Abstract
Utilizing cyanobacteria as a bioenergy resource is difficult due to the cost and energy consuming harvests of microalgal biomass. In this study, an auto-floating system was developed by increasing the photobiological H2 production in the heterocysts of filamentous cyanobacteria. An amount of 1.0 μM of diuron, which inhibited O2 production in cyanobacteria, resulted in a high rate of H2 production in heterocysts. The auto-floating process recovered 91.71% ± 1.22 of the accumulated microalgal biomass from the liquid media. Quantification analysis revealed that 0.72–1.10 μmol H2 per mg dry weight microalgal biomass was necessary to create this auto-floating system. Further bio-conversion by using anaerobic digestion converted the harvested microalgal biomass into biogas. Through this novel coupled system of photobiological H2 production and anaerobic digestion, a high level of light energy conversion efficiency from solar energy to bioenergy was attained with the values of 3.79% ± 0.76.
The conversion of solar energy into clean chemical energy has attracted much attention in recent years, due to the fossil fuel crisis and the emission of greenhouse gases1,2. Photobiological H2 production via cyanobacteria is an important strategy for converting solar energy into renewable and sustainable energy3,4,5; however, a major obstacle in the production of renewable bioenergy from cyanobacteria is its relatively low light energy conversion efficiency (LCE). The theoretical maximal LCE of photosynthesis in cyanobacteria is reported to be approximately 11%6. However, the LCE for renewable energy production has always been relatively low because most of the converted solar energy is stored as biomass7. For example, the LCE for microalgal H2 is 0.042% and 0.075% for Anabaena sp. strain PCC 7120 (henceforth referred to as Anabaena 7120) and Chlamydomonas reinhardtii respectively8,9,10. In these microalgal cells, the electron pathway for hydrogen production is strictly oxygen sensitive, and most of the electrons from water photolysis were utilized to assimilate CO2 and produce biomass6,7. Converting the light energy stored in microalgal biomass into renewable energy is a promising strategy for a new source of bioenergy.
Unfortunately, biomass harvest is still a technical bottleneck for microalgae-driven solar energy conversion11,12. Many strategies have been attempted for the efficient harvest of the biomass, such as centrifugation, filtration, and flocculation11,13. But each one of these harvesting methods are also energy-intensive and expensive14. Bio-flocculation has been utilized successfully in recent years to obtain microalgae sedimentation15, but the recovery of sentimental cells is still a time consuming and costly process. Dissolved air flotation (DAF) is considered to be the primary technology of choice for harvesting of microalgal biomass due to its high efficiency and relatively lower energy consumption compare to centrifugation16,17,18. However, some of the chemical coagulators used in DAF created additional problems, such as excess cost and pollution to the environment15,19. Furthermore, the operational cost of the DAF unit is still high because of the enormous energy required to achieve the necessary air compression of 390 kPa (56 psig)20,21. Therefore, an auto-floating system without the need of coagulants and compressed air will be a promising strategy for an economical and environmental friendly method to harvest microalgae.
Two key features are essential for an auto-floating system. One required element is the ability to auto-flocculate and sediment, and the other is the ability of the microalgae to produce an adequate amount of gas to enable floatation. The heterocyst-containing N2-fixing cyanobacterium Anabaena 7120 is a photoautotrophic microalga that assimilates CO2 from the atmosphere in order to grow. This microalga has a marked flocculation activity attributed to its filamentous cellular structure and excretion of mucilage22,23. Using sunlight as its only source of energy, Anabaena can generate H2 gas under anaerobic conditions24,25,26. Photobiological H2 production of Anabaena 7120 occurs both in vegetative cells and heterocysts. Research has shown that this photobiological activity is mainly catalysed by nitrogenase in the heterocysts27,28,29.
In this study, auto-flotation of Anabaena 7120 was induced by stimulating photobiological H2 production though inhibition of photosynthetic O2 evolution. The relationship between the amount of H2 production and biomass weight was determined by quantification analysis. Following the auto-floating harvest process, an anaerobic digestion of the separated cellular biomass was performed to convert its stored solar energy to H2 and CH4, contributing to a high total LCE value for clean energy production.
Results
Anabaena 7120 rapidly self-flocculated in standing culture
We investigated the self-flocculation ability of Anabaena 7120 and compared it with two other representative photo-H2 producing microalgae, the green alga Chlamydomonas reinhardtii and the cyanobacterium Synechocystis sp. PCC 6803. As clearly shown in figure 1, unlike the unicellular microalgae Chlamydomonas and Synechocystis, the algal biomass of the Anabaena culture aggregated and flocculated rapidly. The culture of Anabaena 7120 settled completely in just 2 hours.
Figure 1. Compared analysis of auto-flocculation process of Anabaena, Chlamydomonas and Synechocystis.
Cells in logarithmic phase (A730 = 0.8–1.0) were equally divided into 60 mL glass tubes. Duplicate tubes of each strain were sampled for the analysis. The self-flocculation of the culture was monitored by taking photographs at the indicated time points.
H2 production was significantly enhanced in DCMU-treated samples
H2 gas can be used as a clean and renewable energy, and also act as an effective force to provide buoyancy for flotation of the microalgal biomass. Anabaena 7120 can produce H2 gas under N-starved conditions by using solar light as the energy resource. Previous reports have shown that O2 is the major inhibitor in the production of photosynthetic H2 in N2-fixing cyanobacteria30,31. Anaerobic conditions induce H2 production within Anabaena 7120 but the amount of H2 produced under these conditions was quite low. As shown in table 1, the highest rate obtained by our laboratory was 1.26 μmol of H2 per mg of chlorophyll a per hour (per mg Chl a.h). Moreover, as seen in Supplementary Fig. S1a, O2 gas (red line) and H2 gas (blue line) were produced simultaneously in the culture. These results corresponded to previous research results presented in Anabaena cylindrica24,32. Although both O2 and H2 evolved in Anabaena 7120, O2 is only produced in vegetative cells. The increase of O2 content (see Supplementary Fig. S1a) revealed the active photochemical function of photosystem II (PSII). As shown in Supplementary Fig. S1b, O2 evolution activity of PSII was quite high in the early stage of H2 production (0–12 h).
