Abstract
The lack of an adequate therapy for Alzheimer's Disease (AD) contributes greatly to the continuous growing amount of papers and reviews, reflecting the important efforts made by scientists in this field. It is well known that AD is the most common cause of dementia, and up-to-date there is no prevention therapy and no cure for the disease, which contrasts with the enormous efforts put on the task. On the other hand many aspects of AD are currently debated or even unknown. This review offers a view of the current state of knowledge about AD which includes more relevant findings and processes that take part in the disease; it also shows more relevant past, present and future research on therapeutic drugs taking into account the new paradigm “Multi-Target-Directed Ligands” (MTDLs). In our opinion, this paradigm will lead from now on the research toward the discovery of better therapeutic solutions, not only in the case of AD but also in other complex diseases. This review highlights the strategies followed by now, and focuses other emerging targets that should be taken into account for the future development of new MTDLs. Thus, the path followed in this review goes from the pathology and the processes involved in AD to the strategies to consider in on-going and future researches.
Keywords: Alzheimer's Disease, Multi-Target-Directed Ligands, Hybrid Molecules, New Molecules Design, Review.
1. INTRODUCTION
There are literally thousands of reviews published on Alzheimer’s disease (AD) in scientific journals. In fact, the huge amount of articles that emerged in a single searching under the title “Alzheimer’s disease review” up to now, is very close to 20.000 in PubMed database. This figure shows that it is a topic of great concern (that it is, indeed!); but the fact that more than thousand articles on the subject appear yearly we can obtain another lecture such as that constantly appears new relevant information about AD.
The purpose of this review is to bring here the well-known and the least recent discoveries involving two aspects of AD: the pathology of the disease and the pharmacological targets that have been investigated recently by different research groups, many of which follow the new paradigm of Multi-Target-Directed Ligands (MTDLs). This therapeutic strategy has been followed not only in the AD research but also in other neurodegenerative diseases. This review focuses mainly on aspects concerning the pathophysiology and medicinal chemistry. Massoud and Gauthier’s review brings a complementary vision of this one, as the authors explore the pharmacological aspects of AD in today’s treatments [1]. The review of Mangialasche et al. [2], focuses on drug development taking into account their mechanism of action and their clinical trial stages, including the different clinical failures that halted the development of encouraging AD therapies. The authors concerns were whether a multifactorial disease could be resolved by a compound targeting a single mechanism of action. Other reviews that are worth mentioning are Huang and Mucke’s review which gives a deep insight into ApoE protein and its relationship with AD [3], and three reviews concerning medicinal chemistry, are from Cavalli et al. [4], Bajda et al. [5], and Leon and Marco-Contelles [6]. Very recently, there are two reviews of designing of drugs with multi-target activity, one of them from Geldenhuys et al. [7]; and the other about the molecular networks paradigm from Csermely et al. [8], this novel paradigm was some time ago proposed for multi-target drug discovery [9].
The economic and social aspects of AD are studied in depth in a recent review from Knapp et al. [10]. These aspects should not be underestimated, as they are of great concern, considering a prevalence of 30 million affected persons worldwide and that it is believed that by 2040 AD will affect more than 80 million of people on our planet [11]. This escalation is parallel to increased life expectancy, as the estimated annual incidence and prevalence of AD increases dramatically with age. In addition, the progression of the co-morbidity with age-related diseases will be enough to threat the sanitary systems in near future. Figures of co-morbidity are significant among the more frequent pathologies affecting elderly people. Co-morbid diseases with increased prevalence in AD patients are (RR versus control subjects): eating disorders (6.4), urinary tract infection (4.9), fracture neck of femur (4.1), pneumonia (2.8), depression (1.8), ischemic stroke (1.3) [12]. There could be additional problems inherent to the fact of patient admission to hospital as the mean stance will increase to 78 days versus 7 days of a non-AD patient, and there is an increased risk of later in-hospital mortality. The costs for AD patients are 34% higher than those of a population without the disease. The mentioned facts have entitled AD the dubious award of “The Challenge of the Second Century” [13].
1.1. Alzheimer’s Disease
AD -the most common neurodegenerative disorder in elderly population- is genetically complex, slowly progressive and irreversible [14]. This disease is characterized clinically not only by an early and progressive memory loss, but also by other cognitive and behavioral disturbances [15, 16]. There are two primary pathological hallmarks of the disease: the deposition of both extracellular parenchymal and cerebrovascular amyloid-β protein (Aβ), and intracellular neurofibrillary tangles (NFTs) of hyperphosphorylated tau (τ) protein [17, 18]. Thus, anomalous protein aggregation is a crucial event in AD and it comprises of the whole processes of the defective protein, its formation, misfolding and defects in the cellular systems responsible for defective products removal [19]. This pathogenic accumulation of proteins is thought to be the cause, or at least the main factor that leads AD to cognitive impairment and, subsequently to a selective and extensive neuronal dysfunction or death in essential areas, as the hippocampus, amygdala, entorhinal cortex, and association cortices of the frontal, temporal and parietal lobes [20]. It has recently been suggested that it could be more the functional loss than the neuronal cell loss which leads to cognitive impairment [21]; and even that the loss of synapses and dendritic spines correlates better with cognitive impairment than the mere loss of neurons [22]. Currently it is proposed that, apart from degeneration of specific neuronal populations and synapses impairment, aberrant neuronal network activity should be considered as one of the main substrates of cognitive decline in AD [3]. Thus, the initial hallmarks considered led to these new ones: cerebrovascular abnormalities and synaptic failure, but also there are other hallmarks that necessarily have to be taken into consideration, namely: oxidative stress and neuroinflammation, all closely related to each other and further related to metabolic changes in the neuronal environment [23].
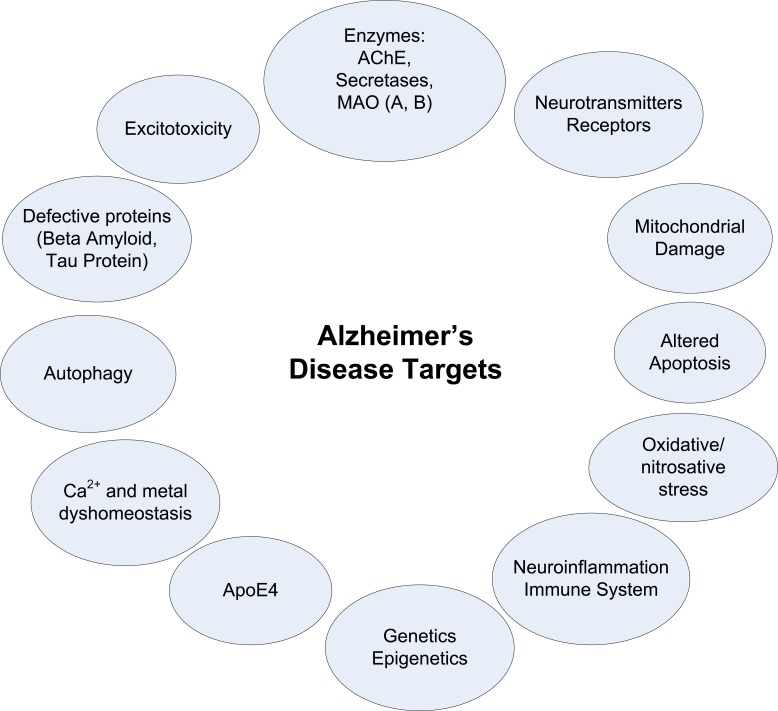
AD is one of the most enigmatic and intractable issues in biomedicine [24]. There are several putative factors in AD disease that could be grouped into endogenous and environmental factors, and these include processes as diverse as age, oxidative stress and free radical formation, defects in cellular bioenergetics and mitochondrial dysfunction, lesion of Golgi apparatus and intracellular transport, molecular chaperones, neurotrophins and neuroinflammation processes, even head injury or use of anesthetics, but little is known about the implications for the pathogenesis of the disease [19], even some factors are not easily considered cause or consequence. Thus, AD is considered as a multifactorial and/or multilevel pathology (Fig. 1), with mechanisms that are complexly interrelated in vicious cycles, leading to neuronal and functional loss in the nervous system [25]. In addition, the contribution of each known factor to the pathology, or even the relevance of other unknown factors to be discovered, marks the difficulty of how to focus the problem.
Fig. (1).
Some of the main current targets in AD research.
1.2. AD Etiology
1.2.1. Aβ Peptides
The pathogenic character of Aβ has given origin to the “amyloid hypothesis” that states that the formation and deposition of small peptides of Aβ forms long insoluble amyloid fibrils, which accumulate in senile plaques [24] in critical regions of the brain, leading to the onset and progression of AD [26] which in the long run will lead to increasing disabilities and finally to death.
The pathogenic Aβ peptides, together with other considered non-pathogenic peptides, are originated by the proteolytic activity on the amyloid precursor protein (APP), a normal naturally occurring transmembrane protein. The activity of secretases (α, β, or γ secretase) excises APP, producing different peptides, depending on two different pathways: the α -or non-amyloidogenic- pathway which yields soluble APPα (sAPPα); and the β secretase -or amyloidogenic- pathway, that is mediated by the sequential action of β-secretase and γ-secretase, and results in the formation of Aβ peptides [27]. The relevance of α-secretase activity on halting the disease progression has been suggested recently, because it prevents the formation of toxic peptides [28, 29]. Since γ-secretase divides its substrate at several neighboring positions, Aβ peptides are a group of peptides differing in length at the C terminus. The dominant species is the Aβ1-40 peptide constituting 80-90% of all Aβ peptides. The second major species is the Aβ1-42 peptide, which constitutes in normal conditions 5-10%, and is considered aggregenic and thus forms the seed for larger oligomers and fibrils and finally for the macroscopic amyloid plaques [30]. From the two identified human β-secretases, the Β-site APP-cleaving enzyme 1 (BACE1), a transmembrane aspartyl protease, is the one significantly expressed in the brain and seem to be relevant by cleaving APP into Aβ [24, 31].
Among other peptide forms, both Aβ peptides mentioned above can interact with themselves forming aggregates, either as cerebral amyloid angiopathy (CAA), as in the case of Aβ1-42 being accumulated in brain vessels [32]; or as senile plaques (SP) in the case of Aβ1-40, which will be deposited later in the disease process [20]. CAA contributes in building up SP aggregates and increases the clinical decline of AD patients [33] as well as leads to an enhanced loss of vessel integrity and functioning [32]. The pathogenic mechanisms, again, are largely unknown.
Extracellular deposited insoluble Aβ plaques are not only the result of the aggregation process of a group of hydrophobic peptides of 39-43 amino acid residues [20], but also the result of the defective degradation of anomalous proteins due to deficiency of the anomalous protein deposits clearance systems, namely the ubiquitin–proteasome–autophagy system and the lysosomal system [34].
1.2.2. Tau Proteins
The other classic pathogenic hallmark of AD, NFTs of τ protein, is constituted mainly by hyperphosphorylated paired helical filaments. Τau protein is a key microtubule-associated protein involved in the axonal trafficking, that in healthy neurons binds and stabilizes microtubules by reversible enzymatically mediated phosphorylation and dephosphorylation processes; but if τ is not dephosphorylated sufficiently, it does not bind adequately to other microtubules and polymerizes into filaments that further form NFTs [20]. The anomalous excessive phosphorylation and subsequent aggregation in intracellular tangles leads to a deadly cascade of impairment of axonal transport, synaptic alterations, microglial and astrocytic activation, progressive neuronal loss associated with multiple neurotransmitter deficiencies, and cognitive failure [24, 35]. Oligomeric and pre-aggregated forms of τ protein have been shown to be toxic in vitro [36]. These facts led to propose the hypothesis that has been named as “Tau hypothesis” [37].
1.2.3. Role of Peptides in the Developing Dementia
Whereas the density of amyloid plaques surprisingly does not correlate well in severity of dementia, NFTs do correlate well with the clinical symptoms, in fact now it is widely believed that there could be presence of amyloid deposits with few or no clinical manifestation [38]. The paradoxical evidences that Aβ plaques do not imply dementia and that the neurotoxic effect of these Aβ senile plaques is independent of its aggregation [39], could be explained by the investigations suggesting that the toxic agent and probable real inductor of AD pathogenesis are not the senile insoluble plaques, but the soluble Aβ peptides and oligomers instead [40]. In supporting this hypothesis, it has been found, that on one side, levels of soluble Aβ correlates with cognitive decline [41]; and on the other side, soluble peptides appear to impair synaptic structure and function [24], and that the accumulation of Aβ peptides leads to synaptic depression and aberrant excitatory neuronal activity [42, 43]. These findings lead to the conclusion that precisely some of the Aβ soluble forms are the pivotal pathogenic agents playing a role in presymptomatic early stages of AD process, well before or during the onset of plaques, although the exact Aβ species implied in the pathogenesis is to be discovered [44]. The relevance of the aggregated forms of Aβ in generating neuron impairment is also discussed, but aggregation could even act in reducing the toxicity of soluble Aβ by recruiting the peptide into the aggregates, and preventing their neurotoxicity [3]. There is however no absolute consensus about the pathogenesis of protofibrils and oligomers of Aβ40 and Aβ42, as indicated by Cerpa et al., suggesting that smaller cleavage products of APP are responsible for the neuronal dysfunction [45].
In summary, whether Aβ peptides and/or NFTs, the molecular mechanisms that conduce to learning and memory deficits remain unknown; however, the evidences point to the fact that the soluble or intermediate forms of these proteins (toxic peptides, protofibrils) which somehow interfere with cellular signaling cascades lead to cognitive impairment [46] and are thought to have cytotoxic effects on neurons [19, 37].
1.2.4. Clearance of Misfolded and Aggregated Peptides
Autophagy, a process that takes part in the cell to degrade damaged organelles and misfolded or aggregated cytoplasmic proteins, comprises of mainly three processes: macroautophagy, microautophagy and chaperone-mediated autophagy, differing in the mode of delivery to the lysosome [47]. The last two processes are relatively unknown in contrast to macroautophagy in which two pathways to induce autophagy have been identified: mammalian target of rapamycin (mTOR)- dependent and mTOR-independent signaling pathways [48]. Failure in clearance mechanisms lead to the accumulation of defective protein (previously formed, misfolded and/or aggregated) which is a crucial hallmark in AD [30]. Clearance of defective proteins implicates the collaboration between molecular chaperones and targeted protein degradation (performed by proteasome-mediated degradation), chaperone-mediated autophagy (CMA) and selective macroautophagy [49]. A failing of misfolded protein removal leads to the building-up of aggregated deposits and the development of the pathogenesis of proteinopathies [50]. The evidences of abnormal protein dynamics due to defective degradation, produced by deficiency of the clearance systems, are overwhelming in AD. Cognitive improvements in different mouse models are studied in recent reports [19, 51]. Prior to mentioning degradation of defective proteins, there are also brain clearance mechanisms of Aβ which follow two main routes [32], the direct way through the Blood-Brain Barrier (BBB), and the drainage via the interstitial fluid (ISF). The progressive impairment of these mechanisms, specially the first one leads, with the aging of the brain vessels, to the enhanced formation of CAA, affecting leptomeningeal arteries, cortical arteries and capillaries [33].
