Abstract
Brassica vegetables are known to contain relatively high concentrations of bioactive compounds associated with human health. A comprehensive profiling of polyphenols from five Brassica species microgreens was conducted using ultra high-performance liquid chromatography photo diode array high-resolution multi-stage mass spectrometry (UHPLC-PDA-ESI/HRMSn). A total of 164 polyphenols including 30 anthocyanins, 105 flavonol glycosides, and 29 hydroxycinnamic acid and hydroxybenzoic acid derivatives were putatively identified.The putative identifications were based on UHPLC-HRMSn analysis using retention times, elution orders, UV/Vis spectra and high resolution mass spectra, in-house polyphenol database, and as well as literature comparisons. This study showed that these five Brassica species microgreens could be considered as good sources of food polyphenols.
Keywords: Microgreen, Brassicaceae, acylated cyanidin 3-sophroside-5-mono- and diglucosides, acylated flavonol glycosides, hydroxycinnamic acid derivatives, UHPLC-PDA-ESI/HRMSn
INTRODUCTION
Microgreens are young edible greens produced from vegetables, herbs, or other plants, ranging in size from five to ten centimeters long including stem and cotyledons (seed-leaves). They are popular for their pretty colors, intense flavors, delicate textures and relatively high nutritional contents (1). The entire plant (seedling) is harvested at the ground level when cotyledon or seed-leaves have fully expanded and before true leaves have fully emerged.
The Brassicacae offers some of the most commonly consumed vegetables worldwide, which can be grown as microgreens. Five Brassica vegetables commonly found in the U.S. market place are red cabbage (B. oleracea var. capitata), purple kohlrabi (B. oleracea var. gongylodes), red and purple mustards (B. juncea), and mizuna (B. rapa var. nipposinica or B. juncea var. japonica). Brassica vegetables are known to be rich sources of ascorbic acid, carotenoids, glucosinolates, polyphenols, and tocopherols (2-4) which have human-health beneficial attributes reportedly involved in preventing cardiovascular diseases and some types of cancers (5-8).
Previous studies have tentatively identified phenolic compounds from 22 mature-leaf Brassica vegetables (9-12) and phenolic compounds have been found in tronchuda cabbage (Brassica oleracia var. costata) seeds (13) mature leaves (14) inter-nodal shoots and roots (15, 16). Twelve specific phenolic compounds have been profiled in two- to twelve-day old seedlings possessing both seed-leaves and true-leaves. The aim of the present study was to characterize and quantify the naturally occurring polyphenols in five commonly consumed Brassicas (mizuna, red cabbage, purple kohlrabi, red mustard and purple mustard) at their microgreen growth stage. The analyses of their native polyphenols and flavonol aglycones were performed using state-of art analytical tools: ultra high-performance liquid chromatography photo diode array high-resolution multistage mass spectrometry (UHPLC-PDA-ESI/HRMS/MSn). Results showed that Brassica microgreens contained notable levels of hydroxyl cinamic acids and may contain different compounds from their true-leaves. A total of 30 anthocyanins, 105 flavonol glycosides, and 29 hydroxycinnamic acid and hydroxylbenzoic acid derivatives were tentatively identified. This is the first known reported study of polyphenol compounds in vegetables at the cotlyledonary-leaf (microgreen) stage of growth of an array of Brassica microgreens.
MATERIALS AND METHODS
Chemicals
Formic acid, HPLC grade Methanol and acetonitrile, were purchased from VWR International, Inc. (Clarksburg, MD). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Lab., Bedford, MA).
Plant Materials and Sample Preparation
Five Brassica species, at the microgreen growth stage, were obtained from Sun Growers Organic Distributors, Inc. (San Diego, CA). All the fresh samples were lyophilized and then powdered. Powdered samples (100 mg) were extracted with 5.00 mL of methanol-water (60:40, v/v) using sonication for 60 min at room temperature, and then centrifuged at 1,000 g for 15 minutes (IEC Clinical Centrifuge, Damon/IEC Division, Needham, MA, USA). The supernatant was filtered through a 17 mm (0.45 μm) PVDF syringe filter (VWR Scientific, Seattle, WA, USA), and 10 μL of the extract was used for each HPLC injection.
UHPLC-PDA-ESI/HRMS/MSn Conditions
The UHPLC-HRMS system used consisted of a LTQ Orbitrap XL mass spectrometer with an Accela 1250 binary Pump, a PAL HTC Accela TMO autosampler, a PDA detector (ThermoFisher Scientific, San Jose, CA), and a G1316A column compartment (Agilent, Palo Alto, CA). Separation was carried out on a Hypersil Gold AQ RP- C18 UHPLC column (200 mm × 2.1 mm i.d., 1.9 μm, ThermoFisher Scientific) with an UltraShield pre-column filter (Analytical Scientific Instruments, Richmond, CA) at a flow rate of 0.3 mL/min. The mobile phase consisted of a combination of A (0.1% formic acid in water, v/v) and B (0.1% formic acid in acetonitrile, v/v). The linear gradient was from 4% to 20% B (v/v) at 40 min, to 35% B at 60 min and to 100% B at 61 min, and held at 100% B to 65 min. The PDA was set at 520, 330 and 280 nm to record the peaks, and UV/Vis spectra were recorded from 200-700 nm.
Both positive and negative ionization modes were used and the conditions were set as follows: sheath gas at 70 (arbitrary units), aux and sweep gas at 15 (arbitrary units), spray voltage at 4.8 kV, capillary temp at 300 °C, capillary voltage at 15 V, and Tube lens at 70 V. The mass range was from 100 to 2000 amu with a resolution of 15,000, FTMS AGC target at 2e5, FT- MS/MS AGC target at 1e5, isolation width of 1.5 amu, and max ion injection time of 500 ms. The most intense ion was selected for the data-dependent scan to offer their MS2 to MS5product ions, respectively, with the a normalization collision energy at 35%.
RESULTS AND DISCUSSION
Strategies for Systematic Identification of Polyphenols from Microgreen Brassica
Brassicaceae polyphenol composition has been extensively investigated. The main flavonols in Brassica vegetables are the O-glycosides of quercetin, kaempferol, and isorhamnetin, (2, 17-22). The sugar moiety found in Brassica vegetables is glucose, occurring as mono-, di-, tri-, tetra-, and pentaglucosides (17-23). They are also commonly found acylated by different hydroxycinnamic acids. Anthocyanins are another main class of flavonoid found in Brassica vegetables and cyanidin is the most common anthocyanidin in colored-leaf Brassica vegetables (2, 24). Hydroxycinnamic acids (C6-C3) are phenolic acids characterized in Brassica vegetables with the most common ones being p-coumaric, caffeic, sinapic and ferulic acids; often found in conjugation with sugars or other hydroxycinnamic acids (2, 17-19, 21, 22).
The five Brassica species microgreen phenolic compounds exhibit absorbance maxima at three wavelengths (280 nm for flavonols and flavonol glycosides, 320 nm for hydroxycinnamic acid derivatives, and 520 nm for anthocyanins(2, 17-19, 21, 22).
