Abstract
Helicobacter pylori-induced gastric inflammation includes induction of inflammatory mediators interleukin (IL)-8 and inducible nitric oxide synthase (iNOS), which are mediated by oxidant-sensitive transcription factor NF-κB. High levels of lipid peroxide (LPO) and increased activity of myeloperoxidase (MPO), a biomarker of neutrophil infiltration, are observed in H. pylori-infected gastric mucosa. Panax ginseng Meyer, a Korean herb medicine, is widely used in Asian countries for its biological activities including anti-inflammatory efficacy. The present study aims to investigate whether Korean Red Ginseng extract (RGE) inhibits H. pylori-induced gastric inflammation in Mongolian gerbils. One wk after intragastric inoculation with H. pylori, Mongolian gerbils were fed with either the control diet or the diet containing RGE (200 mg RGE/gerbil) for 6 wk. The following were determined in gastric mucosa: the number of viable H. pylori in stomach; MPO activity; LPO level; mRNA and protein levels of keratinocyte chemoattractant factor (KC, a rodent IL-8 homolog), IL-1β, and iNOS; protein level of phospho-IκBα (which reflects the activation of NF-κB); and histology. As a result, RGE suppressed H. pylori-induced mRNA and protein levels of KC, IL-1β, and iNOS in gastric mucosa. RGE also inhibited H. pylori-induced phosphorylation of IκBα and increases in LPO level and MPO activity of gastric mucosa. RGE did not affect viable H. pylori colonization in the stomach, but improved the histological grade of infiltration of polymorphonuclear neutrophils, intestinal metaplasia, and hyperplasia. In conclusion, RGE inhibits H. pylori-induced gastric inflammation by suppressing induction of inflammatory mediators (KC, IL-1β, iNOS), MPO activity, and LPO level in H. pylori-infected gastric mucosa.
Keywords: gastric inflammation, Helicobacter pylori, Korean Red Ginseng extract, Mongolian gerbil
1. Introduction
Helicobacter pylori infection leads to gastroduodenal inflammation, peptic ulceration, and gastric carcinoma [1,2]. H. pylori infection is reported to include pathologic changes of the stomach, including edema and congestive surface epithelium [3]. A characteristic event in gastritis is the infiltration of the subepithelial gastric lamina propria by phagocytes, mainly neutrophils and macrophages, that produce large amounts of reactive oxygen species (ROS). ROS activate the oxidant-sensitive transcription factor NF-κB, which induces expression of the inflammatory genes, oncogenes, and cell-cycle regulators [4,5]. H. pylori-induced gastric mucosal injury and inflammation are mediated by proinflammatory cytokines such as interleukin (IL)-8 and IL-1β as well as inflammatory enzymes, including inducible nitric oxide synthase (iNOS). Transcription of these inflammatory mediators is regulated by the oxidant-sensitive transcription factor NF-κB [6–10]. NF-κB is an inducible transcription factor composed of p50/p65 (heterodimer) or p50 (homodimer) [11]. NF-κB is retained in the cytoplasm by binding to the inhibitory protein IκBα. Extracellular stimuli trigger rapid degradation of IκBα by proteasomes, allowing NF-κB to translocate into the nucleus and bind to the DNA sites of target genes, including IL-8, IL-1β, and iNOS [12]. Therefore, degradation of IκBα represents activation of NF-κB.
H. pylori-elicited neutrophils produce ROS, which subsequently injure gastric mucosal cells [13]. ROS cause peroxidation of membrane lipids, thus increasing the level of lipid peroxide (LPO) in the damaged tissues. We previously demonstrated that LPO production increases in parallel with IL-8 production in H. pylori-infected cells [7]. Myeloperoxidase (MPO) is more abundantly expressed in neutrophils than other cells and thus, is used as a biomarker for neutrophil infiltration [14]. In neutrophils, MPO produces hypochlorous acid from hydrogen peroxide and chloride anion during respiratory bursts. Furthermore, it oxidizes tyrosine to form tyrosyl radicals using hydrogen peroxide. Both hypochlorous acid and tyrosyl radicals cause lipid peroxidation sequences [15]. Therefore, high levels of LPO and increased MPO activity could reflect oxidative damage and inflammatory responses of cells.
Korean Red Ginseng, which is the steamed root of a 6-year-old Korean ginseng (Panax ginseng Meyer), is used in Asian countries as a traditional medicine for the treatment of various diseases, including inflammatory disorders [16–18]. The most effective components of Korean Red Ginseng are triterpeneglysides known as ginsenosides [19]. Ginsenosides have anti-inflammatory [20,21] and anticancer effects [22]. An in vitro study showed that Korean Red Ginseng inhibited adhesion of H. pylori to gastric epithelial cells [23]. Korean Red Ginseng extract (RGE) inhibits H. pylori-induced oxidative damage in gastric epithelial cells [24,25]. Previously we showed hepatoprotective effects of Korean Red Ginseng in rats and mouse liver, which may be contributed by its antioxidant activity [26,27]. Therefore, the antioxidant or anti-inflammatory effects of RGE, containing ginsenosides, may protect gastric mucosa from inflammation caused by H. pylori infection.
In the present study, we investigated whether RGE protects against H. pylori-induced gastric inflammation in Mongolian gerbils. Animal models for H. pylori infection have been developed to replicate many features of human gastric inflammation and carcinogenesis in order to test potential therapeutic agents for the prevention and treatment of H. pylori-associated gastric disease. The Mongolian gerbil model is the best animal model for this purpose because H. pylori infection induces chronic gastritis, gastric ulcers, and intestinal metaplasia in these animals. Mongolian gerbils develop gastric neoplasia and gastric cancer after chronic infection by H. pylori strain 7.13 [28,29], as used in the present study. After the infection of gerbils with H. pylori, we determined: the changes in LPO level, which is an index of oxidative membrane damage; the activity of MPO, a biomarker of neutrophil infiltration; the induction of inflammatory mediator keratinocyte chemoattractant factor (KC), an IL-8 homolog in rodents [30]; IL-1β; iNOS; and the phosphorylation of IκBα, which reflects the activation of NF-κB. In addition, viable H. pylori colonization in the stomach, changes in food intake and body weight, stomach weight/total body weight, and histological analysis of gastric mucosa were compared between animals that received RGE and those that did not.