1.2.5. Cholinergic System
AD patients are present with impaired neural transmission of cholinergic, serotoninergic, glutamatergic, dopaminergic, and noradrenergic systems [52, 53]. Specifically the cholinergic system is closely related to the pathology and evolution of the AD disease. In AD, there is a functional impairment of basal forebrain cholinergic neurons linked with structural lesions in the same areas or regions that project to or from those areas [20, 54]. The consequences of these lesions are low levels of acetylcholine (ACh) and a loss of cholinergic transmission, resulting in learning and memory dysfunctioning. It was also found that a reduction of the acetylcholine synthesizing enzyme (choline acetyltrans-ferase, ChAT) correlates with the grade of dementia [55]. These related events led researchers to propose the “cholinergic hypothesis” of AD, that is firmly supported by the fact that nearly all the drugs approved for AD treatment deal in one or another way with this hypothesis [56]. The links between AD disease and cholinergic system are confirmed in newly investigated evidences, and nowadays a presynaptic cholinergic hypofunction is also considered a crucial hallmark in AD [57]. Since acetylcholinesterase (AChE) (EC 3.1.1.7) catalyzes the hydrolysis of ACh into acetate and choline, AChE inhibitors are traditionally used as cholinergic agents, playing an important role in symptomatic AD treatment. In the three-dimensional structure of the enzyme the catalytic active site (CAS), constituted by three amino acids (Ser200-His440-Glu327) lies at the bottom of a long and narrow (20 x5 Å) gorge formed by 14 aromatic amino acids, where tryptophan 84 is the critical constituent of the anionic binding site [58]. The surface of the throat is known as the peripheral anionic side (PAS).
It seems that AChE is also directly implicated in AD pathogenesis influencing Aβ deposition. This has been demonstrated in vitro, incorporating AChE to Aβ peptides [59], and in vivo, injecting AChE in rat hippocampus leading to a significant cognitive impairment [60]. The experimental evidences point out that AChE acts as a molecular chaperone, accelerating the formation and increasing the neurotoxicity of amyloid fibrils and stable complexes, due to PAS, located at the entry side to the active center gorge [61, 62]. It also has been demonstrated that APP processing is regulated by the cholinergic system [20] as well as Aβ which can modulate the cholinergic system [61]. This modulation seems to be age-dependent, as aged neurons appear to be more sensitive to Aβ-mediated inhibition [63]. However, if the degenerative cholinergic loss is primary or secondary to the amyloid plaque pathology remains to be resolved [21].
The influence of neuroreceptors in AD has been intensively studied [64]. Both muscarinic and nicotinic receptors are altered in AD patients [65], and the initial stages of the disease show a clear decrease of ACh levels and nicotinic receptors [54]. The involvement of the muscarinic receptors M1 and M2 in AD has also been well studied [54, 55, 57, 66].
The increase of phosphorylated τ proteins in cell lines or cultured neurons can also be related to the cholinergic system [20, 67]. The APP processing can also be influenced by the cholinergic system, and affected by other endogenous and external factors, such as serotonin, glutamate, estrogen, testosterone, bradykinin, insulin, calmodulin, other neuropeptides and growth factors, copper, statins and some plant extracts [5, 20, 68].
1.2.6. The Connection Among Oxidative/Nitrosative Aberrant Signaling Pathways, Neuronal Excitotoxicity and Neuroinflammation in AD
AD pathology shows other pathogenic hallmarks like oxidative/nitrosative stress, excitotoxicity and neuro-inflammation. The “oxidative stress hypothesis” states that the increase of production of reactive oxygen species (ROS) and free radicals leads to deleterious effects on the cell components that are involved in the pathogenesis of AD [69]. The oxidative stress was initially supposed to be the result of AD pathology, but this premise has been questioned [70, 71], and a more prominent role of oxidative stress in AD pathology is accepted [72]. In fact oxidative stress has been linked to Aβ aggregation through its relationship with BACE1 activity [73]. Nitrosative stress runs in parallel to oxidative stress and also there are products resulting from this activity, namely reactive nitrogen species (RNS), both oxidative and nitrosative stresses are now accepted as central processes in AD pathophysiology [74]. Recently it has been proposed that oxidative/nitrosative stress act as aberrant signaling pathways, resulting in progressive damage to the neuronal network [23]. On the other hand, excitotoxicity as the neuronal tissue status in which there is an excessive stimulation of ionotropic glutamate receptors, namely N-methyl-D-aspartic acid (NMDA) receptors, and an imbalance of neuronal calcium homeostasis, has been shown to conduct neuronal damage by increasing damaging free radicals and also activating nucleases, proteases and phospholipases which are considered the origin of mitochondrial dysfunction and apoptosis [23, 75]. In turn, mitochondria are well known sources of free radicals, and even in mitochondria of non-pathological cells, a low quantity of ROS is produced and maintained at a minimum by cellular defense mechanisms, but ROS can be produced in a quantity that would contribute to synaptic damage [76]. The imbalance of ROS leads to other deleterious effects that may likely end in cellular death [77] and also severely damaging mitochondria [78]. Recently it has been shown that both toxic Aβ and excessive neuronal excitation induce ROS production not only by means of mitochondria but also by the activation of NADPH oxidase, and that ROS would trigger changes in various signaling downstream pathways as mitogen-activated protein kinases (MAPK). Also, NADPH oxidase would lead to the activation of MEK1/2 or ERK1/2 and cytosolic phospholipase A2 (cPLA2) [79]. Phospholipases A2 are enzymes that hydrolyze fatty acids from membrane phospholipids, cPLA2 is found in diverse nervous tissue cells and besides calcium is regulated by receptor mediated signaling pathways. In addition, the role of cPLA2 in neurodegenerative diseases through its implication in oxidative/nitrosative signaling pathways has been clearly shown [80, 81]. Thus, there is a close relationship among Aβ toxic oligomers and excessive activation of NMDA receptors with calcium influx, cPA2 activation, and oxidative/nitrosative aberrant signaling pathways, but also there is a direct influence of Aβ oligomers as they act on NMDA receptor trafficking [82]. In addition, oxidative/nitrosative stress contribute to protein misfolding and aggregation, therefore it is considered a pathogenic trigger of neurodegenerative processes; specifically, RNS acting through the S-nitrosylation of protein-disulphide isomerase (PDI) blocks the protective effect that this enzyme has on neurodegenerative disorders [83]. Nitrosative stress not only has been related to protein misfolding and mitochondrial dysfunction [84] but also to the mediation of cell injury and cell death after excitotoxicity [85].
Neuroinflammation is another feature of neuro-degenerative disorders, but there is little knowledge about factors determining susceptibility to neurodegeneration [86]. During neuroinflammation, nitric oxide (NO), second messenger in inflammatory signaling, is increased, producing and leading, among others, to tyrosine nitration in proteins as τ [87, 88]. Both ROS and RNS act together in neurodegeneration [89] in a vicious cycle, leading to a pro-inflammatory status, with the release of pro-inflammatory cytokines including interleukins (IL-1β, IL-6), and tumor necrosis factor α (TNFα) [90], activating microglia and astrocytes that can produce excess of NO [91]. In NO synthesis intervenes the enzyme nitric oxide synthase (NOS) of which there are three subtypes: neuronal, endothelial and inducible. In spite of the known upregulation in AD of inducible NOS (iNOS), the significance of the other subtypes of NOS (nNOS and eNOS) in AD is largely unknown [23], although a correlation has been found between nNOS in neuronal cells and neurodegeneration [92]. There are evidences of the possible synergism among nNOS and cPLA2 in Aβ neurotoxicity and cyclooxygenase-2 (COX-2) should be included in the group [23].
1.2.7. Main Risk Factors for AD: Age and Genetics
AD is a genetically complex disease, thus it is quite difficult to assess the genetic influence on the apparition and progress of the disease [14]. However, in spite of its inherent difficulties this knowledge area is one of the most studied and promising in AD research till date.
One relevant aspect of AD etiology is the relationship between the age when the first symptoms appear, and the underlying genetics burden on the patient, resulting in two forms of the disease. When symptoms appear after the age of 60, patients are said to suffer from “sporadic late-onset AD” (SAD or LOAD) [3]. Besides aging, the presence of some APOE gene polymorphisms (namely ε4 allele) is one of the most important risk factors for developing LOAD [93, 94]. In a small number of AD patients the onset of dementia is before the age of 60, and they are classified as “early onset familial AD” (EO-FAD) patients; further, they present mutations in APP (amyloid precursor protein gen), PSEN1, and PSEN2 (presenilin genes) [95]. However, there are many unanswered questions in AD genetics, as up to 50% of the heritability of AD remains unexplained by the known genes; and the question, if LOAD or EO-FAD are Mendelian transmitted or not, is still debated [93].
Epigenetics and AD
Suffering from AD for a given genetic burden is not always a certainty, it has been found that human monozygotic twins are likely to differ in developing the disease [96]; thus, environmental risk factors do matter significantly. Epigenetics comprise the mechanisms involved in transient and reversible changes to the chromatin regardless of the cellular differentiation status, and also those modifications concerning gene expression altering transcriptional activity in a coherent manner [97]; bringing the opportunity to environmental factors to influence how genetics will be expressed [98]. Major epigenetic mechanisms namely chromatin remodeling and histone modifications, DNA methylation, and micro RNA (miRNA) are recently described [98, 99]. With the help of those mechanisms heritable and non-heritable traits become modified without altering the DNA sequence, achieving the repression or silencing the expression of specific genes. In turn, the release from a given epigenetic repression can enhance gene expression [98]. The implication of epigenetics has been shown in the development of many diseases, among them neurodegenerative diseases, has become progressively more evident. In the case of AD the early influence on the genome to the later developing of the disease has been proposed; for instance, the ‘‘LEARn” (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of AD [100].
1.2.8. ApoE4
Regardless of the aforementioned evidences about the implication of ApoE on AD risk, ApoE4 is considered an important factor in developing AD and other types of neurological pathogenesis [101]. ApoE is a polymorphic protein, with three human isoforms ApoE2, ApoE3 and ApoE4, that revealed to be crucial in neurobiological functions. Depending on the isoform considered, ApoE has either physiological functions (neurite remodeling, membrane repairing and remyelination), or pathophysiological functions (related to dendritic and synaptic alterations, Aβ clearance or deposition, glutamate receptor function or mitochondrial function) [102]. There are not only acute differences between the neurobiology and pathologic roles of different ApoE isoforms, the different cellular sources also lead to both, physiological and pathophysiological processes [103]. Thus, astrocytes and neurons express ApoE, however, ApoE expression in astrocytes is increased in the course of aging and also in response to estrogen stimulus and/or activation of liver X or NF-κB receptors, the CNS neurons appear to respond mainly to the stimulus of stress and injuries [103]. The differences also extend to the action of isoforms expressed by one or another type of cell, thus ApoE3 expression protected neuronal synapses and dendrites from excitotoxic damage in ApoE-deficient mice, regardless of its cellular origin, whereas neuronal expression of ApoE4 in cortical neurons led to cell death, after excitotoxic challenge [104]. Furthermore, ApoE isoforms respond differentially to AD hallmarks, namely Aβ and NFTs; in both cases ApoE4, on the contrary to other isoforms, increases Aβ accumulation and building up amyloid plaques [3], as well as increases τ phosphorylation and accumulation in the neurons [105]. The negative roles of ApoE4 in AD pathogenesis were also found in age-dependent and excitotoxin-induced neurodegeneration [104], impaired synaptogenesis [106] and neurogenesis [107], mitochondrial dysfunction and neurotoxicity [102], they also caused age- and τ-dependent impairment of hilar GABAergic interneurons, leading to reduced hippocampal neurogenesis and to learning and memory deficits [105].
1.3. Today’s AD Therapeutic Research
Since the dawn of first symptomatic drugs some 15 years back, it can be said that currently there is no effective cure or prevention of Alzheimer's disease available and that the therapeutic approaches are symptomatic and of modest efficacy [14]. The only approved drugs that may produce real improvements in cognitive performance, fall in two categories: AChE inhibitors (AChEI), and NMDA antagonists [108], with respectively 4 and 1 drug each group [109]. The cholinergic drugs tend to restore or increase the cholinergic system deficiency by means of inhibiting acetylcholinesterase (AChE), the enzyme that degrades acetylcholine, and donepezil is a prominent component of this group [110]. There are several evidences that inhibition of AChE not only restores the cholinergic system, but also interferes with the progression of the disease [111]. On the other side, memantine attenuates the excessive NMDA glutamate receptor activity observed in AD, acting as a non-competitive, low-affinity, open-channel blocker, and it is frequently used in combination with AChE inhibitors [14].
The aforementioned drugs are clearly insufficient for an adequate AD therapy, and real solutions for the disease are eagerly awaited; unfortunately promising solutions -as the vaccination therapy attempts up to now have been, either failed in clinical phase application or are still in developing stages. So far, none of the disease modifying drugs recently developed, has demonstrated adequate efficacy in phase III studies, reducing Aβ production, preventing its aggregation, promoting Aβ clearance or targeting τ protein [112]. The poor outcomes of some Aβ targeting therapeutic agents has complicated the current advances in AD [14], although Selkoe argued that the cause of the problem might be on the trial design or the agent used, and not on the target itself [113]. Considering immunotherapy as a case of study, the first attempts to use antibodies against Aβ apparently worked well in mice, but in humans, the last clinical assay phase showed meningoencephalitis in some patients [114]. Nevertheless, immunotherapy approach is continuing, and currently studies about Aβ peptide immunotherapy and against τ protein pathology are ongoing [115]. Apart from the initial limitations of immunotherapy, cases of phase III clinical trial halting were also described for γ-secretase inhibitors, where patients showed cognitive worsening [116], or for pioglitazone, a PPARγ agonist and β-secretase inhibitor, where cardiac side effects and/or lack of efficacy, due to poor brain blood-barrier permeability, were reported in phase III trials [117, 118].
Currently, the trend of research studies is devoted to reduce these impairments or improve their causative origin [119]. Consequently, Hampel et al.’s disease modifying therapeutic solutions are being pursued against Aβ or τ deposition, inflammation and oxidative stress, among others [14]. On the other hand, as Standridge suggests “the paradigm that AD is pharmacologically unresponsive is shifting” and as the understanding of the molecular mechanisms of neurodegeneration progresses AD will progress, it will allow the development of more specific targets by achieving the interruption of the events that lead to this dementia [120]. However, the discovery of a remedy capable to prevent the disease progression will produce its positive results within a relatively delayed time, thus, it can be said that if a drug able to delay the disease 5 years gets approved by 2015, then it is predicted to reduce the clinical symptoms to about 40% by 2050 [117]. According to Holtzman et al., a major challenge for the development of therapies lies in identifying those patients at high risk for moving from a cognitively normal to an impaired status over a 3-4 year window, and to target this population for clinical trials [13].
