier study (Riede and Titze, 2008). Results for species comparisons were identical using male or female elk or pooled data; therefore, data from male and female elk were pooled.
Fig. 6.
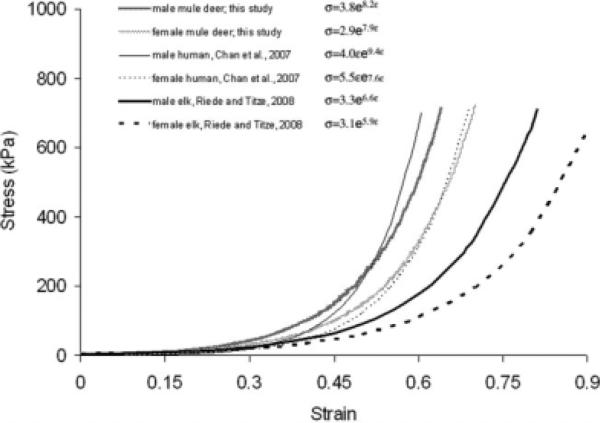
Comparative stress–strain relationship for three different species. Human data after Chan et al. (Table 4 in Chan et al. 2007); elk data from Riede and Titze (2008). Note that the exponential model used by Chan et al. is different than the one used in the elk and in this study.
The linear strain limit was 12% for elk partial lamina propria but 16% to 23% for mule deer partial lamina propria, a significant difference (Table 2). The linear models for the laminae propriae and the vocal fold epithelium were similar in elk and mule deer (Table 2); however, the exponential model for strains above ε1 was not. Constants A and B for the lamina propria and constant A for the vocal fold epithelium were significantly different between elk and mule deer (Table 3).
Table 3 shows that hysteresis was 14.1% in elk lamina propria, but ranged from 16% to 21% in mule deer, a significant difference. Hysteresis in epithelium tissue between elk and mule deer were not different.
DISCUSSION
Our data suggest that viscoelastic properties of vocal folds in two cervid species are different. However, in vitro tensile testing of excised tissue is susceptible to numerous errors and, thus, quantitative data must be interpreted cautiously. Three major sources of errors exist in vocal fold testing: tissue treatment before and during the experiment, setup problems, and measurement errors. We have designed our study in an attempt to bypass the general issues in each of these three sources. Nevertheless, at this stage, the results should be viewed as a starting point for further comparative work on the functional morphology of vocal folds.
Tissue that is removed from the body undergoes changes due to enzymatic processes which sooner or later affect the biomechanical properties of the tissue. To delay and/or minimize such effects, earlier studies of vocal folds kept the tissue frozen between dissection and experiment. However, even freezing can affect tissue properties (Chan and Titze, 2003; Hirpara et al., 2008). Because we had a constant supply of fresh cadavers over a period of 6 weeks, we chose to experiment with unfrozen tissue. Both samples (elk and mule deer vocal folds) were collected and treated identically.
One major setup problem is the mounting of tissue to a laboratory frame. By using the natural anchor points of the lamina propria and the epithelium at the thyroid and the arytenoid cartilage, respectively, this error was limited. However, one set of mule deer vocal folds as well as the elk sample were tested on a partial segment, demanding the clamping of one tissue end without cartilage. We attempted to minimize the possibility of slippage by wrapping the clamped tissue end in dry cellulose paper, and did so in both samples identically.
Finally, a number of nonsystematic errors can be introduced when calculating stress and strain. In an earlier study, measurement errors of tissue mass and tissue length have been identified as having the most deteriorating effect for stress calculations (Hunter et al., 2007). We attempted to minimize such effects by using a relatively large sample size. Furthermore, all experiments in both species were performed by the same person.
Species Differences in Viscoelastic Properties of Vocal Folds
The vocal folds, specifically the lamina propria of two cervid species that produce very different vocal repertoires, demonstrate different visco-elastic properties. A mule deer vocal fold stretched by a certain amount develops a higher stress than an elk vocal fold stretched by the same relative amount (see Fig. 6). Acoustically, this different response should have several predictable effects, which we outline here. These should be tested further in the future.
