Abstract
Discriminating between two herbal medicines (Panax ginseng and Panax quinquefolius), with similar chemical and physical properties but different therapeutic effects, is a very serious and difficult problem. Differentiation between two processed ginseng genera is even more difficult because the characteristics of their appearance are very similar. An ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF MS)-based metabolomic technique was applied for the metabolite profiling of 40 processed P. ginseng and processed P. quinquefolius. Currently known biomarkers such as ginsenoside Rf and F11 have been used for the analysis using the UPLC-photodiode array detector. However, this method was not able to fully discriminate between the two processed ginseng genera. Thus, an optimized UPLC-QTOF-based metabolic profiling method was adapted for the analysis and evaluation of two processed ginseng genera. As a result, all known biomarkers were identified by the proposed metabolomics, and additional potential biomarkers were extracted from the huge amounts of global analysis data. Therefore, it is expected that such metabolomics techniques would be widely applied to the ginseng research field.
Keywords: discrimination, metabolomics, Panax ginseng, Panax quinquefolius, UPLC-QTOF MS
1. Introduction
Ginseng has been considered one of the most valuable medicinal herbs in oriental countries for the past 2,000 yr, and now it is widely used as an alternative medicine and health food [1]. At present, ginseng production is pegged at approximately 8,000 tons/yr; traditional therapeutic herbs are consumed in 35 countries around the world, and its global market was estimated to be about $2,000 million (US dollars) [2]. Most of this production is limited to two genera of ginseng (Panax ginseng and Panax quinquefolius), and four countries—South Korea, China, Canada, and the United States—are the world's biggest ginseng producers. The roots of P. ginseng (Korean ginseng) and P. quinquefolius (American ginseng), two closely related herbal species belonging to the Panax genus, are two of the most commonly used medicinal herbs. However, aside from its wide use as traditional medicine, ginseng is also used for other purposes. Therefore, discrimination and differentiation between these two herbal genera are of importance in terms of food safety and pharmaceutical value. As the characteristics, morphology, and chemical compositions of P. ginseng and P. quinquefolius are very similar, use of traditional methods based on morphological and physicochemical characteristics for identification of these two genera is rather problematic. The study of the currently known most reliable method is based on chromatographic separation of isomeric compounds of ginsenoside Rf and 24(R)-pseudoginsenoside F11, two potential markers present in P. ginseng and P. quinquefolius [3–5].
In recent years, attempts have been made to solve this problem using metabolomics [6]. Metabolomics is a relatively new field of “omics” research concerned with the high-throughput identification and quantification of small-molecule metabolites in the metabolome. It has emerged as an important tool in many disciplines such as human diseases and nutrition, drug discovery, and plant physiology [7–12]. The metabolome of an organism is a compilation of all of its metabolites. Metabolites are small molecules; polymeric biomolecules, such as polysaccharides, lignin, peptides, proteins, DNA, and RNA, are excluded from this category. For this reason, metabolomics is called “a snapshot of an organism,” showing which compounds are present and in what quantities at a given time point.
Analysis of a large number of samples might facilitate the identification of patterns or metabolite markers that are characteristic for a species, a cultivar, a certain stage of development, or conditions, such as disease state, stress, or daily and seasonal changes. Thus, the high-throughput global analysis of metabolome is a key factor of this field. For this reason, NMR (Nuclear Magnetic Resonance spectroscopy)-based metabolite profiling/metabolomics was first used in pioneering studies for the rapid multicomponent analysis of biological samples [13]. Mass spectrometry (MS) is currently the most widely applied technology in metabolomics studies [14]. This research trend is reflected in the research area of ginseng. The metabolomics research for ginseng has been published in numerous reports. In the work of Dan et al [15], the metabolite profiling of the different parts of P. notoginseng was carried out, and metabolic profiling of five Panax genera has been performed by Xie et al [16]. In the study of Zhang et al [17], metabolomics research was applied for the holistic quality evaluation of white and red ginseng. Differences in the chemical composition of ginseng according to cultivation ages have also been investigated using metabolomics as a research tool [18–23]. Most recently, determination of the geographical origins of Korean P. ginseng was studied as a metabolomic approach [24].
In this paper, an ultraperformance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-QTOF MS)-based metabolomic approach was developed to differentiate between processed P. ginseng (red ginseng) and processed P. quinquefolius (red ginseng). This nontargeted global analysis method was confirmed by targeted analysis of ginsenosides, including well-known potential marker substances [ginsenoside Rf and 24(R)-pseudoginsenoside F11].
2. Materials and methods
2.1. Ginseng samples
Processed P. ginseng (good grade red ginseng, 38 roots per 600 g size) was supplied by the Korea Ginseng Corporation (Daejeon, Korea). Processed P. quinquefolius (cultivated red, large size) was purchased from Hsu's Ginseng Enterprises, Inc. (Marathon County, Wisconsin, U.S.A, http://www.hsuginseng.com).