2. Materials and methods
2.1. Animals
Five-wk-old male specific-pathogen-free Mongolian gerbils (MGS/Sea) with an average weight of approximately 40 g were purchased from Charles River Laboratories (Wilmington, MA, USA). Gerbils were housed in polypropylene cages on hard wood chip bedding in groups of five/cage. Food and water were provided ad libitum. The animals were maintained in a temperature-controlled room (22 ± 2°C) with a 12-h light–dark cycle. The animal experiments were performed in accordance with institutional guidelines. Protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Yonsei University Medical Center (Seoul, Korea; Permit No.: 10-107). Ten gerbils were included in each group. Histological observations are reported for 10 gerbils/group. All animals were maintained in the specific pathogen-free facility at Yonsei University Medical Center.
2.2. Bacterial inoculation
H. pylori strain 7.13 was maintained as frozen stock at –80°C in brain–heart infusion medium supplemented with 20% glycerol and 10% fetal bovine serum. Bacteria were grown on horse blood agar plates containing 4% Columbia agar base (Oxoid, Basingstoke, Hampshire, UK), 5% defibrinated horse blood (HemoStat Labs, Dixon, CA, USA), 0.2% β-cyclodextrin, 10 μg/mL vancomycin, 5 μg/mL cefsulodin, 2.5 U/mL polymyxin B, 5 μg/mL trimethoprim, and 8 μg/mL amphotericin B at 37°C under microaerophilic conditions. A microaerobic atmosphere was generated using a CampyGen sachet (Oxoid) in a gas pack jar. For liquid culture, H. pylori was grown in brucella broth (Difco & BBL Diagnostics, Franklin Lakes, NJ, USA) containing 10% FBS (Gibco-BRL, Grand Island, NY, USA). Cultures were shaken in a microaerobic environment. According to the growth curve, 108 bacteria were collected and resuspended in 500 μL of brucella broth for the infection of each animal.
2.3. Preparation of RGE
A standardized water extract of Korean Red Ginseng was prepared and supplied by the Korea Ginseng Corporation (Daejeon, Korea) as described previously [31]. The content of crude saponin in RGE is approximately 7%, and it is composed of the following ginsenosides: 8.27 mg/g of Rb1, 3.22 mg/g of Rb2, 3.90 mg/g of Rc, 1.09 mg/g of Rd, 2.58 mg/g of Re, 1.61 mg/g of Rf, 2.01 mg/g of Rg1, 1.35 mg/g for (20S)-Rg2, 1.04 mg/g for (20S)-Rg3, and 0.95 of Rh1, respectively [31].
2.4. Experimental design
One wk after inoculation with H. pylori, Mongolian gerbils were fed control AIN76A diet (Research Diets, Inc, New Brunswick, NJ, USA) or a diet containing RGE (200 mg RGE/each gerbil) for 6 wk. As a negative control, Mongolian gerbils that were not inoculated with H. pylori were fed the control diet AIN76A. Gerbils that were inoculated with H. pylori were fed the control diet AIN76A and considered as a positive H. pylori control.
This level of RGE supplementation (200 mg RGE/gerbil) was adapted from previous studies showing the protective effect of RGE against oxidative stress-mediated epithelial damage [32,33]. Body weight and food intake were measured every wk during the experimental period. At the end of experimental period, gastric mucosal tissues were examined histologically and H. pylori colonization was confirmed. For biochemical analyses, gastric mucosal samples were homogenized in 10 mM Tris buffer (pH 7.4). The homogenates were used for determining LPO level, MPO activity, and protein levels of KC, iNOS, phospho-specific IκBα and IκBα. For mRNA level of KC, IL-1β, and iNOS, total RNA was isolated from a gastric mucosal sample by the guanidine thiocyanate extraction method. RGE supplementation had no effect on any of these parameters in animals not infected with H. pylori, determined in our preliminary study.
2.5. Determination of the number of viable H. pylori in the stomach
The number of viable H. pylori in the animal stomach was determined as previously described [34]. After the animals were fasted for 24 h, they were euthanized, and their stomachs excised. The stomach was dissected along the greater curvature and washed with 0.01 M phosphate-buffered saline (PBS, pH 7.4) and then divided longitudinally into two halves. One half of each stomach was homogenized in 10 mL of PBS using a Polytron. The diluted homogenates were applied to Helicobacter-selective agar plates. The plates were incubated at 37°C under microaerobic conditions for 5 d. The colonies were counted and the number of viable H. pylori was expressed as colony forming units/g of tissue.
2.6. Histological observation
The other half of each stomach was fixed in 10% neutral buffered formalin and embedded in paraffin. Paraffin sections were cut into 4-μm slices and stained with hematoxylin and eosin for morphological observation. Gastric pathology was blindly evaluated according to published criteria [35]. Morphological features of the gastric antrum and body were graded using the following four-point scale: Grade 0 (normal), Grade 1 (mild), Grade 2 (moderate), and Grade 3 (severe). Four aspects of gastric lesions were recorded according to the updated Sydney system: polymorphonuclear leukocytes (PMNs) infiltration; chronic inflammation, such as mononuclear cell infiltration and lymphoid nodule formation; intestinal metaplasia and hyperplasia; and formation of heterotopic proliferative glands [36]. Microscopic images were obtained at a magnification of ×200.