According to Eq. 1, a string with the lower stress response for a given strain that would reflect the elk's vocal fold, would produce the lower F0. Furthermore, the elk has a longer vocal fold (elk: 3.0 ± 0.42 cm, mean ± std dev.; Riede and Titze, 2008; male mule deer: 2.4 ± 0.73 cm, mean ± std dev., N = 15; female mule deer: 2.1 ± 0.57 cm, mean ± std dev., N = 16) which should also contribute to a comparatively lower F0 (Eq. 1). However, in reality, the male elk can produce sounds with F0 up to 2.5 kHz. Neither adult nor juvenile mule deer have been reported to emit such a high frequency sound. Although the anatomical arrangement invites comparison of vocal fold oscil lation to that of a string, the vocal fold is not a string instrument.
To explain how the viscoelastic differences can be functionally relevant, we offer a hypothesis of how these differences translate to the dynamical processes of sound production. The lamina propria of the vocal fold in mule deer (this study) and elk (Riede and Titze, 2008) is very thick, much thicker than for example in humans (Hirano, 1974). It is possible that a thick lamina propria was originally evolved for nonvocal functions, such as supporting the larynx gating function (Negus, 1924). For example, a tight closure is often spontaneously performed during strength exercises (e.g., Miyamoto et al., 1999). Stotting, a jumping movement that is used by mule deer during vigorous and prolonged battles with predators (Lingle and Pellis, 2002) could be such an exercise. The glottal closure maneuver may help in maintaining a certain pressure in the thorax, thereby adding axial stability (Miyamoto et al., 1999; Negus, 1924). A thick lamina propria might be better suited to fulfill this function.
A thick lamina propria, however, does not self-oscillate well when stiffened and high subglottic pressure is applied (Titze, 1988). Rather a low-frequency sound is easier to produce and is probably achieved with a lax vocal fold. In fact, other species, like the European Red deer (Cervus elaphus scoticus) produce calls with a low F0. Furthermore, species producing low frequency calls, like large cats, are well-known for vocal folds with a thick lamina propria (Hast, 1986).
On the other hand, it is likely that male elk produce bugle calls with very high subglottic pressures because signs of a strong abdominal press are visible (external bulging of the Fossa paralumbalis and raising of the abdomen). We hypothesize that to get part of the vocal fold to vibrate at high tension and high-subglottic pressure, a less stiff superficial lamina propria layer is favored. It is critical to note that not the entire vocal fold is oscillating but only the cover. The compliance of the cover is low enough to respond to the passing air stream. At this stage, we are uncertain about the thickness of the cover or the structural composition of the lamina propria. However, it is likely that the cover is variable, depending on the vocal fold elongation. The more the vocal fold is stretched, the thinner the cover.
The lamina propria of the elk is less stiff. The lower stiffness, however, is an overall measure and could be further differentiated. For example, in human vocal folds, the lamina propria is differentiated in three layers, depending on the content and the orientation of elastic fibers and collagen (Gray et al., 2000). Each of these three layers shows a different viscoelastic response, making a prediction of the cover thickness and the effective mass and tension of the vibrating portion very difficult. Currently, it is unknown if the elk or mule deer vocal fold can be further differentiated.
The hypothesis that a differentiated and overall more compliant elk vocal fold is favored to vibrate at high-tension and high subglottic pressure can be tested in excised larynx experiments by measuring oscillation amplitude while controlling air flow, pressure, and vocal fold setting. We predict that a more compliant elk vocal fold produces a wider F0 range than the mule deer vocal fold. A long and relaxed vocal fold would be helpful in producing low F0 and a larger elk vocal fold is naturally prone to that. Higher F0s could be produced by elongating and tensioning the vocal fold, and the elk vocal fold will probably remain oscillating at high pressure and at maximum strain. Furthermore, more histological work on cervid vocal folds is necessary to confirm the thick lamina propria as common feature in this taxon.