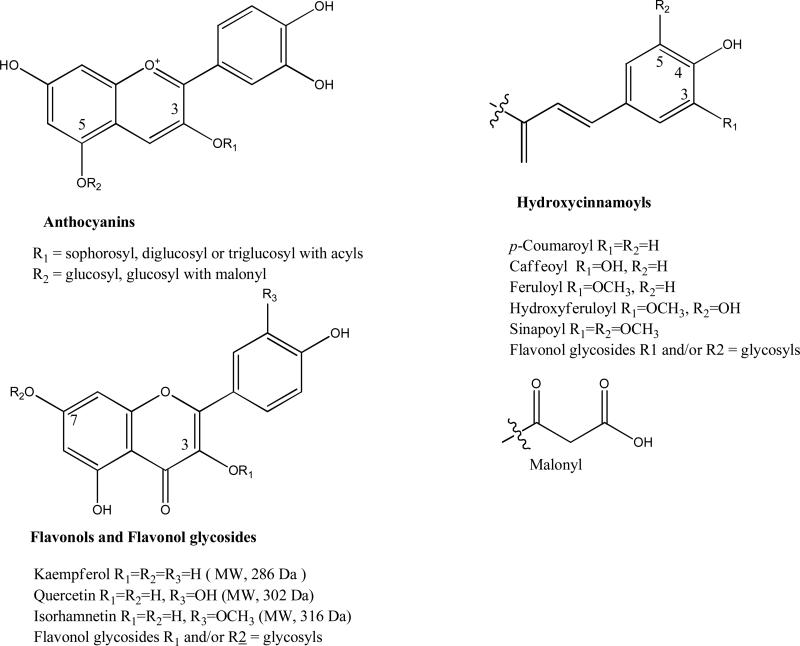
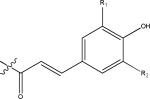
HRMS was used for the determination of chemical formulas. Neutral loss information from MS was used for identification of sugar moiety and acyl groups. In MS analysis, cleavage of the first glycosidic linkage is expected to take place at the O-glycosidic bond at the 7-position of the flavonols and the 5-position of the anthocyanins, leading to the fragmentations [(M-H)-162]− for monohexosides and [(M-H)-324]− for dihexosides (23, 25, 26). The remaining glucose moieties of the flavonoid molecule are expected to be linked to the hydroxyl group at the 3-position of the aglycone. The disaccharide moieties of the flavonoids in Brassica species are mainly sophorosides (2). The MS fragmentation behavior can be used for the determination of interglucoside linkage and neutral losses of 180, 162, and 120 amu indicate a sophoroside with a 1→2 inter glucoside linkage, while loss of 324 amu, and in some cases low abundance of 162 amu, corresponds to a diglucoside with a 1→6 linkage like gentiobioside (27). The saccharides (mono-, di-, tri-saccharides) and acyl groups of flavonol glycoside and their possible neutral losses in CID MS/MS analysis are listed in Table 1, and the basic structures of the phenolic compounds found in these five Brassica species microgreens are shown in Figure 1.
Table 1.
Typical substitutional groups and common neutral losses of polyphenols in five Brassica species microgreens.
| Substitutional groups | Name | Neutral Loss in HRMS |
|---|---|---|
| mono-saccharides | pentose (xylose, arabinose) | 132.0422 (C5H8O4) |
| methyl-pentose (rhamnose) hexose (glucose, galactose) |
146.0579 (C6H10O4) 162.0528 (C6H10O5) |
|
| di-saccharides | sophorose=2-β-D-glucopyranosyl-D-glucose gentiobiose=6-β-D-glucopyranosyl-D-glucose |
324.1056 (C12H20O10) |
| tri-saccharides | sophorotriose (2‴- β-D-glucopyranosyl-2″-β-D-glucopyranosyl-D-glucose) gentiotriose (6‴- β-D-glucopyranosyl-6″-β-D-glucopyranosyl-D-glucose) | 486.1584 (C18H30O15) |
| hydroxycinnamoyls |
p-coumaroyl R1=H R2=H caffeoyl R1=OH R2=H |
146.0347 (C9H6O2) 162.0317 (C9H6O3) |
|
feruloyl R1=OCH3 R2=H sinapoyl R1=OCH3 R2=OCH3 |
176.0473 (C10H8O3) 206.0579 (C11H10O4) |
| hydroxybenzoyls | p-hydroxybenzoyl R1=H R2=H | 120.0211 (C7H4O2) |
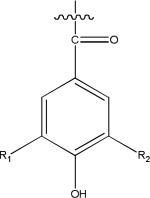
|
galloyl R1= OH R2=OH | 152.0109 (C7H4O4) |
| di-carboxylic acid acyls | malonyl | 86.0004 (C3H2O3) |
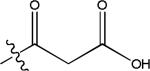
|
Figure 1.
Basic chemical structures identified from five Brassica species microgreens.
Anthocyanins
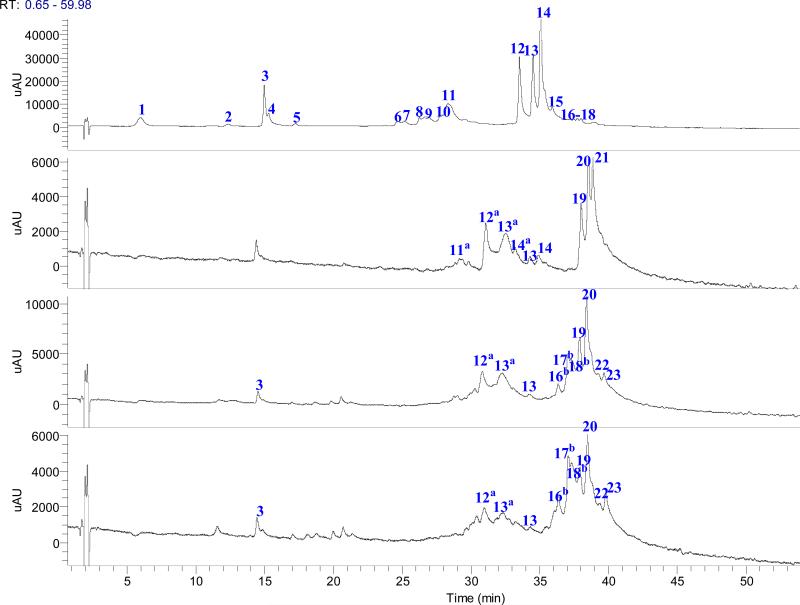
Among the five Brassica species microgreen red cabbage, red mustard, purple mustard, and purple kohlrabi have red to purple colored seed-leaves. UHPLC chromatograms at 520 nm revealed 30 different anthocyanins are likely responsible for this coloration (Figure 2). The retention times (tR), HRMS masses [M]+ molecular formulas, errors (ppm) between theoretical and measured values, and major MS2 and MS3 product ions are summarized in Table 2.
Figure 2.
The UHPLC chromatogram from five Brassica species microgreens: red cabbage (A), purple kohlrabi (B), red mustard (C) and purple mustard (D) under 520 nm.
Table 2.
UHPLC-HRMS-Data of anthocyanins from combines four Brassica species Microgreens: red cabbage, red mustard, purple mustard, mizuna and purple kohlrabi
| Peak # | tR (min) | [M]+ | Formula | Error (mmu) | major and important MS2 ions | major MS3 ion | Tentative Identification |
|---|---|---|---|---|---|---|---|
| 1 | 5.97 | 773.2106 | C33H41O21 | −1.36 | 611(29), 449(40), 287(100) | 287(100) | cy 3-diglucoside-5-glucoside* |
| 2 | 12.34 | 965.2528 | C43H49O25 | −2.12 | 803(100), 641(20), 287(60) | 287(100) | cy 3-hydroxyferuloyl-5-glucoside* |
| 3 | 14.98 | 979.2699 | C44H51O25 | −1.53 | 817(71), 449(46), 287(100) | 287(100) | cy 3-(sinapoyl)-diglucoside-5-glucosides* |
| 4 | 15.29 | 979.2708 | C44H51O25 | −0.61 | 817(82), 449(52), 287(100) | 287(100) | cy 3-(sinapoyl)-diglucoside-5-glucoside* |
| 5 | 17.21 | 1141.3246 | C50H61O30 | 0.34 | 979(100), 449(54) | 287(100) | cy 3-(glucopyranosyl-sinapoyl)diglucoside-5-glucoside* |
| 6 | 24.63 | 919.249 | C42H47O23 | −1.38 | 757(100), 449(19), 287(50) | 287(100) | cy 3-(coumaroyl)sophoroside-5-glucoside* |
| 7 | 25.23 | 1287.3597 | C59H67O32 | −1.01 | 1125(100), 449(6) | 963(100), | cy-3(glucosyl)(sinapoyl)(p-coumaroyl)sophorside-5-glucoside* |
| 8 | 26.31 | 1317.369 | C60H69O33 | −1.94 | 1185(100), 1155(35), 449(2) | 1023(100), 449(3) | cy-3(glucosyl)(sinapoyl)(feruloyl)sophorside-5-glucoside* |
| 9 | 26.97 | 919.249 | C42H47O23 | −1.38 | 757(100), 449(19), 287(50) | 287(100) | cy 3-(coumaroyl)sophoroside-5-glucoside* |
| 10 | 27.71 | 949.2602 | C43H49O24 | −0.66 | 787(100), 449(18), 287(49) | 287(100) | cy 3-(feruloyl)sophoroside-5-glucoside* |
| 11 | 28.31 | 1141.3016 | C53H57O28 | −1.30 | 979(100), 449(11) | 287(100) | cy 3-(caffeoyl)(sinapoyl)diglucoside-5-glucoside |
| 11a | 29.14 | 1005.2492 | C45H49O26 | −1.46 | 757(22), 535(100), 491(10), 287(73) | 287(100) | cy 3-(coumaroyl)sophoroside-5-(malonyl)glucoside |
| 12 | 33.37 | 1125.307 | C53H57O27 | −1.04 | 963(100), 449(13) | 287(100) | cy 3-diferuloylsophoroside-5-glucoside* |
| 12a | 31.07 | 1211.3088 | C56H59O30 | 0.23 | 963(100), 535(81), 521(9) | 287(100) | cy 3-(coumaroyl)(sinapoyl)diglucoside-5-(malonyl)glucoside* |
| 13 | 34.50 | 1125.307 | C53H57O27 | −1.04 | 963(100), 449(13) | 287(100) | cy 3-diferuloylsophoroside-5-glucoside* |
| 13a | 32.56 | 1035.2599 | C46H51O27 | −1.32 | 992(7), 787(40), 780(5), 535(100), 492(12), 449(6), 287(5) | 287(100) | cy 3-(feruloyl)glucoside-5-(malonyl)-glucoside* |