2. NEW THERAPEUTIC STRATEGIES
2.1. Changing Paradigms
It is not so far away that the poly-pharmacology approach (that is administering a drug combination to treat a disease) was criticized in pharmacy schools because of the plethora of side effects that these drugs could produce in patients, with little or no improvement. This induced a tendency toward more specific, more fitted-to-disease drugs. Thus, drug research has moved from a human phenotype-based endeavor to a “target-centric” or “reductionist” approach, trying to reduce drug action to individual genes, single proteins, and one modulating molecule [121], known as the term “one-disease-one-target” [122] or even “one gene, one target, one drug” [123]. The research approach in AD has followed the same trend, targeting each pathological aspect of the disease as specifically and precisely as it could be done, in the hope that it would cope or even reverse the disease. However, the fact that AD is a complex neurodegenerative disorder with a multifaceted pathogenesis [124] (Fig. 1), complicates it to a great extent in choosing the most adequate targets, thereby restricting it to discussion only than to seek clear-cut solutions. The currently available drugs targeting as AChEs inhibitors (AChEI) and NMDA receptor antagonists (memantine), have turned out to be more palliative rather than curative [108]. According to Mudher and Lovestone, the dichotomy in selecting Aβ or τ protein as targets, has led to two kinds of “adepts”, namely “baptists” (those concerned with Aβ), and “tauists” (τ protein research) [125], but in any of the cases promising drugs were not found. On the other hand, antagonizing AChE were acting not only against this target, but also on other aspects of the disease. It has been shown that AChEIs were better solutions than specific one-action drugs. Not only AD but other neurological disorders can be treated by this approach, several diseases having a multifactorial etiology are better treated with a combination of drugs (depression, schizophrenia, allergies, hypertension, inflammation conditions and metabolic diseases) [124]. Experimental studies have shown that such drug combinations also work in AD. As an example, the combination of galantamine and melatonin at sub-effective concentrations (30 and 0.3 nM, respectively) induced a synergistic protection, that was similar to the highest activity obtained separately with effective doses of melatonin or galantamine [126]. It is now well-known that the synergic effect obtained by combining different compounds, interfering simultaneously in a defined biological circuit, may increase the bioavailability of each compound at the same target [127]. There are more attempts to obtain ‘multiplicative’ effects by adding two drugs. Among those association of molecules there are: rivastigmine plus memantine [128], donepezil plus SLV330, a CB1 antagonist, [129], and memantine plus vitamin D [130].
2.2. Multi-Target-Directed Ligands (MTDLs)
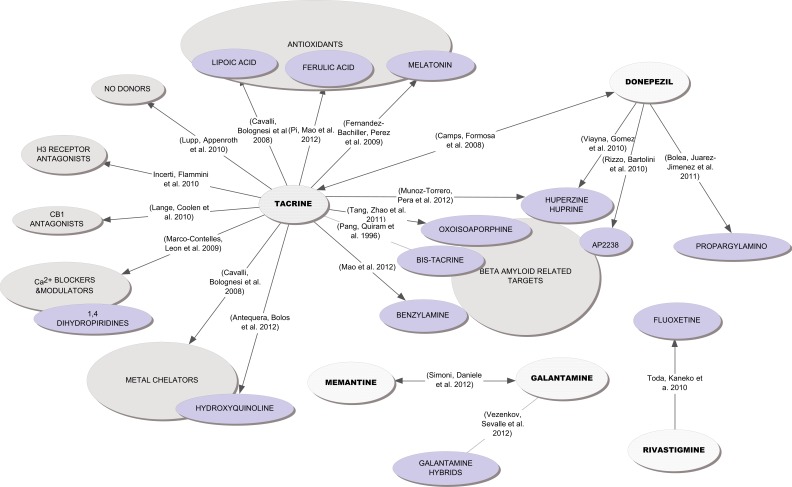
However, despite the new hopes for healing AD, and other neurodegenerative diseases, by drug combinations acting at different levels of the neurotoxic cascade, the researchers are endeavoring to go deeper than that, and ultimately have proposed a new paradigm, emerging from a new concept -therapeutic molecules acting at different levels or targets of the disease- with different denominations: network pharmacology [8, 9, 123], multi-modal therapies [30], multifaceted [131], and more widespread MTDLs [5] or the more recently proposed term “multi-targeted designed drugs” (MTDDs) [7]. A related but more restricted concept, is that of “hybrid compounds” (Fig. 3). Muller-Schiffmann et al. introduce an excellent definition for “hybrid compounds” as ”the combination of two different and independently acting compounds into one covalently linked hybrid compound” that “can convey synergy from the effects of both independently acting moieties to the new composite compound, leading to a pharmacological potency greater than the sum of each individual moiety’s potencies”, taken into account that the moieties can be a wide variety of substances such as small molecules, polypeptides or nucleic acids [127]. Thus, hybrid compounds are designed to be MTDL molecules but not all these compounds originate through the hybrid compound strategy. This new approach in medicinal chemistry, MTDL design strategy, will develop through of single chemical compounds which are able to modulate multiple targets simultaneously (in principle with comparable affinities), with superior efficacy and safety profiles. Acting in a synergic manner on different targets, a single multifunctional drug will interfere with the networked (no matter whether sequential or not) multifactorial etiology of the disease, obtaining a real improvement throughout. In order to seek opportunity to reduce the side effects some authors [4, 121] suggested the possibility of achieving even more attractive therapies in the future. MTDLs are originated not only by the ingenuity of researchers, but also are generated in nature with examples as botulinum toxin, the prime illustration of multi-modular cooperation and site-directed activity; and bleomycin which is another natural MTDL [127, 132]. Cannabinoids, with their multi-level neuroprotective effects, can also be included as nature-generated MTDL [133].
Fig. (3).
Conceptual map showing main trends in constructing hybrid molecules from approved molecules and well known pharmacophores, to obtain new multi-target-directed ligands.
Once the advantage of multi-targeting is clear, the next step is to decide how to combine multi-targeting in a single therapy solution. There are two possibilities, the election of molecules with in-built capacity to act on two or more targets, and/or to combine two or more pharmacophores in a single molecule. Other difficulties that are to be resolved in the MTDL strategy are to choose the most appropriate therapeutic targets (by now a question without a clear answer) and to select an adequate lead molecule to start with.
This last challenge, following previously exposed concepts, points to the AChEIs as a good option, since many AChEIs really found to be good multi-targets (Aβ inhibition, nicotinic receptor modulation, etc.), and are actually one of the first choices of researchers.
2.3. Examples of MTDL
MTDL have been present in one or another form in some compounds used in AD therapy. Thus, galantamine was found to be not only a competitive and reversible acetylcholinesterase inhibitor but also an allosteric modulator at nicotinic acetylcholine receptors [134]. Apart from its AChEI activity, donepezil has been shown to inhibit Aβ self-aggregation and BACE1 [135] and to interact with sigma-1 receptors, known for their anti-amnesic effects [136].
One of the most prominent sources of MTDL is the area of “natural products” and within them, botanical compounds are perhaps not only greater in number but also the most relevant group of chemical compounds that are considered relevant to develop new substances (Table 1).
Table 1.
Examples of Multi-Target-Directed Ligands with Origin on Natural Products and Semi Synthetic Analogs
| Compound | AChE Inhibitor | Tau Hyperphos-Phorylation Inhibitor |
β-amyloid Anti-Aggregation, Clearance, or Secretion | Antioxidant | Other Activities | Clinical Status (Phase) |
References |
|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||
| Physostigmine | ♦ | ♦nicotinic receptor agonist | [265] | ||||
| Phenserine | ♦ | ♦ | ♦ | ♦neurotrophic ukinase modulator uNMDA receptor modulator | I/III | [266] [267] [268] | |
| Galantamine | ♦ | ♦nicotinic receptor allosteric modulator uanti-apoptotic | IV | [134] [269] | |||
| Huperzines A, B | ♦ | ♦NMDA receptor modulator uneuroprotective uanti-apoptotic | II | [270] [271] | |||
| Berberine | ♦ | ♦ | ♦ | ♦ IMAO uanti-neuroinflammatory ucholesterol regulator ♦ insulin regulator | [272] [273] | ||
| Epiberberine | ♦ | ♦ | ♦ | [274] | |||
| Coptisine | ♦neurotrophic ♦ IMAO-A | [274] | |||||
| Groenlandicine | ♦ | ♦ | ♦ | ♦ | [274] [275] | ||
| Jatrorrhizine | ♦ | ♦ | [274] | ||||
| Manzamine | ♦ | ♦anti-neuroinflammatory ukinase modulator | [276] | ||||
| Harmine, Harmaline | ♦ | ♦ | ♦ | ♦ increase dopamine release uIMAOs uNMDA receptor modulators ukinase modulators | [277] [278] | ||
| (10-hydroxy)-Infractopicrin | ♦ | ♦ | [279] | ||||
| Trigonelline | ♦ | ♦neurotrophic umemory enhancement | [280] [281] | ||||
| Nantenine | ♦ | ♦ | ♦adrenergic α1 and serotonin 5-HT2A receptors antagonist | [282] | |||
| Crebanine | ♦ | ♦ α7-nACh receptor modulator | [283] | ||||
| Nicotine | ♦ | ♦ | ♦nicotinic receptor agonist ♦ neuroprotective ♦ anti-apoptotic | [271] | |||
| Caffeine | ♦adenosine A2 receptor antagonist uIMAO-B | [284] [285] [286] | |||||
| Alkaloids | |||||||
| Vincamine | ♦ brain circulation modulator ♦ voltage Na+ channel modulator | [271] | |||||
| Cyclobuxidine F derivatives |
♦ | ♦ | [287] | ||||
| Polyphenols | |||||||
| Luteolin | ♦ | ♦ | ♦ | ♦ | ♦kinase modulator umitochondrial protector | [288] [289] [290] | |
| Myricetin | ♦ | ♦ | ♦anti-neuroinflammatory uNMDA receptor modulator uBACE-1 inhibitor | [291] [292] [293] | |||
| Plant derived Coumarins: Decursinol, Mesuagenin |
♦ | ♦ | ♦ | ♦anti-neuroinflammatory uIMAOs | [294] [295] [296] | ||
| Ensaculin | ♦ | ♦serotonin 5-HT1A agonist ♦ NMDA receptor modulator (antag.) udopamine D2 receptor antagonist | II | [297] [298] | |||
| Epigallocatechin gallate | ♦ | ♦ | ♦BACE-1 inhibitor (?) uα-secretase inhibitor ukinase modulator uα-synuclein inhibitor umetal chelator uanti-inflammatory | II -III | [299] [300] [301] [302] [303] | ||
| Ferulic acid | ♦ | ♦ | ♦BACE-1 inhibitor uprotective against PSEN1 expression (transgenic mice) | [304] [305] [306] | |||
| Rosmarinic acid | ♦ | ♦ | ♦ | ♦ | ♦anti-apoptotic uneuroprotective ubinds to muscarinic M1, nicotinic, serotonin 5-HT1A, serotonin 5-HT2A and histamine H3 receptors | [291] [307] [293] [271] | |
| Nordihydroguaiaretic acid | ♦ | ♦ | [293] | ||||
| Curcumin | ♦ | ♦ | ♦ | ♦anti-inflammatory uBACE-1 inhibitor uα-secretase inhibitor utau dimerization inhibitor umetal chelator uneuroprotective ♦ NMDA receptor modulator (antag.) | II | [308] [309] [310] [311] [312] [313] | |
| Polyphenols | |||||||
| Resveratrol | ♦ | ♦ | ♦ | ♦ SIRT1-ROCK1 signaling pathway regulator uBACE-1 inhibitor uapoptosis modulator uanti-inflammatory | III (n) | [314] [310] [315] [316] [317] [318] | |
| Vitisin A, Heyncanol A | ♦ | ♦ | [319] | ||||
| Hopeahainol | ♦ | ♦ | ♦ | ♦neuroprotective | [320] [321] | ||
| Apocynin | ♦ | ♦anti-inflammatory uNADPH oxidase inhibitors | [225] | ||||
| Honokiol, Magnolol | ♦anti-apoptotic uneuroprotective | [322] | |||||
| Cannabinoids | |||||||
| Cannabidiol | ♦ | ♦neuroprotective usedative | [271] | ||||
| Terpenoids | |||||||
| Niloticane | ♦ | ♦ COX-1 inhibitor ♦ COX-2 inhibitor | [323] | ||||
| Timosaponin AIII & BII | ♦ (AIII) | u(BII) | u(BII) | ♦anti-inflammatory uBACE-1 inhibitor (BII) | [324] [325] | ||
| Withanolide | ♦ | ♦ | ♦ | ♦neurotrophic uBACE-1 inhibitor uα-secretase inhibitor uLDL receptor-related protein enhancer | [326] [327] [328] [329] | ||
| Asiatic acid | ♦ | ♦ | ♦ | ♦BACE-1 inhibitor uanti-apoptotic uanti-inflammatory uα-secretase inhibitor ukinase modulator | [330] [329] [331] | ||
| Sage monoterpenoids | ♦ | ♦ | ♦anti-inflammatory | [271] | |||
| Ginsenosides | ♦ | ♦ | ♦ | ♦neuroprotective ♦ NMDA receptor modulator (antag.) | [271] [275] | ||
| Diverse Compounds | |||||||
| Bryostatin | ♦ | ♦α-secretase inhibitor ukinase modulator | I-II | [332] | |||
| Epothilone | ♦ | ♦ microtubule stabilizer | [333] | ||||
| Diverse Compounds | |||||||
| Geldanamycin | ♦ | ♦ proteasome activator | [208] | ||||
| Rapamycin | ♦ | ♦immunosuppressant | [334] | ||||
| Zeatin | ♦ | ♦ | ♦neuroprotective | [335] [336] | |||
| Butylphtalide (NBP) | ♦ | ♦ | ♦ | ♦αAPPs increased release uanti-inflammatory ukinase modulator uanti-apoptotic | [337] | ||
| Minocycline | ♦ | ♦ | ♦ | ♦BACE-1 inhibitor uanti-apoptotic uanti-inflammatory uneurogenic | [338] [339] [340] [341] | ||
| Geniposide, Gardenoside | ♦ memory enhancement uanti-apoptotic uneurogenic | [342] [343] | |||||
| WIN-026 (KRWAR-026; INM-176) | ♦ | ♦ | ♦ | III | [344] [345] | ||
2.3.1. An Example of Designed MTDL: the Path from Selegiline to Ladostigil
There are also interesting examples of MTDL design based on the combination of two or more pharmacophores acting on different targets of the disease. A good illustrative example is the case of a very promising drug for AD treatment, ladostigil (Table 2), which emerged from selegiline [137]. Selegiline, a selective and irreversible monoamine oxidase B inhibitor (IMAO-B) that failed as antidepressant, but led to the discovery of a new therapy in (PD), also showed neuroprotective properties and is the precursor of rasagiline, [138] a less neurotoxic drug also used in PD therapy. The inhibition of MAO avoids hydrogen peroxide generation and the formation of neurotoxic free radical species. The incorporation of a carbamate moiety, the pharmacophore group of the AChE inhibitor rivastigmine, on the molecule of rasagiline, led to the design of ladostigil, which resulted in an inhibitor of both cholinesterases (AChE and BuChE) and both brain MAO (MAO-A and MAO-B). [139-141]. In addition to this inhibitory effects, ladostigil (but also rasagiline) presented other neuroprotective actions, such as to stimulate the processing of APP to the neuro-protective sAPPα. Furthermore, ladostigil protected against oxidative stress-induced neuronal apoptosis, increases antioxidant enzymes’ expression and catalase activity [142]. Finally ladostigil also increased the brain-derived nerve factor (BDNF) mRNA expression, leading to improved BDNF formation and, consequently, to enhanced neuro-protective activity [143]. Thanks to the wide MTDL profile, ladostigil is not only considered as a promising candidate for AD treatment but also for other neural diseases, namely PD and amyotrophic lateral sclerosis (ALS). The last step in the path followed by Youdim’s group is the attempt to incorporate into the molecule an iron chelator moiety [144], the way is paved to continue the path.
