Introduction
Immunosurveillance in the lymphoid system and in peripheral tissues involves key nonredundant contributions by γδ T cells (1, 2). The widespread implication of γδ T cells is often attributed to the cells’ rapid and abundant production of IFN-γ in mice and in humans (3–5). However, γδ T cells are also a critical source of IL-17A (referred to hereafter as IL-17) in animal models of infection (6–8) or autoimmune disorders (9–12). There is therefore a pressing need to understand what signals regulate the activation and functional response of γδ T cells.
Although our ignorance of most TCRγδ ligands confounds the full dissection of peripheral γδ T cell activation, some γδ T cells have been shown to respond in vivo to innate receptor ligands, notably those for the activating NKG2D receptor (13). This concept was recently extended by reports of TCR-independent stimulation of IL-17 production by IL-1β plus IL-23 (12) and TLR2 or Dectin-1 ligands (14). Collectively, these data have raised the question of the relative importance of innate immunity-associated receptors versus the somatically recombined TCR and its accessory receptors for the activation of γδ T cells in vivo.
We have investigated this key question by employing mouse infection models, coupled with our recently described classification of thymic and peripheral γδ T cell populations (15). We show that for the IFN-γ–producing, CD27+ γδ (γδ27+) subset, CD27 costimulation synergizes with the TCR to provide survival and proliferative signals that determine their expansion upon viral or parasitic infection in vivo. By contrast, the peripheral pool size of IL-17–producing γδ T cells, which lack CD27 expression (γδ27−), depends on TLR/MyD88-dependent innate immune signals during malaria infection. Thus, discrete functional subsets of γδ T cells can be differentially controlled by adaptive and innate immune receptors.
Materials and Methods
Mice
All mice were adults 4–10 wk of age. C57BL/6 (B6), B6.Thy1.1, B6.TCRα-deficient, B6.TCRδ-deficient mice were obtained from The Jackson Laboratory (Bar Harbor, ME). B6.CD27-deficient mice (16) were a kind gift from Dr. Jannie Borst (The Netherlands Cancer Institute, Amsterdam, The Netherlands). Mice were bred and maintained in the specific pathogen-free animal facilities of Instituto de Medicina Molecular (Lisbon, Portugal), Instituto Gulbenkian de Ciência (Oeiras, Portugal), and Cancer Research UK (London, U.K.). All experiments involving animals were performed in compliance with the relevant laws and institutional guidelines and have been approved by the local ethics committees.
mAbs
Anti-mouse mAbs specific for TCRδ (GL3), IFN-γ (XMG1.2), CD3ε (145.2C11), and Vγ4 (UC3-10A6) were purchased from BD Pharmingen (San Diego, CA). Anti-CD27 (LG.7F9), CD19 (eBio1D3), IL-17A (eBio17B7), CD11b (M1/70), and CD11c (N418) mAbs were purchased from eBioscience (San Diego, CA). Anti-Vγ1 (2.11) mAb was a kind gift from Pablo Pereira (Institut Pasteur, Paris, France).
Flow cytometry
All FACS-based assays were performed as previously described (15). Cells were sorted on an FACSAria (BD Biosciences, San Jose, CA) and analyzed on FACSCanto or FACSCalibur (BD Biosciences).
Cell culture and in vitro assays
Cells were cultured as described (15) and stimulated with anti-CD3ε mAb, either plate-bound (1 or 10 μg/ml) or soluble (0.5 μg/ml), in the presence of 105 mitomycin C-treated (Sigma-Aldrich, St. Louis, MO) splenocytes. Where indicated, the following reagents were added to the medium: soluble rCD70 (kindly provided by Dr. Jannie Borst) or LPS from Salmonella Minnesota R595 (10 μg/ml; Alexis Biochemicals, Plymouth Meeting, PA).
Immunoblotting
Cells were lysed in RIPA buffer complemented with a complete Protease Inhibitor Cocktail (Roche Mini Tablet, Roche Applied Science, Burgess Hill, U.K.). Total protein lysates were boiled in sample loading buffer (LDS, Invitrogen, Carlsbad, CA) containing 100 mM DTT. Proteins were subjected to electrophoresis using NuPage 4–12% Bis-Tris precasted gels (Invitrogen) and transferred onto a polyvinylidene fluoride membrane (GE Healthcare, Piscataway, NJ). Membranes were stained with rabbit anti-p100/p52 (4882, Cell Signaling Technology, Beverly, MA), mouse anti-GAPDH (MAB374, Millipore, Bedford, MA), donkey anti-rabbit IgG (31458, Thermo Scientific, Waltham, MA), or goat anti-mouse IgG + IgM (31446, Thermo Scientific). Proteins were detected using Super Signal West Femto substrate (Thermo Scientific).