In contrast to elk (Riede and Titze, 2008) and human (Chan et al., 2007) vocal folds, we found no significant sex differences in the biomechanical properties of mule deer vocal folds. This absence may reflect the overall low-vocal activity in both sexes of mule deer. However, there is a significant sex difference in maximum fundamental frequency in juvenile mule deer contact calls (Lingle et al., 2007a). Whether these vocal differences are reflected in significant biomechanical differences at this early age in mule deer vocal folds remain to be investigated.
The compliance of ligaments and tendons of the skeletomuscular apparatus is adjusted to match the particular architecture and role of the various subunits (muscle-tendon unit or bone/cartilage-ligament unit; Biewener, 2008; Roberts, 2002). We must consider that species differences in vocal fold lamina propria compliance may be an adaptive adjustment to the particular architecture encasing the vocal fold, i.e., the laryngeal framework. The range in which vocal fold length can be altered depends on the flexibility of the arytenoid cartilage pivoting around the cricoarytenoid joint and the thyroid cartilage pivoting around its connection with the cricoid cartilage. Differences between elk and mule deer in those moving units are unknown. Another reason for interpreting stress response of vocal folds in relation to overall larynx anatomy is the glottal aerodynamic forces at the sound source. The interaction of the sound source with the vocal tract filter is an important factor that affects the acoustics of voice production (Titze et al., 2008). For example, there is no lateral ventricle and no vestibular fold or vestibular ligament in cervids (Köhler, 1982). Also, the epilaryngeal tube (or vestibulum laryngis, i.e., the intralaryngeal space from the vocal folds to the cranial edge of the larynx) is relatively long in elk and mule deer (5 to 7 cm long in elk, 4 to 5 cm in mule deer, 2 to 3 cm in human). Both structures, lateral ventricle (Finnegan and Alipour, 2007) and epilaryngeal tube (Döllinger et al., 2006; Titze and Story, 1997) can affect vocal fold vibration.
The emerging picture is that there are biomechanical differences in the viscoelastic properties of vocal folds at two levels: a) species differences and b) sex differences. A number of questions remain about the extent to which these differences influence the functional morphology of the vocal folds as a sound source. A combination of various experimental in vitro techniques and in vivo studies in species allowing the observation of vocal folds in action will bring us closer to an understanding of this very interesting instrument and its evolution.
ACKNOWLEDGMENTS
The authors are very grateful to Ivy LeVan, Dr. Laurie Baeten, and Michael Miller, Colorado Division of Wildlife, Fort Collins, CO, for their help with the tissue retrieval.
Contract grant sponsor: The National Institute on Deafness and Other Communication Disorders; Contract grant number: R01 DC008612; Contract grant sponsor: Deutsche Akademie der Naturforscher Leopoldina; Contract grant number: BMBF-LPD 9901/8-127.
LITERATURE CITED
- Alipour F, Jaiswal S. Glottal airflow resistance in excised pig, sheep, and cow larynges. J Voice. 2009;23:40–50. doi: 10.1016/j.jvoice.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Bartlett D, Remmers JE, Gautier H. Laryngeal regulation of respiratory airflow. Respir Physiol. 1973;18:194–204. doi: 10.1016/0034-5687(73)90050-9. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Tendons and ligaments: Structure, mechanical behavior and biological function. In: Fratzl M, editor. Collagen: Structure and Mechanics. Springer; New York: 2008. pp. 269–284. [Google Scholar]
- Bowyer RT, Kitchen DW. Sex and age class differences in vocalizations of Roosevelt elk during rut. Am Midland Nat. 1987;118:225–235. [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sinauer Associates; Sunderland, MA: 1998. [Google Scholar]