Table 2.
Examples of Designed Multi-Target-Directed Ligands
| Compound | Merged Pharmacophores | Additional Activities | References |
|---|---|---|---|
| Dual Acetylcholinesterase and β-amyloid inhibitors (Drugs interacting with Acetyl and/or Butyrylcholinesterases binding simultaneously to the catalytic anionic site (CAS) and the peripheral anionic site (PAS) or only to PAS) | |||
| Xanthostigmine | Rivastigmine - xanthone hybrid | [4] | |
| AP2238 | Coumarin - dibenzylamine hybrid | [346] | |
| IQM-622 | Tacrine - 8-hydroxyquinoline hybrid | [347] | |
| Indanone-tacrines | Tacrine - donepezil hybrid | [348] | |
| Pyrano[3,2-c]quinoline tacrines | Tacrine - pyrano[3,2.c]quinoline hybrid | [349] | |
| NP-61 (NP-0361) (Structure not disclosed) | Probable tacrine - indole propionamide hybrid | [350, 351, 352] |
|
| Bis(7)-tacrines | Bis-tacrines | BACE1 inhibitors | [353, 354] |
| Tacrine multimers | [355] | ||
| Huprine-tacrines | Tacrine - huprine hybrids | BACE1 inhibitors | [356, 357] |
| Donepezil-huprine derivatives | Huprine - Donepezil hybrids | BACE1 inhibitors | [358] |
| Indanone-dibenzylamines | AP2238 - Donepezil hybrids | [359] | |
| N-benzylpiperidine purine derivatives | Donepezil analogues | [360] | |
| 9-substituted berberines | [361] | ||
| 9-substituted berberines containing tiazole | [362] | ||
| Isaindigotone derivatives | [363] | ||
| Oxoisoaporphines | [364, 365] | ||
| Oxoisoaporphine-tacrines | Tacrine - oxoisoaporphine hybrids | [366] | |
| Multialkoxybenzene-tacrines | Tacrine – multialkoxybenzene hybrids | [367] | |
| 2,4-disubstituted pyrimidines | [368, 369] | ||
| Piperidinium-type and 1,4-dihydropiridine derivatives | [167, 166] | ||
| Piperidine linked derivatives | [370] | ||
| Glutamic acid linked derivatives | Neuroprotective against free radicals | [371] | |
| Benzofuran-based hybrids | [372] | ||
| Benzophenone linked derivatives | [373] | ||
| Bis-nor-meptazinols | [168] | ||
| Dual Acetylcholinesterase inhibitors and antioxidants | |||
| Lipocrine | 6-Chlorotacrine - α-lipoic acid hybrid | β-amyloid inhibitor | [374] |
| Memoquin and derivatives | Caproctamine - 1,4-benzoquinone hybrid | β-amyloid inhibitor Muscarinic M2 receptor antagonist BACE1 inhibitor |
[375, 376, 377, 378, 379] |
| Benzoquinone curcumin hybrid Benzoquinone SKF64346 hybrid |
[380] | ||
| Tacrine melatonin hybrids | [381, 382] | ||
| Dual Acetylcholinesterase inhibitors and antioxidants | |||
| Tacrine ferulic acid hybrids | [383] | ||
| Cystamine-tacrine dimer | [384] | ||
| N-acylaminophenothiazines | [385, 386] | ||
| Dual Acetylcholinesterase inhibitors and metal chelators | |||
| HLA20A | Carbamoylated 8-hydroxyquinolines | [387, 388] | |
| Indanone derivatives | [389] | ||
| Tacrine-8-hydroxyquinoline hybrids PBT2 | Neuroprotective Antioxidant |
[347, 390] | |
| Dual Acetylcholinesterase and β-secretase (BACE1) inhibitors | |||
| Memoquin | Caproctamine - 1,4-benzoquinone hybrid | β-amyloid inhibitor Muscarinic M2 receptor antagonist Antioxidant | [375, 376, 377, 378, 379] |
| Coumarin derivatives | AP2238 derivatives | [391] | |
| Bis(7)-tacrine | Tacrine homodimers | Calcium channel blocker | [353, 392, 393, 354] |
| Tacrine-chromene hybrids | β-amyloid inhibitor | [394] | |
| Huprine-tacrines | Tacrine - huprine hybrids | [356, 357] | |
| Donepezil-huprine derivatives | Huprine - Donepezil hybrids | [358] | |
| N-benzylpiperidines with BACE1 inhibitory moieties | Donepezil - BACE1 inhibitor hybrid | [395] | |
| Quinoxaline based hybrids | Histamine H3 receptor antagonist | [396] | |
| Dual Acetylcholinesterase and monoaminooxidase B (MAO-B) inhibitors | |||
| Ladostigil | Rasagiline - rivastigmine hybrid | Neuroprotective metal chelator β-amyloid modulator |
[397] [398] [141] |
| Propargylamino -benzylpiperidine hybrids | MAOB pharmacophore – CAS AChE pharmacophore hybrid | [399] | |
| Dual Acetylcholinesterase inhibitors and acetylcholine receptor ligands | |||
| Caproctamine | Muscarinic M2 receptor antagonist | [400] | |
| Huprine X (3-Chloro-9-ethyl) | Tacrine – huperizine A hybrids | Muscarinic M1 and M2 receptors agonist | [401] [402] |
| Bis(12)hupyridone | Huperizine A dimer | α7 nicotinic receptor modulator | [403] |
| Ro-46-5934 | Neostigmine derivative | Muscarinic M2 receptor antagonist | [404] |
| Dual Acetylcholinesterase inhibitors and histamine H3 receptor antagonists | |||
| FUB833 | Tacrine-piperidine hybrids | Histamine N-methyltransferase inhibitor (HNMT) | [405] [406] |
| Dual Acetylcholinesterase inhibitors and histamine H3 receptor antagonists | |||
| Quinoxaline derivatives | Quinoxaline-piperidine hybrids | BACE1 inhibitor | [396] |
| Tacrine – piperidine hybrids | [406] | ||
| Dual Acetylcholinesterase inhibitors and N-methyl-D-aspartic acid (NMDA) receptor channel blockers | |||
| Carbacrine | Tacrine – carvedilol hybrid | β-amyloid inhibitor Antioxidant |
[407] |
| Bis(7)-tacrine | Tacrine homodimers | BACE1 inhibitor | [408] [392] [353] [393] [354] |
| Bivalent β-carbolines | β-carboline homodimers | [409] | |
| Dual Acetylcholinesterase inhibitors and serotonin 5-HT6 antagonists | |||
| Latrepirdine (dimebon) | IMAO-B NMDA receptor inhibitor |
[410, 411, 412, 413] | |
| Dual Acetylcholinesterase inhibitors and serotonin 5-HT3 receptor antagonists | |||
| Tacrine – Arylpiperazine hybrids | [414] | ||
| Dual Acetylcholinesterase inhibitors and cannabinoid receptor antagonists | |||
| Diaryl-pyrazolines and diaryl-imidazolines | Tacrine – CB1 antagonist scaffolds | [415] | |
| Arylbenzofuran-based derivatives | [372] | ||
| Dual Acetylcholinesterase and serotonin transporter inhibitors | |||
| RS-1259 (BCG-20-1259) | Rivastigmine – fluoxetine hybrid | [174] | |
| Dual Acetylcholinesterase and σ 1 receptor inhibitors | |||
| SP-04 | [416] | ||
| Dual Acetylcholinesterase inhibitors and calcium channel blockers | |||
| Bis(7)-tacrine | Tacrine homodimers | BACE1 inhibitor NMDA channel blocker |
[392, 353, 393, 408, 354] |
| Tacripyrines: ITH-4012 | Tacrine – dihydropyridine hybrid | [417] | |
| Tacripyrines: ITH-12118 | Tacrine – dihydropyridine hybrid | [418, 419, 420] | |
| Tacripyrines | [421] | ||
| Dihydropyrimidoquinolinediones | [422] | ||
| Dual Acetylcholinesterase inhibitors and platelet activating factor (PAF) antagonists | |||
| PMS-777 | [423] | ||
| PMS-1339 | [424] | ||
| Dual monoaminooxidase B (MAOB) inhibitors and iron-chelating agents | |||
| VAR 10200 (HLA-20A) | 8-hydroxyquinoline – propargylamino pharmacophore hybrid | [387, 388] | |
| VAR 10300 (M-30D) | 8-hydroxyquinoline – propargylamino pharmacophore hybrid | [425, 426, 427, 398] | |
| Dual monoaminooxidase B (MAOB) inhibitors and adenosine A2 receptor antagonists | |||
| Istradefylline (KW-6002) | Caffeine derivative | [428, 429, 430] | |
| Dual monoaminooxidase B (MAOB) inhibitors and peroxisome proliferator-activated receptor gamma (PPARg) modulators | |||
| Pioglitazone, Rosiglitazone | [431, 432, 433] | ||
| Dual histamine H3 receptor and presynaptic acetylcholine muscarinic M2 receptor antagonists | |||
| 4,4’-bispiperidines | [434] | ||
| Dual NMDA receptor channel and L-type calcium channel blockers | |||
| NGP1-01 (polycyclic cage amines) | Neuroprotective Reduces endothelial iron accumulation |
[435, 436, 437] | |
| Dual NMDA receptor channel blocker and serotonin 5-HT3 antagonist | |||
| Memantine | Tau protein phosphorylation inhibition | [438, 439] | |
| Dual phosphodiesterase-4 inhibitor and α-secretase activator | |||
| Etazolate | GABAA receptor modulator | [440] | |
| Dual β-secretase (BACE1) inhibitor and metal chelator | |||
| 1,3-Diphenylurea derivatives | [441] | ||
| Tryptoline and tryptamine triazole derivatives | Anti-amyloid aggregation | [442, 443] | |
| Dual γ-secretase and peroxisome proliferator-activated receptor gamma (PPARg) modulators | |||
| 2-(bis(phenethoxy)pyrimidine-2-ylthio)hexanoic acid and derivatives | [176, 444] | ||
| Dual glycogen synthase kinase-3β (GSK-3) and phosphodiesterase-7 (PDE7) inhibitor | |||
| VP1.15 (2,3-diphenyl-1,2,4-thiadiazole derivative) | [445] | ||
| Dual protein kinase C (PCK) activators and histone deacetylase (HDAC) inhibitors | |||
| Hexahydrobenzo[e]1,4diazocin-3-ones | [446] | ||
| Dual β amyloid oligomerization inhibitors and free radical scavengers | |||
| Phenolic bis-styrylbenzenes | [447] | ||
| Curcumin - cholesterol bivalent ligands | [448] | ||
| Metal chelators which also target β amyloid | |||
| EDTA-2-phenylbenzothiazol-derivatives | [449] | ||
| Benzimidazol-derivatives | [450] | ||
| Dual metal chelators and antioxidants | |||
| Deferiprone – BHT hybrids | [451] | ||
| Glucopyranosyl conjugates of deferiprone and of tetrahydrosalen | Pro-drugs | [452, 453] | |
2.3.2. Natural Origin MTDL: Cannabinoids in AD Therapy
Among the substances with the ability to reduce or mitigate neurodegenerative symptoms, the group of cannabinoids has to be taken into consideration [145, 146]. The connection between the endocannabinoid system and AD has been reported consistently [119, 147-149].
The therapeutic potential of cannabinoids [150] in AD becomes apparent in several mechanisms: non-competitive AChE inhibition [151]; anti-aggregation of Aβ peptides mediated by AChE PAS inhibition or by phagocytosis of Aβ peptides mediated by CB2 receptors [152]; anti-glutamatergic effect [153]; anti-oxidant activity [147]; anti-neuroinflammatory properties [154]. It is also noteworthy
that cannabidiol exerts an inhibitory effect on τ phosphorylation [155]. Recently it has been described that CB1 activation could rescue rat Hippocampal CA1 Pyramidal Neurons from Aβ deleterious action [156].
In this context neuroprotective effects have been described for: HU-211, acting in neurons under neurotoxic environment of glutamate [157]; palmytoyletanolamide, an endocannabinoid that increases survival of cerebellum granular neuron [158]; WIN 55212-2 exerts a protector effect on hippocampus neurons [159]; Δ9-THC protects spinal medulla neurons against excitotoxicity mediated by CB1 receptor [160]. In addition, several in vivo studies reveal that cannabinoids, as Δ9-THC and anandamide, protect neonatal rat brain [161, 162]. Also, Iuovone et al. reported neuroprotective, anti-oxidative and anti-apoptotic effects of cannabidiol against the neurotoxic action of βA [163].
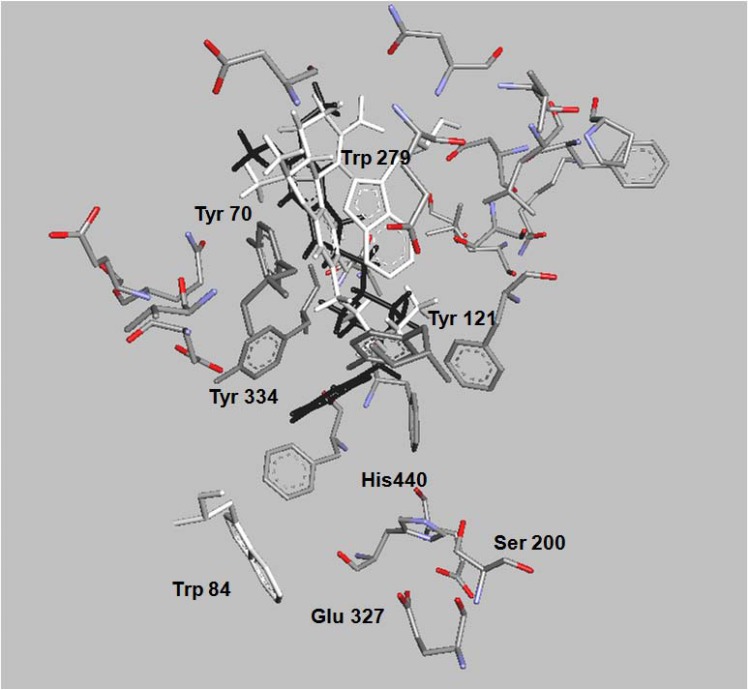
Docking and in vitro studies have shown, that Δ9-THC might bind to AChE with a similar or even higher affinity than reported PAS binders [149], being the AChE inhibitory effect of Δ9-THC and other synthetic cannabinoids as modest as referred for other PAS binders [149]. To this end the Sánchez-Montero group follows a new strategy trying to bind together the described neuroprotective properties of cannabinoids with enhanced AChE inhibition in a single molecule that could pave a new way in the treatment of AD and other neurodegenerative diseases [133, 164]. Blocking both, CAS and PAS of AChE, might not only alleviate the cognitive deficit of AD patients by elevating acetylcholine (ACh) levels through their AChEI activity, but might also act as disease modifying agents via the inhibition of Aβ aggregation. Successful attempts in this sense are reported in the examples of bis-nor-meptazinol derivatives [165] and benzylidene-hydrazono-dihydropyridines, [166], where the dual behavior as AChEI and Aβ fibril formation inhibitors, among others, is observed. In both cases docking studies illustrate the interaction of these compounds with amino acids of the PAS. In the same way, and following a similar methodology, molecular docking of Δ9-THC (white) shows a similar fit with both sites, CAS and PAS, where the interaction with Tyr 121 is noteworthy, (Fig. 2) [164]. It has to be remarked that the fitting of Δ9-THC and donepezil (black) with the amino acids of the PAS, shown in the figure, are very similar [167, 168].