2.7. Determination of LPO level and MPO activity
LPO levels were measured by colorimetric assay as thiobarbituric acid reactive substances [37] and the results were expressed as pg/mg protein. The protein concentration was determined by the method described previously [38]. MPO activity was also determined colorimetrically [39]. One unit of MPO activity was defined as the activity required to degrade 1 μmol of peroxide/min at 25°C. MPO activity is expressed as units/mg protein.
2.8. Real-time reverse transcription-polymerase chain reaction analysis for mRNA expression of KC, IL-1β and iNOS
mRNA expression of iNOS and KC was assessed using real-time reverse transcription-polymerase chain reaction (RT-PCR) analysis. Total RNA isolated from mucosal homogenate was reverse transcribed into cDNA and used for PCR with Mongolian gerbil-specific primers for KC, IL-1β, iNOS, and β-actin. Sequences of KC primers were CACCCGCTCGCTTCTTC (forward primer) and ATGCTCTTGGGGTGAATCC (reverse primer). For IL-1β the forward primer was TGACTTCACCTTGGAATCCGTCTCT and the reverse primer was GGCAACAAGGGAGCTCCATCAC. For iNOS, the forward primer was GCATGACCTTGGTGTTTGGGTGCC and the reverse primer was GCAGCCTGTGTGAACCTGGTGAAGC. For β-actin, the forward primer was ACCAACTGGGACGACTGGAG and the reverse primer was GTGAGGATCTTCATGAGGTAGTC. Real-time RT-PCR reactions were prepared using Taqman reagents (Applied Biosystems, Foster City, CA, USA) for iNOS, KC, and β-actin. A DNA Engine (PTC-200) and its system interface software (MJ Research, Waltham, MA, USA) were used to run samples and analyze data. The β-actin gene was amplified in the same reaction and served as the reference gene. KC and iNOS mRNA levels were reported relative to those of animals not inoculated with H. pylori that were fed the control diet. KC and iNOS mRNA values for the negative control group were set equal to 1.
2.9. Enzyme-linked immunosorbent assay for KC
The level of KC in gastric mucosal tissues was measured using an enzyme-linked immunosorbent assay and a mouse KC assay kit (IBL, Gunma, Japan).
2.10. Western blot analyses for iNOS, phospho-specific IκBα, and ΙκBα
Total cell extracts were prepared from gastric mucosa and separated by SDS-polyacrylamide gel electrophoresis under reducing conditions. Samples were then transferred onto membranes (Amersham Inc., Arlington Heights, IL, USA) by electroblotting. After blocking using 5% nonfat dry milk, the membranes were incubated with anti-iNOS (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phospho-IκBα, anti-IκBα (Cell Signaling Technology, Inc., Beverly, MA, USA), and anti-actin antibodies (Santa Cruz Biotechnology). The immunoreactive proteins were visualized using anti-mouse secondary antibody conjugated to horseradish peroxidase, followed by enhanced chemiluminescence (Amersham). Actin was used as a loading control.
2.11. Statistical analysis
Statistical analyses were carried out using SAS version 9.1 (SAS Inc., Cary, NC, USA). Statistical differences between groups were determined using one-way analysis of variance and Newman–Keuls test. All values were expressed as the mean ± standard deviation for 10 gerbils in each group. Histological observations were reported for 10 gerbils/group. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Effect of RGE on viable H. pylori colonization in the stomach and the stomach weight of Mongolian gerbils
In order to examine gross changes of H. pylori-infected Mongolian gerbils consuming RGE dietary supplements, food intake and body weight change were determined every wk during the experimental period. The weight gain and food intake were similar in all three groups (data not shown). This finding was supported by previous studies showing that H. pylori infection did not affect either body weight or food intake in Mongolian gerbils [40,41].
To determine whether RGE inhibits H. pylori colonization in gastric mucosa, the number of viable H. pylori in the stomachs of gerbils infected with H. pylori were determined after 6 wk of dietary supplementation with RGE (Fig. 1A). In addition, stomach wet weights were compared between groups at the end of the experiment (Fig. 1B). Animals infected with H. pylori had significantly more H. pylori colonization and greater stomach weight than noninfected animals. RGE supplementation had no effect on the number of viable H. pylori in the stomach. H. pylori-induced increases in the stomach weight tended to be smaller in the RGE-treatment group than in the control-diet group, but this difference was not significant. RGE had no antibacterial effect and did not reduce pathologic changes of the stomach, such as edema, in animals infected with H. pylori.
Fig. 1.
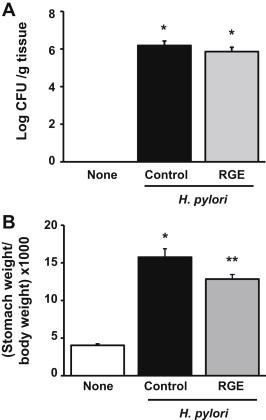
Effect of Korean Red Ginseng extract (RGE) on viable Helicobacter pylori colonization in stomach and stomach weight of Mongolian gerbils. (A) Viable cell numbers of H. pylori are determined and expressed as colony forming units (CFU)/g tissue. (B) Stomach weight to body weight ratio at the end of the experiment. None, animals without H. pylori infection that were fed the control diet; H. pylori control, animals with H. pylori infection that were fed the control diet; and H. pylori + RGE, animals with H. pylori infection that were fed a diet supplemented with RGE. *p < 0.05 versus none. **p < 0.05 versus H. pylori control.