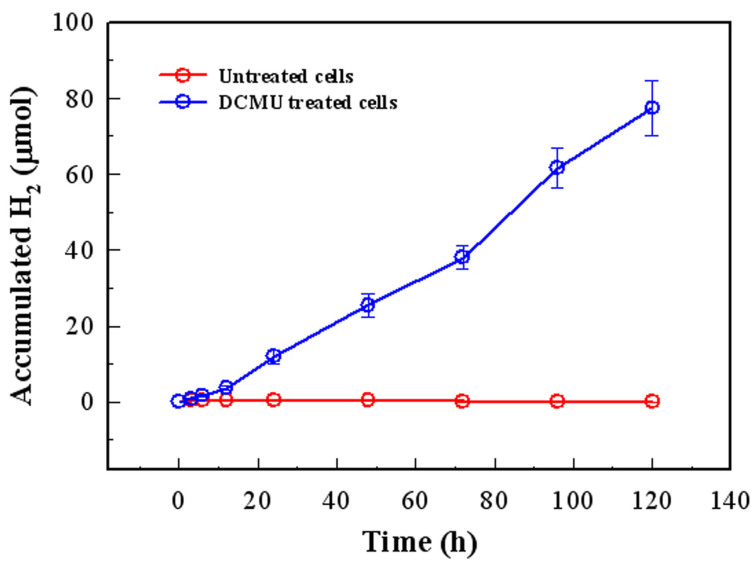
Table 1. The rates and accumulation of H2 production in untreated cells and DCMU treated cells of Anabaena 7120.
| Sample | Accumulated H2 (μmol) 1 | Maximal rate (μmol/mg Chl.a/h) |
|---|---|---|
| Untreated cells | 0.42 ± 0.03 | 1.26 |
| DCMU treated cells | 77.41 ± 7.36 | 11.58 |
1, The data is the average value of triplicate biological texts of sample tubes containing 40 mL new Anabaena 7120 cell cultures (A730 = 0.8–1.0), and all samples were illuminated for 120 hours to produce H2.
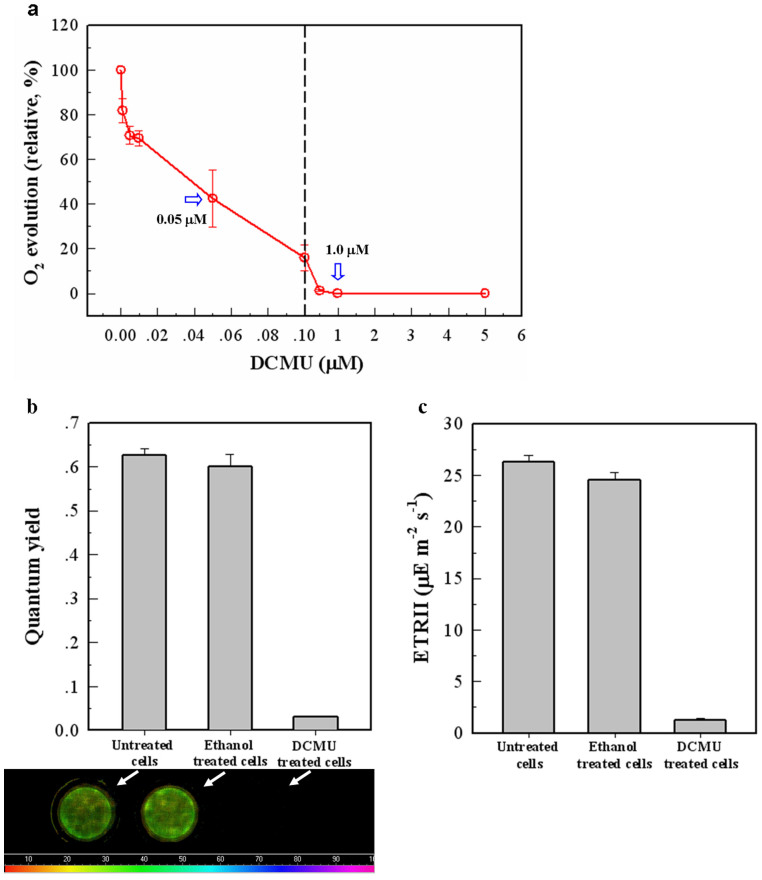
To prevent O2 evolution, diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea; DCMU), an obligate inhibitor of PSII was added to Anabaena 7120 cultures (Fig. 2a). The results showed that the O2 evolution in the Anabaena cells was inhibited by 60% of the original level with the addition of 0.05 μM DCMU, and a 100% suppression was achieved when treated with 1.0 μM DCMU. To investigate the effects of 1.0 μM DCMU on the photochemical properties of PSII, the effective photochemical quantum yield of PSII in dark-adapted samples (Fig. 2b) and the electron transport rate of PSII (ETR II) (Fig. 2c) were also analyzed. These data revealed that the photochemical activity of the PSII reaction center was almost completely suppressed and that electron transfer in PSII was significantly blocked from 26.25 ± 0.65 to 1.25 ± 0.15 μmol electrons m−2s−1. Therefore, a 1.0 μM concentration of DCMU efficiently interrupted photosynthetic O2 evolution in the Anabaena 7120 cells.
Figure 2. Photosystem II (PSII) activity assays using O2 evolution rate measurements and chlorophyll fluorescence analyses.
Cells in the logarithmic phase (A730: 0.8–1.0) were treated with DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea) for 10 minutes prior to the analysis. (a) Photosynthetic O2 evolution rates of the Anabaena 7120 cells were analysed after treatment with DCMU using various concentrations ranging from 0.005 μM to 5.0 μM. BQ was supplemented as an electron acceptor at a final concentration of 2.0 μM. The O2 evolution rate of untreated (0 μM DCMU) cells was set as 100%. (b, c) The photochemical activity of PSII was represented as the change in chlorophyll fluorescence between untreated cells and 1.0 μM DCMU-treated cells. All of the tested samples were adjusted to 20 μg of chlorophyll per mL to measure their fluorescence. The images of fluorescence detected a visible decline in the quantum yield of PSII in DCMU-treated cells, which was detected by Imaging-PAM (see the Methods section). During this experiment, the ethanol-treated cells were added as a control because ethanol was used as a solvent for the DCMU treatment experiments. Error bars indicate s.d. values calculated from the average of triplicate experiments.
To determine the photobiological H2 production in Anabaena 7120 treated with 1.0 μM DCMU, the treated cells were grown with illumination at 100 μE m−2 s−1. As shown in figure 3, the H2 production increased drastically in DCMU-treated cultures and maintained a relative high level for more than 120 hours under continues illumination. Hydrogen production in untreated and treated cells is presented in table 1. DCMU-treated cultures accumulated H2 about 200 folds more and around 10 times faster. These results demonstrated that under illumination, Anabaena 7120 cells produced a high rate of H2 in the absence of O2.
Figure 3. Photobiological H2 production in DCMU-treated cells under anaerobic conditions with continuous illumination.
The tested cells were equally divided into 60 mL glass tubes and then sealed with rubber stoppers after being sparged with argon gas. All of the samples were incubated in a 30°C culture box with 100 μE·m−2·s−1 illumination. The H2 content in the untreated samples and the DCMU-treated samples was then measured by a GC system at specific time points. Compared to the untreated cells (red symbol and line), H2 accumulation in the DCMU-treated samples increased quickly and lasted more than 120 hours. The 0 hour, 3 hour, 6 hour, 12 hour and 24 hour time points were recorded as the early phase, and after the 24 hour point, the H2 accumulation was recorded every 24 hours. Error bars indicate s.d. values calculated from the average of triplicate experiments.