Quantitative RT-PCR
Quantitative RT-PCR was performed as previously described (15) using the following primers: Bcl2a1-Fwd, 5′-AATAACACAGGAGAATGGATACGG-3′ and Bcl2a1-Rev, 5′-TCTCTGGTCCGTAGTGTTACTTGA-3′; Efa1-Fwd, 5′-ACACGTAGATTCCGGCAAGT-3′ and Efa1-Rev, 5′-AGGAGCCCTTTCCCATCTC-3′; Ccnd2-Fwd, 5′-CACCGACAACTCTGTGAAGC-3′ and Ccnd2-Rev, 5′-TCCACTTCAGCTTACCCAACA-3′; Cdk4-Fwd, 5′-GCCAGAGATGGAGGAGTCTG-3′ and Cdk4-Rev, 5′-CCTTGTGCAGGTAGGAGTGC-3′; Cdk6-Fwd, 5′-CGAGTGCAGACCAGTGAGG-3′ and Cdk6-Rev, 5′-TGTGCACACATCAAACAACCT-3′; Il1b-Fwd, 5′-GGACAGAATATCAACCAACAAGTGATA-3′ and Il1b-Rev, 5′-GTGTGCCGTCTTTCATTACACAG-3′; and Il23-Fwd, 5′-TCCCTACTAGGACTCAGCCAAC-3′ and Il23-Rev, 5′-GCTGCCACTGCTGACTAGAA-3′.
Adoptive cell transfers
FACS-sorted cells were injected i.v. into B6.TCRδ-deficient mice (3 × 105 cells/mouse). Mice were sacrificed, and splenocytes were collected after 5 d.
Injection of TCR or TLR agonists
Mice were injected i.p. with 50–100 μg anti-CD3 mAb (145.2C11) or 50 μg LPS from Salmonella Minnesota R595 (Alexis Biochemicals) or 50 μg Pam3CysSerLys4 (PAM; InvivoGen, San Diego, CA). Cells from the peritoneal cavity (PEC) were collected for analysis after 3 d.
Malaria infection
Mice were infected i.v. with 106 GFP-transgenic Plasmodium berghei ANKA-infected RBCs and monitored as previously described (15). Splenocytes were collected for analysis after 3 d.
Murid herpesvirus-4 infection
Mice were infected i.p. with 106 PFU murid herpesvirus-4 (MuHV-4), and cells from the PEC were collected after 8 d. Infection was confirmed by ex vivo reactivation assays, as previously described (17).
Statistical analysis
Statistical significance of differences between populations was assessed using Student t test and is indicated as NS (p ≥ 0.05), *p < 0.05, **p < 0.01, and ***p < 0.001.
Results and Discussion
CD27 costimulation provides survival and proliferative signals to γδ T cells
When purified TCRγδ+ CD3+ CD27+ (γδ27+) and TCRγδ+ CD3+ CD27− (γδ27−) lymph node (LN) and splenic cells were activated in vitro with anti-CD3ε mAb, γδ27+ cells proliferated substantially, displaying a cycling profile and a blasting morphology, whereas γδ27− cells remained quiescent and were highly prone to TCR/CD3-induced apoptosis in vitro and in vivo (Fig. 1A, 1B, Supplemental Fig. 1). Of note, TCR stimulation did not significantly modify the repertoire of either γδ T cell subset (Supplemental Fig. 2).
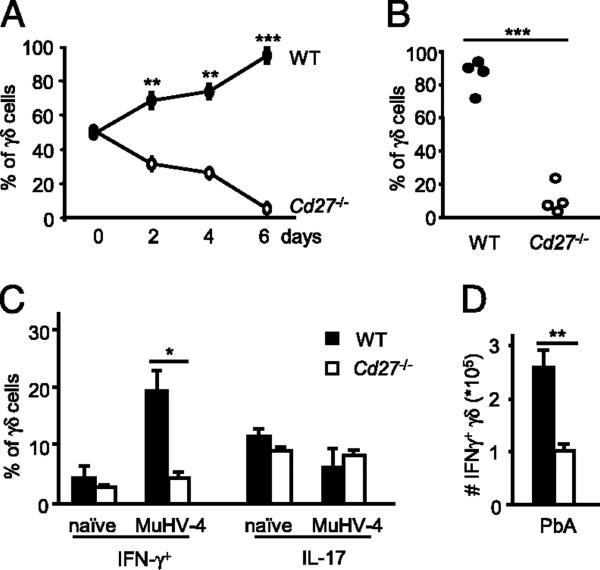
FIGURE 1.