| 14 | 35.07 | 1155.3192 | C54H59O28 | 0.40 | 993(100), 449(9) | 287(100) | cy 3-sinapoylferuloylsophoroside-5-glucoside* |
| 14a | 33.21 | 1065.2702 | C47H53O28 | −1.59 | 817(73), 535(100), 492(2), 449(3) | 287(100) | cy 3-(sinapoyl)glucoside-5-(malonyl)-glucoside* |
| 15 | 35.91 | 1155.3192 | C54H59O28 | 0.40 | 993(100), 449(9) | 287(100) | cy 3-(sinapoyl)(feruloyl)sophoroside-5-glucoside* |
| 16 | 37.14 | 1185.3298 | C55H61O29 | 0.50 | 1023(100), 449(10) | 287(100) | cy 3-(sinapoyl)(sinapoyl)sophoroside-5-glucoside* |
| 16b | 36.34 | 1373.3585 | C62H69O35 | −2.89 | 963(100), 697(66), 653(28) | 287(100) | cy 3-(sinapoyl)(coumaroyl)-triglucoside-5-(malonyl)-glucoside* |
| 17 | 37.55 | 1155.3192 | C54H59O28 | 0.40 | 993(100), 449(9) | 287(100) | cy 3-(sinapoyl)(feruloyl)sophoroside-5-glucoside* |
| 17b | 37.08 | 1197.2902 | C55H57O30 | −2.72 | 949(18), 860(3), 535(100), 517(3), 491(9) | 287(100) | cy 3-(caffeoyl) (sinapoyl)-xylglu-5-(malonyl) glucoside* |
| 18 | 37.99 | 1185.3298 | C55H61O29 | 0.49 | 1023(100), 449(10) | 287(100) | cy 3-(smapoyl)(sinapoyl)sophoroside-5-glucoside* |
| 18b | 37.37 | 1227.3008 | C56H59O31 | −2.68 | 979(82), 535(100), 491(10) | 287(100) | cy 3-(p-coumaroyl)(sinapoyl)diglucoside-5-O-(malonyl)glucoside |
| 19 | 38.00 | 1211.3082 | C56H59O30 | −1.46 | 963(91), 535(100), 491(3) | 287(100) | cy-3-(feruloyl)(feruloyl)diglucoside-5-(malonyl)glucoside* |
| 20 | 38.56 | 1241.3192 | C57H61O31 | −1.03 | 1206(15), 1198(30), 993(100), 535(88), 449(8) | 287(100) | cy 3-(sinapoyl)(feruloyl)diglucoside-5-(malonyl)glucoside* |
| 21 | 38.85 | 1271.3296 | C58H63O32 | −0.10 | 1023(100), 535(51), 491(7) | 287(100) | cy 3-(sinapoyl)(sinapoyl)diglucoside-5-(malonyl)glucoside* |
| 22 | 39.35 | 1241.3190 | C57H61O31 | −0.13 | 993(100), 535(70), 492(13) | 287(100) | cy3-(sinapoyl)(feruloyl)diglucoside-5-(malonyl)glucoside* |
| 23 | 39.81 | 1211.3078 | C56H59O30 | −0.77 | 963(86), 535(100) | 287(100) | cy 3-(p-coumaroyl)(sinapoyl)diglucoside-5-(malonyl)glucoside* |
compared with literature data, cy-cyanidin
In these five Brassica species microgreens, only cyanidin (cy) derivatives were found, which is in accordance with the other studies on Brassicas (24, 28-30). The anthocyanins found in red cabbage microgreens were cy-3-diglucoside-5-glucoside derivatives acylated with different hydroxycinnamic acids at the diglucosyl moiety in the 3-position. High resolution mass spectroscopic analysis with multi stage mass fragmentation was used as an important tool for anthocyanin characterization. Among the 30 cy glycosides found in red cabbage, red mustard, purple mustard and purple kohlarabi microgreens, peak 1 at m/z 773.2106 (C33H41O21, −1.36 mmu) was the lowest molecular weight anthocyanin and the losses of three hexosyl units were observed in MS2 spectra, suggesting cy-3-diglucoside-5-glucoside, a typical compound reported in red cabbage.The major acylated anthocyanins were cy-3-diglucoside-5-glucoside derivatives with various acylated groups e.g. coumaroyl, feruloyl and sinapoyl connected to the diglucoside. The MS/MS of most of the molecular ions of acylated anthocyanins gave the major product ions at m/z 449, a cy 5-glucoside residue, and at m/z 611, a cy-3-diglucoside residue. The MS/MS fragments of the acylated anthocyanins allow for a rough determination of the location of the acylating groups. Peaks 12, 13 and 14 are the major anthocyanins in microgreen red cabbage, and they were identified as cyanidin 3-di-feruloyl-sophoroside-5-glucoside, cy 3-(sinapoyl)(sinapoyl)sophoroside-5-glucoside and cy 3-(sinapoyl)(feruloyl)sophoroside-5-glucoside, respectivelyUsing peak 12 as an example, HRMS gave the [M]+ ion at 1125.3070, corresponding to the formula of C53H57O27. Fragmentation of ion at m/z 1125 in positive mode produced ions at m/z 963 by loss of a glucosyl residue (162 amu) from the 5-position. The ion at m/z 449 was produced by a total loss of 676 amu, corresponding to a di-feruloyl-diglucosyl residue (176 + 176 + 324 amu), from the terminal 3-position
In previous study of purple kohlrabi, 12 anthocyanins have been identified. The major ones are cy 3-(feruloyl)(sinapoyl) diglucoside-5-glucoside, cy 3-(feruloyl) diglucoside-5-glucoside and cy 3-(sinapoyl)(sinapoyl) diglucoside-5-glucoside (31). In our study, the acylated anthocyanins with one malonyl group attached to the hexose of C-5 and other aromatic groups (caffeic, p-coumaric, sinapic or ferulic acid) attached to the C-3 glycosidic substituent were found. In the MS2 spectra, the fragment ions at (m/z 1023, 993 and 963), with the two acyl groups attached to the di-hexose of C-3 are usually observed as the base peak. This fragmentation pattern was evidenced with most anthocyanins analyzed and lead to the tentative identification of cy-3-(feruloyl)(feruloyl)diglucoside-5-(malonyl)-glucoside (m/z 1211, peak 19), cy 3-O-(sinapoyl)(feruloyl)diglucoside-5-O-(malonyl)glucoside (m/z 1241, peak 20), and cy 3-O-(sinapoyl)(sinapoyl)diglucoside-5-O-(malonyl)glucoside (m/z 1271, peak 21). Peaks 11a-14a were identified as cy 3-p-(coumaroyl)sophoroside-5-(malonyl)glucoside, cy 3-O-(pcoumaroyl)(sinapoyl) diglucoside-5-O-(malonyl) glucoside, cy 3-O-(feruloyl)glucoside-5-O-(malonyl)-glucoside and cy 3-O-(sinapoyl)glucoside-5-O-(malonyl)glucoside in red mustard microgreen (10). Peaks 19 and 20, were two major anthocyanins identified in red and purple mustard. Peaks 16b, 17b and 18b were identified as cy 3-(sinapoyl)(coumaroyl)-triglucoside-5-(malonyl)-glucoside, and cy 3-(coumaroyl)(sinapoyl)diglucoside-5-(malonyl)glucoside, respectively.