2.2. Chemicals and reagents
Ginsenoside Rg1, Re, Rf, 20(S)-Rh1, Rb1, Rc, Rb2, Rd, 20(S)-Rg3, and 20(R)-Rg3 standards were purchased from Chromadex (Irvine, CA, USA), and ginsenoside Ro, 20(S)-Rg2, 20(R)-Rg2, 20(S)-Rh2, 20(R)-Rh2, F2, F4, Ra1, Rg6, Rh4, Rk3, Rg5, Rk1, Rb3, Rk2, Rh3, notoginsenoside R1, 24(R)-pseudoginsenoside F11, and gypenoside XVII standards were obtained from Ambo Institute (Seoul, South Korea). Phosphoric acid was purchased from Junsei Chemical Co., Ltd (Tokyo, Japan). HPLC-grade acetonitrile and methanol were purchased from Merck (Darmstadt, Germany). All distilled water used in this experiment was purified by the Milli-Q gradient system (Millipore, Bedford, MA, USA), and the resistance value was measured as 18 MΩ prior to use.
2.3. Sample preparation
Sample preparation was performed in a similar manner as described in our previous studies, using the ultrasonic extraction method [25,26]. A half-gram of dried and ground processed ginseng sample was weighed in a centrifugal tube (15 mL, PP-single use; BioLogix Group, Jinan, Shandong, China) and shaken vigorously after the addition of 10 mL of 50% methanol. Next, extraction was performed in an ultrasonic cleaner (60 Hz; Wiseclean, Seoul, Korea) for 30 min. The solution was centrifuged (Legand Mach 1.6R; Thermo, Frankfurt, Germany) at 3000 × g rate/min speed for 10 min, and an aliquot of supernatant solution was filtered (0.2 μm; Acrodisk, Gelman Sciences, Ann Arbor, MI, USA) and injected into the UPLC system (Waters Co., Milford, MA, USA).
2.4. Liquid chromatography
The instrumental analysis was performed with UPLC using an ACQUITY BEH C18 column (100 mm × 2.1 mm, 1.7 μm; Waters Co.) on a Waters ACQUITY UPLC system with a binary solvent manager, sample manager, and photodiode array detector (PDA). The column temperature was 40°C. The binary gradient elution system consisted of 0.001% phosphoric acid in water (A) and 0.001% phosphoric acid in acetonitrile (B). The separation was achieved using the following protocol: 0–0.5 min (15% B), 14.5 min (30% B), 15.5 min (32% B), 18.5 min (38% B), 24.0 min (43% B), 27.0 min (55% B), 27.0–31.0 min (55% B), 35.0 min (70% B), 38.0 min (90% B), 38.1 min (15% B), and 38.1–43.0 min (15% B). The flow rate was set 0.6 mL/min and the sample injection volume was 2.0 μL. The individual ginsenosides in the eluents were determined at a UV wavelength of 203 nm using a PDA.
2.5. Mass spectrometry
The metabolite profiling of P. ginseng and P. quinquefolius was performed by coupling the Waters ACQUITY UPLC system to the Waters Xevo Q-TOF mass spectrometer (Waters MS Technologies, Manchester, UK) with an electrospray ionization (ESI) interface. The source and desolvation gas temperature were maintained at 400°C and 120°C, respectively. The nebulizer and desolvation gas used was N2. The flow rate of nebulizer gas and cone gas were set at 800 L/h and 50 L/h, respectively. The capillary and cone voltages were adjusted to 2300 V and 40 V, separately. The mass accuracy and reproducibility were maintained by infusing lockmass (leucine–enkephalin, 200 pg/L) thorough Lockspray at a flow rate of 20 μL/min. Centroided data were collected for each sample from 150 Da to 1300 Da, and the m/z value of all acquired spectra was automatically corrected during acquisition based on lockmass and dynamic range enhancement. The accurate mass and molecular formula assignments were obtained with the MassLynx 4.1 software (Waters MS Technologies).
2.6. Multivariate analysis
To evaluate the potential characteristic components of processed P. ginseng and processed P. quinquefolius, the ESI− raw data of all samples was calculated with the MassLynx application manager version 4.1 (Waters MS Technologies). The method parameters were as follows: retention time range, 2–37 min; mass range, 150–1300 Da; and mass tolerance, 0.07 Da. The parameters of peak width at 5% height and peak-to-peak baseline noise were automatically calculated for peak integration. Noise elimination level was set to 0.10, and the retention time tolerance was set to 0.2 min. Any specific mass or adduct ions was not excluded, but isotopic peaks were removed in the multivariate analysis. For data analysis, a list of the intensities of the detected peaks was generated using a pair of retention time (tR) and mass data (m/z) as the identifier of each peak. A temporary ID was assigned to each of these tR–m/z pairs for data adjustment that was based on their chromatographic elution order of UPLC. Upon completion, the correct peak intensity data for each tR–m/z pair for all samples were sorted in a table. Ions from different samples were considered to be the same when they showed the identical tR and m/z value. MarkerLynx (Waters MS Technologies) was used for normalization of each detected peak against the sum of the peak intensities within that sample. The resulting data consisted of a peak number (tR–m/z pair), sample name, and ion intensity. Then, the consequent data sets were analyzed by principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) using MarkerLynx.
