Abstract
Cigarette smoke is considered a major risk factor for vascular diseases. There are many toxic compounds in cigarette smoke, including acrolein and other α,β-unsaturated aldehydes, which are regarded as mediators of inflammation and vascular dysfunction. Furthermore, recent studies have revealed that acrolein, an α,β-unsaturated aldehyde in cigarette smoke, induces inflammatory mediator expression, which is known to be related to vascular diseases. In this study, we investigated whether Korean Red Ginseng (KRG) water extract suppressed acrolein-induced cyclooxygenase (COX)-2 expression in human umbilical vein endothelial cells (HUVECs). Acrolein-induced COX-2 expression was accompanied by increased levels of phosphorylated p38 in HUVECs and KRG inhibited COX-2 expression in HUVECs. These results suggest that KRG suppresses acrolein-induced COX-2 expression via inhibition of the p38 mitogen-activated protein kinase signaling pathway. In addition, KRG exhibited an inhibitory effect on acrolein-induced apoptosis, as demonstrated by annexin V–propidium iodide staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay. Consistent with these results, KRG may exert a vasculoprotective effect through inhibition of COX-2 expression in acrolein-stimulated human endothelial cells.
Keywords: cyclooxygenase-2, endothelial cells, oxidative stress, Panax ginseng, vascular disease
1. Introduction
Cigarette smoke (CS) is associated with the development of inflammation-related diseases such as chronic obstructive pulmonary disease and vascular diseases, including atherosclerosis and stroke [1,2]. Several studies have revealed that CS is a major contributor to vascular diseases because it accelerates the development of atherosclerotic plaques [3,4]. The relationship between CS and the increased incidence of atherosclerosis has been reported [5–7], which may be a consequence of direct endothelial damage, increased proliferation of smooth muscle in atherosclerotic lesions, and/or decreased vasodilation [8].
Endothelial damage has also been suggested as the initial cause of development of vascular diseases. In a previous study, it was shown that inhibition of oxidative stress exerts protection in human endothelial cells, which could be an effective strategy in the treatment of vascular diseases [9]. A number of studies support that reactive oxygen species (ROS) causing oxidative stress may play an essential role in mediating endothelial cell death. Oxidative stress is a major factor in vascular diseases such as hypertension, stroke, and atherosclerosis. Several studies have reported that α,β-unsaturated aldehyde acrolein in CS induces intracellular ROS generation [10,11]. An increase in intracellular ROS levels causes cellular dysfunction.
Korean Red Ginseng (KRG) is a popular traditional herbal medicine that has been widely used to treat several diseases such as cancer and vascular diseases. Recent research shows that ginseng may have therapeutic potential in the treatment of Alzheimer's disease, diabetes, cancer, and cardiovascular diseases, through its antioxidant, antithrombotic, antihyperlipidemic, and anticancer effects [12–15]. In endothelial cells, KRG simulates NO production in vivo and in vitro, suggesting that KRG has antihypertensive effects [16,17]. KRG also promotes angiogenesis through the activation of the signaling pathway, indicating that KRG can be implicated in potential angiogenic therapies for improving tissue repair, wound healing, and cardiovascular diseases [18]. In addition, our previous study suggested that KRG exerts a cytoprotective effect through the induction of heme oxygenase (HO)-1 expression, suggesting a possible therapeutic mechanism of KRG in cardiovascular diseases [19].
It is well known that chronic inflammation contributes to the pathogenesis of many human diseases such as atherosclerosis. Accumulating evidence suggests that KRG is involved in the regulation of inflammatory responses [20,21], suggesting an anti-inflammatory effect of KRG. Cyclooxygenase (COX) catalyzes the conversion of arachidonic acid to prostaglandins that play vital roles in multiple physiological and pathophysiological processes, including inflammation. There are two distinct isoforms of COX in mammalian cells. In particular, COX-2 is normally undetectable in most tissues and is induced in response to numerous stimuli. Vascular diseases may, in part, be caused by COX-2 upregulation at sites of inflammation and vascular injury. COX-2 plays an important role in inflammation, therefore, inhibition of COX-2 expression may participate in the treatment of inflammation-related diseases such as vascular diseases.
The objective of our study was to investigate the vascular protective effect of KRG in acrolein-stimulated human umbilical vein endothelial cells (HUVECs). Therefore, we examined the involvement of COX-2 expression via p38 mitogen-activated protein kinase (MAPK), intracellular ROS, and apoptosis in acrolein-stimulated HUVECs.
