Abstract
The single oral administration of red ginseng oil (5000 mg/kg) to Sprague–Dawley rats induced no changes in behavioral patterns, clinical signs, and body weight, and hepatotoxicity parameters such as aspartate aminotransferase and alanine aminotransferase for 14 d. Therefore, these results suggest that the red ginseng oil is safe and nontoxic acutely.
Keywords: Panax ginseng, red ginseng oil, safety, single oral dose
The use of traditional and herbal medicine is practiced in the prevention, diagnosis, and treatment of diseases, and maintenance of health, and numerous studies have reported the benefits of traditional herb medicines [1–5]. Despite the worldwide use of traditional medicine, there have been concerns about the lack of safety information. An important role of safety is to identify the poison that induces the adverse effects involved in the interaction between toxicants and the cells. The target organs that are affected may vary depending on the chemical properties of the toxicants and the cells [6]. Hence, evaluation of safety studies helps us decide whether or not a new herbal medicine should be adopted for clinical use. Therefore, an acute oral safety study is vitally needed not only to identify the range of doses that could be used subsequently, but also to reveal the possible clinical signs elicited by the substances under investigation.
Ginseng (Panax ginseng Meyer) is a widely used traditional herb medicine [7–10]. There are several types of ginseng depending on the processing methods, including fresh ginseng, white ginseng, and red ginseng. Red ginseng is a type of steamed and dried ginseng that shows enhanced pharmacological effects compared with nonsteamed ginseng [11–14]. However, these diverse beneficial effects of red ginseng have been reported mainly with its water-soluble fractions, probably because red ginseng is widely consumed in the form of hot-water extract or its concentrates. The biological effects of the lipid soluble moiety of red ginseng have been little studied. We have recently demonstrated various biological activities and the underlying molecular mechanisms of red ginseng oil that was prepared by a supercritical CO2 extraction of marc generated after hot water extraction of red ginseng [15,16]. Red ginseng marc oil (RMO) has been shown to have potent antioxidant, hepatoprotective, and anti-inflammatory effects in cells and mice. Recently, several studies have demonstrated the nontoxic effects of ginseng in animals and human studies [17–20]. Lee et al [21] reported that black ginseng produced by heat processing is nontoxic in an acute oral toxicity study. However, little is known about the safety and/or toxicity of red ginseng oil.
In this study, a single oral dose safety on RMO in Sprague–Dawley (SD) rats was conducted as the first step of safety evaluation, which will provide preliminary safety information regarding red ginseng oil.
Five-wk-old male and female SD rats from Hyochang Science (Daegu, South Korea) were used after a 1-wk acclimation to the laboratory environment. The experiment was performed in the animal laboratory under the following conditions: temperature 25 ± 2°C, relative humidity 50 ± 5%, and 12-h light/dark cycle. Drinking water and food were provided ad libitum throughout the experiment. All procedures were approved by the Animal Care and Used Committee of Inje University, Gimhae, South Korea. The animals were divided into four groups of five rats each upon receipt. As no toxicological data were available regarding the safety of red ginseng oil, the highest dosage level was selected as 5,000 mg/kg according to the recommendations of the Korea Food and Drug Administration Guidelines and the Organization for Economic Co-Operation and Development (OECD) Guidelines [22,23]. Both male and female rats were orally administered once at a dose of 5,000 mg/kg of RMO. In general, a nontoxic compound is recommended to be tested up to 2,000 mg/kg or 5,000 mg/kg for acute toxicity. Red ginseng is used as functional food and 5,000 mg/kg is deemed to be a better choice for the current study rather than 2,000 mg/kg, which is recommended as a maximum dose for drugs. Based on many previous reports, the oral route administration is probably the most convenient and commonly used one when studying single oral dose safety [24–26].