3. Results and discussion
3.1. Targeted analysis
The first step of the experimental procedures used in this study involved gathering information about a number of the processed ginseng (red ginseng) samples and confirmation of known biomarkers in the literature [3–5]. Therefore, ginsenosides analysis was performed as part of the targeted analysis. Ginsenoside analysis was performed in the same manner as described in our previous studies [25,26]. The UPLC chromatograms of the processed P. ginseng [Korean red ginseng (KRG)] and processed P. quinquefolius [American red ginseng (ARG)] are shown in Fig. 1, and the contents of ginsenosides involved in the two processed ginseng (red ginseng) genera are presented in Table 1. In summary, ginsenoside Ro, Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, 20(S)-Rg2, 20(R)-Rg2, 20(S)-Rg3, 20(R)-Rg3, 20(S)-Rh1, F4, Ra1, Rg6, Rh4, Rk3, Rg5, Rk1, Rb3, and notoginsenoside R1 were found in KRG samples, and in the case of ARG, ginsenoside Ro, Rb1, Rb2, Rc, Rd, Re, Rg1, 20(S)-Rg2, 20(R)-Rg2, 20(S)-Rg3, 20(R)-Rg3, 20(S)-Rh1, F2, F4, Rg6, Rh4, Rk3, Rg5, Rk1, Rb3, and notoginsenoside R1 were found.
Fig. 1.
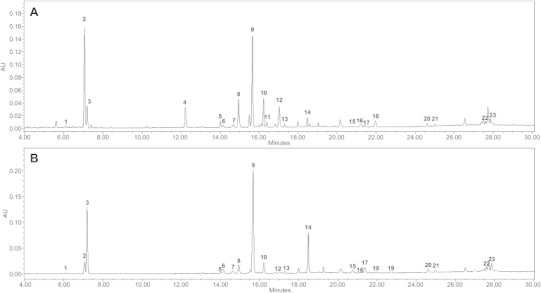
Representative UPLC chromatogram of (A) processed Panax ginseng and (B) processed Panax quinquefolius. NG-R1; 1, G-Rg1; 2, G-Re; 3, G-Rf; 4, 20(S)-G-Rh1; 5, 20(S)-G-Rg2; 6, 20(R)-G-Rg2; 7, G-Ro; 8, G-Rb1; 9, G-Rc; 10, G-Ra1; 11, G-Rb2; 12, G-Rb3; 13, G-Rd; 14, G-Rg6; 15, G-Rk3; 16, G-F4; 17, G-Rh4; 18, G-F2; 19, 20(S)-G-Rg3; 20, 20(R)-G-Rg3; 21, G-Rk1; 22, G-Rg5; and 23. UPLC, ultraperformance liquid chromatography.
Table 1.
The Contents of ginsenosides in two processed Panax ginseng genus (n = 20)
| Analytes | Processed Panax ginseng (mg/g) | Processed Panax quinquefolius (mg/g) | p |
|---|---|---|---|
| NG-R1 | 0.153 ± 0.285 | 0.009 ± 0.023 | 3.7 × 10−2 |
| G-Rg1 | 3.339 ± 2.116 | 0.965 ± 0.584 | 7.8 × 10−5 |
| G-Re | 1.518 ± 0.609 | 6.775 ± 3.621 | 3.0 × 10−6 |
| G-Rf | 0.746 ± 0.502 | ND | 2.3 × 10−6 |
| 20(S)-G-Rh1 | 0.207 ± 0.138 | 0.109 ± 0.089 | 1.2 × 10-2 |
| 20(S)-G-Rg2 | 0.157 ± 0.053 | 0.827 ± 0.303 | 4.8 × 10−9 |
| 20(R)-G-Rg2 | 0.111 ± 0.071 | 0.711 ± 0.269 | 2.2 × 10-9 |
| G-Ro | 1.640 ± 0.748 | 2.865 ± 1.392 | 1.7 × 10−3 |
| G-Rb1 | 4.700 ± 3.428 | 26.575 ± 11.936 | 7.6 × 10−8 |
| G-Rc | 1.177 ± 0.603 | 1.021 ± 0.363 | 3.3 × 10−1 |
| G-Ra1 | 0.692 ± 0.725 | ND | 4.2 × 10−4 |
| G-Rb2 | 1.326 ± 0.794 | 0.146 ± 0.051 | 2.4 × 10−6 |
| G-Rb3 | 0.218 ± 0.124 | 0.279 ± 0.104 | 9.7 × 10−2 |
| G-Rd | 0.243 ± 0.204 | 0.322 ± 1.646 | 6.5 × 10−8 |
| G-Rg6 | 0.031 ± 0.016 | 0.486 ± 0.197 | 3.4 × 10−9 |
| G-Rk3 | 0.058 ± 0.044 | 0.046 ± 0.031 | 3.1 × 10−1 |
| G-F4 | 0.073 ± 0.036 | 0.947 ± 0.381 | 3.7 × 10−9 |
| G-Rh4 | 0.116 ± 0.079 | 0.092 ± 0.065 | 3.0 × 10−1 |
| G-F2 | ND | 0.145 ± 0.158 | 5.9 × 10−4 |
| 20(S)-G-Rg3 | 0.111 ± 0.062 | 0.937 ± 0.321 | 3.9 × 10−10 |
| 20(R)-G-Rg3 | 0.075 ± 0.045 | 0.597 ± 0.214 | 6.3 × 10−10 |
| G-Rk1 | 0.032 ± 0.023 | 0.390 ± 0.154 | 2.0 × 10−9 |
| G-Rg5 | 0.052 ± 0.045 | 0.894 ± 0.308 | 1.2 × 10−10 |
G, ginsenoside; ND, not detected; NG, notoginsenoside.