3.2. Effect of RGE on H. pylori-induced histological changes in gastric mucosal regions of Mongolian gerbils
In H. pylori-infected animals, moderate to severe gastritis was accompanied by PMN infiltration, mainly neutrophil infiltration, and by lymphoid follicle formation in the mucosa and submucosa. The hyperplasia and mucous-gland metaplasia of epithelial cells in infected animals were obvious (Fig. 2A, middle panel) in comparison with the normal gastric mucosal regions of noninfected animals (Fig. 2A, left panel). The gastric mucosal lesions of RGE-supplemented animals showed less evidence of inflammatory cell infiltration, hyperplasia, and intestinal metaplasia than those of infected animals fed the control diet (Fig. 2A, right panel). H. pylori-induced chronic inflammation was reduced by RGE treatment. However, none of these differences between H. pylori-infected animals that were supplemented with RGE and those that were fed the control diet were significant. Taken together, RGE improved the histological grade of PMN infiltration, intestinal metaplasia, and hyperplasia in Mongolian gerbils, which suggests that RGE has an anti-inflammatory effect against H. pylori-induced gastric inflammation.
Fig. 2.
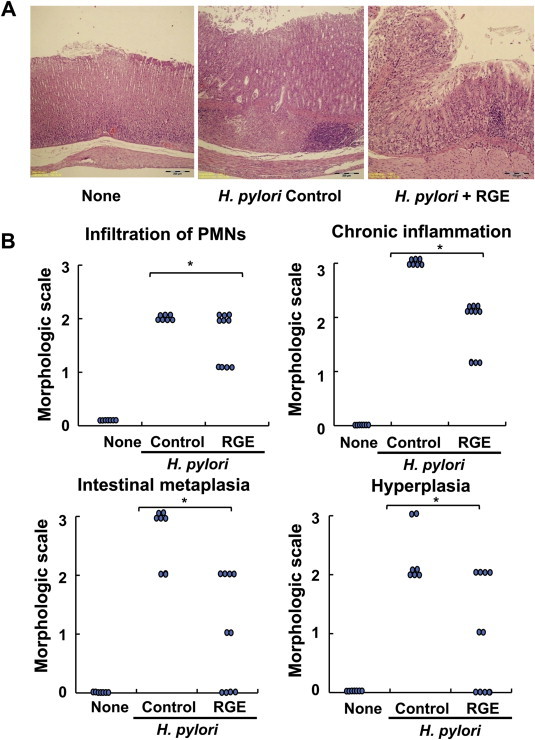
Effect of Korean Red Ginseng extract (RGE) on Helicobacter pylori-induced histological changes in gastric mucosal regions of Mongolian gerbils. (A) Gastric mucosal sections stained with hematoxylin and eosin. Microscopic images were obtained at magnification of ×200. (B) The inflammatory responses of gastric mucosa were graded according to morphologic criteria: Grade 0, normal; Grade 1, mild; Grade 2, moderate; and Grade 3, severe. The following characteristics of gastric lesions were recorded: polymorphonuclear leukocytes (PMNs) infiltration, chronic inflammation such as mononuclear cells infiltration and lymphoid nodules formation, intestinal metaplasia, and hyperplasia and formation of heterotopic proliferative glands. None, animals without H. pylori infection that were fed the control diet; H. pylori control, animals with H. pylori infection that were fed the control diet; and H. pylori + RGE, animals with H. pylori infection that were fed a diet supplemented with RGE. *Statistically significant difference (p < 0.05) between H. pylori control and H. pylori + RGE.
3.3. Effect of RGE on H. pylori-induced increases in LPO level and MPO activity in gastric mucosal tissues of Mongolian gerbils
As shown in Fig. 3A, MPO activity in gastric mucosa was increased by H. pylori infection, and was attenuated by RGE supplementation. The reduced MPO activity in the gastric mucosal tissues of the RGE-treatment group was associated with reduced infiltration by neutrophils (Fig. 2). RGE supplementation inhibited H. pylori-induced neutrophil infiltration in the gastric mucosal lesions of Mongolian gerbils. The level of LPO, an oxidative damage index, was higher in the gastric mucosal tissues of H. pylori-infected animals than that in noninfected animals (Fig. 3B). RGE supplementation suppressed the H. pylori-induced increase in the LPO level of gastric mucosal tissues.
Fig. 3.
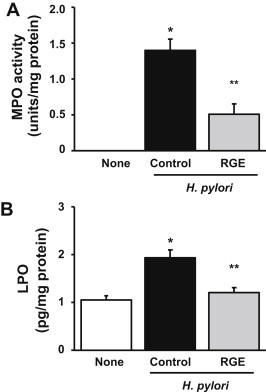
Effect of Korean Red Ginseng extract (RGE) on Helicobacter pylori-induced increases in myeloperoxidase (MPO) activity and lipid peroxide (LPO) level in gastric mucosal tissues of Mongolian gerbils. (A) The MPO activity in the gastric mucosal tissues is expressed as units/mg protein. (B) LPO levels were measured as thiobarbituric acid reactive substances and are expressed as pg/mg protein. None, animals without H. pylori infection that were fed the control diet; H. pylori control, animals with H. pylori infection that were fed the control diet; and H. pylori + RGE, animals with H. pylori infection that were fed a diet supplemented with RGE. *p < 0.05 versus none. **p < 0.05 versus H. pylori control.
3.4. Effect of RGE on H. pylori-induced expression of KC and iNOS, phosphorylation of IκBα, in the gastric mucosal tissues of Mongolian gerbils
To investigate the inhibitory effects of RGE against H. pylori-induced inflammation, the expression levels of important inflammatory mediators (KC, IL-1β, iNOS) were determined in the gastric mucosal tissues of animals infected with H. pylori that were and were not supplemented with RGE. As shown in Fig. 4, the mRNA expression of KC, IL-1β, and iNOS in gastric mucosal tissues was greater in H. pylori-infected animals than in non-infected animals. H. pylori-induced mRNA expression of KC, IL-1β, and iNOS was significantly lower in the RGE-treatment group than in the control-diet group. Protein levels of KC and iNOS induced by H. pylori infection were also lower in the RGE-treatment group than in the control-diet group, as determined by enzyme-linked immunosorbent assay and Western blotting, respectively (Fig. 5A).