Fig. (2).
The binding pattern of dronabinol (∆9-THC; (tetrahydrocannabinol) (white) and donepezil (black) to acetylcholinesterase is shown. The represented amino acids showed in the binding site are part of PAS, CAS and the active catalytic site of the enzyme.
3. AD THERAPEUTIC TARGETS
Targeting the AD appears to be very elusive, although some general pathways –not exclusive to AD- are recognized in different pathogenic cascades, each of them has conducted various “hypothesis” of AD. Researchers are following different approaches to find the optimal molecules. Strategies that according to Grill and Cummings can be classified into two groups, depending on where is the focus for the drug targets is that is, symptomatic therapies, and disease-modifying therapies [169].
3.1. Symptomatic Targets
This strategy is involved in targeting neurotransmission either on neurotransmitters and receptors as ACh [170], GABA and opioid receptors [171], opioid receptor ligands [172], glutamate and NMDA [173], 5-HT (or the serotonin transporter, SERT) [174], H3 (histamine) [175], peroxisome proliferator-activated receptor γ (PPARγ) [176]; or on the enzymes involved in their metabolism. Whereas disease-modifying targets strategy is related to Aβ, τ protein and different aspects of neuroprotection. However, the molecules obtained do not fall exclusively into one or another group, and it has been found that compounds targeting neurotransmission also are involved in many other aspects. As an example of the situation targeting synaptic dysfunction [177, 178] will pertain to both targeting neuro-transmission and to disease-modifying targets, as Aβ oligomers induce synaptic dysfunction [24]. Thus, multi-targeting surpasses the classifying capacity of the initial two-group classification.
3.1.1. Targeting Cholinergic System and Glutamatergic Neurotransmission
Among the drugs for the treatment of AD symptoms, currently they target either cholinergic or glutamatergic neurotransmission, and the first group constitutes the majority of the approved drugs. Memantine, is up to date the only approved drug that antagonizes NMDA-type glutamate receptors, thus preventing excitotoxicity by an excess of aberrant neuronal stimulation [42]. On the other hand, according to cholinergic hypothesis, AChE hydrolyze ACh, leading to decreased levels of the neurotransmitter, then inhibiting CAS on the enzyme will raise ACh levels, recovering the loss of cholinergic transmission and synaptic density in AD patients. This was for a long time the premise that led to optimize the search for molecules that could inhibit AChE with fewer side effects [179]. Recently, it has been pointed out that H3 antagonists can also elevate ACh levels in some cerebral areas, enhancing memory preclinical models; it is thought that there are multiple biochemical pathways involved in the mechanisms [180]. On the other hand, Inestrosa et al. [61] reported that AChE is also implicated in AD pathology by accelerating Aβ aggregation, due to the activity of enzyme PAS, therefore AChE became an enzyme that could be targeted by two means. AChE also has some non-catalytic trophic functions whose relevance as a valuable therapeutic target –if any- is not yet defined [181, 182].
Cholinergic brain receptors are another target that has been researched [183]; both muscarinic and nicotinic acetylcholine receptors are implicated in the pathophysiology of AD, although the relevance of the different subtypes in the disease is not yet clear [20, 57]. Activation of M1 muscarinic receptor was reported by Nitsch et al. [184] as a stimulator of the non-amyloidogenic processing of the APP preventing the formation of AB, via PKC activation and MAPK dependent pathways, and decrease τ hyper-phosphorylation via GSK-3β inhibition [185]. More recently, Fisher argues that cholinergic hypofunction could be expected to be restored to normal, specifically via selective activation of M1 muscarinic receptors, and then alter the onset or progression of AD [57].
Finally, targeting butyrylcholinesterase (BChE), a serine hydrolase related to acetylcholinesterase that also catalyzes the hydrolysis of acetylcholine, has been considered relevant because of its implication in the pathogenesis of AD [186] and is considered a possible responsible of the inefficacy of AChE selective inhibitors [69].
3.2. Disease Modifying Targets
The other targeting strategy, namely disease-modifying tries to intervene in the different pathologic processes developed in AD; taking them as targets: defective proteins, ApoE4, oxidative/nitrosative stress, mitochondrial damage, altered apoptosis, autophagy impairment, neuroinflammation and activation of immune system, Ca2+ and metal dyshomeostasis, and prostacyclin and endocannabinoid system signaling. In addition to these targets, recently it is been proposed the possibility to modulate epigenetic modifications what could be preventive and also curative target.
3.2.1. Targeting Defective Protein: Aβ and τ
Aberrant accumulation of misfolded proteins is double targeted, Aβ [187] and τ protein aggregation [188], although there are many confluences and overlapping characteristics between the two targets.
In the endoplasm reticulum (ER) modulation of the stress pathways and their interactions with mitochondria are being investigated as feasible targets of Aβ production, as ER is not only the main organelle for the synthesis and processing of proteins as Aβ peptides -but also the main cellular source of Ca2+- [189]. Also, targeting retromers -proteins that modulate the endosomal residency time of secretases- can be modulated through the production of Aβ peptides, by mediating the retrieval of transmembrane proteins from endosomes to the trans-Golgi network [190].
Another crucial step in peptides processing path is molecular recognition and self-assembly events targeted by using hybrid molecules such as α-aminoisobutyric acid plus an aromatic moiety, that could inhibit amyloid fibrils and oligomers formation [191].
There are also attempts to disrupt aberrant APP processing, by targeting β- or γ-secretase either by inhibiting their activities [192] or -more effectively- modulating its activity. In this way, it has been found that some non-steroidal anti-inflammatory drugs (NSAID) reduce oligomer formation by modulating γ-secretase activity indirectly [193], as inhibiting secretases directly is somehow dangerous given the importance of other crucial substrates that could be affected [24, 194]; thus, the search for more selective compounds is continued [195]. Inhibiting secretase compounds are found in natural origin as stilbenoids, from the ethyl acetate soluble fraction of Smilax china L., and show an intense BACE1-inhibiting activity [196]. Chemically related compound stilbenes (compounds related to resveratrol) have been suggested as neuroprotective agents against AD; however, the mechanism for this remains unclear [197]. In the other way around, targeting α-secretase is also under scrutiny as its activity generates sAPPα that, as described previously, has neuroprotective effects [198]. Regarding this effect sirtuins –a family of histone deacetylases involved in numerous cellular signaling pathways- and more specifically SIRT1, has been shown to direct APP processing by activating expression of the α-secretase gene ADAM10; upregulation of SIRT1 can also induce the Notch pathway which has the capacity to repair neuronal damage [199] and also inhibit mTOR (mammalian target of rapamycin, see 3.2.3) signaling [200].
Other ways to eliminate pathologic Aβ peptides could be by means of immunotherapy either vaccinating with Aβ active-peptide or passive infusion of anti-Aβ monoclonal antibodies but the failures with vaccination -mentioned earlier- has imposed more cautious procedures as in the trial where 6% of treated patients developed autoimmune menin-goencephalitis [114]. Another approach to eliminate oligomers could be the stimulation of its selective degradation by proteases as plasmin or cathepsin B [24]; resveratrol would also enhance the clearance of Aβ peptides [201], although some authors showed concern on the capacity of resveratrol to prevent oligomer formation [202]. Perhaps, the most promising way of removing Aβ up to date is by modulating APOE expression (see below targeting ApoE4).
Regarding targeting τ protein, τ kinases, including CDK5, GSK-3β, MARK, MAPK, are considered reliable targets for AD therapy as modulators of τ phosphorylation, although there are serious concerns about the safety of their use, and it is thought that it would be better to reduce τ protein levels than τ phosphorylation [3]. Adding a touch of overlapping, GSK-3β has also been related with presenilins and with Aβ [203, 204]. Methylene blue, whose mechanism of action was first believed to be an inhibition of τ-τ interactions, later has been shown to possess other actions as reducing soluble τ protein and also other activities [205]. Recently the relationship among Aβ, τ protein and mTOR has been pointed out [206]. Also, misfolded τ protein of neurofibrillary tangles constitutes a primary target struggled by geldanamycin through the inhibition of heat shock protein 90 (HSp90), a molecular chaperone with important roles in regulating protein folding related to pathogenic transformation [207]. Thus, geldanamycin would activate proteasome resulting in an accelerated degradation of misfolded τ protein [208].
Another protein that can be targeted is TAR-DNA-binding protein-43 (TDP-43), which is a predominantly nuclear protein identified as a regulator of crucial trans-criptional events in the central nervous system and is strongly associated with neurodegeneration [209]. Aggregates of TDP-43 in patients with ALS and frontotemporal dementia, and also a correlation with τ protein accumulation has been found [30].
3.2.2. Targeting ApoE4
Targeting ApoE4 has been studied from its dependent and independent effects on Aβ. The anti-aggregation and clearance action of ApoE4 on Aβ has led to the search ways of reducing its expression. ApoE expression is transcriptionally regulated by the ligand-activated nuclear receptor PPARγ that acts with liver X receptor, and both form heterodimers with retinoid X receptors (RXRs), leading to microglial activation and suppressing the inflammatory status of the brain [118]. It has been shown that chronic administration of agonists of both receptors reduces Aβ levels and also improves cognitive function in mouse models of AD. Although PPARγ agonists phase III clinical trial outcome was negative [118] hopes have not decreased and bexarotene, a blood-barrier permeant RXR agonist, is the new promising compound which has displayed its efficacy in preclinical models of AD [210, 211]. Another approach to target ApoE4 is by means of identifying the so called “small-molecule structure correctors” that are capable of disrupting ApoE4 domain interaction (altered protein conformation) that makes it highly susceptible to proteolytic cleavage and the generation of neurotoxic fragments [101, 102].
3.2.3. Other Disease Modifying Targets
Classic AD hallmarks are closely accompanied by other pathologic processes: increased oxidative/nitrosative stress, neuroinflammation, microglia accumulation, mitochondrial damage, autophagy impairment, increased apoptosis, altered integrity and function of BBB, aberrant cholesterol metabolism, and Ca2+ and metal dyshomeostasis. In addition, very recently it has been shown that prostaglandins and endocannabinoid signaling are impaired in AD [212]. These events are under scrutiny for research that can be both directly or indirectly targeted. Despite the fact that these processes are considered secondary and less relevant, they are guiding the search for new MTDLs and also incorporating specific moieties with capacity to cope each of them. In the following paragraphs their relevance to AD therapy and the way they can interact with each other and with other targets will be shown.
Oxidative/nitrosative stress is one of the central pathologic processes present in AD, albeit previous, consequence or simultaneous to the disease, some researchers have put their efforts to target them by using antioxidants [213], and to actively incorporate them to MTDL for e.g. with retinoids [214], or lipoic acid [215]. The relationship between oxidative stress and immune function and its relevance for searching targets has been studied by some authors [216, 217]. Another recent field of research of new MTDL is of PLA2 inhibitors, which has been discussed in 1.2.6. cPLA2 is involved in oxidative/nitrosative aberrant signaling pathways that are linked to excessive NMDA activation and to NADPH oxidase activity, and also seems to play important roles in the pathophysiology of neuro-degenerative diseases such as AD [218-221]. However, it should be determined whether to activate [222] or to inhibit [223, 224] PLA2. To this respect botanical phenolics are considered as valuable substances within these novel therapeutic strategies for AD [23, 225].
Another pathological process present in AD patients is neuroinflammation that raised some interest as a possible target. In this regard NSAIDs were proposed as therapeutic preventing compounds [226] (see 3.2.1). Neuroinflammation is related to an increased microglia accumulation that has been observed nearby amyloid plaques, but interestingly increasing Aβ clearance, has led to a novel therapeutic strategy [227].
Mitochondria play a crucial role in cell survival and death through multiple functions; mitochondrial dysfunction and altered dynamics are present early in AD and are crucial in its pathogenesis [228]. Among the mitochondrial dysfunctions, results of induction of oxidative damage are crucial in AD pathology [229], Ca2+ homeostasis and neuro-inflammation [152]. In addition, it has been shown that ER is linked to mitochondria both physically and biochemically, via a specialized subcompartment of ER named mitochondria-associated ER membranes (MAM), where APP would be processed, as MAM reveals PS1, PS2 and γ-secretase activity [230]. Recently, specific mitochondria therapeutic drugs as mitochondria-directed antioxidants and Szeto–Schiller peptides has been proposed, whereas small cell permeable peptide antioxidants target mitochondria in a potential-independent manner [231, 232]. Methylene blue which has been discussed above due to its inhibiting activity of τ protein also appeared to protect mitochondrial function in addition to enhance brain metabolism and hemodynamics [233].
Autophagy is a crucial mechanism for eliminating defective cellular products, and whenever this function is defective or insufficient will lead to cellular dysfunctions as neurodegeneration that is found in AD and other diseases as PD or ALS. On the contrary, an increase of autophagy function will conduce to the clearance of those products and subsequently to the cellular recovery, if not too late. Thus, there has been put in a lot of efforts to obtain compounds with the ability to enhance this cellular function; Sarkar et al. in their review show an exhaustive list of 30 autophagy enhancers [234]. The authors differentiate among autophagy enhancers that are mTOR dependent and those that are not. mTOR is an evolutionarily conserved Ser/Thr protein kinase that plays a crucial role in several biological processes as regulation of cell growth and survival. Recently, mTOR has been proposed as a valuable target in obtaining compounds for AD and other neurodegenerative diseases [235]. Synaptic plasticity is another process under mTOR influence as there is a correlation of its impairment with the inhibition of mTOR signaling [236]. On the contrary, inhibition of mTOR reduces Aβ in vivo [237], and increases autophagy through the control of autophagy-lysosome protein degradation [238]. Also changes in mTOR levels in lymphocytes are related to AD [239]. Small molecule enhancers of rapamycin (SMERs) related to mTOR, also induced autophagy, improved cell viability and neurodegenerative proteins clearance in cellular models [240].