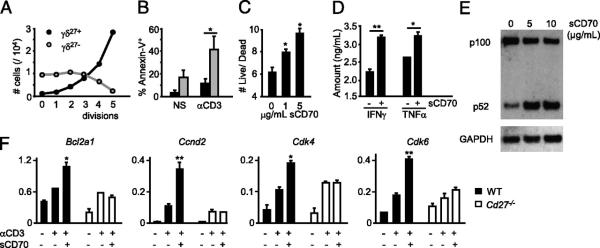
CD27 costimulation provides antiapoptotic and proliferative signals to γδ T cells. Peripheral γδ27+ (black filling) and γδ27− (gray filling) cells (A, B) or total γδ cells (C, D) were FACS-sorted from pooled spleen and LNs of C57BL/6 mice, stained with CFSE, cultured with allophycocyanin without (nonstimulated [NS]) or with anti-CD3ε mAb (αCD3) for 72 h, and then stained with Annexin V. A, Absolute numbers per cell division calculated based on CFSE dilution kinetics. B, Percentages of Annexin V+ (apoptotic) cells. C, Ratio of Annexin V− (live)/Annexin V+ (dead) cells among divided (CFSElo) cells when sCD70 was added to the cultures. D, Cytokine bead array analysis for IFN-γ and TNF-α in the cultures’ supernatants, with or without sCD70. E, FACS-sorted γδ27+ cells were cultured for 16 h with anti-CD3 (1 μg/ml) and 5–10 μg/ml sCD70 or 10 μg/ml human IgG1 control. Immunoblotting analyses were performed on total cellular extracts with indicated Abs. F, γδ cells, FACS-sorted from Cd27+/+ or Cd27−/− mice, were cultured for 6 h with or without anti-CD3 (1 μg/ml, plate-bound) and sCD70 (5 μg/ml). Quantitative real-time PCR data for Bcl2a1, Cyclin D2, CDK4, and CDK6 in arbitrary units normalized to the housekeeping gene Efa1. Significant increases relative to anti-CD3 samples are indicated. Data in A–F are representative of three independent experiments (each involving four to six animals) with consistent results. Error bars represent SD (n = 3). *p < 0.05; **p < 0.01.
To directly test whether CD27 regulates the response to TCRγδ stimulation, we supplemented anti-CD3–treated cultures of total γδ cells with soluble rCD70 (sCD70), which acts as a CD27 agonist (18). There was a dose-dependent increase in the live/dead cell ratio among proliferating γδ cells (Fig. 1C), and this was associated with an accumulation of IFN-γ and TNF-α (Fig. 1D), signature cytokines of γδ27+ cells (15).
To gain mechanistic insight into this effect, biochemical and transcriptional events implicated in lymphocyte survival and proliferation were investigated. In B cells, the TNF ligand family member, BLyS (also known as BAFF), is known to decrease cell death by cleaving NF-κB p100, which is induced by Ag receptor signaling, to p52 (19, 20). Likewise, the high levels of p100 in TCR-stimulated γδ27+ cells were reduced by sCD70 treatment, with concomitant accumulation of p52 (Fig. 1E). Furthermore, sCD70 potentiated TCR-mediated induction of antiapoptotic (Bcl2a1) and cell cycling (Cyclin D2, CDK4, and CDK6) genes in Cd27+/+, but not in Cd27−/− γδ cells, whereas sCD70 treatment in the absence of TCR stimulation had no effect (Fig. 1F and data not shown). These results point to the TCR–CD27 axis as a fundamental regulator of the survival and proliferation of γδ27+ cells and imply a striking parallel between CD27 costimulation of γδ T cells and BAFF-R costimulation of B cells.