Microalgal biomass automatically floated with high level of H2
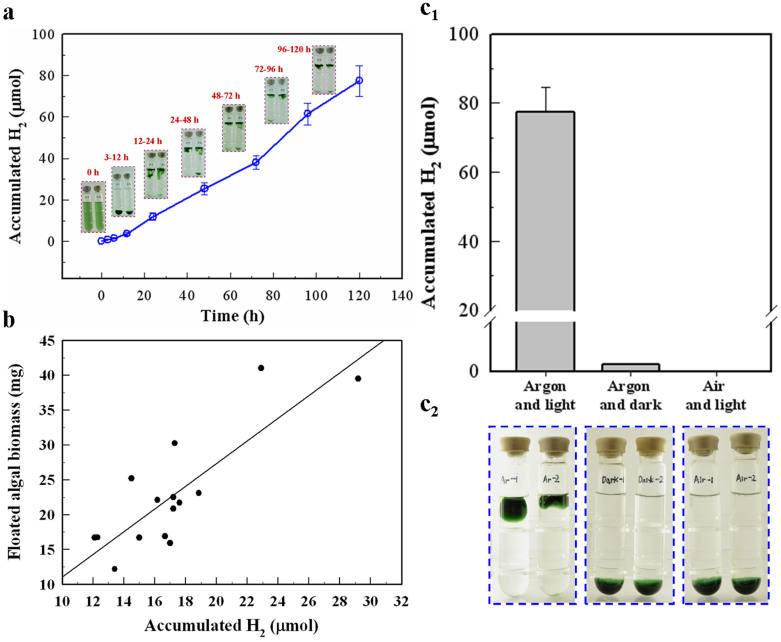
From figure 1, Anabaena 7120 self-flocculated rapidly in standing culture. The flocculated microalgal biomass cluster treated with DCMU was observed floating within 12 hours (Fig. 4a). The relationship between H2 accumulation and the amount of floating biomass were investigated (Fig. 4b). Under our experimental conditions, 0.72–1.10 μmol of H2 per mg of dry weight microalgal biomass was necessary for the biomass to float. Thus, auto-flotation occurred with a relatively high level of H2. Additional evidence was provided by the observation that the auto-floating process was disrupted when H2 production ceased under dark or anaerobic conditions (Fig. 4c). Together, these results demonstrated that auto-flotation of the biomass was a process that depended on a high level of hydrogen. The floating microalgae biomass was harvested at an efficiency of 91.71% ± 1.22.
Figure 4. Auto-flotation induced in DCMU treated cells.
Logarithmic growth cells were used for the assay. (a) The monitoring of microalgal biomass auto-flotation during the photobiological H2 production of DCMU-treated samples. The 0, 3, 6, 12 and 24 hour time points were recorded as the early phase, and after the 24 hour time point the H2 accumulation was recorded every 24 hours. (b) The analysis of the quantitative relation between H2 accumulation and the amount of auto-floated algal biomass. Cells in various concentrations (A730 = 0.6–1.2) were sampled for analysis. Auto-flotation occurred between 12–24 h after treated by 1.0 μM DCMU in anaerobic conditions in these sealed tubes with various amount of algal biomass. After measurement of H2 accumulation in tubes, the floated biomass was immediately transferred for dry weight analysis. (c) The analysis of the relationship between H2 production (c1) and auto-flotation of biomass (c2). Each group of cells at the 120 hour time point (DCMU-treated samples under argon with light, under argon in the dark and under air with light, respectively) were recorded and compared. Error bars indicate the s.d. values that were calculated from the average of triplicate experiments.
Heterocyst was required for high level of H2 production and self-flotation
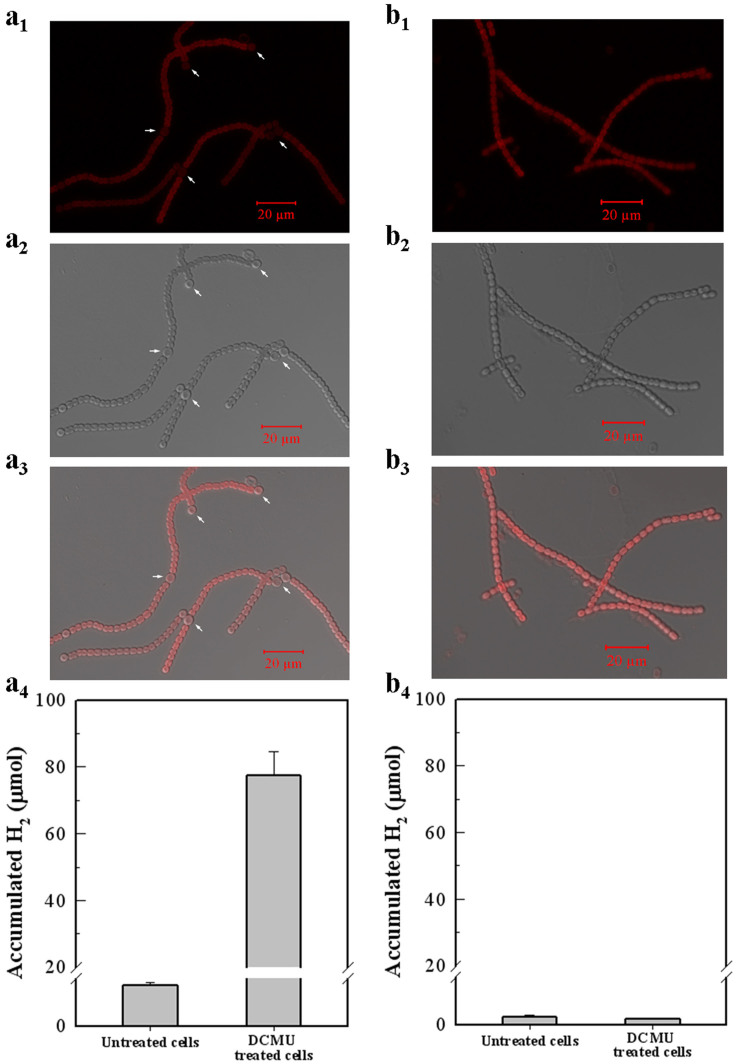
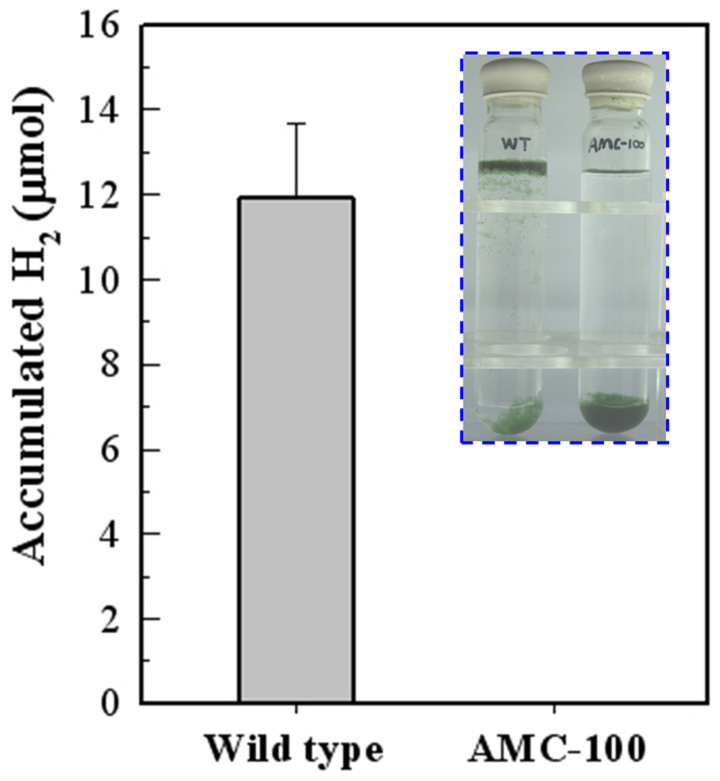
In untreated Anabaena 7120 cells, heterocysts are the major contributors to photobiological H2 production, however their contribution is unclear in DCMU-treated cultures31,33. Because heterocysts differentiation in Anabaena 7120 is dependent on nitrogen starvation, the H2 production of 1.0 μM DCMU-treated cultures under BG-II (−N) and BG-II (+N) media conditions were evaluated and compared (Fig. 5). Forty mL Anabaena 7120 cultures in the logarithmic growth phase were treated with DCMU. During a 120-hour period, H2 accumulation in DCMU-treated cultures grown in BG-II (−N) containing heterocysts increased significantly from 0.42 μmol to 77 μmol (Fig. 5a). In contrast, DCMU-treated cultures grown in BG-II (+N) without heterocysts accumulated only baseline volume of H2 gas (0.08 μmol in untreated cells and 0.06 μmol in DCMU-treated cells) shown in figure 5b. To determine whether the high level of H2 production and auto-flotation was dependent on heterocyst in BG-II (−N) medium, we tested the H2 production and flotation feature of a heterocyst