Ginsenosides Rf and Ra1 are present in KRG, whereas ginsenoside F2 is found only in ARG samples, which is in good agreement with previous reports [3–5,27]. The biomarker of KRG, ginsenoside Rf, is also confirmed in our result, in addition to ginsenoside Ra1, whereas ginsenoside F2 was found as a potential biomarker of ARG. However, 24(R)-pseudoginsenoside F11 was not detected in ARG because it does not absorb light at 203 nm.
The content of ginsenoside Ra1 in KRG was 0.692 ± 0.725 mg/g and that of ginsenoside F2 in ARG was 0.145 ± 0.158 mg/g. For the analysis of these ginsenosides, a high level of technology should be supported because of their low contents in ginseng samples and the chemical similarity of ginsenosides' moiety. Therefore, for delicate instrumental analysis conditions, high resolution and low background signal are required. These requirements can be fully satisfied by using a UPLC system, as has been thoroughly explored in our previous studies [26].
3.2. Nontargeted analysis
To obtain more information on the components of the two processed genera, the UPLC-QTOF MS data were used for nontargeted component analysis. The chromatograms of different kinds of processed ginseng genera were generated with an analysis time of 43 min, as in our previous research. The gradient elution mode was used in a UPLC system to acquire the maximized chromatographic performance such as simultaneous data acquisition and appropriate retention time and integration value. Then, these chromatographic data were extracted for multivariate analysis. Fig. 2 shows the total ion chromatograms of KRG and ARG. The accurate mass measurement was established by the simultaneous but independent acquisition of reference ions of leucine–enkephalin (m/z 556.2771) via the LockSpray interface. This system offers several advantages for nontargeted metabolite profiling, including minimization of ion suppression according to the reference ions and prevention of fluctuations in reference ionization efficiency according to the gradient elution. Using this system, highly improved mass accuracy data were acquired in the range of 0.1–20 ppm, and the acquired exact mass significantly reduced the number of possible structures of metabolites.
Fig. 2.
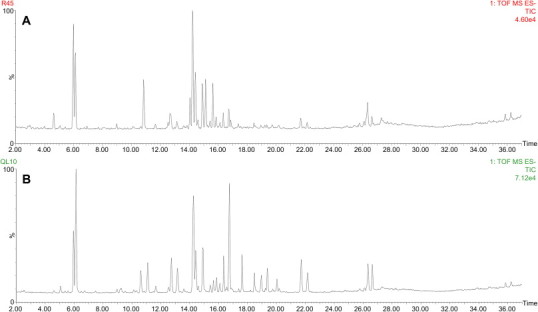
Representative total ion chromatogram (TIC) of ginseng samples. (A) Processed Panax ginseng; (B) processed Panax quinquefolius.
In order to find novel discrimination marker ions between KRG and ARG, unsupervised PCA and supervised OPLS-DA were performed using the UPLC-QTOF MS data. After creating a process for mean centering and pareto scaled data set, the data were displayed as score plots (Fig. 3). As shown in Fig. 3, most KRG and ARG samples were clearly clustered into two groups, KG and AG groups. This means that the holistic qualities of KRG and ARG were consistent with each other and indeed different in the levels or occurrences of their components.
Fig. 3.
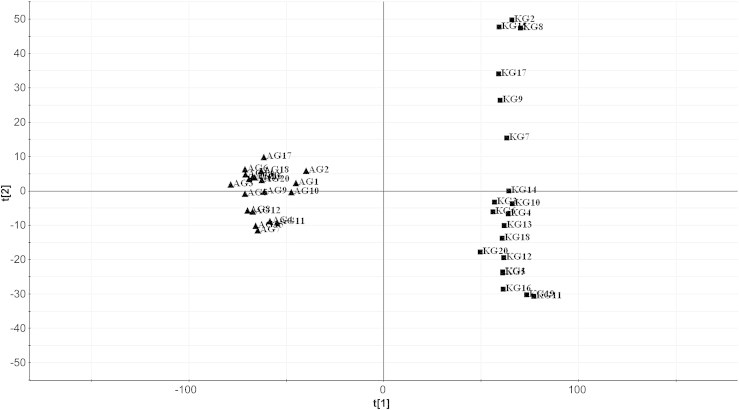
PCA/score plot of processed Panax ginseng and processed Panax quinquefolius samples using Pareto scaling with mean centering. PCA, principal component analysis; ■, processed P. ginseng (KG); ▲, processed P. quinquefolius (AG).