Apoptosis, a characteristic event of the neuro-degenerative diseases [241], has not been properly investigated as a target; however, Kobayashi et al. proposed increased astrocyte apoptosis as a new histological hallmark of white matter degeneration in AD [242]. Dyshomeostasis of both Ca2+ and metals have been lately used in MTDL research [4, 6, 243], in order to obtain anti-oxidative and anti-apoptotic activities among others.
Recently in AD, the event of the breakdown of BBB, and a deficient clearance of Aβ through it have been discovered. One of the disease modifying targets that has been proposed is RAGE (receptors for advanced glycation end products), which interacts with BBB and lead to oxidative stress, inflammation response and reduced cerebral blood flow. RAGE also interacts with circulating Aβ and mediates its transport across BBB into the brain [244]. Deane discusses the structure-function relation of RAGE-ligand interaction and suggests that is a potential target for the research of new molecules for treatment of AD [244]. In addition, Kook et al. identified a potential molecular pathway that underlies Aβ-RAGE interaction-induced breakdown of BBB [245]. Gelsolin is a molecule that could be inversely related to Aβ deposition in BBB, which is putatively produced in choroid plexus, although it is also expressed in a wide variety of organs. It has been found that gelsolin levels in brain are decreased in AD [246], and its administration could control the accumulation of Aβ in brain vessels walls, which eventually controls CAA progression [247]. In this way, a novel therapeutic target is tissue transglutaminase that is a Ca2+-dependent enzyme which presents covalent extracellular matrix protein cross-linking activity associated with CAA pathology. Thus, inhibiting this activity could also prevent CAA [32]. Megalin receptor has been also implicated in Aβ clearance from CNS across the choroid plexus epithelium, thus increasing its expression is an interesting target in the struggle against AD [248].
Another recently defined target for AD is monoacylglycerol lipase (MAGL), an enzyme of the serine hydrolase superfamily which is known for metabolizing lipids, but its relevancy as a target in AD therapy comes from its role of hydrolyzing the endocannabinoid molecule 2-arachidonoylglycerol (2-AG). 2-AG has many functions as retrograde messenger, such as modulating synaptic transmission and plasticity [249], and as neuroprotective. Consequently, MAGL inhibition has been shown to suppress Aβ production and accumulation, as well as to reduce BACE1 expression, and prevents neuroinflammation and neurodegeneration in AD in vivo models [250].
There are more targets under study such as those related to latrepirdine and its relationship with protein catabolism pathway [251], P53 and its functional cross-talk linking presenilins and Pen-2 [252], inhibition of the de novo synthesis of cholesterol by statins, that might decrease the risk of developing AD [253], toll-like receptors signaling in plasticity, neurogenesis and disease [254], MTDL based on iron chelating compounds with the ability to regulate the transcriptional activator hypoxia-inducible factor 1 (HIF-1), that could compensate for oxidative stress [144], or compounds that inhibit primary amine oxidase (PrAO) such as phenelzine, an enzyme which has been found to be implicated in the etiology of AD [131]. In addition, there are therapies related to nutritional supplements [255], such as proprietary diets that contain both polyphenols and polyunsaturated fatty acids [256]. Hormonal therapy, namely estrogen or testosterone treatments, is proposed to struggle against AD and may also reduce the risk or delay the onset of dementia [257, 258].
3.2.4. Epigenetics as a Possible Preventive and/or Therapeutic Target
In spite of the specificity of gene repression by epigenetic mechanisms it has been shown that the treatment with unspecific inhibitors could lead to good clinical outcomes. One of the modifiers of gene are histone deacetylases (HDACs) that induce decreasing or silenced gene transcription by transferring acetyl groups from acetylated histone protein back to CoA. Substances that show HDAC inhibiting activity (HDAC-I) have demonstrated their capacity to revert previous gene repression and reinstate, for instance, learning behavior in animal models, and to decrease Aβ [259]. Valproic acid, with HADC-I activity, seems to have the Aβ reducing capacity [260] but phenyl butyrate, another compound with HADC-I activity, did not act on the Aβ levels albeit normalized phosphorylated τ protein was present in hippocampus [261].
DNA methylation is another mechanism by which some genes are silenced or repressed in the living cell. To this respect, some nutrients namely folate, vitamin B12, vitamin B6, riboflavin, methionine, choline and betaine, are related to the epigenetic mechanism of DNA methylation [262], by acting on the levels of the universal methyl donor S-adenosylmethionine and methyltransferase inhibitor S-adenosylhomocysteine. Thus, folate/methionine/homocysteine metabolism has been proved to be highly relevant in this epigenetic mechanism. Folate deficiency results in a reversible hypomethylation in both animal models and humans, but alterations in the methionine/homocysteine are also related to the Aβ deposition [263].
In spite of the promising results obtained by the cited compounds, it is difficult to assume that new drugs developed to specifically modulate epigenetic changes could act without side effects [264]. On the other hand, this amplitude gives a framework for the diverse genetic and environmental factors interacting in the development of AD.
3.3. Targets and Lead Moieties
The multifactorial etiology of AD put forward the aptitude of the new therapy strategy of MTDL. However, the best way to follow is a maze, not only there are a multitude of mechanisms implied but also the selection of a lead molecule is not clearly seen, be it from natural or synthetic origin. The amount of hybrid molecules resulting from randomly joined moieties is out of capacity nowadays, and the number of possible candidates is large enough even for joining two different chemical groups, so the possible combinations using three or more moieties make the effort almost unfeasible.
The “tangled web of targets” [38] makes it difficult to show an organized taxonomy of the candidate molecules. One of the ways to classify them could be by grouping different chemical compounds within a target. Each group of molecules within the same target could further be grouped into a chemical lead, and then incorporated into different molecules or moieties that bring the additional properties of the original molecule. The amount of candidate molecules from natural origin suggests that they should be presented separately from the synthetic ones. Table 1 shows those natural origin compounds and some of their properties that could be useful in designing new molecules. In Table 2 are presented some useful non-natural origin compounds that are also good candidates to be considered in designing new MTDL. Fig. (3), materializes the concept that hybrid molecules will form a web of relationships among them, representing a conceptual map with hybrid molecules constructed from approved therapeutic drugs that are being investigated to maximize the benefits of two or more moieties grouped in a molecule.
Published reviews on MTDL classify the new molecules based on one chemical lead and then list the modifications or moieties addition that could be done on the scaffold to maximize the pharmacological parameters measured in it [5, 8, 69, 123, 124].
CONCLUSIONS
The question arises as to whether the search to cure AD is a kind of a search for a pot of gold. Every announcement with the final solution on it is followed by the news about its failure in one or another of its development steps. From years’ perspective this behavior seems to be the rule till date, thus the problem has remain unresolved and is most likely to remain unresolved through traditional approaches. It is a point of concern whether the reviewed targets lead towards the final solution. May be, but in spite of the enormous and crucial advancements in the knowledge of the mechanisms and implications in AD, multifactoriality of the disease perhaps would need a multifactorial answer. Multi-Target-Directed Ligands are believed to be precisely the right answer to the conundrum; or perhaps because it is probably that idiosyncrasy that people carry in their lives that would need to design more acutely the solutions, depending not only on the genetics, but also on the epigenetics and environment differences.
ACKNOWLEDGEMENTS
Authors acknowledge the valuable contribution to the elaboration of this review of David Muñoz-Solano and Wilma Villaro, members of the research team. Without their help, support and critical view; this review simply would not have been completed.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Massoud F, Gauthier S. Update on the pharmacological treatment of Alzheimer's disease. Curr. Neuropharmacol. 2010;8(1):69–80. doi: 10.2174/157015910790909520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. Lancet Neurol. 2010;9(7):702–716. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148(6):1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli A, Bolognesi ML, Minarini A, Rosini M, Tumiatti V, Recanatini M, Melchiorre C. Multi-target-directed ligands to combat neurodegenerative diseases. J. Med. Chem. 2008;51(3):347–372. doi: 10.1021/jm7009364. [DOI] [PubMed] [Google Scholar]
- 5.Bajda M, Guzior N, Ignasik M, Malawska B. Multi- target-directed ligands in Alzheimer's disease treatment. Curr. Med. Chem. 2011;18(32):4949–4975. doi: 10.2174/092986711797535245. [DOI] [PubMed] [Google Scholar]
- 6.Leon R, Marco-Contelles J. A step further towards multitarget drugs for Alzheimer and neuronal vascular diseases: targeting the cholinergic system, amyloid-beta aggregation and Ca(2+) dyshomeostasis. Curr. Med. Chem. 2011;18(4):552–576. doi: 10.2174/092986711794480186. [DOI] [PubMed] [Google Scholar]
- 7.Geldenhuys WJ, Van der Schyf CJ. Designing drugs with multi-target activity: the next step in the treatment of neurodegenerative disorders. Expert Opin. Drug Discov. 2013;8(2):115–129. doi: 10.1517/17460441.2013.744746. [DOI] [PubMed] [Google Scholar]
- 8.Csermely P, Korcsmaros T, Kiss HJ, London G, Nussinov R. Structure and dynamics of molecular networks: a novel paradigm of drug discovery: a comprehensive review. Pharmacol. Ther. 2013;138(3):333–408. doi: 10.1016/j.pharmthera.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol. Sci. 2005;26(4):178–182. doi: 10.1016/j.tips.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review. Int. J. Geriatr. Psychiatry. 2013;28(6):551–561. doi: 10.1002/gps.3864. [DOI] [PubMed] [Google Scholar]
- 11.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heun R, Schoepf D, Potluri R, Natalwala A. Alzheimer's disease and co-morbidity: Increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-up. Eur Psychiatry. 2013;28(1):40–48. doi: 10.1016/j.eurpsy.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci. Transl. Med. 2011;3(77): 0. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampel H, Prvulovic D, Teipel S, Jessen F, Luckhaus C, Frolich L, Riepe MW, Dodel R, Leyhe T, Bertram L, Hoffmann W, Faltraco F. The future of Alzheimer's disease: the next 10 years. Prog. Neurobiol. 2011;95(4):718–728. doi: 10.1016/j.pneurobio.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Terry RD, Katzman R. Senile dementia of the Alzheimer type. Ann. Neurol. 1983;14(5):497–506. doi: 10.1002/ana.410140502. [DOI] [PubMed] [Google Scholar]
- 16.Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10(10):1055–1063. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 17.Tiraboschi P, Hansen LA, Thal LJ, Corey-Bloom J. The importance of neuritic plaques and tangles to the development and evolution of AD. Neurology. 2004;62(11):1984–1989. doi: 10.1212/01.wnl.0000129697.01779.0a. [DOI] [PubMed] [Google Scholar]
- 18.van Marum RJ. Current and future therapy in Alzheimer's disease. Fund. Clin. Pharmacol. 2008;22(3):265–274. doi: 10.1111/j.1472-8206.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 19.Jellinger KA. Recent advances in our understanding of neuro- degeneration. J. Neural Transm. 2009;116(9):1111–1162. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- 20.Kar S, Slowikowski SP, Westaway D, Mount HT. Inter- actions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J. Psychiatry Neurosci. 2004;29(6):427–441. [PMC free article] [PubMed] [Google Scholar]
- 21.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011;221(2):555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 22.Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443(7113):768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- 23.Sun GY, He Y, Chuang DY, Lee JC, Gu Z, Simonyi A, Sun AY. Integrating cytosolic phospholipase A(2) with oxidative/ nitrosative signaling pathways in neurons: a novel therapeutic strategy for AD. Mol. Neurobiol. 2012;46(1):85–95. doi: 10.1007/s12035-012-8261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haass C, Selkoe DJ. Soluble protein oligomers in neuro- degeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 25.Standridge JB. Vicious cycles within the neuropathophysiologic mechanisms of Alzheimer's disease. Curr. Alzheimer Res. 2006;3(2):95–108. doi: 10.2174/156720506776383068. [DOI] [PubMed] [Google Scholar]
- 26.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 27.De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol. Rev. 2010;90(2):465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- 28.Endres K, Fahrenholz F. Regulation of alpha-secretase ADAM10 expression and activity. Exp. Brain Res. 2012;217(3-4):343–352. doi: 10.1007/s00221-011-2885-7. [DOI] [PubMed] [Google Scholar]
- 29.Prox J, Rittger A, Saftig P. Physiological functions of the amyloid precursor protein secretases ADAM10. BACE1 and Presenilin. Exp. Brain Res. 2012;217(3-4):331–341. doi: 10.1007/s00221-011-2952-0. [DOI] [PubMed] [Google Scholar]
- 30.Ubhi K, Masliah E. Alzheimer's Disease: Recent Advances and Future Perspectives. J. Alzheimers Dis. 2013;33 (Suppl 1 ):S185–194. doi: 10.3233/JAD-2012-129028. [DOI] [PubMed] [Google Scholar]
- 31.Stockley JH, O'Neill C. The proteins BACE1 and BACE2 and beta-secretase activity in normal and Alzheimer's disease brain. Biochem. Soc. Trans. 2007;35(Pt 3):574–576. doi: 10.1042/BST0350574. [DOI] [PubMed] [Google Scholar]
- 32.Wilhelmus MM, de Jager M, Drukarch B. Tissue transglutaminase: a novel therapeutic target in cerebral amyloid angiopathy. Neurodegener. Dis. 2012;10(1-4):317–319. doi: 10.1159/000333224. [DOI] [PubMed] [Google Scholar]