O-Glycosylated Flavonols and Their Acylated Derivatives
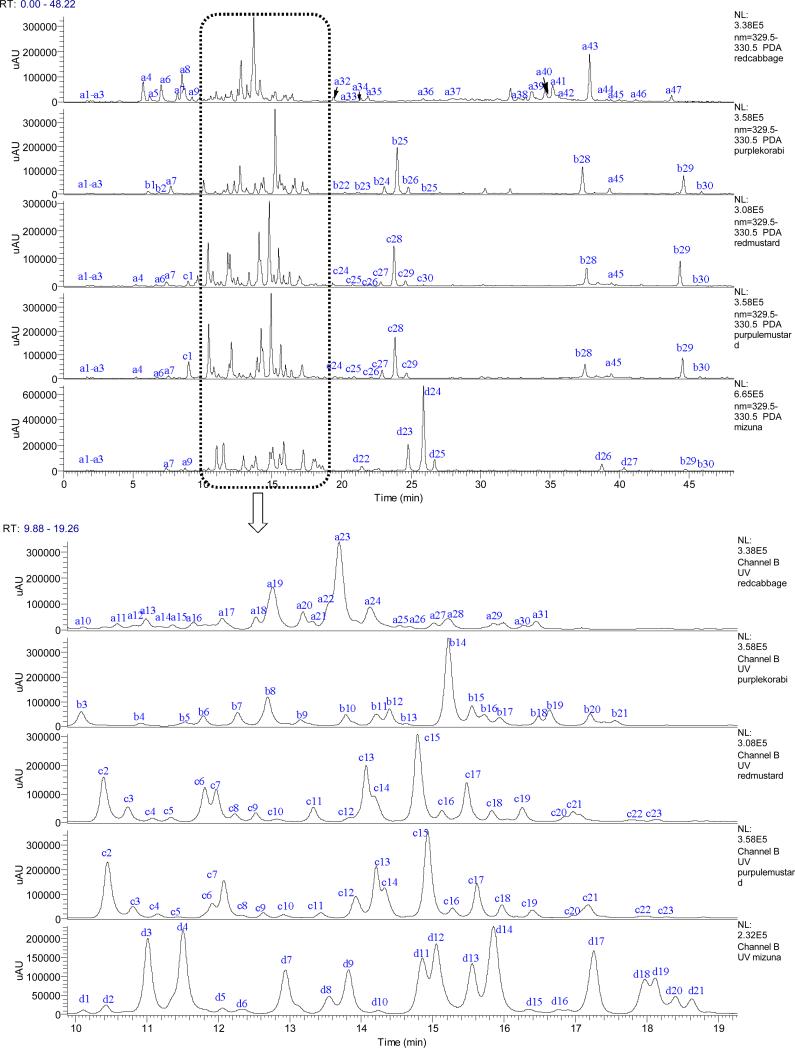
Acylated flavonoid glycosides were easily identified based on the increased mass of the parent ions and the wavelength maxima (330-336 nm) of their UV spectra (Figure 3). According to the MSn (n=2-5) data, the aglycones of the flavonol glycosides were quercetin (Qn), kaempferol (Km), and isorhamnetin (Is). Using the strategy described previously, 105 flavonol glycosides were characterized in five microgreen vegetables (Figure 3). Among them, 18 were non-acylated flavonoid glycosides, and 87 were acylated flavonoid glycosides. The compound distribution in these 5 microgreens is shown in Table 3. Qn 3-sophoroside-7-glucoside, Qn 3-hydroxyferuloylsophoroside-7-glucoside, Km 3-hydroxyferuloylsophoroside-7-glucoside, Km 3-sinapoylsophoroside-7-glucoside and Is 3-caffeoylsophoroside-7-glucoside, are common peaks in all five brassica species microgreens. Is 3-O-glucoside, Qn 3,7-di-O-glucoside, Km 3-pcoumaroyldiglucoside, Qn 3-caffeoylsophoroside, Qn 3-feruloylsophoroside, Qn 3-feruloylsophoroside-7-glucoside, and Km 3-sinapoylsophoroside were found only in mizuna microgreen, while Km 3-sinapoylsophoroside-7-glucoside and Qn 3-sinapoylsophorotrioside were found only in purple kohlrabi. Red cabbage microgreens had, Km 3-pcoumaroylsophorotrioside, Km 3-p-coumaroylsophoroside-7-diglucoside, km 3-hydroxyferuloylsophorotrioside-7-glucoside, Km 3-disinapoyldiglucoside-7-glucoside, Km 3-sinapoylferuloylsophoroside-7-glucoside and Qn 3-disinapoylsophorotrioside which were also found in mature red cabbage. Km 3-sophorotrioside-7-glucoside, Qn 3-caffeoylsophorotrioside-7-glucoside and Qn 3-hydroxyferuloylsophorotioside-7-glucoside only existed in microgreen of red mustard and purple mustard.
Figure 3.
The UHPLC chromatogram of five Brassica species microgreens: red cabbage (A), purple kohlrabi (B), red mustard (C), purple mustard (D) and mizuna (E) under 330 nm.
Table 3.
UHPLC-HRMS-Data of flavonol glycosides and derivatives of hydroxycinnamic acids and hydroxybenzoic acids from five Brassica speies microgreens: red cabbage, red mustard, purple mustard, mizuna and purple kohlrabi.
| Peak.No. | tR(min) | [M-H]- | Formula | Error (mmu) | major and important MS2 ions | MS3 ion | Tentative Identification |
|---|---|---|---|---|---|---|---|
| a1 | 1.66 | 133.0143 | C4H5O5 | 0.40 | 241(83), 153(100) | 115(100) | malic acid* |
| a2 | 1.98 | 191.0192 | C6H7O7 | −0.53 | 173(22), 111(100) | 67(100) | citric acid* |
| a3 | 3.37 | 205.0349 | C7H9O7 | −0.48 | 173(61), 159(6), 143(11), 111(100) | methyl citric acid | |
| b1 | 5.56 | 503.1398 | C21H27O14 | −0.83 | 341(100), 179(9) | 179(100) | courmaroyl-di-glucoside |
| a4 | 5.71 | 355.1029 | C16H19O9 | −1.56 | 217(59), 193(100), 175(40) | 134(100) | feruloyl-glucose |
| b2 | 5.93 | 353.0870 | C16H17O9 | −0.80 | 191(100), 179(43), 135(8) | 173(100) | caffeoyl-quinic acid |
| 5a | 6.12 | 299.0768 | C13H15O8 | −0.44 | 239(90), 179(71), 137(100) | salicyloyl-glucose* | |
| a6 | 6.21 | 547.1671 | C23H31O15 | 0.47 | 223(100) | 208(100) | sinapoyl-gentiobiose* |
| a7 | 7.18 | 447.0557 | C20H15O12 | −1.12 | 357(38), 275(55), 259(100) | 139(100) | rhamnosyl-ellagic acid |
| a8 | 8.32 | 787.1942 | C33H39O22 | 1.45 | 625(100) | 300(100) | qn 3-diglucoside-7-glucoside* |
| a9 | 9.20 | 787.1920 | C33H39O22 | −1.85 | 625(100) | 300(100) | qn 3-diglucoside-7-glucoside* |
| c1 | 9.68 | 933.2486 | C39H49O26 | −0.79 | 771(100) | 591(100) | km 3-sophorotrioside-7-glucoside |
| b3 | 10.06 | 787.1916 | C33H39O22 | −2.25 | 625(100) | 300(100) | qn 3-sophoroside-7-glucoside* |
| d1 | 10.09 | 845.2113 | C39H41O21 | −3.28 | 683(100), 477(15), 315(6) | 353(100) | is 3-sinapoylglucoside-7-glucoside* |
| a10 | 10.11 | 771.1978 | C33H39O21 | −1.13 | 609 (100) | 285(100) | km 3-sophoroside-7-glucoside* |
| d2 | 10.43 | 817.2015 | C34H41O23 | −2.91 | 609(100), 447(34) | 447(100) | km 3-diglucoside-7-glucoside with HCOOH |
| c2 | 10.45 | 1141.2889 | C49H57O31 | 1.07 | 979 (100), 949(93), 787(72) | 787(100) | qn 3-hydroxyferuloylsophorotioside-7-glucoside* |
| a11 | 10.59 | 979.2349 | C43H47O26 | −1.23 | 817(98), 787(100), 625(59) | 625(100) | qn 3 hydroxyferuloylsophoroside-7-glucoside* |
| c3 | 10.80 | 1111.2760 | C48H55O30 | −2.13 | 949(100), 787(30) | 787(100) | qn 3-caffeoylsophorotrioside-7-glucoside* |
| a12 | 10.82 | 979.2333 | C43H47O26 | −2.80 | 817(98), 787(100), 625(59) | 625(100) | qn 3 hydroxyferuloylsophoroside-7-glucoside* |
| b4 | 10.92 | 787.1906 | C33H39O22 | −3.25 | 625(100) | 300(100) | qn 3-sophoroside-7-glucoside* |
| d3 | 10.97 | 979.2359 | C43H47O26 | −0.20 | 817(92), 787(100), 625(51) | 625(100) | qn 3 hydroxyferuloylsophoroside-7-glucoside* |
| a13 | 10.99 | 949.2256 | C42H45O25 | 0.06 | 787(100), 625(22) | 625(100) | qn 3-caffeoylsophoroside-7-glucoside* |
| c4 | 11.11 | 1111.2749 | C48H55O30 | −3.46 | 949(100), 787(29) | 787(100) | qn 3-caffeoylsophorotrioside-7-glucoside* |
| a14 | 11.12 | 949.2231 | C42H45O25 | −2.44 | 787(100), 625(20) | 625(100) | qn 3-caffeoylsophoroside-7-glucoside* |
| c5 | 11.31 | 1111.2762 | C48H55O30 | −2.16 | 949(100), 787(29) | 787(100) | qn 3-caffeoylsophorotrioside-7-glucoside* |
| a15 | 11.36 | 1111.2780 | C48H55O30 | −0.36 | 949(100), 787(30) | 787(100) | qn 3-caffeoylsophorotrioside-7-glucoside* |
| d4 | 11.47 | 949.2234 | C42H45O25 | −2.14 | 787(100), 625(20) | 625(100) | qn 3-caffeoylsophoroside-7-glucoside* |