To explore the potential chemical markers that contributed most to the differences between two groups, UPLC-QTOF MS data from these samples were processed by supervised OPLS-DA. As shown in Fig. 4A (S-plot), the first six ions—a (tR 16.74 min, m/z 945.5520), b (tR 11.08 min, m/z 799.4848), c (tR 16.74 min, m/z 991.5507), d (tR 6.12 min, m/z 945.5508), e (tR 6.12 min, m/z 991.5513), and f (tR 11.08 min, m/z 845.4691)—at the lower left of the “S” were the ions from ARG that contributed most to the differences between the two processed ginseng groups. Analogously, as shown in Fig. 4A, six ions—g (tR 15.64 min, m/z 1077.5826), h (tR 10.83 min, m/z 799.4848), i (tR 5.92 min, m/z 845.4995), j (tR 4.61 min, m/z 961.5509), k (tR 15.64 min, m/z 1123.6045), and l (tR 14.90 min, m/z 1077.5825)—at the top right corner of the “S” were the ions from KRG that contributed most to the differences between the two groups.
Fig. 4.
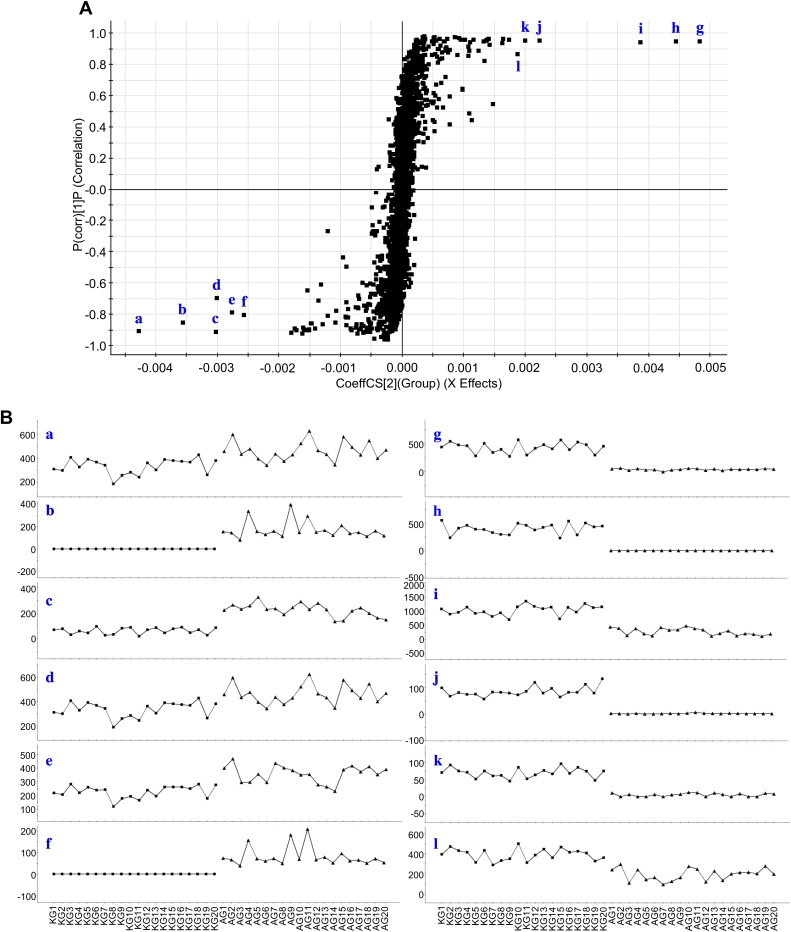
(A) OPLS-DA/S-plot and (B) selected ion intensity trend plots of processed Panax ginseng (■, KG) and processed Panax quinquefolius (▲, AG) samples. a (tR 16.74 min, m/z 945.5520), b (tR 11.08 min, m/z 799.4848), c (tR 16.74 min, m/z 991.5507), d (tR 6.12 min, m/z 945.5508), e (tR 6.12 min, m/z 991.5513), f (tR 11.08 min, m/z 845.4691), g (tR 15.64 min, m/z 1077.5826), h (tR 10.83 min, m/z 799.4848), i (tR 5.92 min, m/z 845.4995), j (tR 4.61 min, m/z 961.5509), k (tR 15.64 min, m/z 1123.6045), and l (tR 14.90 min, m/z 1077.5825). OPLS-DA, orthogonal partial least squares discriminant analysis.
The ion intensity trends of these ions in the tested samples are provided in Fig. 4B. The intensities of ions b and f were relatively high in all ARG samples, but they were undetectable in the KRG samples. Ions a, c, d, and e were detected in most of the samples, but the intensities of these ions were relatively higher in all ARG samples than in the KRG group. The ion intensity trends suggested that components related to ions a–f could be used as potential chemical markers of ARG to distinguish it from KRG. The intensities of ions h and j were relatively high in all KRG samples, but they were undetectable in ARG. And ions g, i, k, and l were mainly detected in KRG as relatively higher intensities than in another group. These ion intensity trends suggested that components related to ions g–l could be used as potential chemical markers of KRG to distinguish it from ARG.