2. Materials and methods
2.1. Materials
KRG powder was obtained from the Korea Ginseng Corporation (Daejeon, Korea). M199 medium and fetal bovine serum were purchased from Welgene (Daegu, Korea). TRIzol reagent was supplied by Invitrogen (Carlsbad, CA, USA). All other chemicals and reagents were of analytical grade.
2.2. Preparation of KRG water extract
For preparation of KRG water extract, we modified a method used in a previous study [22]. KRG powder was soaked in water (1:25, w/w) for 3 h, and boiled for 40 min. Following centrifugation at 1,900 g for 60 min, supernatants of ginseng extract were further centrifuged at 10,000 g for 30 min and lyophilized. Ginseng extracts were dissolved in pure water immediately prior to the experiment. The general composition of the product offered by the Korea Ginseng Corporation is as follows: moisture 36%, solid volume 64%, ash 2.5%, total fat 0.05%, total crude saponin 70 mg/g, and total ginsenosides 20 mg/g.
2.3. Cell culture
HUVECs were maintained in M199 medium and supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin, 10 ng/mL human fibroblast growth factor, and 18 mU/mL heparin. The cells were incubated at 37°C under a 5% CO2 atmosphere. HUVECs were grown to ∼80% confluence, maintained with fresh medium described above, and subcultured every 2 or 3 d. The cells were used within nine passages during these experiments [23].
2.4. Western blot analysis
We applied 20 or 40 μg of the whole cell lysate proteins to each lane and analyzed them with western blotting. Western blotting was performed using primary antibodies as follows: anti-COX-2, p38 MAPK, phopho-p38, cyclic AMP-responsive element-binding protein (CREB), phospho-CREB (Cell Signaling, Danvers, MA, USA) and anti-glyceraldehyde 3-phosphate dehydrogenase (AbFrontier, Seoul, Korea). Horseradish-peroxidase-conjugated anti-IgG antibodies were used as the secondary antibody to detect the above-mentioned protein bands by enhanced chemiluminescence WESTSAVE-Up (Abfrontier).
2.5. RNA isolation and reverse transcriptase-polymerase chain reaction
RNA extraction was achieved using 1 mL TRIzol reagent. The RNA pellets were washed in 70% ethanol, dried completely, and dissolved in diethylpyrocarbonate to inhibit RNase. Total RNA was quantified using a ND-100 spectrometer (NanoDrop Technologies, Wilmington, DE, USA). Polymerase chain reaction (PCR) was performed using the synthesized cDNA as a template and using specific primers for COX-2 or β-actin as a loading control. The primer sequence for human COX-2 was 5′-GACAGTCCACCAACTTACAAT-3′ (forward) and 5′-CATCTCTCCATCAATTATCTGAT-3′ (reverse). The amplified products were resolved by 1% agarose gel electrophoresis, stained with ethidium bromide, and photographed under ultraviolet light.
2.6. Immunofluorescence staining
HUVECs were cultured in a glass culture chamber slide (Falcon Plastics, London Ontario, Canada) and processed for immunofluorescence analysis. Immunofluorescence was performed as described previously [24].
2.7. Measurement of prostaglandin E2
The amount of prostaglandin (PG)E2 in the culture medium was measured using the PGE2 EIA kit according to the manufacturer's protocol (Cayman Chemical Company, Ann Arbor, MI, USA). Samples as well as standards were applied to a 96-well plate, precoated with goat anti-mouse IgG, and incubated with PGE2 acetylcholinesterase tracer and PGE2 antiserum. All the wells were emptied, rinsed five times, and incubated with Ellman's reagent for 60 min in the dark with gentle rocking to produce 5-thio-2-nitrobenzoic acid, which has a strong absorbance at 405 nm; the plate was read at 405 nm in an enzyme-linked immunosorbent assay reader (EL 800; Bio-Tek, Winooski, VT, USA). We calculated the results using the standard curve, which were expressed as picograms per milliliter.
2.8. Measurement of intracellular ROS generation
Intracellular ROS in acrolein-stimulated HUVECs is analyzed using a fluorescent dye, 2′,7′-dichlorofluorescein diacetate (DCF/DA). In the presence of oxidants, DCFH was converted to the highly fluorescent DCF. After 18 h incubation with 25 μM acrolein in the presence or absence of KRG, cells were stained with 10 μM DCF/DA, and fluorescence was analyzed by a FACS Vantage flow cytometer (Becton Dickinson, San Jose, CA, USA) and fluorescence microscopy (Eclipse 50i; Nikon, Japan) [25].