- Brown C, Alipour F, Berry DA, Montequin D. Laryngeal biomechanics and vocal communication in the squirrel monkey (Saimiri boliviensis). J Acoust Soc Am. 2003;113:2114–2126. doi: 10.1121/1.1528930. [DOI] [PubMed] [Google Scholar]
- Chan RW, Titze IR. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482–491. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- Chan RW, Fu M, Young L, Tirunagari N. Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Ann Biomed Eng. 2007;35:1471–1483. doi: 10.1007/s10439-007-9314-x. [DOI] [PubMed] [Google Scholar]
- Cowan IM, Geist V. Aggressive behavior in deer of the genus Odocoileus. J Mammal. 1961;42:522–526. [Google Scholar]
- Döllinger M, Berry DA, Montequin DA. The influence of epilarynx area on vocal fold dynamics. Otolaryngol Head Neck Surg. 2006;135:724–729. doi: 10.1016/j.otohns.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Feighny JJ, Williamson KE, Clarke JA. North American elk bugle vocalizations: Male and female bugle call structure and context. J Mammal. 2006;87:1072–1077. [Google Scholar]
- Finnegan A, Alipour F. Phonatory effects of supraglottic structures in excised canine larynges. J Voice. 2007;23:51–61. doi: 10.1016/j.jvoice.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist V. Behavior: Adaptive strategies in mule deer. In: Wallmo OC, editor. Mule and Black-Tailed Deer of North America. University of Nebraska Press; Lincoln: 1981. pp. 157–223. [Google Scholar]
- Gray SD, Titze IR, Alipour F, Hammond TH. Biomechanical and histological observations of vocal fold fibrous proteins. Ann Otol Rhinol Laryngol. 2000;109:77–85. doi: 10.1177/000348940010900115. [DOI] [PubMed] [Google Scholar]
- Hahn MS, Kobler JB, Starcher BC, Zeitels SM, Langer R. Quantitative and comparative studies of vocal fold extracellular matrix. I. Elastic fibers and hyaluronic acid. Ann Otol Rhinol Laryngol. 2006a;115:156–164. doi: 10.1177/000348940611500213. [DOI] [PubMed] [Google Scholar]
- Hahn MS, Kobler JB, Zeitels SM, Langer R. Quantitative and comparative studies of vocal fold extracellular matrix. II. Collagen. Ann Otol Rhinol Laryngol. 2006b;115:225–232. doi: 10.1177/000348940611500311. [DOI] [PubMed] [Google Scholar]
- Hammond TH, Gray SD, Butler J, Zhou R, Hammond E. Age- and gender-related elastin distribution changes in human vocal folds. Otolaryngol Head Neck Surg. 1997;119:314–322. doi: 10.1016/S0194-5998(98)70071-3. [DOI] [PubMed] [Google Scholar]
- Harrison DFN. The Anatomy and Physiology of the Mammalian Larynx. Cambridge University Press; Cambridge, UK: 1995. [Google Scholar]
- Hast MH. The larynx of roaring and non-roaring cats. J Anat. 1986;149:221–222. [PMC free article] [PubMed] [Google Scholar]
- Hirano M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr (Basel) 1974;26:89–94. doi: 10.1159/000263771. [DOI] [PubMed] [Google Scholar]
- Hirpara KM, Paul MB, Sullivan J, O'sullivan ME. The effects of freezing on the tensile properties of repaired porcine flexor tendon. J Hand Surg. 2008;33:353–358. doi: 10.1016/j.jhsa.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Hollien H. Vocal pitch variation related to changes in vocal fold length. J Speech Hear Res. 1960;3:150–156. [Google Scholar]
- Hunter EJ, Titze IR. Refinements in modeling the passive properties of laryngeal soft tissue. J Appl Physiol. 2007;103:206–219. doi: 10.1152/japplphysiol.00892.2006. [DOI] [PubMed] [Google Scholar]
- Hunter EJ, Titze IR, Alipour F. A three-dimensional model of vocal fold adduction/abduction. J Acoust Soc Am. 2004;115:1747–1759. doi: 10.1121/1.1652033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter EJ, Alipour F, Titze IR. Sensitivity of elastic properties to measurement uncertainties in laryngeal muscles with implications for voice fundamental frequency prediction. J Voice. 2007;21:641–650. doi: 10.1016/j.jvoice.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M. The vocalizations of ungulates, their causation and function. Z Tierpsychol. 1972;31:171–222. doi: 10.1111/j.1439-0310.1972.tb01764.x. [DOI] [PubMed] [Google Scholar]