| b5 | 11.53 | 609.1447 | C27H29O16 | −1.41 | 489(7), 447(100), 285(10) | 285(100) | km 3-diglucoside |
| a16 | 11.65 | 1111.2761 | C48H55O30 | −2.26 | 949(100), 788(34), 625(36) | 625(100) | qn 3-caffeoylsophorotrioside-7-glucoside* |
| b6 | 11.81 | 771.1976 | C33H39O21 | −1.33 | 609(100) | 285(100) | km 3-sophoroside-7-glucoside* |
| c6 | 11.92 | 787.1942 | C33H39O22 | 1.45 | 625(100) | 300(100) | qn 3-sophoroside-7-glucoside* |
| a17 | 12.05 | 963.2385 | C43H47O25 | −2.69 | 801(100), 609(2) | 609(100) | km 3-hydroxyferuloylsophoroside-7-glucoside* |
| d5 | 12.07 | 1111.2766 | C48H55O30 | −1.76 | 949(100), 787(38) | qn 3-caffeoylsophorotrioside-7-glucoside* | |
| c7 | 12.08 | 979.2349 | C43H47O26 | −1.23 | 817(98), 787(100), 625(59) | 625(100) | qn 3 hydroxyferuloylsophoroside-7-glucoside* |
| b7 | 12.25 | 979.2329 | C43H47O26 | −3.21 | 817(95), 787(100), 625(55) | 625(100) | qn 3 hydroxyferuloylsophoroside-7-glucoside* |
| c8 | 12.28 | 1125.2937 | C49H57O30 | −0.28 | 963(100) | 771(100) | km 3-hydroxyferuloylsophorotrioside-7-glucoside* |
| d6 | 12.37 | 949.2234 | C42H45O25 | −2.14 | 787(100), 625(20) | 625(100) | qn 3-caffeoylsophoroside-7-glucoside* |
| a18 | 12.53 | 977.2541 | C44H49O25 | −2.80 | 831(43), 771(100), 625(21) | 301(100) | qn 3 sophoroside-7-sinapoylrhamoside |
| c9 | 12.63 | 1095.2826 | C48H55O29 | −0.28 | 975(2), 933(100), 809(7) | 771(100) | km 3-caffeoylsophorotrioside-7-glucoside* |
| b8 | 12.69 | 949.2236 | C42H45O25 | −1.94 | 787(100), 625(20) | 625(100) | qn 3-caffeoylsophoroside-7-glucoside* |
| a19 | 12.72 | 933.2289 | C42H45O24 | −1.73 | 771(100) | 609(100) | km 3-caffeoyldiglucoside-7-glucoside |
| c10 | 12.91 | 547.1671 | C23H31O15 | 0.47 | 223(100) | 208(100) | sinapoylgentiobiose* |
| d7 | 12.94 | 963.2381 | C43H47O25 | −3.09 | 801(100) | 609(100) | km 3-hydroxyferuloylsophoroside-7-glucoside* |
| b8 | 13.15 | 1111.2760 | C48H55O30 | −2.13 | 949(100), 787(30) | 787(100) | qn 3-caffeoylsophorotrioside-7-glucoside* |
| a20 | 13.18 | 1095.2797 | C48H55O29 | −3.42 | 949(84), 933(100), 787(45) | 787(100) | qn 3-p-coumaroyltriglucoside-7-glucoside |
| a21 | 13.32 | 1095.2826 | C48H55O29 | −0.28 | 975(2), 933(100), 809(7) | 771(100) | km 3-caffeoylsophorotrioside-7-glucoside* |
| c11 | 13.44 | 625.1410 | C27H29O17 | −0.04 | 463(8), 343(16), 301(100) | 179(100) | qn diglucoside* |
| a22 | 13.52 | 1155.3022 | C50H59O31 | −2.38 | 993(100), 950(41), 787(39) | qn 3-sinapoyltriglucoside-7-glucoside | |
| d8 | 13.58 | 961.2592 | C44H49O24 | −2.84 | 623(72), 609(100), 592(27) | 257(100) | km 3-sophoroside-7-sinapoylrhamnoside |
| a23 | 13.69 | 993.2493 | C44H49O26 | −2.45 | 801(13), 787(100) | 607(100) | qn 3-sinapoylsophoroside-7-glucoside* |
| d9 | 13.77 | 933.2275 | C42H45O24 | −3.13 | 771(100) | 609(100) | km 3-caffeoyldiglucoside-7-glucoside |
| b10 | 13.78 | 963.2391 | C43H47O25 | −2.09 | 801(100) | 609(100) | km 3-hydroxyferuloylsophoroside-7-glucoside* |
| c12 | 13.92 | 355.1029 | C16H19O9 | −1.56 | 217(59), 193(100), 175(40) | 134(100) | feruloylglucose* |
| a24 | 14.12 | 1125.2937 | C49H57O30 | −0.28 | 963(100) | 771(100) | km 3-hydroxyferuloylsophorotrioside-7-glucoside* |
| b11 | 14.21 | 355.1029 | C16H19O9 | −1.56 | 217(59), 193(100), 175(40) | 134(100) | feruloylglucose* |
| d10 | 14.21 | 933.2283 | C42H45O24 | −2.24 | 787(10), 771(100), 625(11) | 625(100) | km 3-caffeoyldiglucoside-7-glucoside |
| c13 | 14.21 | 1155.3028 | C50H59O31 | −1.78 | 993(100), 950(29), 788(30) | qn 3-sinapoyltriglucoside-7-glucoside | |
| c14 | 14.33 | 1155.3023 | C50H59O31 | −2.28 | 993(100), 950(29), 788(30) | qn 3-sinapoyltriglucoside-7-glucoside | |
| b12 | 14.39 | 933.2306 | C42H45O24 | −0.03 | 787(14), 771(100), 625(11) | 625(100), 607(8) | qn 3-p-coumaroyldiglucoside-7-glucoside* |
| a25 | 14.52 | 1095.2810 | C48H55O29 | −2.45 | 949(100), 933(38), 771(62), 625(40) | km 3-caffeoyl-triglucoside-7-glucoside | |
| b13 | 14.61 | 933.2280 | C42H45O24 | −2.63 | 771(100) | 609(100) | km 3-caffeoyl-diglucoside-7-glucoside |
| a26 | 14.67 | 1095.2811 | C48H55O29 | −2.35 | 949(100), 933(38), 932(6), 787(6), 771(62) | km 3-caffeoyl-triglucoside-7-glucoside | |
| d11 | 14.89 | 385.1137 | C17H21O10 | −0.32 | 247(52), 223(100), 205(55) | 164(100) | sinapic acid-glucose |
| c15 | 14.91 | 993.2486 | C44H49O26 | −3.15 | 831(99), 787(100), 769(6), 625(44) | 625(100) | qn 3-sinapoylsophorotrioside* |
| a27 | 15.01 | 1139.3093 | C50H59O30 | −0.32 | 977(100) | 771(100) | km 3-sinapoylsophorotrioside-7-glucoside* |
| d12 | 15.04 | 993.2481 | C44H49O26 | −3.65 | 831(100), 787(94), 769(6), 625(45) | qn 3-sinapoyldiglucoside-7-glucoside | |
| a28 | 15.21 | 977.2535 | C44H49O25 | −2.24 | 815(100), 609(3) | 609(100) | km 3-sinapoylsophoroside-7-glucoside* |
| b14 | 15.22 | 993.2496 | C44H49O26 | −2.15 | 831(99), 787(100), 769(6), 625(44) | 625(100) | qn 3-sinapoyltriglucoside |
| c16 | 15.27 | 963.2387 | C43H47O25 | −2.49 | 801(100), 609(2) | 609(100) | km 3-hydroxyferuloylsophoroside-7-glucoside* |
| b15 | 15.55 | 963.2381 | C43H47O25 | −3.09 | 801(100), 787(45), 625(26) | 625(100) | km 3-hydroxyferuloylsophoroside-7-glucoside* |
| d13 | 15.55 | 963.2374 | C43H47O25 | −3.79 | 801(100), 787(47), 625(25) | km 3-hydroxyferuloylsophoroside-7-glucoside* | |
| c17 | 15.63 | 1139.3103 | C50H59O30 | 0.56 | 977(100), 771(3) | 771(100) | km 3-sinapoylsophorotrioside-7-glucoside* |
| b16 | 15.72 | 963.2391 | C43H47O25 | −2.09 | 801(100), 787(45), 625(26) | 625(100) | km 3-hydroxyferuloylsophoroside-7-glucoside* |