2.9. Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling assay
To clarify whether KRG-mediated inhibition of acrolein-induced COX-2 expression plays a significant role in cytoprotection against oxidative stress, acrolein-stimulated cells were pretreated with KRG (1 mg/mL) or untreated, and cell death was measured by in situ terminal transferase dUTP nick end labeling (TUNEL) assay. To measure DNA fragmentation, the commercially available in situ death detection kit (Roche Diagnostics, Mannheim, Germany) was utilized. HUVECs were cultured in a glass culture chamber slide and fixed for 30 min in 10% neutral buffered formalin solution at room temperature. A TUNEL assay system was used, according to the manufacturer's instructions, for examination under a fluorescence microscope, with excitation at 488 nm and emission at 525 nm [26].
2.10. Apoptosis assay
Cell death was detected by annexin V–fluorescein isothiocyanate (FITC) (BD PharMingen, San Diego, CA, USA) and propidium iodide (PI) staining of necrotic and apoptotic cells. Cells were washed in PBS, resuspended in 100 μL binding buffer containing 5 μL annexin V–FITC and 1 μg/mL PI, and incubated for 10 min at room temperature in the dark. Cells were analyzed using a FACScan (Becton Dickinson). Data were analyzed using CELLQuest software (Becton Dickinson). Positioning of quadrants on the Annexin V/PI dot plots was performed as previously described [25].
2.11. Statistical analysis
Data were expressed as mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance (GraphPad Prism version 4; GraphPad Software, San Diego, CA, USA) followed by Bonferroni's multiple comparison test.
3. Results
3.1. Effects of COX-2 inhibition by KRG in acrolein-stimulated HUVECs
Upregulation of COX-2 expression plays a key role in inflammation. A previous study found that acrolein in CS induces COX-2 expression in human endothelial cells [24]. We demonstrated the effect of KRG on COX-2 induction in acrolein-stimulated HUVECs. KRG inhibited acrolein-induced COX-2 protein expression in a concentration-dependent manner (Fig. 1A). KRG also inhibited the COX2 mRNA level (Fig. 1B).
Fig. 1.
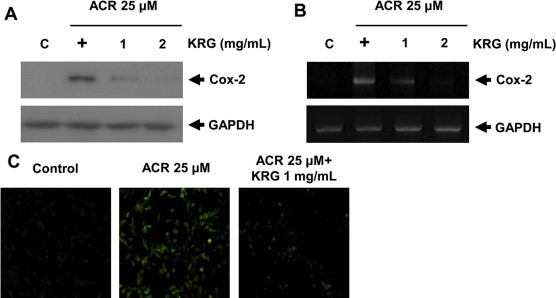
Inhibition of COX-2 by KRG in acrolein-stimulated HUVECs. Cell lysates were prepared after pretreatment with various concentrations of KRG in 25 μM acrolein-stimulated HUVECs for 18 h (protein) or 1 h (total RNA). COX-2 was determined by western blotting (A). The amplified reverse transcriptase polymerase chain reaction product was visualized on 1% agarose gel (B). Representative data from 3 independent experiments are shown. COX-2 localization was determined by immunofluorescence staining with an anti-COX-2 antibody followed by a fluorescent-tagged secondary antibody (C). COX-2, cyclooxygenase-2; HUVECs, human umbilical vein endothelial cells; KRG, Korean Red Ginseng.
3.2. Immunofluorescence staining of COX-2
After pretreatment of acrolein-stimulated cells with KRG, the cells were fixed, and COX-2 localization in HUVECs was observed by immunofluorescence staining with an anti-COX-2 antibody followed by a fluorescence-tagged secondary antibody. Immunofluorescence analysis showed that acrolein-induced COX-2 protein levels were inhibited in HUVECs after treatment with KRG (Fig. 1C).
3.3. Inhibition of PGE2 secretion by KRG in acrolein-stimulated HUVECs
The induction of COX-2 expression is known to be responsible for PGE2 release in the culture medium of cells stimulated with acrolein. Acrolein increased PGE2 secretion, which was dramatically reduced by KRG (Fig. 2). This result indicates that KRG leads to the reduction of COX-2 protein expression and subsequently PGE2 biosynthesis in acrolein-stimulated HUVECs.
Fig. 2.