differentiation deficient mutant AMC-100 (published as LW1) in which the nifHDK promoter was deleted from the chromosome34. AMC-100 was grown in BG-II (+N) medium and then induced for photobiological H2 production in BG-II (−N) medium with 1.0 μM DCMU. As shown in figure 6, the photobiological H2 production is quite low in AMC-100 culture and no self-flotation occurred. These results strongly suggested that heterocysts were required for the high rate and sustained photobiological H2 production in 1.0 μM DCMU-treated cultures. Also shown in Supplementary Fig. S2, other important filamentous cyanobacteria that differentiate into heterocysts also exhibited this auto-floating capacity when treated with DCMU. The flotation behaviours revealed in figure 6 and Supplementary Fig. S2 indicated that heterocyst differentiation is an important component for a microalgal self-flotation harvesting system.
Figure 5. Morphological and physiological investigation of the relationship between heterocyst development and photobiological H2 production in Anabaena 7120 cells.
Cells in the logarithmic growth phase were used for the assay. (a1–a3) A confocal microscopy image of the Anabaena 7120 cells grown in BG-II (−N) medium showed normal development of heterocysts. (b1–b3) The confocal microscopy image shows Anabaena 7120 cells grown in BG-II (+N) medium without heterocysts. The cells were immobilised with 1% (w/v) agar in BG-II (−N) medium before observation and graphic analysis. A number of heterocysts (white arrows) were observed at the frequencies between 1/10 to 1/20 (heterocyst/vegetative cell). a1, b1-a bright field micrograph of the cells; a2, b2- an auto fluorescence (exited at 488 nm) micrograph of the cells; a3, b3-a combined micrograph of the cells. (a4) Physiological analysis of the photobiological H2 accumulation in Anabaena 7120 cells with normal heterocysts. (b4) Physiological analysis of the photobiological H2 accumulation in Anabaena 7120 cells without heterocysts.
Figure 6. Characterization of photo-H2 production and auto-flotation of wild type Anabaena 7120 and the heterocyst deficient mutant AMC-100.
The AMC-100 strain was grown in non-diazotrophic BG-II (+N) medium. The logarithmic phase cells were than washed and suspended in BG-II (−N) medium and treated by 1.0 μM DCMU. The accumulated H2 gas in the sample tubes was detected after 24 h of treatment. Error bars indicate the s.d. values that were calculated from the average of triplicate experiments.
The harvested microalgal biomass was further converted into H2 and CH4 by anaerobic digestion
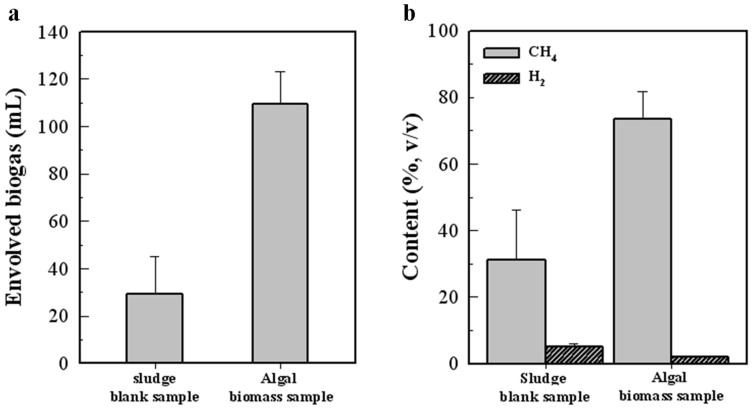
During high levels of photobiological H2 production, the microalgal biomass was separated from liquid media via the auto-floating process (Fig. 4). The microalgal biomass was anaerobically digested to produce biogas (H2 and CH4) by waste anaerobic sludge. As shown, the harvested microalgal biomass further degraded into biogases, yielding 266.3 mL biogas per g of dry weight (Fig. 7). The LCE for direct photobiological H2 production in Anabaena 7120 cells increased to as high as 1.18% with DCMU addition. Furthermore, the conversion of the auto-floating microalgal biomass increased the LCE of the whole system to a maximum value of 4.77% and an average value of 3.79%, shown in table 2.
Figure 7. Biogas production from the microalgal biomass substrate after photobiological H2 production.