CD27 signals are required for the expansion of IFN-γ–producing γδ 27+ cells
To assess if CD27 costimulation was necessary for optimal γδ T cell expansion in vitro and in vivo, competition assays were performed between CD27-deficient (Thy1.2) and wild-type (WT) Thy1.1 congenic γδ splenocytes. Cells were mixed at a 1:1 ratio and either cultured with anti-CD3 in the presence of APCs or injected into TCRδ-deficient hosts. There was a marked advantage of Cd27+/+ γδ cells in expansion in both scenarios (Fig. 2A, 2B, Supplemental Fig. 3). Next, the impact of CD27 deficiency on the γδ T cell response to infection was examined. Given that γδ27+ cells are functionally committed to IFN-γ production (15), immune responses to herpesviruses are critically dependent on IFN-γ (21), and γδ lymphocytes have been repeatedly implicated in controlling infections with human (22) and murine (23) herpesviruses, we infected mice with MuHV-4. MuHV-4 infection resulted in the accumulation of IFN-γ–producing, but not of IL-17–producing, γδ cells in the PEC of WT animals (Fig. 2C). Critically, this was dependent on CD27 (Fig. 2C), as was a similar expansion of IFN-γ–producing γδ splenocytes in a malaria infection model (Fig. 2D). Of note, CD27 expression was essentially stable postinfection in adoptively transferred γδ cells (Supplemental Fig. 4). Collectively, these data establish the importance of CD27 for determining the peripheral pool size of IFN-γ–producing γδ cells upon infection. The general significance of these findings is indicated by evidence that CD27 signaling likewise selectively promotes αβ Th1 cell differentiation (24).
FIGURE 2.
CD27 signals are required for the expansion of IFN-γ–producing γδ27+ cells. A and B, Total γδ cells were FACS-sorted from pooled spleen and LN of Cd27+/+.Thy1.1 (WT) and Cd27−/−.Thy1.2 mice. A, Cells were cocultured at a 50:50 ratio, stimulated with anti-CD3 in the presence of allophycocyanin, and stained for Thy1.2 at indicated time points. B, Cells were coinjected at a 50:50 ratio into TCRd−/− mice, and after 5 d, splenocytes were harvested and stained for CD3ε, TCRδ, and Thy1.2 to discriminate WT and Cd27−/− γδ cells. Each dot represents one recipient mouse (n = 4). C, WT and Cd27−/− mice were infected with MuHV-4 i.p., and cells were harvested from the PEC after 8 d. Percentage of IFN-γ+ or IL-17+ cells upon intracellular cytokine staining of total γδ cells, FACS-purified from PEC of WT or Cd27−/− mice. D, WT and Cd27−/− mice were immunized i.v. with P. berghei ANKA (PbA)-infected RBCs. Absolute numbers of IFN-γ+ γδ cells in the spleen at day 4 postinfection. Data in A–D are representative of three independent experiments with similar results. Error bars represent SD (n = 3). *p < 0.05; **p < 0.01; ***p < 0.001.
Innate TLR/MyD88-dependent signals selectively expand IL-17–producing γδ 27− cells in vivo
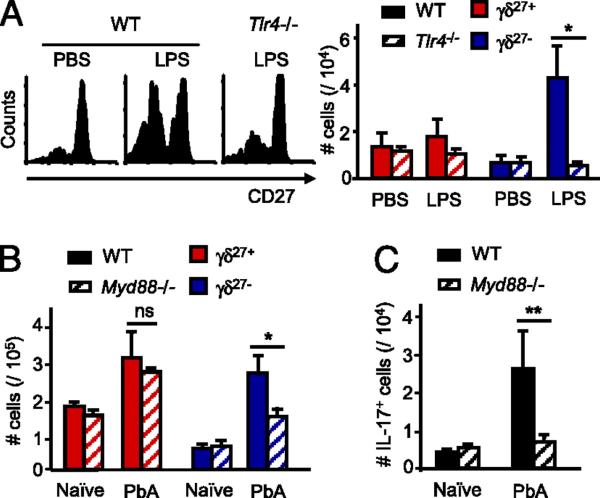
Because IL-17–producing γδ T cells do not express CD27 (15), other signals must account for their documented expansion in response to various infectious organisms (6–8, 15). In this regard, Mills and collaborators (12) recently showed that TLR agonists, in particular LPS, can stimulate dendritic cells to produce IL-1β and IL-23, which jointly promote IL-17 production by γδ cells in vitro, in the absence of TCR activation (12). To examine whether these results might apply in vivo, LPS was initially injected into the PEC of WT or TLR4-deficient mice, and γδ cells were retrieved and analyzed 72 h later. LPS treatment led to a marked and selective proliferation of γδ27− cells in WT but not TLR4-deficient mice (Fig. 3A, Supplemental Fig. 5). Similar data were obtained using the TLR2 agonist PAM, with a concomitant increase of γδ PEC cells producing IL-17, but not IFN-γ (Supplemental Fig. 6). Of note, we obtained no evidence for direct stimulation of γδ27− cells by LPS or PAM in vitro (Supplemental Fig. 7), whereas isolated IL-17–producing γδ27− cells responded strongly to IL-1β and IL-23, for which candidate sources in vivo (postinfection) were CD11bhiCD11clo myeloid cells (Supplemental Fig. 8).