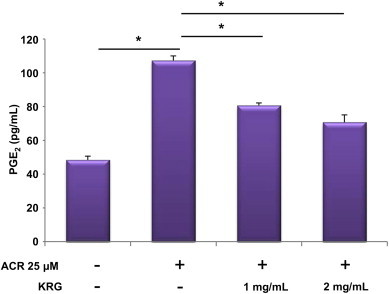
Inhibition of PGE2 secretion by KRG in acrolein-stimulated HUVECs. After pretreatment with various concentrations of KRG (1 and 2 mg/mL) in 25 μM acrolein-stimulated HUVECs for 18 h, the release of PGE2 was measured in the supernatants. The values shown for PGE2 production are mean ± standard deviation of three independent experiments. *p < 0.001. HUVECs, human umbilical vein endothelial cells; KRG, Korean Red Ginseng; PGE2, prostaglandin E2.
3.4. Protection against acrolein-mediated oxidative stress provided by KRG
Elevation of intracellular ROS levels causes cellular dysfunction. Thus, we examined the effect of KRG on ROS production in acrolein-stimulated cells. The shift to the right of the curve due to increased fluorescence indicates an increase in the intracellular levels of ROS. The results indicate that ROS generation in cells treated with acrolein increased compared to untreated cells, whereas KRG inhibited acrolein-induced ROS generation (Fig. 3A and B). These results indicate that KRG may play a role in the inhibition of COX-2 expression via reduction of acrolein-generated ROS in acrolein-stimulated HUVECs.
Fig. 3.
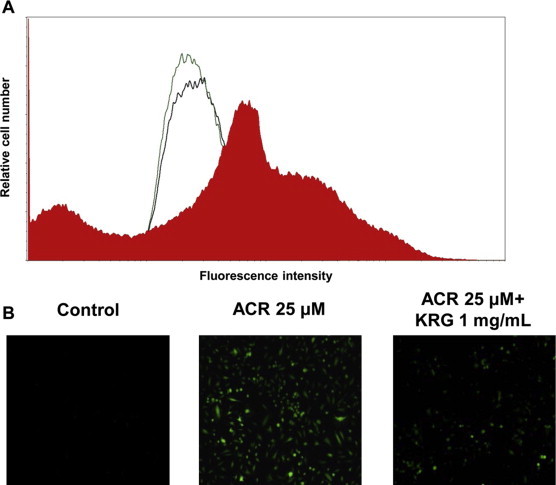
Modulation of intracellular ROS generation by KRG in acrolein-stimulated HUVECs. ROS levels as measured by fluorescence-activated cell sorting after staining with the fluorescence probe DCF. The curves correspond to cells cultured in the presence of vehicle (control: green line), 25 μM acrolein treatment (red area), and pretreatment with KRG in acrolein-stimulated cells (black line) (A). Cells were exposed to vehicle, 25 μM acrolein, and 25 μM acrolein + 1 mg/mL KRG in the presence of DCF diacetate and measured by fluorescence microscopy (B). DCF, 2′,7′-dichlorofluorescein; HUVECs, human umbilical vein endothelial cells; KRG, Korean Red Ginseng; ROS, reactive oxygen species. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Involvement of p38 MAPK–CREB pathways in the inhibition of acrolein-induced COX-2 expression by KRG
A previous study demonstrated that acrolein induces COX-2 expression via the p38 MAPK–CREB pathway [24]. Thus, to determine the upstream signaling pathway involved in KRG-mediated COX-2 inhibition, we measured the activation of p38 and CREB by detecting increased phospho-p38 and phospho-CREB levels in acrolein-stimulated cells and found that phosphorylation of p38 and CREB was strongly reduced by KRG in acrolein-stimulated cells (Fig. 4). These results demonstrate the role of p38 and CREB signaling in the inhibition of acrolein-mediated COX-2 induction.
Fig. 4.
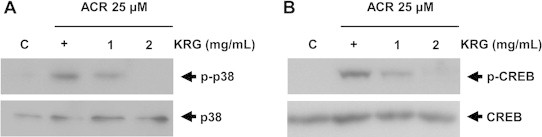
Central role of p38 and CREB signaling pathway in KRG-mediated inhibition of COX-2 expression in acrolein-stimulated cells. Cells were pretreated with KRG (1 and 2 mg/mL) in 25 μM acrolein-stimulated HUVECs. Whole cell lysates were prepared and subjected to western blot analysis with antibodies against anti-phosph-p38 (A) and CREB (B). Representative western blots of three independent experiments are shown. COX-2, cyclooxygenase-2; CREB, cyclic AMP-responsive element-binding protein; HUVECs, human umbilical vein endothelial cells; KRG, Korean Red Ginseng.