| d14 | 15.82 | 933.2283 | C42H45O24 | −2.33 | 787(10), 771(100), 625(11) | 625(100) | km 3-caffeoyldiglucoside-7-glucoside |
| a29 | 15.87 | 947.2429 | C43H47O24 | −2.28 | 827(2), 785(100), 609(2) | 609(100) | km 3-feruloylsophoroside-7-glucoside* |
| b17 | 15.93 | 933.2280 | C42H45O24 | −2.63 | 788(10), 771(100), 625(11) | 625(100) | km 3-caffeoyldiglucoside-7-glucoside |
| c18 | 15.93 | 1109.2946 | C49H57O29 | −4.06 | 947(100) | 771(100) | km 3-feruloylsophorotrioside-7-glucoside |
| a30 | 16.28 | 917.2318 | C42H45O23 | −4.26 | 755(100) | 609(100) | km 3-p-coumaroylsophoroside-7-glucoside* |
| d15 | 16.36 | 1095.2805 | C48H55O29 | −2.95 | 933(100), 787(28) | km 3-caffeoyltriglucoside-7-glucoside | |
| c19 | 16.39 | 977.2535 | C44H49O25 | −2.24 | 815(100), 609(3) | 609(100) | km 3-sinapoylsophoroside-7-glucoside* |
| a31 | 16.47 | 1079.2852 | C48H55O28 | −3.33 | 755(100), 609(12) | 609(100) | km 3-p-coumaroylsophoroside-7-diglucoside |
| b18 | 16.48 | 1139.3065 | C50H59O30 | −3.16 | 977(100) | 771(100) | km 3-sinapoylsophorotrioside-7-glucoside* |
| b19 | 16.63 | 977.2542 | C44H49O25 | −2.64 | 815(100) | 609(100) | km 3-sinapoylsophoroside-7-glucoside* |
| d16 | 16.76 | 609.1441 | C27H29O16 | −2.01 | 489(13), 447(100), 285(19) | 284(100) | km 3-glucoside-7-glucoside** |
| c20 | 16.93 | 947.2429 | C43H47O24 | −2.28 | 827(2), 785(100), 609(2) | 609(100) | km 3-feruloylsophoroside-7-glucoside* |
| c21 | 17.17 | 639.1566 | C28H31O17 | 2.08 | 519(10), 477(100), 315(12) | 314(100) | is 3-glucoside-7-glucoside* |
| b20 | 17.20 | 947.2439 | C43H47O24 | −2.38 | 785(100) | 609(100) | km 3-feruloylsophoroside-7-glucoside* |
| d17 | 17.25 | 977.2535 | C44H49O25 | −3.34 | 815(100), 771(10) | 609(100) | km 3-sinapoylsophoroside-7-glucoside* |
| b21 | 17.55 | 917.2328 | C42H45O23 | −2.91 | 755(100) | 609(100) | km 3-p-coumaroylsophoroside-7-glucoside |
| d18 | 17.97 | 947.2429 | C43H47O24 | −3.38 | 785(100) | 609(100) | km 3-feruloylsophoroside-7-glucoside* |
| c22 | 18.03 | 551.1753 | C26H31O13 | −1.71 | 389(100), 341(6) | 341 (100) | ferulic acid-rhamnosylglucose with a 48 amu group |
| d19 | 18.12 | 947.2449 | C43H47O24 | −1.38 | 785(100) | 609(100) | km 3-feruloylsophoroside-7-glucoside* |
| c23 | 18.28 | 993.2473 | C44H49O26 | −4.49 | 801(13), 787(100) | 607(100) | qn 3-sinapoylsophoroside-7-glucoside* |
| d20 | 18.36 | 639.1548 | C28H31O17 | −1.87 | 519(11), 477(100), 315(12) | 314(100) | is 3-glucoside-7-glucoside |
| d21 | 18.63 | 917.2330 | C42H45O23 | −2.71 | 755(100) | 609(100) | km 3-p-coumaroyldiglucoside-7-glucoside |
| b22 | 19.13 | 625.1382 | C27H29O17 | −2.82 | 505(21), 463(37), 445(55), 301(60), 300(100) | qn 3-diglucoside | |
| a32 | 19.40 | 935.2444 | C42H47O24 | −1.88 | 773(100), 755(29), 663(52), 285(30) | 285(100) | km aglycone with 7 glucoside and 3 acyl glucosyls |
| a33 | 20.43 | 625.1414 | C27H29O17 | 0.60 | 505(18), 463(17), 445(54), 300(100) | 271(100) | qn 7-sophoroside* |
| b23 | 21.28 | 639.1548 | C28H31O17 | −2.9 | 315(100), 300(16) | is 3-diglucoside | |
| a34 | 21.40 | 965.2516 | C49H41O21 | 1.02 | 803(100), 785(24), 693(48), 667(9), 285(21) | 285(100) | km 3-caffeoyldiglucoside-7-glucoside |
| d22 | 21.48 | 933.2271 | C42H45O24 | −3.53 | 787(10), 771(100), 625(11) | 625(100) | km 3-caffeoyldiglucoside-7-glucoside |
| a35 | 21.90 | 935.2436 | C42H47O24 | −2.68 | 773(100), 756(31), 663(55), 637(10), 285(24) | 285(100) | km aglycone with 7 glucoside and 3 acyl glucosyls |
| c24 | 22.16 | 831.1997 | C38H39O21 | 0.93 | 625(100) | 300(100) | qn 3-sinapoylsophoroside* |
| b24 | 23.13 | 193.0506 | C10H9O4 | −0.03 | 178(19), 149(50), 134(100) | 106(100) | ferulic acid** |
| b25 | 24.01 | 223.0607 | C11H11O5 | −2.23 | 208(8), 179(11), 164(100) | 149(100) | sinapic acid** |
| d23 | 24.79 | 193.0502 | C10H9O4 | −0.43 | 178(25), 149(55), 134(100) | 106(100) | ferulic acid** |
| b26 | 24.92 | 593.1503 | C27H29O15 | −1.51 | 447(100) | 284(100) | km 3-glucoside-7-rhanmoside |
| d24 | 25.86 | 223.0607 | C11H11O5 | −0.50 | 208(8), 179(11), 164(100) | 149(100) | sinapic acid isomer |
| b27 | 26.17 | 977.2536 | C44H49O25 | −3.24 | 815(100), 653(14) | 653(100) | km 3-sinapoylsophoroside-7-glucoside* |
| d25 | 26.69 | 223.0607 | C11H11O5 | −2.23 | 208(8), 179(11), 164(100) | 149(100) | sinapic acid isomer |
| a36 | 32.77 | 753.2253 | C34H41O19 | 0.73 | 529(100) | 205(100) | disinapoylgentiobiose* |
| a37 | 33.68 | 1123.2886 | C53H55O27 | −4.47 | 961(100), 755(20) | 755(100) | km 3-hydroxyferuloylsophorotrioside-7-glucoside* |
| a38 | 34.63 | 1153.2981 | C54H57O28 | 1.32 | 991(100), 785(20) | 785(100) | km 3-sinapoylferuloylsophoroside-7-glucoside* |
| a39 | 35.20 | 1183.3081 | C55H59O29 | −5.62 | 1021(100), 816(19) | 815(100) | km 3-disinapoyldiglucoside-7-glucoside |
| a40 | 35.52 | 1183.3086 | C55H59O29 | −5.20 | 977(22), 959(7), 815(100), 609(14), 591(7) | 609(100) | km 3-sinapoyldiglucoside-7-sinapoylglucoside |
| b28 | 37.34 | 753.2253 | C34H41O19 | 0.73 | 529(100) | 205(100) | disinapoylgentiobiose* |
| a41 | 37.86 | 753.2253 | C34H41O19 | 0.73 | 529(100) | 205(100) | disinapoylgentiobiose* |
| d26 | 38.81 | 753.2258 | C34H41O19 | 1.39 | 529(100) | 223(100) | disinapoylgentiobiose* |
| a42 | 39.11 | 723.2144 | C33H39O18 | 0.29 | 529(100), 499(21) | 223(100) | sinapoyl-feruloylgentiobiose* |
| a43 | 39.35 | 723.2125 | C33H39O18 | −1.69 | 529(100), 499(21) | 223(100) | sinapoyl-feruloylgentiobiose* |
| a44 | 39.99 | 1199.3057 | C55H59O30 | −3.96 | 993(100, -206), 787(12) | 787(100) | qn 3-disinapoylsophorotrioside |