- 33.Thal DR, Ghebremedhin E, Orantes M, Wiestler OD. Vascular pathology in Alzheimer disease: correlation of cerebral amyloid angiopathy and arteriosclerosis/lipohyalinosis with cognitive decline. J. Neuropathol. Exp. Neurol. 2003;62(12):1287–1301. doi: 10.1093/jnen/62.12.1287. [DOI] [PubMed] [Google Scholar]
- 34.Ihara Y, Morishima-Kawashima M, Nixon R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harb Perspect. Med. 2012;2(8):2:a006361. doi: 10.1101/cshperspect.a006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riemer J, Kins S. Axonal Transport and Mitochondrial Dysfunction in Alzheimer's Disease. Neurodegener. Dis. 2012;12(3):111–124. doi: 10.1159/000342020. [DOI] [PubMed] [Google Scholar]
- 36.Khlistunova I, Biernat J, Wang Y, Pickhardt M, von Bergen M, Gazova Z, Mandelkow E, Mandelkow EM. Inducible expression of Tau repeat domain in cell models of tauopathy: aggregation is toxic to cells but can be reversed by inhibitor drugs. J. Biol. Chem. 2006;281(2):1205–1214. doi: 10.1074/jbc.M507753200. [DOI] [PubMed] [Google Scholar]
- 37.Maccioni RB, Farias G, Morales I, Navarrete L. The revitalized tau hypothesis on Alzheimer's disease. Arch Med Res. 2010;41(3):226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Gravitz L. Drugs: a tangled web of targets. Nature. 2011;475(7355):S9–11. doi: 10.1038/475S9a. [DOI] [PubMed] [Google Scholar]
- 39.Heinitz K, Beck M, Schliebs R, Perez-Polo JR. Toxicity mediated by soluble oligomers of beta-amyloid(1-42) on cholinergic SN56. 5.G4 cells. J. Neurochem. 2006;98(6):1930–1945. doi: 10.1111/j.1471-4159.2006.04015.x. [DOI] [PubMed] [Google Scholar]
- 40.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat. Rev. Neurosci. 2007;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 41.Fukumoto H, Tokuda T, Kasai T, Ishigami N, Hidaka H, Kondo M, Allsop D, Nakagawa M. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010;24(8):2716–2726. doi: 10.1096/fj.09-150359. [DOI] [PubMed] [Google Scholar]
- 42.Lipton SA. Pathologically-activated therapeutics for neuro- protection: mechanism of NMDA receptor block by memantine and S-nitrosylation. Curr. Drug Targets. 2007;8(5):621–632. doi: 10.2174/138945007780618472. [DOI] [PubMed] [Google Scholar]
- 43.Pham E, Crews L, Ubhi K, Hansen L, Adame A, Cartier A, Salmon D, Galasko D, Michael S, Savas JN, Yates JR, Glabe C, Masliah E. Progressive accumulation of amyloid-beta oligomers in Alzheimer's disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277(14):3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao LN, Long H, Mu Y, Chew LY. The Toxicity of Amyloid beta Oligomers. Int. J. Mol. Sci. 2012;13(6):7303–7327. doi: 10.3390/ijms13067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerpa W, Dinamarca MC, Inestrosa NC. Structure-function implications in Alzheimer's disease: effect of Abeta oligomers at central synapses. Curr. Alzheimer Res. 2008;5(3):233–243. doi: 10.2174/156720508784533321. [DOI] [PubMed] [Google Scholar]
- 46.Oddo S. The role of mTOR signaling in Alzheimer disease. Front Biosci (Schol Ed) 2012;4:941–952. doi: 10.2741/s310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim. Biophys. Acta. 2009;1792(1):3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 48.Zhu XC, Yu JT, Jiang T, Tan L. Autophagy Modulation for Alzheimer's Disease Therapy. Mol. Neurobiol. 2013 doi: 10.1007/s12035-013-8457-z. [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Terpstra EJ. Ubiquitin Receptors and Protein Quality Control. J. Mol. Cell Cardiol. 2013;55:73–84. doi: 10.1016/j.yjmcc.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCray BA, Taylor JP. The role of autophagy in age-related neurodegeneration. Neurosignals. 2008;16(1):75–84. doi: 10.1159/000109761. [DOI] [PubMed] [Google Scholar]
- 51.Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, Ohno M, Schmidt SD, Wesson D, Bandyopadhyay U, Jiang Y, Pawlik M, Peterhoff CM, Yang AJ, Wilson DA, St George-Hyslop P, Westaway D, Mathews PM, Levy E, Cuervo AM, Nixon RA. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer's disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134 (Pt 1):258–277. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bell KF, Claudio Cuello A. Altered synaptic function in Alzheimer's disease. Eur. J. Pharmacol. 2006;545(1):11–21. doi: 10.1016/j.ejphar.2006.06.045. [DOI] [PubMed] [Google Scholar]
- 53.Xu Y, Yan J, Zhou P, Li J, Gao H, Xia Y, Wang Q. Neurotransmitter receptors and cognitive dysfunction in Alzheimer's disease and Parkinson's disease. Prog. Neurobiol. 2012;97(1):1–13. doi: 10.1016/j.pneurobio.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ladner CJ, Lee JM. Pharmacological drug treatment of Alzheimer disease: the cholinergic hypothesis revisited. J. Neuropathol. Exp. Neurol. 1998;57(8):719–731. doi: 10.1097/00005072-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003;289(2):210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- 57.Fisher A. Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer's disease. J. Neurochem. 2012;120 Suppl 1:22–33. doi: 10.1111/j.1471-4159.2011.07507.x. [DOI] [PubMed] [Google Scholar]
- 58.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic-structure of acetylcholinesterase from torpedo-californica - a prototypic acetylcholine-binding protein. Science. 1991;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 59.Bartolini M, Bertucci C, Cavrini V, Andrisano V. beta-amyloid aggregation induced by human acetylcholinesterase: inhibition studies. Biochem. Pharmacol. 2003;65(3):407–416. doi: 10.1016/s0006-2952(02)01514-9. [DOI] [PubMed] [Google Scholar]
- 60.Chacon MA, Reyes AE, Inestrosa NC. Acetylcholinesterase induces neuronal cell loss, astrocyte hypertrophy and behavioral deficits in mammalian hippocampus. J. Neurochem. 2003;87(1):195–204. doi: 10.1046/j.1471-4159.2003.01985.x. [DOI] [PubMed] [Google Scholar]
- 61.Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, Casanueva OI, Soto C, Garrido J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez A, Alarcon R, Opazo C, Campos EO, Munoz FJ, Calderon FH, Dajas F, Gentry MK, Doctor BP, De Mello FG, Inestrosa NC. Stable complexes involving acetylcholinesterase and amyloid-beta peptide change the biochemical properties of the enzyme and increase the neurotoxicity of Alzheimer's fibrils. J. Neurosci. 1998;18(9):3213–3223. doi: 10.1523/JNEUROSCI.18-09-03213.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaucher E, Aumont N, Pearson D, Rowe W, Poirier J, Kar S. Amyloid beta peptide levels and its effects on hippocampal acetylcholine release in aged cognitively-impaired and -unimpaired rats. J. Chem. Neuroanat. 2001;21(4):323–329. doi: 10.1016/s0891-0618(01)00120-x. [DOI] [PubMed] [Google Scholar]
- 64.Nordberg A. Neuroreceptor changes in Alzheimer disease. Cerebrovasc. Brain Metab. Rev. 1992;4(4):303–328. [PubMed] [Google Scholar]
- 65.Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J. Neurosci. Res. 1992;31(1):103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez-Puertas R, Pascual J, Vilaro T, Pazos A. Autoradiographic distribution of M1 M2 M3 and M4 muscarinic receptor subtypes in Alzheimer's disease. Synapse. 1997;26(4):341–350. doi: 10.1002/(SICI)1098-2396(199708)26:4<341::AID-SYN2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Zheng WH, Bastianetto S, Mennicken F, Ma W, Kar S. Amyloid beta peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115(1):201–211. doi: 10.1016/s0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 68.Mills J, Reiner PB. Regulation of amyloid precursor protein cleavage. J Neurochem. 1999;72(2):443–460. doi: 10.1046/j.1471-4159.1999.0720443.x. [DOI] [PubMed] [Google Scholar]
- 69.Rampa A, Belluti F, Gobbi S, Bisi A. Hybrid-based multi-target ligands for the treatment of Alzheimer's disease. Curr. Top. Med. Chem. 2011;11(22):2716–2730. doi: 10.2174/156802611798184409. [DOI] [PubMed] [Google Scholar]
- 70.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 71.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence?. Nat. Med. 2004;10 Suppl:S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 72.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chem. Res.Toxicol. 2008;21(1):172–188. doi: 10.1021/tx700210j. [DOI] [PubMed] [Google Scholar]
- 73.Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J. Neurochem. 2008;104(3):683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mangialasche F, Polidori MC, Monastero R, Ercolani S, Camarda C, Cecchetti R, Mecocci P. Biomarkers of oxidative and nitrosative damage in Alzheimer's disease and mild cognitive impairment. Ageing Res. Rev. 2009;8(4):285–305. doi: 10.1016/j.arr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 75.Simonyi A, He Y, Sheng W, Sun AY, Wood WG, Weisman GA, Sun GY. Targeting NADPH oxidase and phospholipases A2 in Alzheimer's disease. Mol. Neurobiol. 2010;41(2-3):73–86. doi: 10.1007/s12035-010-8107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pagani L, Eckert A. Amyloid-Beta interaction with mitochondria. Int J Alzheimers Dis. 2011;2011(925050) doi: 10.4061/2011/925050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Azarashvili T, Stricker R, Reiser G. The mitochondria permeability transition pore complex in the brain with interacting proteins - promising targets for protection in neurodegenerative diseases. Biol. Chem. 2010;391(6):619–629. doi: 10.1515/BC.2010.070. [DOI] [PubMed] [Google Scholar]
- 78.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J Neurosci. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008;106(1):45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 80.Sun GY. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J. Lipid Res. 2003;45(2):205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 81.Sun GY, Horrocks LA, Farooqui AA. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J. Neurochem. 2007;103(1):1–16. doi: 10.1111/j.1471-4159.2007.04670.x. [DOI] [PubMed] [Google Scholar]
- 82.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat. Neurosci. 2005;8(8):1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 83.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neuro- degeneration. Nature. 2006;441(7092):513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 84.Bossy-Wetzel E, Talantova MV, Lee WD, Scholzke MN, Harrop A, Mathews E, Gotz T, Han J, Ellisman MH, Perkins GA, Lipton SA. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron. 2004;41(3):351–365. doi: 10.1016/s0896-6273(04)00015-7. [DOI] [PubMed] [Google Scholar]
- 85.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J. Neurosci. 1997;17(23):9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Piehl F, Olsson T. Inflammation and susceptibility to neuro- degeneration: the use of unbiased genetics to decipher critical regulatory pathways. Neuroscience. 2009;158(3):1143–1150. doi: 10.1016/j.neuroscience.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 87.Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J. Struct. Biol. 2000;130(2-3):184–208. doi: 10.1006/jsbi.2000.4274. [DOI] [PubMed] [Google Scholar]
- 88.Horiguchi T, Uryu K, Giasson BI, Ischiropoulos H, LightFoot R, Bellmann C, Richter-Landsberg C, Lee VMY, Trojanowski JQ. Nitration of tau protein is linked to neuro- degeneration in tauopathies. Am. J. Pathol. 2003;163(3):1021–1031. doi: 10.1016/S0002-9440(10)63462-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Floyd RA. Neuroinflammatory processes are important in neurodegenerative diseases: An hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radical Biol. Med. 1999;26(9-10):1346–1355. doi: 10.1016/s0891-5849(98)00293-7. [DOI] [PubMed] [Google Scholar]
- 90.Benveniste EN, Benos DJ. TNF-alpha- and IFN-gamma-mediated signal transduction pathways: effects on glial cell gene expression and function. FASEB J. 1995;9(15):1577–1584. doi: 10.1096/fasebj.9.15.8529837. [DOI] [PubMed] [Google Scholar]
- 91.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 2010;41(2-3):242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 92.Thorns V, Hansen L, Masliah E. nNOS expressing neurons in the entorhinal cortex and hippocampus are affected in patients with Alzheimer's disease. Exp. Neurol. 1998;150(1):14–20. doi: 10.1006/exnr.1997.6751. [DOI] [PubMed] [Google Scholar]
- 93.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68(2):270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 94.De Jager PL, Shulman JM, Chibnik LB, Keenan BT, Raj T, Wilson RS, Yu L, Leurgans SE, Tran D, Aubin C, Anderson CD, Biffi A, Corneveaux JJ, Huentelman MJ, Alzheimer's Disease Neuroimaging I, Rosand J, Daly MJ, Myers AJ, Reiman EM, Bennett DA, Evans DA. A genome-wide scan for common variants affecting the rate of age-related cognitive decline. Neurobiol. Aging. 2012;33(5):e1–15. doi: 10.1016/j.neurobiolaging.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tanzi RE. The genetics of Alzheimer disease. Cold Spring Harb Perspect. Med. 2012;2(10):2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS One. 2009;4(8):e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Babenko O, Kovalchuk I, Metz GA. Epigenetic programming of neurodegenerative diseases by an adverse environment. Brain Res. 2012;1444:96–111. doi: 10.1016/j.brainres.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 98.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic mechanisms in Alzheimer's disease. Neurobiol. Aging. 2011;32(7):1161–1180. doi: 10.1016/j.neurobiolaging.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conaway JW. Introduction to theme "Chromatin epigenetics and transcription". Annu Rev Biochem. 2012;81:61–64. doi: 10.1146/annurev-biochem-090711-093103. [DOI] [PubMed] [Google Scholar]
- 100.Lahiri DK, Maloney B. The "LEARn" (Latent Early-life Associated Regulation) model integrates environmental risk factors and the developmental basis of Alzheimer's disease and proposes remedial steps. Exp. Gerontol. 2010;45(4):291–296. doi: 10.1016/j.exger.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahley RW, Huang Y. Small-Molecule Structure Correctors Target Abnormal Protein Structure and Function: Structure Corrector Rescue of Apolipoprotein E4-Associated Neuro- pathology. J. Med. Chem. 2012;55(21):8997–9008. doi: 10.1021/jm3008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen HK, Liu Z, Meyer-Franke A, Brodbeck J, Miranda RD, McGuire JG, Pleiss MA, Ji ZS, Balestra ME, Walker DW, Xu Q, Jeong DE, Budamagunta MS, Voss JC, Freedman SB, Weisgraber KH, Huang Y, Mahley RW. Small molecule structure correctors abolish detrimental effects of apolipoprotein E4 in cultured neurons. J. Biol. Chem. 2012;287(8):5253–5266. doi: 10.1074/jbc.M111.276162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang Y. Molecular and cellular mechanisms of apolipoprotein E4 neurotoxicity and potential therapeutic strategies. Curr. Opin. Drug Discov. Devel. 2006;9(5):627–641. [PubMed] [Google Scholar]