In the present study, general symptoms, clinical signs, and mortality rates were examined at the given RMO dose and then daily for 14 d after the treatment. The clinical symptom is one of the major important observations to indicate the side effects on organs in the treated groups [27]. All groups treated with RMO did not show any symptoms or abnormal clinical sings, and no deaths were observed during the study period (Table 1), indicating that the administration of the RMO has a negligible level of toxicity on the growth of the animals. In addition, no deaths or adverse clinical signs were observed due to gavage of RMO at a dose of 5,000 mg/kg (Tables 2 and 3). Also, food intake and water consumption were not affected by the administration of RMO (data not shown); moreover, it did not induce appetite suppression and had no deleterious effects, indicating that there was no disturbance in carbohydrate, protein, or fat metabolism.
Table 1.
Mortality of Spraguee-Dawley (SD) rats orally treated with red ginseng marc oil (RMO) in a single-dose safety
| Sex | Group/Dose (mg/kg) | No. of animals | Days after dosing |
Mortality, % (dead/total) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||
| Male | RMO 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0/5) |
| RMO 5,000 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0/5) | |
| Female | RMO 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0/5) |
| RMO 5,000 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0/5) | |
Table 2.
Summary of clinical signs 1 in rats exposed to red ginseng marc oil (RMO)
| Sex | Group/Dose (mg/kg) | No. of animals | Clinical signs | Hours (Day 0) after dosing |
||||
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2 | 4 | 6 | ||||
| Male | RMO 0 | 5 | NOA | 5 | 5 | 5 | 5 | 5 |
| RMO 5,000 | 5 | NOA | 5 | 5 | 2 | 2 | 2 | |
| Soft stool | 0 | 0 | 2 | 2 | 2 | |||
| Compound-colored stool | 0 | 0 | 1 | 1 | 1 | |||
| Female | RMO 0 | 5 | NOA | 5 | 5 | 5 | 5 | 5 |
| RMO 5,000 | 5 | NOA | 5 | 4 | 4 | 4 | 4 | |
| Soft stool | 0 | 1 | 1 | 1 | 1 | |||
| Compound -colored stool | 0 | 0 | 0 | 0 | 0 | |||
NOA, No Observable Abnormality.
Table 3.
Summary of clinical signs 2 in rats exposed to red ginseng marc oil (RMO)
| Sex | Group/ Dose (mg/kg) | No. of animals | Clinical signs | Days after dosing |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||||
| Male | RMO 0 | 5 | NOA | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| RMO 5,000 | 5 | NOA | 2 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Soft stool | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Compound-colored stool | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Female | RMO 0 | 5 | NOA | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| RMO 5,000 | 5 | NOA | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Soft stool | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Compound-colored stool | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
NOA, No Observable Abnormality.
Body weights were measured on the day of dosing (Day 0) prior to treatment, 1 d, 2 d, 7 d, 13 d, and 14 d after dosing. Typically, changes in body weight are one of the indicators of adverse effects of testing substances, and it is considered significant when body weight loss is more than 10% from the initial weight [28]. In this study, the body weight data indicated that there were no statistically significant differences between RMO-treated groups and the control groups throughout the experimental period (Fig. 1). Furthermore, any decrease in body weight gain was not found in the male and female rats treated with RMO. The above results for single oral dose safety test suggest that RMO is safe and nontoxic to rats at the dose of 5,000 mg/kg.
Fig. 1.
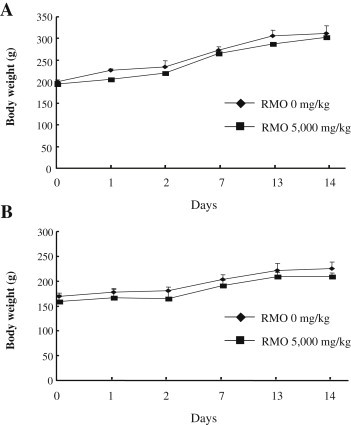
Body weight changes of rats exposed to a single-dose toxicity of red ginseng marc oil (RMO). (A) Male and (B) female Sprague–Dawley rats were administered orally with RMO at doses of 5,000 mg/kg (■) on the day after dosing. Control animals received the same amount of vehicle alone (♦). Data are presented as mean ± SD (n = 5). There was no significant difference in body weight between RMO-treated group and control group (p > 0.05). RMO, red ginseng marc oil; SD, standard deviation.