| d27 | 40.41 | 723.2120 | C33H39O18 | −2.19 | 529(100), 499(21) | 223(100) | sinapoyl-feruloylgentiobiose |
| a45 | 43.75 | 959.2830 | C45H51O23 | 0.35 | 735(100), 529(7), 511(11) | 529(100) | trisinapoylgentionbiose* |
| a47 | 44.67 | 959.2798 | C45H51O23 | −2.87 | 735(100), 529(10), 511(13) | 223(100) | trisinapoylgentionbiose* |
| b30 | 45.98 | 929.2695 | C44H49O22 | −2.63 | 705(100), 511(6) | 499(100) | feruloyl-disinapoyl-gentionbiose |
km- kaempferol, qn-quercetin, is-isorhamnetin
identified with literature data
with reference standards
Using MS analysis of peak a19, as an example, the deprotonated molecular ion at m/z 933 (C42H45O24) lost a hexosyl group from position 7, giving the product ion at m/z 771. The MS3 product ion revealed a loss of 162 amu, corresponding to a caffeoyl group, and a loss of dihexoxyl group at the 3-position (324 amu), leading to the Km aglycone (m/z 285). Thus, peak a19 was tentatively identified as Km 3-caffeoyldiglucoside-7-glucoside. Peak b13 also exhibited the deprotonated ion at m/z 933 but showed different fragmentation pathways. During the MS fragmentation of peak 18a, loss of 162 amu, corresponding to a hexosyl moiety at the terminal 7-position was observed. Further fragmentation of the acylated ion, m/z 625, gave the loss of pcoumaroyl group and the loss of a dihexosyl group, producing the Qn aglycone ion (m/z 301). Thus, peak b13 was assigned as Qn 3-p-coumaroyldiglucoside-7-glucoside. Using this strategy, the remaining flavonols were identified on the basis of HRMS, MS fragmentation pattern, UV maxima, and retention times as flavonols, previously characterized in the five Brassica species microgreens.
Derivatives of Hydroxycinnamic Acids and Hydroxybenzoic acids
Hydroxycinnamic acids and hydroxybenzoic acids are considered non-flavonoid phenolics and are characterized by their C6-C3 and C6-C structures, respectively. Most of the hydroxycinnamic acids and hydroxybenzoic acid derivatives detected in mature vegetables (17-19, 21) were also detected in our five Brassica species microgreens. However, our five Brassica species microgreens contained a greater variety and higher concentrations of cinamic acids than their mature leaf counterpart. The retention times, HRMS molecular ions [M-H]−, diagnostic MS2 and MS3 product ions, UV λmax and identification of the hydroxycinnamates, arranged by molecular weight, are listed in Table 3. Their peaks are eluted with the flavanol glycoside peaks, as shown in Figure 3. The hydroxycinnamic acids, hydoxycinnamoylquinic acids, hydroxycinnamoylmalic acids, and hydroxycinnamoyl saccharides with one to three glucosides were identified using reference compounds (designated by **) or from the literature (designated by *) Table 3. Sixteen of the hydroxycinnamoylsaccharides were formed from di- or triglucoses, mainly gentiobiose, with one to three hydroxycinnamoyl units. By direct comparison with reference compounds in mustard greens, peaks a36, a41, b28 and d26 (Figure 3) were identified as disinapoylgentiobioses. Peaks a4, b11, and c12 were identified as feruloyl-glucosides. Peaks a42, d27, and a43 were identified as sinapoyl-feuloylgentiobioses. Peak a47 and b30, identified as trisinapoylgentionbiose and feruloyl-disinapoyl-gentionbiose, are peaks common to microgreens of mizuna, purple kohlrabi, red mustard, and purple mustard. Peak d11 is only found in mizuna and was tentatively identified as sinapic acid-glucose.
Other organic acids, such as caffeoylquinic acid, ferulic acid, sinapic acid, citric acid, malic acid, and caffeoylquinic acid are organic acids common in these five microgreens. There were a number of organic acid isomers found in the five Brassisa microgreens and identification was based on their similar MS2 and MS3 spectra. However, they exhibited different retention times based on species. For example, the peaks a42, a43and d27 all had the same [M-H]− at m/z 723. HRMS measurements suggested the formula of C33H39O18, with the main MS2 product ion at m/z 529 (M - 194, neutral loss of ferulic acid) and the main MS3 product ions at m/z 223 sinapic acid). These compounds were identified as sinapoyl-ferulic acid and its isomers. Similarly, peaks a36, b28, and a41 ([M - H]− at m/z 753, with a main MS2 product ion at 529 and main MS3 product ions at 205) was identified as disinapoylgentiobiose and its isomers.
In summary, this is the first study characterizing phenolic profiles specifically in Brassisca species microgreens. A total of 165 phenolic compounds were tentatively identified using complementary information from UHPLC-PDA-HRMSn in negative and positive modes, revealing a large number of highly glycosylated and acylated quercetin, kaempferol, cyanidin aglycones and complex hydroxycinnamic and benzoic acids. The results showed that the Brassica species microgreens tended to have more complex polyphenols profiles and to contain more varieties of polyphenols compared to their mature plant counterpart. Thus, Brassica species microgreens could be considered a good source for polyphenols. This compositional study should serve as reference base for these five Brassica species microgreens and enhance their value to health agencies and consumers
ACKNOWLEDGEMENTS
This research is supported by the Agricultural Research Service of the U.S. Department of Agriculture and an Interagency Agreement with the Office of Dietary Supplements of the National Institutes of Health.