Fig. 4.
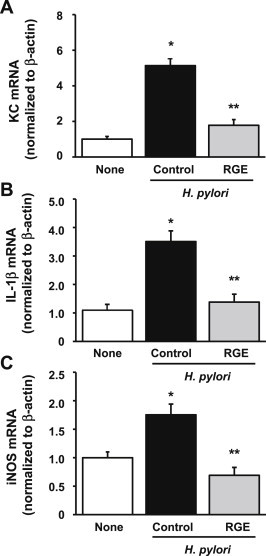
Effect of Korean Red Ginseng extract (RGE) on Helicobacter pylori-induced mRNA expression of keratinocyte chemoattractant factor (KC), interleukin (IL)-1β, and inducible nitric oxide synthase (iNOS), in gastric mucosal tissues of Mongolian gerbils. Real-time quantitative RT-PCR was performed on reverse-transcribed RNA isolated from gastric mucosa. mRNA level of (A) KC, (B) IL-1β, and (C) iNOS was normalized to β-actin. None, animals without H. pylori infection that were fed the control diet; H. pylori control, animals with H. pylori infection that were fed the control diet; and H. pylori + RGE, animals with H. pylori infection that were fed a diet supplemented with RGE. *p < 0.05 versus none. **p < 0.05 versus H. pylori control.
Fig. 5.
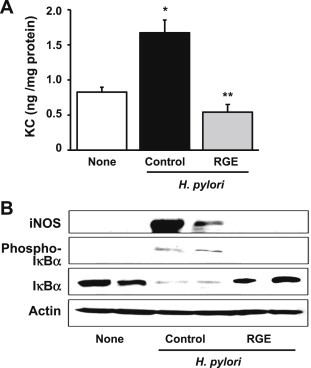
Effect of Korean Red Ginseng extract (RGE) on protein levels of, keratinocyte chemoattractant factor (KC), inducible nitric oxide synthase (iNOS), phospho-specific form of IκBα, and IκBα in Helicobacter pylori-infected gastric mucosal tissues of Mongolian gerbils. (A) The levels of KC in gastric mucosal tissues, as measured by enzyme linked immunosorbent assay. (B) iNOS, phospho-IκBα, IκBα, and actin protein levels in gastric mucosal tissues, as determined by Western blotting. Two representative bands per group are shown. None, animals without H. pylori infection that were fed the control diet; H. pylori control, animals with H. pylori infection that were fed the control diet; and Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds H. pylori + RGE, animals with H. pylori infection that were fed a diet supplemented with RGE.
As shown in Fig. 5B, the level of phospho-IκBα was greater in the H. pylori-infected groups than in the noninfected group, and was lower in the RGE-treatment group than in the control-diet group. IκBα, which was lower in the H. pylori-infected groups than in the noninfected group, was maintained in the RGE-treatment group. This suggests that RGE supplementation may inhibit NF-κB activation by suppressing phosphorylation of IκBα in the gastric mucosal tissues of H. pylori-infected Mongolian gerbils.
4. Discussion
The present study demonstrates that dietary supplementation of RGE fed to Mongolian gerbils for 6 wk improves H. pylori-induced gastric lesions, as determined by histological observation. RGE moderated the H. pylori-induced increase in neutrophil infiltration, MPO activity, LPO level, and the expression of inflammatory mediators (KC, IL-1β, iNOS). RGE was also associated with a reduction in IκΒα phosphorylation relative to that measured in animals fed the control diet. This demonstrates that RGE has an anti-inflammatory effect on H. pylori-induced gastric inflammation in Mongolian gerbils.
However, the number of viable bacteria obtained from the gastric mucosal tissues of H. pylori-infected animals fed a diet supplemented with RGE was not different from that obtained from animals receiving a control diet without RGE. RGE may not have an antibacterial effect on H. pylori colonization in the gastric mucosa of Mongolian gerbils. A previous study demonstrated that panaxytriol isolated from ginseng was effective in inhibiting H. pylori growth with an MIC of 50 μg/mL [42]. However, our preliminary study using gastric epithelial AGS cells showed that RGE did not affect the growth of H. pylori for 24 h culture (data not shown). Further study should be performed to determine anti-H. pylori activity of individual components of RGE. In addition, long-term exposure of RGE to the cells and animals infected with H. pylori is necessary to determine whether RGE has bactericidal/bacteriostatic effect.
Even though RGE has no cytotoxic effect on the bacterium, RGE may be beneficial for preventing and inhibiting the development of the gastric inflammation induced by H. pylori infection by reducing oxidative stress and suppressing the expression of inflammatory mediators in gastric mucosa.
KC, an IL-8 homolog, is a neutrophil chemoattractant that is involved in murine inflammation by stimulating neutrophil infiltration into infected tissues [30,43]. Increased activity of MPO represents neutrophil infiltration to the infected tissues and propagation of inflammation [14]. H. pylori-associated gastric mucosal injuries, including inflammation, are attributed to the activated neutrophils that adhere to postcapillary venules and subsequently migrate into the interstitium [44,45]. We found that H. pylori infection increased KC expression and MPO activity, suggesting increased infiltration of neutrophils into gastric mucosal tissues of Mongolian gerbils. The results are supported by histological observation showing neutrophil infiltration in H. pylori-infected gastric mucosa in the present study. Because RGE supplementation reduced KC expression, RGE may attenuate gastric inflammation by suppressing KC-mediated neutrophil infiltration into H. pylori-infected gastric mucosal tissues of Mongolian gerbils.