All animals survived during the experiment and were subjected to terminal necropsy at the end of the experiment on Day 14. Necropsy is a key procedure of most safety and/or toxicity studies, and remarkable changes in tissues and organs are recorded during this process [29]. No remarkable abnormalities were observed in animal organs including the naked eyes, liver, kidneys, lung, heart, thymus, spleen, adrenal glands, and reproductive organs (Table 4). Therefore, we concluded that the lethality of RMO after a single oral administration could be higher than 5,000 mg/kg in both male and female rats under current experimental conditions. According to the study of Jothy et al [27], substances with LD50 values higher than 5,000 mg/kg by oral route are regarded as safe or practically nontoxic [27]. Similar results were found for a single oral dose of Coriolus versicolor water extract (5,000 mg/kg) that was shown to be nontoxic to the tested SD rats [30]. Meanwhile, a study performed by Fujii et al [31], in which Oligonol was used, revealed that the extract did not cause any mortality up to 2,000 mg/kg and was thus considered safe [31]. However, acute safety studies are hampered by limitations in detecting test substance-related effects on vital functions of cardiovascular, central nervous, and respiratory systems, which should be evaluated prior to human exposure [32]. Further studies should be conducted to clarify the systemic safety of RMO using repeated-dose safety pharmacology studies.
Table 4.
Summary of necropsy findings in rats exposed to red ginseng marc oil (RMO)
| Sex |
Male |
Female |
||
|---|---|---|---|---|
| Group | G1 | G2 | G1 | G2 |
| Dose (mg/kg) | 0 | 5,000 | 0 | 5,000 |
| No. of animals | 5 | 5 | 5 | 5 |
| Unremarkable findings | 5 | 5 | 5 | 5 |
| No. of examined | 5 | 5 | 5 | 5 |
External surface and all organs in body cavity were unremarkable.
The enzymatic activities of serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were used as biochemical markers for hepatotoxicity. Blood samples from rats were centrifuged at 3,000 × g at 4°C for 10 min, and the AST and ALT activities in serum supernatants were determined using a commercially available kit (Youndong Pharm, Yong-In, South Korea). Hepatocellular damage is characterized by a mutual rise in serum levels of AST and ALT [33]. Liver is the main target organ of acute toxicity in exposure to foreign substances being absorbed in intestines and metabolized to other compounds that may or may not be hepatotoxic to the mice [34]. There were no significant changes in the serum levels of AST and ALT after RMO administration, demonstrating that liver function was preserved in male and female rats exposed to RMO for 14 d (Fig. 2). Moreover, lipid peroxidation was slightly decreased by the treatment of RMO (5,000 mg/kg), but not significantly. These data do not exhibit significant differences compared with the control group. Our results demonstrate that RMO caused no hepatotoxic effects in male and female rats up to 5,000 mg/kg acutely.
Fig. 2.
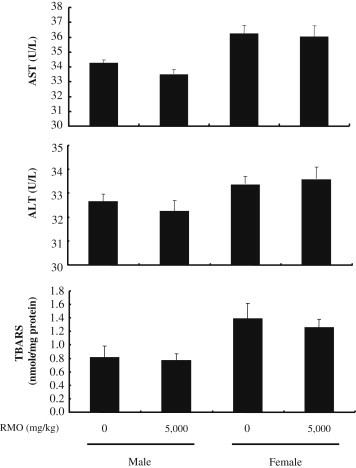
Hepatic parameters of rats exposed to a single-dose toxicity of red ginseng marc oil (RMO). Male and female Sprague–Dawley rats were administered orally with RMO (5,000 mg/kg), and hepatotoxicity parameters including serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), and hepatic thiobarbituric acid reactive substances (TBARS) values were determined at the end of the experiment on Day 14. Data are presented as mean ± SD (n = 5). There was no significant difference in the hepatic parameters between RMO-treated group and control group (p > 0.05). SD, standard deviation.