REFERENCES
- 1.Xiao Z, Lester GE, Luo Y, Wang Q. Assessment of vitamin and carotenoid concentrations of emerging food products: edible microgreens. J Agric Food Chem. 2012;60:7644–51. doi: 10.1021/jf300459b. [DOI] [PubMed] [Google Scholar]
- 2.Cartea ME, Francisco M, Soengas P, Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16:251–80. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guzman I, Yousef GG, Brown AF. Simultaneous extraction and quantitation of carotenoids, chlorophylls, and tocopherols in brassica vegetables. J Agric Food Chem. 2012;60:7238–44. doi: 10.1021/jf302475d. [DOI] [PubMed] [Google Scholar]
- 4.Verkerk R, Schreiner M, Krumbein A, Ciska E, Holst B, Rowland I, De Schrijver R, Hansen M, Gerhauser C, Mithen R, Dekker M. Glucosinolates in Brassica vegetables: the influence of the food supply chain on intake, bioavailability and human health. Mol Nutr Food Res. 2009;53(Suppl 2):S219. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- 5.Terry P, Wolk A, Persson I, Magnusson C. Brassica vegetables and breast cancer risk. JAMA. 2001;285:2975–7. doi: 10.1001/jama.285.23.2975. [DOI] [PubMed] [Google Scholar]
- 6.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–48. [PubMed] [Google Scholar]
- 7.Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132:2991–4. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- 8.Cox DN, Melo L, Zabaras D, Delahunty CM. Acceptance of health-promoting Brassica vegetables: the influence of taste perception, information and attitudes. Public Health Nutr. 2012;15:1474–82. doi: 10.1017/S1368980011003442. [DOI] [PubMed] [Google Scholar]
- 9.Lin LZ, Harnly JM. Phenolic component profiles of mustard greens, yu choy, and 15 other brassica vegetables. J Agric Food Chem. 2010;58:6850–7. doi: 10.1021/jf1004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin L, Sun J, Chen P, Harnly J. UHPLC-PDA-ESI/HRMS/MSn Analysis of Anthocyanins, Flavonol Glycosides and Hydroxycinnamic Acid Derivatives in Red Mustard Green (Brassica juncea Coss variety). J Agric Food Chem. 2011 doi: 10.1021/jf202556p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L, Sun J, Chen P, Harnly JM. LC-PDA-ESI/MSn Identification of New Anthocyanins in Purple Bordeaux Radish (Raphanus sativus L. variety). J Agric Food Chem. 2011 doi: 10.1021/jf200571a. [DOI] [PubMed] [Google Scholar]
- 12.Lin LZ, Harnly JM. Identification of the Phenolic Components of Collard Greens, Kale, and Chinese Broccoli. J Agr Food Chem. 2009;57:7401–7408. doi: 10.1021/jf901121v. [DOI] [PubMed] [Google Scholar]
- 13.Ferreres F, Fernandes F, Sousa C, Valentao P, Pereira JA, Andrade PB. Metabolic and bioactivity insights into Brassica oleracea var. acephala. J Agric Food Chem. 2009;57:8884–92. doi: 10.1021/jf902661g. [DOI] [PubMed] [Google Scholar]
- 14.Ferreres F, Valentao P, Llorach R, Pinheiro C, Cardoso L, Pereira JA, Sousa C, Seabra RM, Andrade PB. Phenolic compounds in external leaves of tronchuda cabbage (Brassica oleracea L. var. costata DC). J Agric Food Chem. 2005;53:2901–7. doi: 10.1021/jf040441s. [DOI] [PubMed] [Google Scholar]
- 15.Sousa C, Lopes G, Pereira DM, Taveira M, Valentao P, Seabra RM, Pereira JA, Baptista P, Ferreres F, Andrade PB. Screening of antioxidant compounds during sprouting of Brassica oleracea L. var. costata DC. Combinatorial chemistry & high throughput screening. 2007;10:377–86. doi: 10.2174/138620707781662817. [DOI] [PubMed] [Google Scholar]
- 16.Taveira M, Pereira DM, Sousa C, Ferreres F, Andrade PB, Martins A, Pereira JA, Valentao P. In vitro cultures of Brassica oleracea L. var. costata DC: potential plant bioreactor for antioxidant phenolic compounds. J Agric Food Chem. 2009;57:1247–52. doi: 10.1021/jf803496x. [DOI] [PubMed] [Google Scholar]
- 17.Olsen H, Aaby K, Borge GI. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J Agric Food Chem. 2009;57:2816–25. doi: 10.1021/jf803693t. [DOI] [PubMed] [Google Scholar]
- 18.Ferreres F, Valentao P, Pereira JA, Bento A, Noites A, Seabra RM, Andrade PB. HPLC-DAD-MS/MS-ESI screening of phenolic compounds in Pieris brassicae L. Reared on Brassica rapa var. rapa L. J Agric Food Chem. 2008;56:844–53. doi: 10.1021/jf072657a. [DOI] [PubMed] [Google Scholar]
- 19.Harbaum B, Hubbermann EM, Wolff C, Herges R, Zhu Z, Schwarz K. Identification of flavonoids and hydroxycinnamic acids in pak choi varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC-ESI-MSn and NMR and their quantification by HPLC-DAD. J Agric Food Chem. 2007;55:8251–60. doi: 10.1021/jf071314+. [DOI] [PubMed] [Google Scholar]
- 20.Rochfort SJ, Imsic M, Jones R, Trenerry VC, Tomkins B. Characterization of flavonol conjugates in immature leaves of pak choi [Brassica rapa L. Ssp. chinensis L. (Hanelt.)] by HPLC-DAD and LC-MS/MS. J Agric Food Chem. 2006;54:4855–60. doi: 10.1021/jf060154j. [DOI] [PubMed] [Google Scholar]
- 21.Romani A, Vignolini P, Isolani L, Ieri F, Heimler D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. Subsp. sylvestris L.). J Agric Food Chem. 2006;54:1342–6. doi: 10.1021/jf052629x. [DOI] [PubMed] [Google Scholar]
- 22.Llorach R, Gil-Izquierdo A, Ferreres F, Tomas-Barberan FA. HPLC-DAD-MS/MS ESI characterization of unusual highly glycosylated acylated flavonoids from cauliflower (Brassica oleracea L. var. botrytis) agroindustrial byproducts. J Agric Food Chem. 2003;51:3895–9. doi: 10.1021/jf030077h. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo F, Tomas-Barberan FA, Ferreres F. Characterisation of flavonols in broccoli (Brassica oleracea L. var. italica) by liquid chromatography-uV diode-array detection-electrospray ionisation mass spectrometry. J Chromatogr A. 2004;1054:181–93. doi: 10.1016/j.chroma.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 24.Lin LZ, Sun J, Chen P, Harnly J. UHPLC-PDA-ESI/HRMS/MS(n) analysis of anthocyanins, flavonol glycosides, and hydroxycinnamic acid derivatives in red mustard greens (Brassica juncea Coss variety). J Agric Food Chem. 2011;59:12059–72. doi: 10.1021/jf202556p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X, Prior RL. Identification and characterization of anthocyanins by high- performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: vegetables, nuts, and grains. J Agric Food Chem. 2005;53:3101–13. doi: 10.1021/jf0478861. [DOI] [PubMed] [Google Scholar]
- 26.Wu X, Prior RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005;53:2589–99. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 27.Ferreres F, Llorach R, Gil-Izquierdo A. Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2004;39:312–21. doi: 10.1002/jms.586. [DOI] [PubMed] [Google Scholar]
- 28.Arapitsas P, Sjoberg PJR, Turner C. Characterisation of anthocyanins in red cabbage using high resolution liquid chromatography coupled with photodiode array detection and electrospray ionization-linear ion trap mass spectrometry. Food Chem. 2008;109:219–226. doi: 10.1016/j.foodchem.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Arapitsas P, Turner C. Pressurized solvent extraction and monolithic column-HPLC/DAD analysis of anthocyanins in red cabbage. Talanta. 2008;74:1218–23. doi: 10.1016/j.talanta.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 30.McDougall GJ, Fyffe S, Dobson P, Stewart D. Anthocyanins from red cabbage--stability to simulated gastrointestinal digestion. Phytochemistry. 2007;68:1285–94. doi: 10.1016/j.phytochem.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Park WT, Kim JK, Park S, Lee SW, Li X, Kim YB, Uddin MR, Park NI, Kim SJ, Park SU. Metabolic Profiling of Glucosinolates, Anthocyanins, Carotenoids, and Other Secondary Metabolites in Kohlrabi (Brassica oleracea var. gongylodes). J Agric Food Chem. 2012;60:8111–6. doi: 10.1021/jf301667j. [DOI] [PubMed] [Google Scholar]