RGE supplementation inhibited the expression of the inflammatory mediators (iNOS, KC, and IL-1β) that was induced by H. pylori infection. Increased activity of iNOS and high levels of KC and IL-1β have been observed in the gastric mucosa of patients with chronic gastritis and gastric adenocarcinoma [46]. Neutrophil infiltration is positively correlated with the expression of iNOS and inflammatory cytokines in gastric mucosa [47]. These studies showed that the upregulation of iNOS, KC, and IL-1β by H. pylori infection might be associated with neutrophil infiltration. ROS are produced from the activated neutrophils in H. pylori-infected gastric mucosa. ROS activate oxidant-mediated transcription factors such as NF-κB, which induces the expression of iNOS, KC, and IL-1β. Therefore, RGE inhibits the expression of inflammatory cytokines including iNOS, KC, and IL-1β by suppressing the neutrophil infiltration caused by H. pylori infection in the gastric mucosa of Mongolian gerbils. Because the expressions of inflammatory mediators are critical for gastric inflammation and carcinogenesis, RGE may prevent the development of the gastric inflammation and gastric cancer that is associated with H. pylori infection.
Phosphorylation of IκBα is required for NF-κB activation, which regulates the expression of KC, IL-1β, and iNOS. Phosphorylation of IκBα acts as a trigger for IκB degradation, allowing the nuclear translocation of NF-κB and the expression of NF-κB target genes. Even though the Mongolian gerbil model is good for studying gastric inflammation and gastric cancer induced by H. pylori infection, there are few antibodies reported in the studies using Mongolian gerbils. Due to lack of antibodies, it is difficult to examine the serum levels of inflammatory mediators such as cytokines, which is a noninvasive way to confirm gastritis. Therefore, we assessed the phospho-specific form of IκBα as a biomarker of NF-κB activation in the present study. Several studies have demonstrated that H. pylori induces the expression of proinflammatory mediators such as KC, IL-1β, and iNOS through the activation of NF-κB [9,10]. In the present study, RGE decreased the phosphorylation of IκBα that was induced by H. pylori infection. The results suggest that RGE inhibits the expression of KC, IL-1β, and iNOS in the H. pylori-infected gastric mucosal tissues of Mongolian gerbils by suppressing the phosphorylation of IκBα, and thus inhibits NF-κB activation.
ROS are known to cause peroxidation of membrane lipids. Lipid peroxidation is involved in the pathogenesis of gastric diseases, including gastritis, that are associated with H. pylori infection. In the present study, the LPO level in the gastric mucosal tissues of Mongolian gerbils was increased by H. pylori infection. RGE supplementation reduced this increase in LPO level. The inhibitory effect of RGE on increases in LPO levels induced by H. pylori infection may be related to a reduction in MPO activity in the gastric mucosal tissues of animals supplemented with RGE. LPO level is directly correlated with ROS production and neutrophil infiltration [48,49]. The main source of ROS production may be host neutrophils that are activated by H. pylori [50,51]. Therefore, RGE may decrease the production of ROS and lipid peroxidation through inhibition of KC-mediated neutrophil infiltration in H. pylori-infected gastric mucosa. Previously, we found that H. pylori itself activates NADPH oxidase to produce ROS in gastric epithelial cells, resulting in the induction of NF-κB-mediated expression of IL-8, IL-1β, and iNOS [6–8]. Therefore, RGE may inhibit NADPH oxidase and thus suppress the ROS production that activates NF-κB and induces expression of IL-8, IL-1β, and iNOS in gastric epithelial cells. Further study should be undertaken to determine whether RGE inhibits ROS production by suppressing NADPH oxidase in H. pylori-infected gastric epithelial cells or gastric mucosal tissues.
The present study suggests that RGE attenuates H. pylori-induced expression of inflammatory mediators without affecting the number of viable H. pylori. Therefore, it is assumed that RGE suppresses H. pylori-induced inflammation including NF-κB activation and expression of inflammatory mediators, without direct action on H. pylori. Even though the first choice of the H. pylori therapy is eradication of the bacteria by antibiotics, the complete clearance of the bacteria is difficult in most patients. The inhibition of H. pylori-induced expression of inflammatory mediators by RGE may be useful for prevention of inflammation and possibly carcinogenesis mediated by the H. pylori infection.
Our findings demonstrate that H. pylori induced oxidative stress (determined by LPO levels in gastric mucosa), inflammation (examined by expressions of cytokines and iNOS, histologic observation of neutrophil infiltration, and MPO activity), and proliferation (observed by histologic hyperplasia), which were inhibited by RGE treatment. The precise mechanism of RGE on proliferation, mucosal destruction, inflammation, oxidative stress, and any presence of dysplasia or metaplasia should be determined to evaluate the anti-inflammatory effect of RGE using various gastric epithelial cells infected with H. pylori.
In conclusion, RGE supplementation inhibits neutrophil infiltration and lipid peroxidation, determined by MPO activity and LPO level, and attenuates the induction of inflammatory mediators (KC, IL-1β, iNOS), which results in suppression of H. pylori-induced gastric inflammation in Mongolian gerbils. Therefore, RGE may be beneficial for the prevention and treatment of H. pylori-associated gastric inflammation.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by a 2010 grant from the Korean Society of Ginseng.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Jeong-Heon Cha, Email: jcha@yuhs.ac.
Hyeyoung Kim, Email: kim626@yonsei.ac.kr.