The present results show that RMO does not induce any apparent in vivo damage in the current single oral dose safety study. No death or signs of damage were observed in rats treated with RMO at a dose of 5,000 mg/kg, thus establishing its safety in use. A detailed experimental analysis of its chronic toxicity is essential for further support of RMO.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgments
We thank Korea Ginseng Corporation for the preparation of red ginseng oil. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MEST; No. 20100014447).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Rahimi R., Shams-Ardekani M.R., Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J Gastroenterol. 2010;16:4504–4514. doi: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X.M., Srivastava K. Traditional Chinese medicine for the therapy of allergic disorders. Curr Opin Otolaryngol Head Neck Surg. 2006;14:191–196. doi: 10.1097/01.moo.0000193199.40096.f7. [DOI] [PubMed] [Google Scholar]
- 3.Palombo E.A. Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res. 2006;20:717–724. doi: 10.1002/ptr.1907. [DOI] [PubMed] [Google Scholar]
- 4.Wojcikowski K., Johnson D.W., Gobe G. Herbs or natural substances as complementary therapies for chronic kidney disease: ideas for future studies. J Lab Clin Med. 2006;147:160–166. doi: 10.1016/j.lab.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Cohen I., Tagliaferri M., Tripathy D. Traditional Chinese medicine in the treatment of breast cancer. Semin Oncol. 2002;29:563–574. doi: 10.1053/sonc.2002.50005. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh D., Ghosh S., Sarkar S., Ghosh A., Das N., Das Saha K., Mandal A.K. Quercetin in vesicular delivery systems: evaluation in combating arsenic-induced acute liver toxicity associated gene expression in rat model. Chem Biol Interact. 2010;186:61–71. doi: 10.1016/j.cbi.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Ernst E. The risk-benefit profile of commonly used herbal therapies: Ginkgo, St. John's Wort, Ginseng, Echinacea, Saw Palmetto, and Kava. Ann Intern Med. 2002;136:42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- 8.Hofseth L.J., Wargovich M.J. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 9.Vuksan V., Sung M.K., Sievenpiper J.L., Stavro P.M., Jenkins A.L., Di Buono M., Lee K.S., Leiter L.A., Nam K.Y., Arnason J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr Metab Cardiovasc Dis. 2008;18:46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee J.G., Lee Y.Y., Kim S.Y., Pyo J.S., Yun-Choi H.S., Park J.H. Platelet antiaggregating activity of ginsenosides isolated from processed ginseng. Pharmazie. 2009;64:602–604. [PubMed] [Google Scholar]
- 11.Kim J. Protective effects of Asian dietary items on cancers — soy and ginseng. Asian Pac J Cancer Prev. 2008;9:543–548. [PubMed] [Google Scholar]
- 12.Jang D.J., Lee M.S., Shin B.C., Lee Y.C., Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–450. doi: 10.1111/j.1365-2125.2008.03236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quan H.Y., Kim do Y., Chung S.H. Korean red ginseng extract alleviates advanced glycation end product-mediated renal injury. J Ginseng Res. 2013;37:187–193. doi: 10.5142/jgr.2013.37.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung J.H., Kang I.G., Kim D.Y., Hwang Y.J., Kim S.T. The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37:167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFkappaB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bak M.J., Jun M., Jeong W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int J Mol Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aphale A.A., Chhibba A.D., Kumbhakarna N.R., Mateenuddin M., Dahat S.H. Subacute toxicity study of the combination of ginseng (Panax ginseng) and ashwagandha (Withania somnifera) in rats: a safety assessment. Indian J Physiol Pharmacol. 1998;42:299–302. [PubMed] [Google Scholar]