In order to identify the important potential marker ions, such as ginsenoside Rf, Ra1, F2, and 24(R)-pseudoginsenoside F11, a qualitative analysis of ginsenosides present in KRG and ARG was performed. The identifications of marker ions confirmed in samples by individual ginsenoside standard materials were compared with respect to each other, and the results are summarized in Table 2. As a result, ions b and f were the fragment ions from the same molecule, and these ions were [M−H]– and [M−H+HCOOH]– from 24(R)-pseudoginsenoside F11, respectively, and ion h was [M−H]– from ginsenoside-Rf. These two ginsenosides occupy an important position in Fig. 4A (top-right and lower-left corner of “S”). This phenomenon confirmed the fact that ginsenoside-Rf and 24(R)-pseudoginsenoside F11 could be used as marker substances of KRG and ARG, respectively. Ginsenosides Ra1 and F2 were confirmed in all samples, but do not occupy an important position in Fig. 4A. This is because ginsenosides Ra1 and F2 had low values of “factor of change” derived from the low concentration and high standard deviation in samples. This means that these ginsenosides showed a low contribution to the distinction between the processed ginseng genera.
Table 2.
Components that were identified from processed Panax ginseng and processed Panax quinquefolius
| Identity | Chemical formula | tR (min) | Mean measured mass | Theoretical E × act mass | Mass accuracy (ppm) | [M−H]– | [M−H + HCOOH]– |
|---|---|---|---|---|---|---|---|
| Unknown | – | 4.61 | 961.5509 | – | – | (j) 961.5509 | 1007.5630 |
| N-R1 | C47H80O18 | 5.06 | 931.5325 | 931.5266 | 6.3 | 931.5325 | 977.5453 |
| G-Rg1 | C42H72O14 | 5.92 | 799.4965 | 799.4844 | 15.1 | 799.4965 | (i) 845.4995 |
| G-Re | C48H82O18 | 6.12 | 945.5508 | 945.5423 | 8.5 | (d) 945.5508 | (e) 991.5513 |
| G-Rf | C42H72O14 | 10.83 | 799.4848 | 799.4844 | 0.5 | (h) 799.4848 | 835.5026 |
| pG-F11 | C42H72O14 | 11.08 | 799.4848 | 799.4844 | 0.5 | (b) 799.4848 | (f) 845.4691 |
| G-Rh1 | C36H62O9 | 12.54 | 637.4426 | 637.4316 | 17.3 | 637.4426 | 6834336 |
| 20(S)-G-Rg2 | C42H72O13 | 12.71 | 783.4899 | 783.4895 | 0.5 | 783.4899 | 829.5025 |
| 20(R)-G-Rg2 | C42H72O13 | 13.16 | 783.4897 | 783.4895 | 0.3 | 783.4897 | 829.5046 |
| G-Rb1 | C54H92O23 | 14.23 | 1107.5990 | 1107.5951 | 3.5 | 1107.5990 | 1153.619 |
| G-Ro | C48H76O19 | 14.41 | 955.4886 | 955.4903 | −1.8 | 955.4886 | – |
| G-Rc | C53H90O22 | 14.90 | 1077.5825 | 1077.5846 | −1.9 | (l) 1077.5825 | 1123.6079 |
| G-Ra1 | C58H98O26 | 15.12 | 1209.6300 | 1209.6268 | 2.6 | 1209.6300 | – |
| G-Rb3 | C53H90O22 | 15.86 | 1077.5988 | 1077.5846 | 13.2 | 1077.5988 | 1123.6123 |
| G-Rb2 | C53H90O22 | 15.64 | 1077.5826 | 1077.5846 | −1.9 | (g) 1077.5826 | (k) 1123.6045 |
| G-Rd | C48H82O18 | 16.74 | 945.5520 | 945.5423 | 10.3 | (a) 945.5520 | (c) 991.5507 |
| Gy-XVII | C48H82O18 | 17.62 | 945.5453 | 945.5423 | 3.2 | 945.5423 | 991.5512 |
| G-Rg6 | C42H70O12 | 18.93 | 765.4822 | 765.4789 | 4.3 | 765.4822 | 811.4964 |
| G-Rk3 | C36H60O8 | 19.23 | 619.4316 | 619.4210 | 17.1 | 619.4316 | 665.4370 |
| G-F4 | C42H70O12 | 19.38 | 765.4801 | 765.4789 | 1.6 | 765.4801 | 811.4951 |
| G-Rh4 | C36H60O8 | 19.73 | 619.4308 | 619.4210 | 15.8 | 619.4308 | 665.4356 |
| G-F2 | C42H72O13 | 20.03 | 783.5050 | 783.4895 | 16.3 | 783.5050 | 829.5000 |
| 20(S)-G-Rg3 | C42H72O13 | 21.69 | 783.4882 | 783.4895 | −1.7 | 783.4882 | 829.5044 |
| 20(R)-G-Rg3 | C42H72O13 | 22.16 | 783.4903 | 783.4895 | 1.0 | 783.4903 | 829.5070 |
| G-Rk1 | C42H70O12 | 26.32 | 765.4788 | 765.4789 | −0.1 | 765.4788 | – |
| G-Rg5 | C42H70O12 | 26.62 | 765.4794 | 765.4789 | 0.7 | 765.4794 | – |
| 20(S)-G-Rh2 | C36H62O8 | 26.64 | – | 621.4366 | – | – | 667.4510 |
| 20(R)-G-Rh2 | C36H62O8 | 26.81 | – | 621.4366 | – | – | 667.4527 |
| G-Rh3 | C36H60O7 | 29.94 | – | 603.4261 | – | – | 649.4399 |
| G-Rk2 | C36H60O7 | 30.41 | – | 603.4261 | – | – | 649.4395 |
G, ginsenoside; Gy, gypenoside; NG, notoginsenoside; pG, pseudoginsenoside.