FIGURE 3.
TLR/MyD88-dependent innate signals control the pool size of IL-17–producing γδ27− cells in vivo. A, WT and Tlr4−/− mice were injected i.p. with 50 μg LPS or PBS as control and sacrificed after 3 d. CD27 expression in pregated γδ cells (left panel) and absolute numbers of γδ27+ or γδ27− cells (right panel) isolated from PEC. B and C, C57BL/6 (WT) and Myd88−/− mice were immunized i.v. with P. berghei ANKA (PbA)-infected RBCs. Absolute numbers of γδ27+ and γδ27− cells (B) and absolute numbers of IL-17+ γδ cells (C) in the spleen at day 3 postinfection. Data in A–C are representative of two independent experiments (each involving three animals) with similar results. Error bars represent SD (n = 3). ns, p ≥ 0.05; *p < 0.05; **p < 0.01.
Furthermore, γδ27− splenocyte expansion to malaria infection was significantly reduced in MyD88-deficient animals (Fig. 3B), in which both TLR2 and TLR4 pathways are blocked (25). This resulted in a complete failure to expand IL-17–producing γδ cells (Fig. 3C), whereas the pool of IFN-γ–producing γδ cells was unaffected (not shown). Collectively, our results demonstrate that innate TLR/MyD88-dependent signals selectively control the peripheral pool size of murine IL-17–producing γδ cells. The general significance of these findings is implied by recent evidence for a role of TLRs in the differentiation of IL-17–producing CD4+ (26) and CD8+ (27) αβ T cells.
In sum, the γδ cell response to infection constitutes a primary example of how a T cell compartment can be composed of two arms, respectively regulated by innate versus adaptive immunity components. For murine lymphoid γδ T cells, the separation of these two arms begins in the thymus, where commitment to IFN-γ or IL-17 expression occurs (10, 15, 28). This developmental preprogramming of γδ cells may be crucial for their rapid responses upon activation in the periphery, as γδ T cell responses to infectious organisms can immediately deploy differentiated effector cells, which simply need to increase in number (2). This may also explain that CD27, a coreceptor typically associated with the clonal expansion of adaptive αβ T cells (16), can account for the rapid expansion of first line of defense IFN-γ–producing γδ cells. We suspect that the balance between the peripheral pools of IFN-γ– versus IL-17–producing γδ T cells may be substantially influenced by the maturation of CD70+ dendritic cells (24) for γδ27+ cell stimulation and by the activation of monocytes/macrophages that provide IL-1β and IL-23 for γδ27− cell expansion.
A general value in understanding these differential pathways of cell activation is implicit in the longstanding finding that previously activated CD4+ TCRαβ+ helper cell subsets can be reactivated by cytokines, including those of the IL-1 family, in the absence of TCR activation (29). However, it is important to note that our study does not exclude the TCR as being a powerful regulator of IL-17–producing γδ cells in certain scenarios (currently under examination). Rather, this study firmly establishes that during infection by various agents in vivo, different effector subsets of T cells are substantively and differentially regulated by innate and adaptive pathways. Those subsets have both direct and indirect effects on immune responses. For example, their rapid provision of IFN-γ or IL-17 can significantly impact de novo Th1/Th17 differentiation of CD4+ T cells, as has been reported in both infectious (6) and autoimmune (10, 12) contexts. Thus, lymphoid stress surveillance mediated by γδ T cells may be an important component of the priming of Ag-specific immunity (2), a concept that should be further developed in both mice and humans.