References
- 1.Blaser M.J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 2.NIH Consensus Conference Helicobacter pylori in peptic ulcer disease. NIH consensus development panel on Helicobacter pylori in peptic ulcer disease. J Am Med Assoc. 1994;272:65–69. [PubMed] [Google Scholar]
- 3.Amagase K., Nakamura E., Endo T., Hayashi S., Hasumura M., Uneyama H., Torii K., Takeuchi K. New frontiers in gut nutrient sensor research: prophylactic effect of glutamine against Helicobacter pylori-induced gastric diseases in Mongolian gerbils. J Pharmacol Sci. 2010;112:25–32. doi: 10.1254/jphs.09r11fm. [DOI] [PubMed] [Google Scholar]
- 4.Burdon R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Rad Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 5.Handa O., Naito Y., Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997–1003. doi: 10.1007/s00011-010-0245-x. [DOI] [PubMed] [Google Scholar]
- 6.Kim H., Lim J.W., Kim K.H. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001;36:706–716. doi: 10.1080/003655201300191969. [DOI] [PubMed] [Google Scholar]
- 7.Kim H., Seo J.Y., Kim K.H. Inhibition of lipid peroxidation, NF-kappaB activation and IL-8 production by rebamipide in Helicobacter pylori-stimulated gastric epithelial cells. Dig Dis Sci. 2000;45:621–628. doi: 10.1023/a:1005474013988. [DOI] [PubMed] [Google Scholar]
- 8.Lim J.W., Kim H., Kim K.H. NF-κB, inducible nitric oxide synthase and apoptosis by Helicobacter pylori infection. Free Rad Biol Med. 2001;31:355–366. doi: 10.1016/s0891-5849(01)00592-5. [DOI] [PubMed] [Google Scholar]
- 9.Fu S., Ramanujam K.S., Wong A., Fantry G.T., Drachenberg C.B., James S.P., Meltzer S.J., Wilson K.T. Increased expression and cellular localization of inducible nitric oxide synthase and cyclooxygenase 2 in Helicobacter pylori gastritis. Gastroenterology. 1999;116:1319–1329. doi: 10.1016/s0016-5085(99)70496-8. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda T., Tsukamoto T., Takasu S., Shi L., Hirano N., Ban H., Meltzer S.J., Wilson K.T. Anti-inflammatory effects of caffeic acid phenethyl ester (CAPE), a nuclear factor-kappaB inhibitor, on Helicobacter pylori-induced gastritis in Mongolian gerbils. Int J Cancer. 2009;125:1786–1795. doi: 10.1002/ijc.24586. [DOI] [PubMed] [Google Scholar]
- 11.Baeuerle P.A., Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 12.Thanos D., Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M., Miura S., Mori M., Kai A., Suzuki H., Fukumura D., Suematsu M., Tsuchiya M. Rebamipide, a novel antiulcer agent, attenuates Helicobacter pylori induced gastric mucosal cell injury associated with neutrophil derived oxidants. Gut. 1994;35:1375–1378. doi: 10.1136/gut.35.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebanoff S.J. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 15.Heinecke J.W., Li W., Francis G.A., Goldstein J.A. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91:2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi K.S., Song H., Kim E.H., Choi J.H., Hong H., Han Y.M., Hahm K.B. Inhibition of hydrogen sulfide-induced angiogenesis and inflammation in vascular endothelial cells: Potential mechanisms of gastric cancer prevention by Korean Red Ginseng. J Ginseng Res. 2012;36:135–145. doi: 10.5142/jgr.2012.36.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun T.K. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 18.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. [PubMed] [Google Scholar]
- 19.Shibata S., Fujita M., Itokawa H., Tanaka O., Ishii T. Studies on the constituents of Japanese and Chinese crude drugs. XI. Panaxadiaol, a sapogenin of ginseng roots. Chem Pharm Bull (Tokyo) 1963;11:759–761. doi: 10.1248/cpb.11.759. [DOI] [PubMed] [Google Scholar]
- 20.Ro J.Y., Ahn Y.S., Kim K.H. Inhibitory effect of ginsenoside on the mediator release in the guinea pig lung mast cells activated by specific antigen-antibody reactions. Int J Immunopharmacol. 1998;20:625–641. doi: 10.1016/s0192-0561(98)00062-9. [DOI] [PubMed] [Google Scholar]
- 21.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 22.Toh D.F., Patel D.N., Chan E.C., Teo A., Neo S.Y., Koh H.L. Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells. Chin Med. 2011;6:4–12. doi: 10.1186/1749-8546-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee J.H., Shim J.S., Lee J.S., Kim M.K., Chung M.S., Kim K.H. Pectin-like acidic polysaccharide from Panax ginseng with selective antiadhesive activity against pathogenic bacteria. Carbohydr Res. 2006;341:1154–1163. doi: 10.1016/j.carres.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 24.Park S., Yeo M., Jin J.H., Lee K.M., Jung J.Y., Choue R., Cho S.W., Hahm K.B. Rescue of Helicobacter pylori-induced cytotoxicity by red ginseng. Dig Dis Sci. 2005;50:1218–1227. doi: 10.1007/s10620-005-2763-x. [DOI] [PubMed] [Google Scholar]
- 25.Park S., Yeo M., Jin J.H., Lee K.M., Kim S.S., Choi S.Y., Hahm K.B. Inhibitory activities and attenuated expressions of 5-LOX with red ginseng in Helicobacter pylori-infected gastric epithelial cells. Dig Dis Sci. 2007;52:973–982. doi: 10.1007/s10620-006-9440-6. [DOI] [PubMed] [Google Scholar]
- 26.Kim H., Lee Y.H., Kim S.I. Hepatoprotective effect of Panax ginseng against thioacetamide intoxication in rats. Korean Biochem J. 1989;22:12–18. [Google Scholar]