- 18.Park I.D., Yoo H.S., Lee Y.W., Son C.G., Kwon M., Sung H.J., Cho C.K. Toxicological study on MUNOPHIL, water extract of Panax ginseng and Hericium erinaceum in rats. J Acupunct Meridian Stud. 2008;1:121–127. doi: 10.1016/S2005-2901(09)60032-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee N.H., Yoo S.R., Kim H.G., Cho J.H., Son C.G. Safety and tolerability of Panax ginseng root extract: a randomized, placebo-controlled, clinical trial in healthy Korean volunteers. J Altern Complement Med. 2012;18:1061–1069. doi: 10.1089/acm.2011.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess F.G., Jr., Parent R.A., Stevens K.R., Cox G.E., Becci P.J. Effects of subchronic feeding of ginseng extract G115 in beagle dogs. Food Chem Toxicol. 1983;21:95–97. doi: 10.1016/0278-6915(83)90275-2. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.R., Oh C.J., Li Z., Li J.J., Wang C.Y., Wang Z., Gu L.J., Lee S.H., Lee J.I., Lim B.O. Evaluation of the oral acute toxicity of black ginseng in rats. J Ginseng Res. 2011;35:39–44. [Google Scholar]
- 22.Korea Food and Drug Administration . 2005. Testing guidelines for safety evaluation of drugs (Notification NO. 2005-2060, issued by the Korea Food and Drug Administration on October 21, 2005) [Google Scholar]
- 23.Organization for Economic Co-Operation and Development (Ed.) 2001. OECD guideline (420) for the testing of chemicals acute oral toxicity–acute toxic class method. [Google Scholar]
- 24.Bakoma B., Berke B., Eklu-Gadegbeku K., Agbonon A., Aklikokou K., Gbeassor M., Creppy E.E., Moore N. Acute and sub-chronic (28 days) oral toxicity evaluation of hydroethanolic extract of Bridelia ferruginea Benth root bark in male rodent animals. Food Chem Toxicol. 2013;52:176–179. doi: 10.1016/j.fct.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Chanda S., Dave R., Kaneria M., Shukla V. Acute oral toxicity of Polyalthia longifolia var. pendula leaf extract in Wistar albino rats. Pharm Biol. 2012;50:1408–1415. doi: 10.3109/13880209.2012.682117. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y., Zhang S., Li C., Xiao L., Shen J., Yin J. Acute and subchronic toxicity of xylo-oligosaccharide in mice and rats. Toxicol Mech Methods. 2012;22:605–610. doi: 10.3109/15376516.2012.706837. [DOI] [PubMed] [Google Scholar]
- 27.Jothy S.L., Zakaria Z., Chen Y., Lau Y.L., Latha L.Y., Sasidharan S. Acute oral toxicity of methanolic seed extract of Cassia fistula in mice. Molecules. 2011;16:5268–5282. doi: 10.3390/molecules16065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liju V.B., Jeena K., Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L) Food Chem Toxicol. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 29.Hwang Y.H., Ha H., Ma J.Y. Acute oral toxicity and genotoxicity of Dryopteris crassirhizoma. J Ethnopharmacol. 2013;149:133–139. doi: 10.1016/j.jep.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Hor S.Y., Ahmad M., Farsi E., Lim C.P., Asmawi M.Z., Yam M.F. Acute and subchronic oral toxicity of Coriolus versicolor standardized water extract in Sprague–Dawley rats. J Ethnopharmacol. 2011;137:1067–1076. doi: 10.1016/j.jep.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Fujii H., Nishioka H., Wakame K., Magnuson B.A., Roberts A. Acute, subchronic and genotoxicity studies conducted with Oligonol, an oligomerized polyphenol formulated from lychee and green tea extracts. Food Chem Toxicol. 2008;46:3553–3562. doi: 10.1016/j.fct.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Diallo A., Gadegkeku K., Agbonon A., Aklikokou K., Creppy E., Gbeassor M. Acute and sub-chronic (28-day) oral toxicity studies of hydroalcohol leaf extract of Ageratum conyzoides L (Asteraceae) Trop J Pharm Res. 2010;9:463–467. [Google Scholar]
- 33.Aniagu S.O., Nwinyi F.C., Olanubi B., Akumka D.D., Ajoku G.A., Izebe K.S., Agala P., Agbani E.O., Enwerem N.M., Iheagwara C. Is Berlina grandiflora (Leguminosae) toxic in rats? Phytomedicine. 2004;11:352–360. doi: 10.1078/0944711041495155. [DOI] [PubMed] [Google Scholar]
- 34.Rhiouani H., El-Hilaly J., Israili Z.H., Lyoussi B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J Ethnopharmacol. 2008;118:378–386. doi: 10.1016/j.jep.2008.05.009. [DOI] [PubMed] [Google Scholar]