Other potential marker ions were identified by comparing the spectrum of standard materials and selected ions in samples and individual retention times. Ions a and c were the fragment ions from the same molecule, and these ions were [M−H]– and [M−H+HCOOH]– from ginsenoside Rd, respectively. Ions d and e were the fragment ions from ginsenoside-Re with respect to [M−H]– and [M−H+HCOOH]–. Ions g and k were confirmed as [M−H]– and [M−H+HCOOH]– of ginsenoside Rc, and ion i was confirmed as [M−H]– ion of ginsenoside Rg1 by use of standard materials. These ions could not be used as a marker substance; it is only because of the difference between the concentrations of the two groups is a phenomenon. These are called “false-positives” in metabolomics and should be excluded by other verification methods (using standard material).
Finally, in Fig. 4, ion j occupies an important position but could not be confirmed by standard materials. However, it can be assumed that ion j might be 20-gluco-ginsenoside Rf, based on the exact mass and previous studies [27,28].
In the present study, discrimination between two processed ginseng genera and exploration of the characteristic chemical markers of processed ginseng were performed. In targeted analysis, ginsenoside Rf was confirmed as a chemical marker of KRG. Additionally, ginsenoside Ra1 and F2 were extracted as potential chemical markers of KRG and ARG, respectively. An optimized UPLC-Q-TOF MS-based metabolic profiling method was developed for the analysis and evaluation of two processed ginseng genera. All known biomarkers, such as ginsenoside Rf and 24(R)-pseudoginsenoside F11, were identified. And additional potential biomarkers such as 20-gluco-ginsenoside Rf were extracted from huge amounts of global analysis data using the proposed metabolomic approach. Thus, such metabolomics techniques should be frequently applied in ginseng research.
Conflicts of interest
All authors declare no conflicts of interest.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Gyo In, Email: 20109042@kgc.or.kr.
Jeong-Han Kim, Email: kjh2404@snu.ac.kr.
References
- 1.Soldati F. Panax ginseng; standardization and biological activity. In: Cutler S.J., Cutler H.G., editors. Biologically active natural products. CRC Press; New York: 2000. pp. 209–232. [Google Scholar]
- 2.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan T.W.D., But P.P.H., Cheng S.W., Kwok I.M.Y., Lau F.W., Xu H.X. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 4.Leung Kelvin S.Y., Chan K., Bensoussan A., Munroe M.J. Application of atmospheric pressure chemical ionization mass spectrometry in the identification and differentiation of Panax species. Phytochem Anal. 2007;18:146–150. doi: 10.1002/pca.962. [DOI] [PubMed] [Google Scholar]
- 5.Li L., Luo G.A., Liang Q.L., Hu P., Wang Y.M. Rapid qualitative and quantitative analyses of Asian ginseng in adulterated American ginseng preparations by UPLC/Q-TOF-MS. J Pharm Biomed Anal. 2010;52:66–72. doi: 10.1016/j.jpba.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Lai Y.H., So P.K., Lo Samual C.L., Ng Eddy W.Y., Poon Terence C.W., Yao Z.-P. Rapid differentiation of Panax ginseng and Panax quinquefolius by matrix-assisted laser desorption/ionization mass spectrometry. Anal Chim Acta. 2012;753:73–81. doi: 10.1016/j.aca.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 7.Shyur L.F., Yang N.S. Metabolomics for phytomedicine research and drug development. Curr Opin Chem Biol. 2008;12:66–71. doi: 10.1016/j.cbpa.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Wishart D.S. Metabolomics; application to food science and nutrition research. Trends Food Sci Technol. 2008;19:482–493. [Google Scholar]
- 9.Cevallos J.M., Corcuera J.I.R.-D., Etxeberria E., Danyluk M.D., Rodrick G.E. Metabolomics in food science: a review. Trends Food Sci Technol. 2009;20:557–566. [Google Scholar]