- 27.Kim H., Lee Y.H., Kim S.I. Effect of polyacetylene compounds from Panax ginseng on lipid peroxidation in mouse liver. Korean J Toxicol. 1988;4:13–21. [Google Scholar]
- 28.Franco A.T., Johnston E., Krishna U., Yamaoka Y., Israel D.A., Nagy T.A., Wroblewski L.E., Piazuelo M.B., Correa P., Peek R.M., Jr. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco A.T., Israel D.A., Washington M.K., Krishna U., Fox J.G., Rogers A.B., Neish A.S., Collier-Hyams L., Perez-Perez G.I., Hatakeyama M. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiratori Y., Hikiba Y., Mawet E., Niwa Y., Matsumura M., Kato N., Shiina S., Tada M., Komatsu Y., Kawabe T. Modulation of KC/gro protein (interleukin-8 related protein in rodents) release from hepatocytes by biologically active mediators. Biochem Biophys Res Commun. 1994;203:1398–1403. doi: 10.1006/bbrc.1994.2340. [DOI] [PubMed] [Google Scholar]
- 31.Ramesh T., Kim S.W., Hwang S.Y., Sohn S.H., Yoo S.K., Kim S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.S., Kim D.H., Kim B.K., Yoon S.K., Kim M.H., Lee J.Y., Kim H.O., Park Y.M. Effects of topically applied Korean Red Ginseng and its genuine constituents on atopic dermatitis-like skin lesions in NC/Nga mice. Intl Immunopharmacol. 2011;11:280–285. doi: 10.1016/j.intimp.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S., Keto Y., Fujita H., Muramatsu H., Nishino T., Okabe S. Pathological changes in the formation of Helicobacter pylori-induced gastric lesions in Mongolian gerbils. Dig Dis Sci. 1998;43:754–765. doi: 10.1023/a:1018861930068. [DOI] [PubMed] [Google Scholar]
- 35.Cao X., Tsukamoto T., Seki T., Tanaka H., Morimura S., Cao L., Mizoshita T., Ban H., Toyoda T., Maeda H. 4-Vinyl-2,6-dimethoxyphenol (canolol) suppresses oxidative stress and gastric carcinogenesis in helicobacter pylori-infected carcinogen-treated mongolian gerbils. Int J Cancer. 2008;122:1445–1454. doi: 10.1002/ijc.23245. [DOI] [PubMed] [Google Scholar]
- 36.Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. The updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Ohkawa H., Ohnishi N., Yagi K. Assay for lipid peroxides for animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 38.Lowry O.H., Rosenbrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.Krawisz J.E., Sharon P., Stenson W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 40.Takeda K., Utsunomiya H., Kakiuchi S., Okuno Y., Oda K., Inada K., Tsutsumi Y., Tanaka T., Kakudo K. Citrus auraptene reduces Helicobacter pylori colonization of glandular stomach lesions in Mongolian gerbils. J Oleo Sci. 2007;56:253–260. doi: 10.5650/jos.56.253. [DOI] [PubMed] [Google Scholar]
- 41.Shinizaki K., Kamada T., Sugiu K., Kusunoki H., Manabe N., Shiotani A., Hata J., Teramoto F., Haruma K. High-protein diet suppresses corpus atrophic gastritis in Helicobacter pylori infected Mongolian gerbils. Nutr Cancer. 2013;62:1067–1073. doi: 10.1080/01635581.2010.492086. [DOI] [PubMed] [Google Scholar]
- 42.Bae E.A., Han M.J., Baek N.I., Kim D.H. In vitro anti-Helicobacter pylori activity of panaxytriol isolated from ginseng. Arch Pharm Res. 2001;24:297–299. doi: 10.1007/BF02975095. [DOI] [PubMed] [Google Scholar]
- 43.Bozic C.R., Kolakowski L.F., Jr., Gerard N.P., Garcia-Rodriguez C., von Uexkull-Guldenband C., Conklyn M.J., Breslow R., Showell H.J., Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 44.Suzuki M., Miura S., Suematsu M., Fukumura D., Kurose I., Suzuki H., Kai A., Kudoh Y., Ohashi M., Tsuchiya M. Helicobacter pylori-associated ammonia production enhances neutrophil-dependent gastric mucosal cell injury. Am J Physiol. 1992;263:G719–G725. doi: 10.1152/ajpgi.1992.263.5.G719. [DOI] [PubMed] [Google Scholar]
- 45.Crabtree J.E., Lindley I.J. Mucosal interleukin-8 and Helicobacter pylori-associated gastroduodenal disease. Eur J Gastroenterol Hepatol. 1994;6:S33–S38. [PubMed] [Google Scholar]
- 46.Rajnakova A., Moochhala S., Goh P.M., Ngoi S. Expression of nitric oxide synthase, cyclooxygenase, and p53 in different stages of human gastric cancer. Cancer Lett. 2001;172:177–185. doi: 10.1016/s0304-3835(01)00645-0. [DOI] [PubMed] [Google Scholar]
- 47.Kaise M., Miwa J., Iihara K., Suzuki N., Oda Y., Ohta Y. Helicobacter pylori stimulates inducible nitric oxide synthase in diverse topographical patterns in various gastroduodenal disorders. Dig Dis Sci. 2003;48:636–643. doi: 10.1023/a:1022855818944. [DOI] [PubMed] [Google Scholar]
- 48.Drake I.M., Mapstone N.P., Schorah C.J., White K.L., Chalmers D.M., Dixon M.F., Axon A.T. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H. pylori eradication. Gut. 1998;42:768–771. doi: 10.1136/gut.42.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki H., Mori M., Seto K., Kai A., Kawaguchi C., Suzuki M., Suematsu M., Yoneta T., Miura S., Ishii H. Helicobacter pylori-associated gastric pro- and anti-oxidant formation in Mongolian gerbils. Free Rad Biol Med. 1999;26:679–684. doi: 10.1016/s0891-5849(98)00248-2. [DOI] [PubMed] [Google Scholar]
- 50.Mai U.E.H., Perez-Perez G.I., Allen J.B., Wahl S.M., Blaser M.J., Smith P.D. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leukocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig P.M., Territo M.C., Karnes W.E., Walsh J.H. Helicobacter pylori secretes a chemotactic factor monocytes and neutrophils. Gut. 1992;33:1020–1023. doi: 10.1136/gut.33.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]