(a) The detected total biogas volume of the blank sample and the sample with algal biomass as a substrate. (b) The gas composition (H2 and CH4) analysis during the anaerobic digestion stage. Error bars indicate the s.d. values that were calculated from the average of triplicate experiments.
Table 2. The LCE calculation of the two stages and the whole process.
| Stage | light energy absorption (J) | Energy production (J) | LCE (%) |
|---|---|---|---|
| Algae growth | 79619.48 | ––– | ––– |
| Photobiological H2 production | 2948.87 | 18.36 ± 1.75 | 0.62 ± 0.06 |
| Anaerobic dark digestion | ––– | 3112.77 ± 631.47 | ––– |
| Couple process | 82568.35 | 3131.13 ± 631.50 | 3.79 ± 0.76 |
Discussion
For most species of microalgae, much of the solar energy consumed during photosynthesis is converted and stored as cellular biomass6,7. Thus, only a fraction of the converted solar energy is available for photobiological H2 production by microalgae. H2 and CH4 gas produced in microalgal anaerobic digestion is a type of bioenergy that can be readily used. Sulphur-deprived Chlamydomonas and nitrogen-deprived Cyanothece, as unicellular microalgae, have shown high levels of photobiological H2 production under the proper conditions35,36. However, the separation and harvest of the biomass from these species of microalgae is quite difficult and expensive due to the low specific gravity of the individual cells keeping the cells suspended in culture16,37. Furthermore, the LCE for H2 production in most species of cyanobacteria is relatively low, i.e., below 1%8,9. Compared with unicellular microalgae, flocculation is more likely to occur naturally in Anabaena 7120 cultures due to its filamentous cell structure and excretion of mucilage. Figure 1 revealed that a standing culture of Anabaena 7120 flocculated and settled automatically in a very short time period (approximately 60 min). This fast self-flocculation activity provides a significant advantage in the creation of an auto-floating system for the efficient and economic harvest of microalgal biomass. According to the requirements of an auto-floating system, as mentioned above, microalgae floated if an adequate amount of H2 gas was also produced.
In this study, a high level of photobiological H2 production in heterocysts was stimulated and sustained by the inhibition of O2 production in the microalgal cells. Previous research had shown that any trace of O2 ceased the anaerobiosis induced nitrogenase activity38. Even though heterocysts already have low O2 concentration, the strict anaerobic intracellular condition resulting from DCMU treatment was essential. The same conclusion was drawn in an Anabaena strain in which nitrogenase activity within the anaerobic heterocysts was suppressed significantly by exposing to a trace amount of O238. Argon gas and DCMU induced strict anaerobiosis is the most likely explanation for the high level of photo-H2 production in Anabaena 7120. We also investigated the possible reason for such a stimulated long stage of H2 production in 1.0 μM DCMU treated cells. As the major carbohydrate storage in N2-fixing cyanobacteria, glycogen can provide nitrogenase energy and reducing power for N2 fixation and H2 production36. As shown in Supplementary Fig. S3a, the accumulated glycogen was consumed in DCMU treated Anabaena 7120 cells during the H2 production period. In addition, the increase in nitrogenase (NifH) expression suggested that more electrons and reductants from glycogen were transferred to nitrogenase for proton reduction and H2 gas evolving (see Supplementary Fig. S3b).
As a PSII photochemical inhibitor, DCMU addition is an effective strategy to inhibit O2 evolution and improve the long stage H2 production in Anabaena cultures used in this study. However, DCMU utilization may bring some environmental concerns as well. Metal ion inducible gene expression systems, such as Cu and Ni, could also be chosen as a possible alternative strategy for repression of PSII activity and increase photoautotrophic H2 production in microalgae39. The protein such as Nac2 which is required for stable expression of the core polypeptide of PSII could be a possible target39. Beside the metal ions, some environmental factors (light intensity, O2 level and CO2 concentration) inducible gene systems also had been reported in microalgae40,41,42,43, which would eliminate the use of environmentally hazardous inducers.
As shown, Anabaena 7120 cells auto-flocculated rapidly and the high level of H2 gas evolution from the heterocysts induced flotation of the flocculated microalgal biomass. Harvesting efficiency of over 90% was achieved by using this method. This auto-floating system of the filamentous cyanobacterium provided an economical and environmental friendly strategy for microalgal biomass harvests. Moreover, some auto-flocculating microalgae have been utilized successfully to sediment un-flocculating microalgae15. The former research revealed that the sedimentation rate of the un-flocculating microalgae was increased considerably when mixed with auto-flocculating microalgae, leading to a higher recovery percentage. Referring to the recent bio-flocculation research of Salim et al.15, such an auto-floating system has the potential to be widely used for harvesting of the un-flocculating microalgae, such as the unicellular microalga Chlorella.
The LCE for photosynthesis can reach a theoretical maximum of 11% in cyanobacteria, while the LCE for direct photobiological H2 production is always less than 0.1% in Anabaena 7120. By the auto-floating harvest strategy, the further conversion of microalgal biomass into clean bioenergy can become a promising bioenergy resource with a relatively high LCE. The bioenergy obtained by this auto-floating system can be calculated in two steps: the H2 gas energy produced during the first stage and the biogas produced in the second stage (Table 2).
During the first stage, photobiological H2 was produced from the heterocysts. At the second stage, the microalgal biomass was separated from the auto-floating system and further converted by anaerobic digestion into clean energy as H2 and CH4. The auto-floating process combines effective algal photobiological H2 production and residual microalgal biomass digestion for the production of clean energy. The LCE of this couple system for biofuel production from solar energy was obtained with an average value of 3.79%. Moreover, it avoids costly energy-consuming processes, such as centrifugation and filtration, to harvest the microalgal biomass44. The LCE for direct photobiological H2 production increased to an average value of 3.79%.
Photobiological H2 production in cyanobacteria can be further increased through genetic engineering or by optimization of growth factors8,29,45. In addition, strategies to improve clean energy production from the accumulated microalgal biomass continued to be explored46. Based on the auto-floating process described here, commercial applications can be developed for the future production of clean energy from microalgae.
Methods
Species and growth conditions
Filamentous cyanobacteria Anabaena 7120 (FACHB-418), Anabaena variabilis (FACHB-319) and Anabaena cylindrica (FACHB-1038) were purchased from the Institute of Hydrobiology, Chinese Academy of Sciences. The nifHDK operon promoter mutant AMC-100 was provided by Professor Jame W. Golden. The unicellular cyanobacterium Synechocystis 6803 and green alga Chlamydomonas reinhardtii CC503 were kind gifts from Professor Quanxi Wang. Microalgal cells were cultured at 30°C in BG-II media (with or without nitrogen)47 and buffered with Tris-HCl (5 mM, pH 8.0). The liquid culture was bubbled with 2–3% (v/v) CO2 in air under continuous illumination by fluorescent lamps (30 μE m−2s−1).