3.6. Inhibition of endothelial cell death by KRG in acrolein-stimulated cells
Fluorescence-activated cell sorting showed that while the number of apoptotic cells increased following treatment with acrolein, pretreatment with KRG reduced the number of apoptotic cells (Fig. 5A). To confirm this result, we evaluated the presence of dead cells by TUNEL staining, which is widely used in detecting DNA fragmentations in situ. The TUNEL assay indicates cell death, including apoptosis, by detection of the appearance of intensely stained nuclei, which indicates incorporation of labeled dUTP into the 3′-end of fragmented DNA derived from apoptotic nuclei. As illustrated in Fig. 5B, acrolein treatment significantly increased the proportion of TUNEL-positive cells, which was restored by KRG pretreatment. These results revealed that the vascular protective effect of KRG is mediated by the inhibition of COX-2 expression in acrolein-stimulated HUVECs.
Fig. 5.
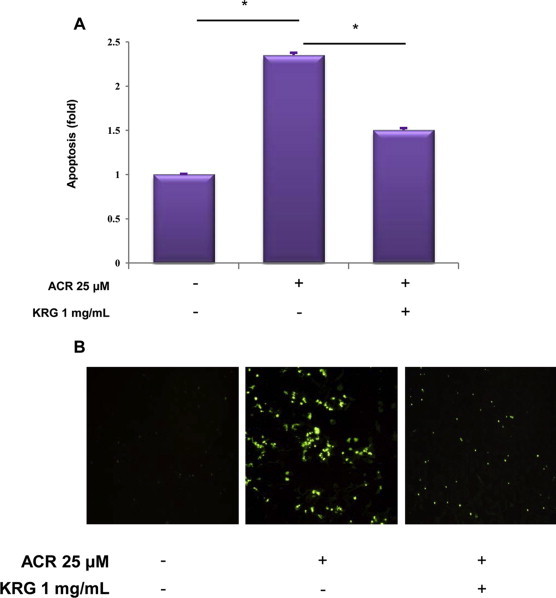
Effect of KRG on apoptosis in acrolein-stimulated HUVECs. Acrolein (25 μM)-stimulated HUVECs were pretreated for 1 h with 1 mg/mL KRG. After 18 h incubation, the protective effect of KRG on acrolein-induced apoptosis in HUVECs was determined by Annexin V/PI staining (A) and the in situ terminal transferase dUTP nick end labeling assay (B). Quantitative data are presented as the fold of dead cells after treatment with annexin V/PI staining. Results are expressed as a dot plot and represent three independent experiments. *p < 0.001. HUVECs, human umbilical vein endothelial cells; KRG, Korean Red Ginseng; PI, propidium iodide.
4. Discussion
In this study, we explored the inhibition of an inflammatory mediator, COX-2, by KRG water extract in HUVECs. We found that KRG inhibited both mRNA and the protein level of COX-2 and its cytoprotective effect in acrolein-stimulated HUVECs.
There is increasing evidence that α,β-unsaturated aldehydes in CS, including acrolein and crotonaldehyde play an important pathophysiological role in vascular diseases such as atherosclerosis and Alzheimer's disease. Exposure to α,β-unsaturated aldehydes is critical to the inflammatory response via activation of the proinflammatory signaling pathway and redox-sensitive transcription factors [27,28]. Furthermore, α,β-unsaturated aldehydes increase oxidative stress [29], which plays a crucial role in the pathogenesis of vascular diseases via direct injury to the endothelium [30].
COX-2, a key enzyme for prostaglandin biosynthesis, is an inducible enzyme that is rapidly induced during inflammatory reactions. Numerous studies have reported the involvement of CS in vascular diseases through COX-2 and endothelial NO synthase activity [31,32]. Increase of COX-2 expression was reported to promote atherosclerotic inflammation [33]. Chronic inflammation plays an important role in vascular diseases, therefore, COX-2 may participate in the development of inflammation-related diseases, including vascular diseases.