- Köhler H. Vergleichend-Anatomische Untersuchungen am Kehlkopf von Cerviden, Dissertation. University of Giessen; Germany: 1982. [Google Scholar]
- Lingle S, Pellis SM. Fight or flight? Antipredator behavior and the escalation of coyote encounters with deer. Oecologia. 2002;131:154–164. doi: 10.1007/s00442-001-0858-4. [DOI] [PubMed] [Google Scholar]
- Lingle S, Rendall D, Pellis SM. Altruism and recognition in the antipredator defence of deer, Part 1: Species and individual variation in fawn distress calls. Anim Behav. 2007a;73:897–905. [Google Scholar]
- Lingle S, Rendall D, Wilson WF, DeYoung RW, Pellis SM. Altruism and recognition in the antipredator defence of deer, Part 2: Why mule deer help nonoffspring fawns. Anim Behav. 2007b;73:907–917. [Google Scholar]
- Min YB, Titze IR, Alipour-Haghighi F. Stress-strain response of the human vocal ligament. Ann Otol Rhinol Laryngol. 1995;104:563–569. doi: 10.1177/000348949510400711. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Iinuma N, Maeda M, Wada E, Shimizu K. Effects of abdominal belts on intra-abdominal pressure, intramuscular pressure in the erector spinae muscles and myoelectrical activities of trunk muscles. Clin Biomech (Bristol, Avon) 1999;14:79–89. doi: 10.1016/s0268-0033(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Negus VE. A hitherto undescribed function of the vocal cords. J Laryngol Otol. 1924;39:1–8. [Google Scholar]
- Negus VE. The Comparative Anatomy and Physiology of the Larynx. Grune & Stratton, Inc.; New York: 1949. [Google Scholar]
- Richardson LW, Jacobson HA, Muncy RJ, Perkins CJ. Acoustics of white-tailed deer (Odocoileus virginianus). J Mammal. 1983;64:245–252. [Google Scholar]
- Riede T, Titze IR. Vocal fold elasticity of the Rocky Mountain elk (Cervus elaphus nelsoni) – producing high fundamental frequency vocalization with a very long vocal fold. J Exp Biol. 2008;211:2144–2154. doi: 10.1242/jeb.017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TJ. The integrated function of muscles and ten-dons during locomotion. Comp Biochem Physiol A. 2002;133:1087–1099. doi: 10.1016/s1095-6433(02)00244-1. [DOI] [PubMed] [Google Scholar]
- Schneider R. Der larynx der saügetiere. In: Helmcke JL, Langerken H, Starck D, Wermuth H, editors. Handbuch der Zoologie. Vol. 8. Walther de Gruyter; Berlin: 1964. pp. 1–128. [Google Scholar]
- Struhsaker TT. The behavior of the elk (Cervus canadensis) during the rut. Z Tierpsychol. 1968;24:80–114. doi: 10.1111/j.1439-0310.1967.tb01229.x. [DOI] [PubMed] [Google Scholar]
- Tembrock G. Akustische Kommunikation bei Saügetieren. Wissenschaftliche Buchgesellschaft; Darmstadt, Germany: 1996. [Google Scholar]
- Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am. 1988;83:1536–1552. doi: 10.1121/1.395910. [DOI] [PubMed] [Google Scholar]
- Titze IR. Mechanical stress in phonation. J Voice. 1994;8:99–105. doi: 10.1016/s0892-1997(05)80302-9. [DOI] [PubMed] [Google Scholar]
- Titze IR, Story BH. Acoustic interactions of the voice source with the lower vocal tract. J Acoust Soc Am. 1997;101:2234–2243. doi: 10.1121/1.418246. [DOI] [PubMed] [Google Scholar]
- Titze IR. The myoelastic aerodynamic theory of phonation. The National Center for Voice and Speech. Denver, CO; Iowa City, IO: 2006. [Google Scholar]
- Titze IR, Riede T, Popollo P. Vocal exercises to determine nonlinear source-filter interaction. J Acoust Soc Am. 2008;123:1902–1915. doi: 10.1121/1.2832339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg J. The aerodynamic myoelastic theory of voice production. J Speech Hearing Res. 1958;3:227–244. doi: 10.1044/jshr.0103.227. [DOI] [PubMed] [Google Scholar]