Determination of cell density and chlorophyll a content
The cell density was calculated using light absorption at 730 nm (A730) in a UV-Vis mini-1240 spectrophotometer (SHIMADZU, Kyoto, Japan) with a 3 mL cuvette and an optical path of 1 cm. Chlorophyll a (Chl a) content was measured as previously described48. In summary, Anabaena 7120 cells (1.5 mL) were collected by centrifugation and washed twice with 1 mL of fresh BG-II medium. The supernatant was discarded after centrifugation; 1.5 mL of methyl alcohol was added to the cell pellet; and the mixture was incubated at 4°C for 6–14 h. The supernatant was then collected by centrifugation and absorbance at 665 nm (A665) was measured. The concentration of Chl a (μg mL−1) was calculated as 13.9 × A66549.
Sample preparation for photobiological H2 production
Anabaena 7120 cells were cultured in 0.5 L BG-II (−N) medium for 5 days (A730 = 0.8–1.0) and were transferred to 60 mL glass tubes (leaving 20 mL of head space). DCMU was added to the cultures for a final concentration of 1.0 μM. The tubes were sparged with Ar gas for 2 minutes to eliminate the air in the samples and then sealed with rubber stoppers. The samples were incubated at 30°C under continuous illumination by fluorescent lamps (100 μE m−2 s−1) to induce photobiological H2 production. The incubated samples were then subjected to H2 and O2 assays at specific time points.
Biogas production
After the Anabaena 7120 microalgal biomass finished producing photobiological H2, it was collected and transferred to the biogas incubator and monitoring system for sludge-based anaerobically digested biogas production. Substrate fermentation was conducted in batch tests as follows: 100 mL biogas batch fermenters were filled with 5.0 g of anaerobic sludge from a mesophilic anaerobic digester in the Gaobeidian Sewage Treatment Plant in Beijing, China. The substrate was equivalent to 9.0 g of wet microalgal biomass per test. It was loaded and the fermenters were then sealed with rubber septa and incubated in a 37°C water bath for dynamic monitoring of biogases production. The incubation and monitoring process were performed in the Automatic Methane Potential Test System (AMPTS) II (Bioprocess Control Sweden AB, Lund, Sweden). The seeding sludge contained 10.8% total solids (TS) and 5.57% volatile solids (VS).
Gas analysis
To measure the amount of photobiological H2 production and O2 evolution from the samples, 500 μL of gas was withdrawn at predetermined time intervals from the bottles with a gas-tight syringe and injected into a gas chromatograph equipped with a thermal conductivity detector (TCD). The column was a 5 Å molecular sieve column (2 m × 1/8 mm), which could assay H2, O2 and N2 simultaneously. Highly pure argon gas (≥99.999%) was used as the carrier gas at a flow rate of 21 mL per minute, which was based on the effective separation and assay of the gas mixture in our samples.
To measure the amount of dark fermentative biogas production, the volume of evolved gas was monitored every 24 hours by AMPTS II. The measured volume was then converted to a volume of gas at standard temperature and pressure using the Ideal Gas Law. The methane content of the biogas was analysed every day using a SP-6890A gas chromatograph, (Lu Nan Analytic Instruments Co., Ltd., Shandong, China) equipped with a TCD. A 1.83 m column was packed with Porapak Q (50/80 mesh). The operating temperatures of the column, injector port and the detector were 90°C, 40°C and 50°C, respectively. Ar gas was used as the carrier gas at a flow rate of 30 mL per minute. A standard gas mixture which was composed of 15.0% H2, 60.1% CH4 and 19.9% CO2 was used to calibrate the system. The H2 and CH4 contents were multiplied by the production of biogas.
Confocal laser scanning microscopic (LSM) analysis
Following the details of methods described in previous studies50,51,52, the Anabaena 7120 filamentous cells grown in BG-II (−N) and BG-II (+N) were sampled for morphological vegetative cell and heterocyst analysis and examined using a Zeiss LSM 710 confocal microscope (Carl Zeiss Microscopy GmbH., Oberkochen, Germany). Bright field and auto fluorescence (exited at 488 nm) micrographs were captured to illustrate the visible differences between the microalgal cells with and without heterocysts.
PSII activity analysis
The PSII activity was determined using the benzoquinone (BQ) photosynthetic evolution rate of O2 and the quantum yield efficiency, which detected the changes in chlorophyll fluorescence. Photosynthetic O2 evolution was measured at 25°C (Hansatech Instruments, Ltd., Norfolk, England) with a Clark-type oxygen electrode, according to the method described by Mi et al. (1995)53. Cells (2 mL) were collected from the sample tube and injected into the reaction chamber, followed by suspension in BQ, to a final concentration of 10 μM. The intensity of the action light (AL) used to measure photosynthetic O2 evolution was 800 μE m−2 s−1. Changes in chlorophyll fluorescence were measured using a pulse amplitude modulated (PAM) chlorophyll fluorometer (Heinz Walz GmbH., Effeltrich, Germany) and an emitter-detector-cuvette assembly (ED-101US; Heinz Walz GmbH) with a unit 101ED, as described elsewhere54,55,56. To detect a visible change between the PSII activity of the treated and untreated cells, the quantum yield of PSII was detected by a new pulse-amplitude modulated imaging system that measures active fluorescence (Heinz Walz GmbH., Germany), which is described in detail elsewhere57,58.
Calculation of light energy conversion efficiency (LCE)
The LCE of the whole conversion process consists of two steps: direct photobiological H2 production and the anaerobic digestion of microalgal biomass for biogas production. The LCE of step 1 was determined based on the supplied light energy during the H2-producing phase; the LCE of step 2 was based on the supplied light energy during the cell growth of the biomass accumulation phase. The efficiency of each step was calculated according to the following equation:
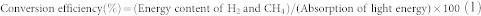 |
In full expression the equation is in form:
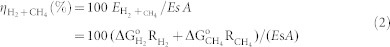 |
where ΔG°H2 and ΔG°CH4 are the standard Gibbs energy for the energy storage reaction generating H2 (237200 J mol−1 at 297 K) and CH4 (890310 J mol−1 at 297 K) respectively, RH2 and RCH4 are the amounts of accumulated H2 and CH4 gas respectively, Es is light energy radiation, and A is the illumination area59,60.
Author Contributions
M.C. performed the experiments with input from C.L. and J.L. M.C., J.L., L.Z. and S.C. designed the experiments, analysed the data and wrote the paper. S.L. and J.W. provided the overall guidance on study design, execution and wrote the paper.