Ginseng has been used as a general tonic for >2000 years in East Asia, and it has become a famous herbal medicine for treatment of various diseases, including vascular disorders. KRG has been reported to have effective pharmacological activities, including antioxidant, anticarcinogenic, and ameliorative effects on blood circulation [34,35]. Recently, the diverse effects of several constituents of KRG, including ginsenoside, on endothelial cells have been extensively studied. Hien et al demonstrated the anti-inflammatory and antiatherosclerotic activities of ginsenoside Rg3 in human endothelial cells, with a decrease of cell adhesion molecules and proinflammatory cytokines [36]. Moreover, the cytoprotective effect of ginsenoside Rb1 in endothelial cell damage mediated by oxidized low-density lipoprotein has been reported [37]. Several constituents of red ginseng have been reported to regulate proliferation and migration and to protect oxidative stress-mediated damage in human endothelial cells [38,39]. There is evidence demonstrating the presence of major ginsenosides including Rb1 and Rg1 in KRG water extract [40]. Thus, these components could also contribute to the diverse retinue of protective actions of KRG.
A previous study showed that the induction of HO-1 expression may exert protective effects in KRG-treated human endothelial cells [19]. The inhibitory effect of KRG on inflammatory responses has also been reported. However, there have been no reports revealing the mechanism underlying KRG-inhibited COX-2 expression in acrolein, α,β-unsaturated aldehydes in CS, stimulated HUVECs. We have established that the major signaling pathway of COX-2 (i.e., p38 MAPK–CREB) and intracellular ROS generation are involved in this inhibition of COX-2 expression in acrolein-stimulated HUVECs by KRG. As mentioned above, KRG also exerts preventive effects on apoptosis induced by acrolein. Therefore, the inhibition of COX-2 expression following KRG water extract treatment may be associated with its strong protective effect in acrolein-stimulated HUVECs.
In conclusion, we propose that the KRG water extract may exert a cytoprotective effect through the inhibition of COX-2 induction and that this reduction of COX-2 in acrolein-stimulated HUVECs is mediated by the p38 MAPK–CREB pathway. This study suggests a possible therapeutic mechanism of KRG in vascular diseases.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
This work was supported by the 2012 grant from the Korean Society of Ginseng.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Campbell S.C., Moffatt R.J., Stamford B.A. Smoking and smoking cessation – the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201:225–235. doi: 10.1016/j.atherosclerosis.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 2.Spira A., Beane J., Shah V., Liu G., Schembri F., Yang X., Palma J., Brody J.S. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101:10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gokkusu C., Ademoglu E., Tamer S., Alkan G. Oxidant-antioxidant profiles of platelet rich plasma in smokers. Addict Biol. 2001;6:325–330. doi: 10.1080/13556210020077046. [DOI] [PubMed] [Google Scholar]
- 4.Ockene I.S., Miller N.H. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation. 1997;96:3243–3247. doi: 10.1161/01.cir.96.9.3243. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.E., Lee S.H., Ryu D.S., Park C.S., Park K.S., Park Y.S. Differentially-expressed genes related to atherosclerosis in acrolein-stimulated human umbilical vein endothelial cells. Biochip J. 2010;4:306–313. [Google Scholar]
- 6.Lee S.H., Lee S.E., Ahn H.J., Park C.S., Cho J.J., Park Y.S. Identification of atherosclerosis related gene expression profiles by treatment of benzo(a)pyrene in human umbilical vein endothelial cells. Mol Cell Toxicol. 2009;5:13–19. [Google Scholar]
- 7.Jeong S.I., Lee S.E., Yang H., Park C.S., Cho J.J., Park Y.S. MicroRNA microarray analysis of human umbilical vein endothelial cells exposed to benzo(a)pyrene. Biochip J. 2012;6:191–196. [Google Scholar]