Supplementary Material
Acknowledgments
We thank Dan Pennington for valuable suggestions; Jannie Borst for CD27-deficient mice and sCD70; Aymen Al-Shamkhani for sCD70; Margarida Saraiva, Margarida Correia-Neves, Marie-Laure Michel, Daniel Correia, Ana deBarros, Gleb Turchinovich, and Victor Peperzak for helpful discussions and technical help; and the Instituto de Medicina Molecular and the Cancer Research UK flow cytometry and animal facilities for technical support.
This work was supported by an installation grant from the European Molecular Biology Organization (to B.S.-S.), Grants PTDC/SAU-MII/104158/2008 (to B.S.-S.) and PTDC/SAU-MII/099314/2008 (to J.P.S.) from Fundação para a Ciência e Tecnologia, and the Wellcome Trust (to A.C.H. and M.W.).
Abbreviations used in this paper
- LN
lymph node
- MuHV-4
murid herpesvirus-4
- NS
nonstimulated
- PAM
Pam3CysSerLys4
- PbA
Plasmodium berghei ANKA
- PEC
peritoneal cavity
- sCD70
soluble rCD70
- WT
wild-type
Footnotes
Disclosures The authors have no financial conflicts of interest.
References
- 1.Hayday A, Tigelaar R. Immunoregulation in the tissues by gammadelta T cells. Nat. Rev. Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J. Exp. Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibbons DL, Haque SF, Silberzahn T, Hamilton K, Langford C, Ellis P, Carr R, Hayday AC. Neonates harbour highly active gammadelta T cells with selective impairments in preterm infants. Eur. J. Immunol. 2009;39:1794–1806. doi: 10.1002/eji.200939222. [DOI] [PubMed] [Google Scholar]
- 5.Ramsburg E, Tigelaar R, Craft J, Hayday A. Age-dependent requirement for gammadelta T cells in the primary but not secondary protective immune response against an intestinal parasite. J. Exp. Med. 2003;198:1403–1414. doi: 10.1084/jem.20030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 8.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Shao H, Lan C, Nian H, O’Brien RL, Born WK, Kaplan HJ, Sun D. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J. Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J. Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, Schpero W, Kaplan DH, Hayday AC, Girardi M. Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat. Immunol. 2008;9:146–154. doi: 10.1038/ni1556. [DOI] [PubMed] [Google Scholar]
- 14.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-gamma-and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat. Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 17.Marques S, Alenquer M, Stevenson PG, Simas JP. A single CD8+ T cell epitope sets the long-term latent load of a murid herpesvirus. PLoS Pathog. 2008;4:e1000177. doi: 10.1371/journal.ppat.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowley TF, Al-Shamkhani A. Stimulation by soluble CD70 promotes strong primary and secondary CD8+ cytotoxic T cell responses in vivo. J. Immunol. 2004;172:6039–6046. doi: 10.4049/jimmunol.172.10.6039. [DOI] [PubMed] [Google Scholar]
- 19.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat. Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 20.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, III, Brezski RJ, Treml LS, Jordan KA, Monroe JG, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat. Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steed A, Buch T, Waisman A, Virgin HW., IV Gamma interferon blocks gammaherpesvirus reactivation from latency in a cell type-specific manner. J. Virol. 2007;81:6134–6140. doi: 10.1128/JVI.00108-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barcy S, De Rosa SC, Vieira J, Diem K, Ikoma M, Casper C, Corey L. Gamma delta+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J. Immunol. 2008;180:3417–3425. doi: 10.4049/jimmunol.180.5.3417. [DOI] [PubMed] [Google Scholar]
- 23.Sciammas R, Kodukula P, Tang Q, Hendricks RL, Bluestone JA. T cell receptor-gamma/delta cells protect mice from herpes simplex virus type 1-induced lethal encephalitis. J. Exp. Med. 1997;185:1969–1975. doi: 10.1084/jem.185.11.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. TLR signaling. Semin. Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, Ma L, Yang XO, Nurieva RI, Tian Q, Dong C. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of utoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loser K, Vogl T, Voskort M, Lueken A, Kupas V, Nacken W, Klenner L, Kuhn A, Foell D, Sorokin L, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010;16:713–717. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 28.Do JS, Fink PJ, Li L, Spolski R, Robinson J, Leonard WJ, Letterio JJ, Min B. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J. Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, Paul W. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA. 2009;106:13463–13468. doi: 10.1073/pnas.0906988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.