- 10.Bictash M., Ebbels T.M., Chan Q., Loo R.L., Yap Ivan K.S., Brown I.J., Iorio M.D., Daviglus M.L., Holmes E., Stamler J. Opening up the “Black Box”: metabolic phenotyping and metabolome-wide association studies in epidemiology. J Clin Epidemiol. 2010;63:970–979. doi: 10.1016/j.jclinepi.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madsen R., Lundstedt T., Trygg J. Chemometrics in metabolomics—a review in human disease diagnosis. Anal Chim Acta. 2010;659:223–233. doi: 10.1016/j.aca.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 12.Putri S.P., Nakayama Y., Matsuda F., Uchikata T., Kobayashi S., Matsubara A., Fukusaki E. Current metabolomics: practical applications. J Biosci Bioeng. 2013;115:579–589. doi: 10.1016/j.jbiosc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Kim H.K., Choi Y.H., Verpoorte R. NMR-based plant metabolomics: where do we stand, where do we go? Trends Biotechnol. 2011;29:267–275. doi: 10.1016/j.tibtech.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Xiao J.F., Zhou B., Ressom H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Anal Chem. 2012;32:1–14. doi: 10.1016/j.trac.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dan M., Su M., Gao X., Zhao T., Zhao A., Xie G., Qiu Y., Zhou M., Liu Z., Jia W. Metabolite profiling of Panax notoginseng using UPLC-ESI-MS. Phytochemistry. 2008;69:2237–2244. doi: 10.1016/j.phytochem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Xie G., Plumb R., Su M., Xu Z., Zhao A., Qiu M., Liu Z., Jia W. Ultra-performance LC/TOF MS analysis of medicinal Panax herbs for metabolomic research. J Sep Sci. 2008;31:1015–1026. doi: 10.1002/jssc.200700650. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H.M., Li S.L., Zhang H., Wang Y., Zhao Z.L., Chen S.L., Xu H.X. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Shin Y.S., Bang K.H., In D.S., Kim O.T., Hyun D.Y., Ahn I.O., Ku B.C., Kim S.W., Seong N.S., Cha S.W. Fingerprinting analysis of fresh ginseng roots of different ages using 1H-NMR spectroscopy and principal components analysis. Arch Pharm Res. 2007;30:1625–1628. doi: 10.1007/BF02977333. [DOI] [PubMed] [Google Scholar]
- 19.Kang J.H., Lee S.Y., Kang S.M., Kwon H.N., Park J.H., Kwon S.W., Park S.H. NMR-based metabolomics approach for the differentiation of ginseng (Panax ginseng) roots from different origins. Arch Pharm Res. 2008;31:330–336. doi: 10.1007/s12272-001-1160-2. [DOI] [PubMed] [Google Scholar]
- 20.Lin W.N., Lu H.Y., Lee M.S., Yang S.Y., Chen H.J., Chang Y.S., Chang W.T. Evaluation of the cultivation age of dried ginseng radix and its commercial products by using 1H-NMR fingerprint analysis. Am J Chinese Med. 2010;38:205–218. doi: 10.1142/S0192415X10007762. [DOI] [PubMed] [Google Scholar]
- 21.Kim N.H., Kim K.O., Choi B.Y., Lee D.H., Shin Y.S., Bang K.H., Cha S.W., Lee J.W., Choi H.K., Jang D.S. Metabolomic approach for age discrimination of Panax ginseng using UPLC-Q-Rof MS. J Agric Food Chem. 2011;59:10435–10441. doi: 10.1021/jf201718r. [DOI] [PubMed] [Google Scholar]
- 22.Kim N.H., Kim K.O., Lee D.H., Shin Y.S., Bang K.H., Cha S.W., Lee J.W., Choi H.K., Hwang B.Y., Lee D.H. Nontargeted metabolomics approach for age differentiation and structure interpretation of age-dependent key constituents in hairy roots of Panax ginseng. J Nat Prod. 2012;75:1777–1784. doi: 10.1021/np300499p. [DOI] [PubMed] [Google Scholar]
- 23.Yang S.O., Shin Y.S., Hyun S.H., Cho S.Y., Bang K.H., Lee D.H., Choi S.P., Choi H.K. NMR-based metabolic profiling and differentiation of ginseng roots according to cultivation ages. J Pharmaceut Biomed. 2012;58:19–26. doi: 10.1016/j.jpba.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Song H.H., Kim D.Y., Woo S., Lee H.K., Oh S.R. An approach for simultaneous determination for geographical origins of Korean Panax ginseng by UPLC-QTOF/MS coupled with OPLS-DA models. J Ginseng Res. 2013;37:341–348. doi: 10.5142/jgr.2013.37.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.In G., Ahn N.G., Bae B.S., Han S.T., Noh K.B., Kim C.S. New method for simultaneous quantification of 12 ginsenosides in red ginseng powder and extract: in-house method validation. J Ginseng Res. 2012;36:205–210. doi: 10.5142/jgr.2012.36.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H.W., In G., Lee M.W., Kim S.Y., Kim K.T., Cho B.G., Han G.H., Chang I.M. Simultaneous determination of 30 ginsenosides in Panax ginseng preparations using ultra performance liquid chromatography. J Ginseng Res. 2013;37:457–467. doi: 10.5142/jgr.2013.37.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christensen L.P. Ginsenoside: chemistry, biosynthesis, analysis and potential health effects. Adv Food Nutr Res. 2008;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 28.Park J.D. Recent studies on the chemical constituents of Korean ginseng. Korean J Ginseng Sci. 1996;20:389–415. [Google Scholar]