- 8.Barnoya J., Glantz S.A. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 9.Aoki M., Nata T., Morishita R., Matsushita H., Nakagami H., Yamamoto K., Yamazaki K., Nakabayashi M., Ogihara T., Kaneda Y. Endothelial apoptosis induced by oxidative stress through activation of NF-kappaB: antiapoptotic effect of antioxidant agents on endothelial cells. Hypertension. 2001;38:48–55. doi: 10.1161/01.hyp.38.1.48. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang J.S., Li Y.P., Li C.Y., Cai C., Chen C.S., Chen S.X., Chen Y.F., Yang L., Xie Y.P. Mitochondrial ROS-K+ channel signaling pathway regulated secretion of human pulmonary artery endothelial cells. Free Radic Res. 2012;46:1437–1445. doi: 10.3109/10715762.2012.724532. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.C., Hsieh C.W., Lai P.H., Lin J.B., Liu Y.C., Wung B.S. Upregulation of endothelial heme oxygenase-1 expression through the activation of the JNK pathway by sublethal concentrations of acrolein. Toxicol Appl Pharmacol. 2006;214:244–252. doi: 10.1016/j.taap.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Park B.J., Lim Y.S., Lee H.J., Eum W.S., Park J., Han K.H., Choi S.Y., Lee K.S. Anti-oxidative effects of Phellinus linteus and red ginseng extracts on oxidative stress-induced DNA damage. BMB Rep. 2009;42:500–505. doi: 10.5483/bmbrep.2009.42.8.500. [DOI] [PubMed] [Google Scholar]
- 13.Jin Y.R., Yu J.Y., Lee J.J., You S.H., Chung J.H., Noh J.Y., Im J.H., Han X.H., Kim T.J., Shin K.S. Antithrombotic and antiplatelet activities of Korean red ginseng extract. Basic Clin Pharmacol Toxicol. 2007;100:170–175. doi: 10.1111/j.1742-7843.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 14.Kwak Y.S., Kyung J.S., Kim J.S., Cho J.Y., Rhee M.H. Anti-hyperlipidemic effects of red ginseng acidic polysaccharide from Korean red ginseng. Biol Pharm Bull. 2010;33:468–472. doi: 10.1248/bpb.33.468. [DOI] [PubMed] [Google Scholar]
- 15.Wong V.K., Cheung S.S., Li T., Jiang Z.H., Wang J.R., Dong H., Yi X.Q., Zhou H., Liu L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse Lewis lung carcinoma via modulation of ERK-p53 and NF-kappaB signaling. J Cell Biochem. 2010;111:899–910. doi: 10.1002/jcb.22778. [DOI] [PubMed] [Google Scholar]
- 16.Jeon B.H., Kim C.S., Kim H.S., Park J.B., Nam K.Y., Chang S.J. Effect of Korean red ginseng on blood pressure and nitric oxide production. Acta Pharmacol Sin. 2000;21:1095–1100. [PubMed] [Google Scholar]
- 17.Sung J.D., Han K.H., Zo J.H., Park H.J., Kim C.H., Oh B.H. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28:205–216. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.M., Namkoong S., Yun Y.G., Hong H.D., Lee Y.C., Ha K.S., Lee H., Kwon H.J., Kwon Y.G., Kim Y.M. Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-Dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1674–1679. doi: 10.1248/bpb.30.1674. [DOI] [PubMed] [Google Scholar]
- 19.Yang H., Lee S.E., Jeong S.I., Park C.S., Jin Y.H., Park Y.S. Up-regulation of heme oxygenase-1 by Korean Red Ginseng water extract as a cytoprotective effect in human endothelial cells. J Ginseng Res. 2011;35:352–359. doi: 10.5142/jgr.2011.35.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K.W., Jung S.Y., Choi S.M., Yang E.J. Effects of ginsenoside Re on LPS-induced inflammatory mediators in BV2 microglial cells. BMC Complement Altern Med. 2012;12 doi: 10.1186/1472-6882-12-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen T., Lee J., Park M.H., Lee Y.G., Rho H.S., Kwak Y.S., Rhee M.H., Park Y.C., Cho J.Y. Ginsenoside Rp(1), a ginsenoside derivative, blocks promoter activation of iNOS and COX-2 genes by suppression of an IKK beta-mediated NF-kappa B pathway in HEK293 cells. J Ginseng Res. 2011;35:200–208. doi: 10.5142/jgr.2011.35.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y.K., Guo Q., Packer L. Free radical scavenging activity of red ginseng aqueous extracts. Toxicology. 2002;172:149–156. doi: 10.1016/s0300-483x(01)00585-6. [DOI] [PubMed] [Google Scholar]
- 23.Jeong S.I., Lee S.E., Yang H., Park C.S., Jin Y.H., Park Y.S. Effect of alpha,beta-unsaturated aldehydes on endothelial cell growth in bacterial cellulose for vascular tissue engineering. Mol Cell Toxicol. 2012;8:119–126. [Google Scholar]