Supplementary Material
Supplementary information
Acknowledgments
We are grateful to Prof. James W. Golden (University of California, San Diego) for his kindly gift of mutant AMC-100; to Prof. Weimin Ma (Research Center of Spore Plant Biology, Shanghai Normal University) for providing us the microalgae species. We thank Dr. Zengjuan Fu (Institute of Botany, CAS) for providing us the excellent technical support on analysis of PSII activity. We thank Dr. Rui Shi (Center of Biomedical Analysis, Tsinghua University) for providing us the excellent microscopy technical service. The authors thank Prof. Feng Chen (Peking University) for his critical reading of the paper. This work was supported by National Key Technology R&D Program (Grant No. 2012 BAC18B01) and International scientific and technological cooperation projects (Grant No. 2010DFB64040) of the Ministry of Science and Technology of China.
References
- Jeffries T. & Lindblad P. We march backwards into the future. Curr. Opin. in Biotechnol. 20, 255–256 (2009). [DOI] [PubMed] [Google Scholar]
- Scholes G. D., Fleming G. R., Olaya-Castro A. & van Grondelle R. Lessons from nature about solar light harvesting. Nat. Chem. 3, 763–774 (2011). [DOI] [PubMed] [Google Scholar]
- Benemann J. R. Hydrogen production by microalgae. J. Appl. Phycol. 12, 291–300 (2000). [Google Scholar]
- Ghirardi M. L., Dubini A., Yu J. & Maness P. C. Photobiological hydrogen-producing systems. Chem. Soc. Rev. 38, 52–61 (2009). [DOI] [PubMed] [Google Scholar]
- Prince R. C. & Kheshgi H. S. The Photobiological Production of Hydrogen: Potential Efficiency and Effectiveness as a Renewable Fuel. Crit. Rev. Microbiol. 31, 19–31 (2005). [DOI] [PubMed] [Google Scholar]
- Ducat D. C., Way J. C. & Silver P. A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 29, 95–103 (2011). [DOI] [PubMed] [Google Scholar]
- Dismukes G. C., Carrieri D., Bennette N., Ananyev G. M. & Posewitz M. C. Aquatic phototrophs: efficient alternatives to land-based crops for biofuels. Curr. Opin. in Biotechnol. 19, 235–240 (2008). [DOI] [PubMed] [Google Scholar]
- Lindblad P. et al. Photoproduction of H2 by wildtype Anabaena PCC 7120 and a hydrogen uptake deficient mutant: from laboratory experiments to outdoor culture. Int. J. Hydrogen Energy 27, 1271–1281 (2002). [Google Scholar]
- Hallenbeck P. C. & Benemann J. R. Biological hydrogen production; fundamentals and limiting processes. Int. J. Hydrogen Energy 27, 1185–1193 (2002). [Google Scholar]
- Berberoğlu H. & Pilon L. Maximizing the solar to H2 energy conversion efficiency of outdoor photobioreactors using mixed cultures. Int. J. Hydrogen Energy 35, 500–510 (2010). [Google Scholar]
- Grima E. M., Belarbi E. H., Acién-Fernández F. G., Robles-Medina A. & Chisti Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol. Adv. 20, 491–515 (2003). [DOI] [PubMed] [Google Scholar]
- Uduman N., Qi Y., Danquah M. K., Forde G. M. & Hoadley A. Dewatering of microalgal cultures: A major bottleneck to algae-based fuels. J. Renew. Sust. Energy 2, 12701–12715 (2010). [Google Scholar]
- Golueke C. G. & Oswald W. J. Harvesting and Processing Sewage-Grown Planktonic Algae. J. Water Pollut. Con. F. 37, 471–498 (1965). [Google Scholar]
- Wijffels R. H. & Barbosa M. J. An Outlook on Microalgal Biofuels. Science 329, 796–799 (2010). [DOI] [PubMed] [Google Scholar]
- Salim S., Bosma R., Vermuë M. & Wijffels R. H. Harvesting of microalgae by bio-flocculation. J. Appl. Phycol. 23, 849–855 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bare W. F. R., Jones N. B. & Middlebrooks E. J. Algae Removal Using Dissolved Air Flotation. Water Pollut. Con. F. 47, 153–169 (1975). [PubMed] [Google Scholar]
- Chung Y., Choi Y. C., Choi Y. H. & Kang H. S. A demonstration scaling-up of the dissolved air flotation. Water Res. 34, 817–824 (2000). [Google Scholar]
- Teixeira M. R. & Rosa M. J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of microcystis aeruginosa: Part I: The key operating conditions. Sep. Purif. Technol. 52, 84–94 (2006). [Google Scholar]
- Lee A., Lewis D. & Ashman P. Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel. J. Appl. Phycol. 21, 559–567 (2009). [Google Scholar]
- Wiley P. E., Brenneman K. J. & Jacobson A. E. Improved Algal Harvesting Using Suspended Air Flotation. Water Environ. Res. 81, 702–708 (2009). [DOI] [PubMed] [Google Scholar]
- Féris L. A. & Rubio J. Dissolved air flotation (DAF) performance at low saturation pressures. Filtr. Sep. 36, 61–65 (1999). [Google Scholar]
- Avnimelech Y., Troeger B. W. & Reed L. W. Mutual Flocculation of Algae and Clay: Evidence and Implications. Science 216, 63–65 (1982). [DOI] [PubMed] [Google Scholar]
- Walsby A. E. Mucilage secretion and the movements of blue-green algae. Protoplasma 65, 223–238 (1968). [Google Scholar]
- Benemann J. R. & Weare N. M. Hydrogen Evolution by Nitrogen-Fixing Anabaena cylindrica Cultures. Science 184, 174–175 (1974). [DOI] [PubMed] [Google Scholar]
- Tsygankov A. A., Serebryakova L. T., Rao K. K. & Hall D. O. Acetylene reduction and hydrogen photoproduction by wild-type and mutant strains of Anabaena at different CO2 and O2 concentrations. FEMS Microbiol. Lett. 167, 13–17 (1998). [Google Scholar]
- Dutta D., De D., Chaudhuri S. & Bhattacharya S. Hydrogen production by Cyanobacteria. Microb. Cell Fact. 4, 36 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R. B. & Burris R. H. Hydrogen metabolism in isolated heterocysts of Anabaena 7120. Arch. Microbiol. 116, 125–132 (1978). [Google Scholar]
- Houchins J. P. & Burris R. H. Occurrence and localization of two distinct hydrogenases in the heterocystous cyanobacterium Anabaena sp. strain 7120. J. Bacteriol. 146, 209–214 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa H., Mochinaru H. & Sakurai H. Disruption of the uptake hydrogenase gene, but not of the bidirectional hydrogenase gene, leads to enhanced photobiological hydrogen production by the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. Appl. Microbiol. Biotechnol. 58, 618–624 (2002). [DOI] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol. Rev. 56, 340–373 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnini P. et al. Hydrogenases and Hydrogen Metabolism of Cyanobacteria. Microbiol. Mol. Biol. Rev. 66, 1–20 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. C. & Benemann J. R. Hydrogen production by nitrogen-starved cultures of Anabaena cylindrica. Appl. Environ. Microbiol. 33, 123–131 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi M. L. et al. Hydrogenases and Hydrogen Photoproduction in Oxygenic Photosynthetic Organisms. Annu. Rev. Plant Biol. 58, 71–91 (2007). [DOI] [PubMed] [Google Scholar]