- 24.Park Y.S., Kim J., Misonou Y., Takamiya R., Takahashi M., Freeman M.R., Taniguchi N. Acrolein induces cyclooxygenase-2 and prostaglandin production in human umbilical vein endothelial cells: roles of p38 MAP kinase. Arterioscler Thromb Vasc Biol. 2007;27:1319–1325. doi: 10.1161/ATVBAHA.106.132837. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.E., Jeong S.I., Kim G.D., Yang H., Park C.S., Jin Y.H., Park Y.S. Upregulation of heme oxygenase-1 as an adaptive mechanism for protection against crotonaldehyde in human umbilical vein endothelial cells. Toxicol Lett. 2011;201:240–248. doi: 10.1016/j.toxlet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee S.E., Yang H., Jeong S.I., Jin Y.H., Park C.S., Park Y.S. Induction of heme oxygenase-1 inhibits cell death in crotonaldehyde-stimulated HepG2 cells via the PKC-delta-p38-Nrf2 pathway. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0041676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong S.I., Lee S.E., Yang H., Park C.S., Cho J.J., Park Y.S. Genome-wide analysis of gene expression by crotonaldehyde in human umbilical vein endothelial cells. Mol Cell Toxicol. 2011;7:127–134. [Google Scholar]
- 28.Lee S.E., Park Y.S. The role of antioxidant enzymes in adaptive responses to environmental toxicants in vascular disease. Mol Cell Toxicol. 2013;9:95–101. [Google Scholar]
- 29.Park Y.S., Misonou Y., Fujiwara N., Takahashi M., Miyamoto Y., Koh Y.H., Suzuki K., Taniguchi N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;327:1058–1065. doi: 10.1016/j.bbrc.2004.12.104. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.E., Park Y.S. Role of lipid peroxidation-derived α, β-unsaturated aldehydes in vascular dysfunction. Oxid Med Cell Longev. 2013;2013:629028. doi: 10.1155/2013/629028. doi: http://dx.doi.org/10.1155/2013/629028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 32.Karaoglu A., Tunc T., Aydemir G., Onguru O., Uysal B., Kul M., Aydinoz S., Oztas E., Sarici U. Role of cyclooxygenase 2 and endothelial nitric oxide synthetase in preclinical atherosclerosis. Fetal Pediatr Pathol. 2012;31:432–438. doi: 10.3109/15513815.2012.659408. [DOI] [PubMed] [Google Scholar]
- 33.Burleigh M.E., Babaev V.R., Oates J.A., Harris R.C., Gautam S., Riendeau D., Marnett L.J., Morrow J.D., Fazio S., Linton M.F. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation. 2002;105:1816–1823. doi: 10.1161/01.cir.0000014927.74465.7f. [DOI] [PubMed] [Google Scholar]
- 34.Nam K.Y. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng C. A. Meyer) J Ginseng Res. 2005;29:1–18. [Google Scholar]
- 35.Yang S.J., Woo K.S., Yoo J.S., Kang T.S., Noh Y.H., Lee J.S., Jeong H.S. Change of Korean Ginseng components with high temperature and pressure treatment. Korean J Food Sci Technol. 2006;38:521–525. [Google Scholar]
- 36.Hien T.T., Kim N.D., Kim H.S., Kang K.W. Ginsenoside Rg3 inhibits tumor necrosis factor-alpha-induced expression of cell adhesion molecules in human endothelial cells. Pharmazie. 2010;65:699–701. [PubMed] [Google Scholar]
- 37.He F., Guo R., Wu S.L., Sun M., Li M. Protective effects of ginsenoside Rb1 on human umbilical vein endothelial cells in vitro. J Cardiovasc Pharmacol. 2007;50:314–320. doi: 10.1097/FJC.0b013e3180cab12e. [DOI] [PubMed] [Google Scholar]
- 38.Leung K.W., Pon Y.L., Wong R.N., Wong A.S. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281:36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- 39.Kwok H.H., Ng W.Y., Yang M.S., Mak N.K., Wong R.N., Yue P.Y. The ginsenoside protopanaxatriol protects endothelial cells from hydrogen peroxide-induced cell injury and cell death by modulating intracellular redox status. Free Radic Biol Med. 2009;48:437–445. doi: 10.1016/j.freeradbiomed.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 40.Shin S., Jang J.Y., Park D., Yon J.M., Baek I.J., Hwang B.Y., Nam S.Y., Yun Y.W., Kim K.Y., Joo S.S. Korean red ginseng extract does not cause embryo-fetal death or abnormalities in mice. Birth Defects Res B Dev Reprod Toxicol. 2010;89:78–85. doi: 10.1002/bdrb.20224. [DOI] [PubMed] [Google Scholar]