- Golden J. W., Whorff L. L. & Wiest D. R. Independent regulation of nifHDK operon transcription and DNA rearrangement during heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 173, 7098–7105 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Zhang L., Forestier M., Ghirardi M. L. & Seibert M. Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green Alga Chlamydomonas reinhardtii. Plant Physiol. 122, 127–136 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A., Stöckel J., Min H., Sherman L. A. & Pakrasi H. B. High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat. Commun. 1, 139 (2010). [DOI] [PubMed] [Google Scholar]
- Craggs R. J., McAuley P. J. & Smith V. J. Wastewater nutrient removal by marine microalgae grown on a corrugated raceway. Water Res. 31, 1701–1707 (1997). [Google Scholar]
- Rippka R. & Stanier R. Y. The Effects of anaerobiosis on nitrogenase synthesis and heterocyst development by nostocacean cyanobacteria. J. Gen. Microbiol. 105, 83–94 (1978). [Google Scholar]
- Surzycki R., Cournac L., Peltier G. & Rochaix J. D. Potential for hydrogen production with inducible chloroplast gene expression in Chlamydomonas. P. Natl. Acad. Sci. 104, 17548–17553 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hihara Y., Kamei A., Kanehisa M., Kaplan A. & Ikeuchi M. DNA Microarray Analysis of Cyanobacterial Gene Expression during Acclimation to High Light. Plant Cell 13, 793–806 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata N., Takahashi S., Nishiyama Y. & Allakhverdiev S. I. Photoinhibition of photosystem II under environmental stress. BBA-Bioenergetics 1767, 414–421 (2007). [DOI] [PubMed] [Google Scholar]
- Sicora C. I., Ho F. M., Salminen T., Styring S. & Aro E. M. Transcription of a “silent” cyanobacterial psbA gene is induced by microaerobic conditions. BBA-Bioenergetics 1787, 105–112 (2009). [DOI] [PubMed] [Google Scholar]
- Hanawa Y., Watanabe M., Karatsu Y., Fukuzawa H. & Shiraiwa Y. Induction of a high-CO2-inducible, periplasmic protein, H43, and its application as a high-CO2-responsive marker for study of the high-CO2-sensing mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol. 48, 299–309 (2007). [DOI] [PubMed] [Google Scholar]
- Greenwell H. C., Laurens L. M. L., Shields R. J., Lovitt R. W. & Flynn K. J. Placing microalgae on the biofuels priority list: a review of the technological challenges. J. R. Soc. Interface 7, 703–726 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows E. H., Chaplen F. W. R. & Ely R. L. Optimization of media nutrient composition for increased photofermentative hydrogen production by Synechocystis sp. PCC 6803. Int. J. Hydrogen Energy 33, 6092–6099 (2008). [Google Scholar]
- Yen H. W. & Brune D. E. Anaerobic co-digestion of algal sludge and waste paper to produce methane. Bioresour. Technol. 98, 130–134 (2007). [DOI] [PubMed] [Google Scholar]
- Allen M. M. Simple conditions for growth of unicellular bule-green algae on plates. J. Phycol. 4, 1–4 (1968). [DOI] [PubMed] [Google Scholar]
- Ma W., Shi D., Wang Q., Wei L. & Chen H. Exogenous expression of the wheat chloroplastic fructose-1,6-bisphosphatase gene enhances photosynthesis in the transgenic cyanobacterium Anabaena PCC 7120. J. Appl. Phycol. 17, 273–280 (2005). [Google Scholar]
- Arnon D. I. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta Vulgaris. Plant Physiol. 24, 1–15 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. et al. Treatment with moderate concentrations of NaHSO3 enhances photobiological H2 production in the cyanobacterium Anabaena sp. strain PCC 7120. Int. J. Hydrogen Energy 35, 12777–12783 (2010). [Google Scholar]
- Lehner J. et al. The morphogene AmiC2 is pivotal for multicellular development in the cyanobacterium Nostoc punctiforme. Mol. Microbiol. 79, 1655–1669 (2011). [DOI] [PubMed] [Google Scholar]
- Sukenik A., Kaplan-Levy R. N., Welch J. M. & Post A. F. Massive multiplication of genome and ribosomes in dormant cells (akinetes) of Aphanizomenon ovalisporum (Cyanobacteria). ISME J. 6, 670–679 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H., Endo T., Ogawa T. & Asada K. Thylakoid Membrane-Bound, NADPH-Specific Pyridine Nucleotide Dehydrogenase Complex Mediates Cyclic Electron Transport in the Cyanobacterium Synechocystis sp. PCC 6803. Plant and Cell Physiol. 36, 661–668 (1995). [Google Scholar]
- Schreiber U., Schliwa U. & Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62 (1986). [DOI] [PubMed] [Google Scholar]
- Schreiber U., Endo T., Mi H. & Asada K. Quenching Analysis of Chlorophyll Fluorescence by the Saturation Pulse Method: Particular Aspects Relating to the Study of Eukaryotic Algae and Cyanobacteria. Plant Cell Physiol. 36, 873–882 (1995). [Google Scholar]
- Campbell D., Hurry V., Clarke A. K., Gustafsson P. & Oquist G. Chlorophyll Fluorescence Analysis of Cyanobacterial Photosynthesis and Acclimation. Microbiol. Mol. Biol. Rev. 62, 667–683 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl M., Chen M., Ralph P. J., Schreiber U. & Larkum A. W. D. Ecology: A niche for cyanobacteria containing chlorophyll d. Nature 433, 820–820 (2005). [DOI] [PubMed] [Google Scholar]
- Schreiber U., Quayle P., Schmidt S., Escher B. I. & Mueller J. F. Methodology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens. and Bioelectron. 22, 2554–2563 (2007). [DOI] [PubMed] [Google Scholar]
- Liu J., Bukatin V. E. & Tsygankov A. A. Light energy conversion into H2 by Anabaena variabilis mutant PK84 dense cultures exposed to nitrogen limitations. Int. J. Hydrogen Energy 31, 1591–1596 (2006). [Google Scholar]
- Yoon J. H., Shin J. H., Kim M. S., Sim S. J. & Park T. H. Evaluation of conversion efficiency of light to hydrogen energy by Anabaena variabilis. Int. J. Hydrogen Energy 31, 721–727 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information