- 104.Buttini M, Masliah E, Yu GQ, Palop JJ, Chang S, Bernardo A, Lin C, Wyss-Coray T, Huang Y, Mucke L. Cellular source of apolipoprotein E4 determines neuronal susceptibility to excitotoxic injury in transgenic mice. Am. J. Pathol. 2010;177(2):563–569. doi: 10.2353/ajpath.2010.090973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons leading to learning and memory deficits in mice. J. Neurosci. 2010;30(41):13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brodbeck J, McGuire J, Liu Z, Meyer-Franke A, Balestra ME, Jeong DE, Pleiss M, McComas C, Hess F, Witter D, Peterson S, Childers M, Goulet M, Liverton N, Hargreaves R, Freedman S, Weisgraber KH, Mahley RW, Huang Y. Structure-dependent impairment of intracellular apolipoprotein E4 trafficking and its detrimental effects are rescued by small-molecule structure correctors. J. Biol. Chem. 2011;286(19):17217–17226. doi: 10.1074/jbc.M110.217380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y. GABAergic interneuron dysfunction impairs hippocampal neuro- genesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5(6):634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Witt A, Macdonald N, Kirkpatrick P. Memantine hydrochloride. Nat. Rev. Drug Discov. 2004;3(2):109–110. doi: 10.1038/nrd1311. [DOI] [PubMed] [Google Scholar]
- 109.Wilkinson D. Pharmacotherapy of Alzheimer's disease. Psychiatry. 2005;4(1):43–47. [Google Scholar]
- 110.Sugimoto H, Ogura H, Arai Y, Limura Y, Yamanishi Y. Research and development of donepezil hydrochloride a new type of acetylcholinesterase inhibitor. Jpn. J. Pharmacol. 2002;89(1):7–20. doi: 10.1254/jjp.89.7. [DOI] [PubMed] [Google Scholar]
- 111.Lleo A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer's disease. Annu. Rev. Med. 2006;57:513–533. doi: 10.1146/annurev.med.57.121304.131442. [DOI] [PubMed] [Google Scholar]
- 112.Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer's disease: focus on disease modifying drugs. Br. J. Clin. Pharmacol. 2012;73(4):504–517. doi: 10.1111/j.1365-2125.2011.04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Selkoe DJ. Alzheimer's disease. Cold Spring Harb Perspect. Biol. 2011;3(7):a004457. doi: 10.1101/cshperspect.a004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman S, Michel BF, Boada M, Frank A, Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 115.Delrieu J, Ousset PJ, Caillaud C, Vellas B. 'Clinical trials in Alzheimer's disease': immunotherapy approaches. J Neurochem. 2012;120 Suppl 1:186–193. doi: 10.1111/j.1471-4159.2011.07458.x. [DOI] [PubMed] [Google Scholar]
- 116.Schor NF. What the halted phase III gamma-secretase inhibitor trial may (or may not) be telling us. Ann Neurol. 2011;69(2):237–239. doi: 10.1002/ana.22365. [DOI] [PubMed] [Google Scholar]
- 117.Palmer AM. What are the prospects of slowing the progression of Alzheimer's disease?. Drug Discov Today. 2012;17(21-22):1157–1159. doi: 10.1016/j.drudis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 118.Mandrekar-Colucci S, Landreth GE. Nuclear receptors as therapeutic targets for Alzheimer's disease. Expert Opin. Ther. Targets. 2011;15(9):1085–1097. doi: 10.1517/14728222.2011.594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Campbell VA, Gowran A. Alzheimer's disease taking the edge off with cannabinoids?. Br. J. Pharmacol. 2009;152(5):655–662. doi: 10.1038/sj.bjp.0707446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Standridge JB. Current status and future promise of pharma- cotherapeutic strategies for Alzheimer's disease. J. Am. Med. Dir. Assoc. 2006;7(3 ) Suppl :S46–51 45. doi: 10.1016/j.jamda.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 121.Bolognesi ML, Matera R, Minarini A, Rosini M, Melchiorre C. Alzheimer's disease: new approaches to drug discovery. Curr. Opin. Chem. Biol. 2009;13(3):303–308. doi: 10.1016/j.cbpa.2009.04.619. [DOI] [PubMed] [Google Scholar]
- 122.Morphy R, Kay C, Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov. Today. 2004;9(15):641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 123.Hopkins AL. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 124.Costantino L, Barlocco D. Designed multiple ligands: basic research vs clinical outcomes. Curr. Med. Chem. 2012;19(20):3353–3387. doi: 10.2174/092986712801215883. [DOI] [PubMed] [Google Scholar]
- 125.Mudher A, Lovestone S. Alzheimer's disease-do tauists and baptists finally shake hands?. Trends Neurosci. 2002;25(1):22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 126.Romero A, Egea J, Garcia AG, Lopez MG. Synergistic neuro-protective effect of combined low concentrations of galantamine and melatonin against oxidative stress in SH-SY5Y neuroblastoma cells. J.Pineal Res. 2010;49(2):141–148. doi: 10.1111/j.1600-079X.2010.00778.x. [DOI] [PubMed] [Google Scholar]
- 127.Muller-Schiffmann A, Sticht H, Korth C. Hybrid compounds: from simple combinations to nanomachines. BioDrugs. 2012;26(1):21–31. doi: 10.2165/11597630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 128.Han HJ, Kim BC, Lee JY, Ryu SH, Na HR, Yoon SJ, Park HY, Shin JH, Cho SJ, Yi HA, Choi MS, Heo JH, Park KW, Kim KK, Choi SH. Response to Rivastigmine Transdermal Patch or Memantine plus Rivastigmine Patch is affected by Apolipoprotein E Genotype in Alzheimer Patients. Dement. Geriatr. Cogn. Disord. 2012;34(3-4):167–173. doi: 10.1159/000342927. [DOI] [PubMed] [Google Scholar]
- 129.de Bruin NM, Prickaerts J, Lange JH, Akkerman S, Andriambeloson E, de Haan M, Wijnen J, van Drimmelen M, Hissink E, Heijink L, Kruse CG. SLV330 a cannabinoid CB1 receptor antagonist, ameliorates deficits in the T-maze object recognition and Social Recognition Tasks in rodents. Neurobiol. Learn Mem. 2010;93(4):522–531. doi: 10.1016/j.nlm.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 130.Annweiler C, Beauchet O. Possibility of a new anti-alzheimer's disease pharmaceutical composition combining memantine and vitamin D. Drugs Aging. 2012;29(2):81–91. doi: 10.2165/11597550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 131.Song MS, Matveychuk D, Mackenzie EM, Duchcherer M, Mousseau DD, Baker GB. An update on amine oxidase inhibitors: Multifaceted drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;44:118–124. doi: 10.1016/j.pnpbp.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 132.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu. Rev. Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 133.Romero J, Martinez-Orgado J. Cannabinoids and Neuro- degenerative Diseases. CNS & Neuro. Disorders-Drug Targets. 2009;8(6):440–450. doi: 10.2174/187152709789824589. [DOI] [PubMed] [Google Scholar]
- 134.Maelicke A, Samochocki M, Jostock R, Fehrenbacher A, Ludwig J, Albuquerque EX, Zerlin M. Allosteric sensitization of nicotinic receptors by galantamine a new treatment strategy for Alzheimer's disease. Biol. Psychiatry. 2001;49(3):279–288. doi: 10.1016/s0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- 135.Mancini F, Naldi M, Cavrini V, Andrisano V. Multiwell fluorometric and colorimetric microassays for the evaluation of beta-secretase (BACE-1) inhibitors. Anal. Bioanal. Chem. 2007;388(5-6):1175–1183. doi: 10.1007/s00216-007-1356-2. [DOI] [PubMed] [Google Scholar]
- 136.Meunier J, Ieni J, Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br. J. Pharmacol. 2006;149(8):998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Youdim MB. The path from anti Parkinson drug selegiline and rasagiline to multifunctional neuroprotective anti Alzheimer drugs ladostigil and m30. Curr. Alzheimer Res. 2006;3(5):541–550. doi: 10.2174/156720506779025288. [DOI] [PubMed] [Google Scholar]
- 138.Lahtinen H, Koistinaho J, Kauppinen R, Haapalinna A, Keinanen R, Sivenius J. Selegiline treatment after transient global ischemia in gerbils enhances the survival of CA1 pyramidal cells in the hippocampus. Brain Res. 1997;757(2):260–267. doi: 10.1016/s0006-8993(97)00227-8. [DOI] [PubMed] [Google Scholar]
- 139.Youdim MB, Gross A, Finberg JP. Rasagiline [N-propargyl-1R(+)-aminoindan], a selective and potent inhibitor of mitochondrial monoamine oxidase B. Br. J. Pharmacol. 2001;132(2):500–506. doi: 10.1038/sj.bjp.0703826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Weinstock M, Bejar C, Wang RH, Poltyrev T, Gross A, Finberg JP, Youdim MB. TV3326 a novel neuroprotective drug with cholinesterase and monoamine oxidase inhibitory activities for the treatment of Alzheimer's disease. J. Neural Transm. Suppl. 2000;(60):157–169. doi: 10.1007/978-3-7091-6301-6_10. [DOI] [PubMed] [Google Scholar]
- 141.Zheng H, Amit T, Bar-Am O, Fridkin M, Youdim MB, Mandel SA. From anti-Parkinson's drug rasagiline to novel multitarget iron chelators with acetylcholinesterase and monoamine oxidase inhibitory and neuroprotective properties for Alzheimer's disease. J. Alzheimers Dis. 2012;30(1):1–16. doi: 10.3233/JAD-2012-120013. [DOI] [PubMed] [Google Scholar]
- 142.Weinreb O, Bar-Am O, Amit T, Drigues N, Sagi Y, Youdim MB. The neuroprotective effect of ladostigil against hydrogen peroxide-mediated cytotoxicity. Chem. Biol. Interact. 2008;175(1-3):318–326. doi: 10.1016/j.cbi.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 143.Van der Schyf CJ, Mandel S, Geldenhuys WJ, Amit T, Avramovich Y, Zheng H, Fridkin M, Gal S, Weinreb O, Bar Am O, Sagi Y, Youdim MB. Novel multifunctional anti-Alzheimer drugs with various CNS neurotransmitter targets and neuroprotective moieties. Curr Alzheimer Res. 2007;4(5):522–536. doi: 10.2174/156720507783018226. [DOI] [PubMed] [Google Scholar]
- 144.Weinreb O, Amit T, Mandel S, Youdim MB. Novel therapeutic approach for neurodegenerative pathologies: multitarget iron-chelating drugs regulating hypoxia-inducible factor 1 signal transduction pathway. Neurodegener. Dis. 2012;10(1-4):112–115. doi: 10.1159/000332597. [DOI] [PubMed] [Google Scholar]
- 145.Bisogno T, Di Marzo V. The role of the endocannabinoid system in Alzheimer's disease: facts and hypotheses. Curr. Pharm. Des. 2008;14(23):2299–3305. doi: 10.2174/138161208785740027. [DOI] [PubMed] [Google Scholar]
- 146.Gowran A, Noonan J, Campbell VA. The multiplicity of action of cannabinoids: implications for treating neurodegeneration. CNS Neurosci. Ther. 2011;17(6):637–644. doi: 10.1111/j.1755-5949.2010.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ, Romero J. Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer's disease brains. J. Neurosci. 2003;23(35):11136–11141. doi: 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pazos MR, Nunez E, Benito C, Tolon RM, Romero J. Role of the endocannabinoid system in Alzheimer's disease: new perspectives. Life Sci. 2004;75(16):1907–1915. doi: 10.1016/j.lfs.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 149.Eubanks LM, Rogers CJ, Beuscher; Koob GF, Olson AJ, Dickerson TJ, Janda KD. A Molecular Link between the Active Component of Marijuana and Alzheimer's Disease Pathology. Mol. Pharm. 2006;3(6):773–777. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Karl T, Cheng D, Garner B, Arnold JC. The therapeutic potential of the endocannabinoid system for Alzheimer's disease. Expert Opin. Ther. Targets. 2012;16(4):407–420. doi: 10.1517/14728222.2012.671812. [DOI] [PubMed] [Google Scholar]
- 151.Nagayama T, Sinor AD, Simon RP, Chen J, Graham SH, Jin KL, Greenberg DA. Cannabinoids and neuroprotection in global and focal cerebral ischemia and in neuronal cultures. J. Neurosci. 1999;19(8):2987–2995. doi: 10.1523/JNEUROSCI.19-08-02987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Eubanks LM, Rogers CJ, Beuscher AE, Koob GF, Olson AJ, Dickerson TJ, Janda KD. A molecular link between the active component of marijuana and Alzheimer's disease pathology. Mol. Pharm. 2006;3(6):773–777. doi: 10.1021/mp060066m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, Klein T, Fernandez F, Tan J, Shytle RD. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflam. 2005;2(29) doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grundy RI, Rabuffetti M, Beltramo M. Cannabinoids and neuroprotection. Mol. Neurobiol. 2001;24(1-3):29–51. doi: 10.1385/MN:24:1-3:029. [DOI] [PubMed] [Google Scholar]
- 155.Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J. Mol. Med (Ber) 2006;84(3):253–258. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- 156.Haghani M, Shabani M, Javan M, Motamedi F, Janahmadi M. CB1 cannabinoid receptor activation rescues amyloid beta-induced alterations in behaviour and intrinsic electrophysiological properties of rat hippocampal CA1 pyramidal neurones. Cell Physiol. Biochem. 2012;29(3-4):391–406. doi: 10.1159/000338494. [DOI] [PubMed] [Google Scholar]
- 157.Eshhar N, Striem S, Biegon A. HU-211 a non-psychotropic cannabinoid rescues cortical-neurons from excitatory amino-acid toxicity in culture. Neuroreport. 1993;5(3):237–240. doi: 10.1097/00001756-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 158.Skaper SD, Buriani A, DalToso R, Petrelli L, Romanello S, Facci L, Leon A. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. Proc. Natl. Acad. Sci. U. S. A. 1996;93(9):3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shen MX, Thayer SA. Cannabinoid receptor agonists protect cultured rat hippocampal neurons from excitotoxicity. Mol. Pharmacol. 1998;54(3):459–462. doi: 10.1124/mol.54.3.459. [DOI] [PubMed] [Google Scholar]
- 160.Abood ME, Rizvi G, Sallapudi N, McAllister SD. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neurosci. Lett. 2001;309(3):197–201. doi: 10.1016/s0304-3940(01)02065-1. [DOI] [PubMed] [Google Scholar]
- 161.van der Stelt M, Veldhuis WB, Bar PR, Veldink GA, Vliegenthart JFG, Nicolay K. Neuroprotection by Delta(9)-tetrahydrocannabinol the main active compound in marijuana, against ouabain-induced in vivo excitotoxicity. Journal of Neuroscience. 2001;21(17):6475–6479. doi: 10.1523/JNEUROSCI.21-17-06475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.van der Stelt M, Veldhuis WB, van Haaften GW, Fezza F, Bisogno T, Bar PR, Veldink GA, Vliegenthart JFG, Di Marzo V, Nicolay K. Exogenous anandamide protects rat brain against acute neuronal injury in vivo. J. Neurosci. 2001;21(22):8765–8771. doi: 10.1523/JNEUROSCI.21-22-08765.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Iuvone T, Esposito G, Esposito R, Santamaria R, Di Rosa M, Izzo AA. Neuroprotective effect of cannabidiol a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004;89(1):134–141. doi: 10.1111/j.1471-4159.2003.02327.x. [DOI] [PubMed] [Google Scholar]
- 164.Sanchez-Montero JM. Spanish National Pharmacy Academy Award (Alcaliber Award). Real Academia Nacional de Farmacia. Available from http://ranf.com/component/easytablepro/concurso-cientifico.html?start=100. 2008.
- 165.Zheng W, Li J, Qiu Z, Xia Z, Li W, Yu L, Chen H, Chen J, Chen Y, Hu Z, Zhou W, Shao B, Cui Y, Xie Q, Chen H. Novel bis-(-)-nor-meptazinol derivatives act as dual binding site AChE inhibitors with metal-complexing property. Toxicol. Appl. Pharmacol. 2012;264(1):65–72. doi: 10.1016/j.taap.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 166.Alptuzun V, Prinz M, Horr V, Scheiber J, Radacki K, Fallarero A, Vuorela P, Engels B, Braunschweig H, Erciyas E, Holzgrabe U. Interaction of (benzylidene-hydrazono)- 1,4-dihydropyridines with beta-amyloid acetylcholine and butyrylcholine esterases. Bioorg. Med. Chem. 2010;18(5):2049–2059. doi: 10.1016/j.bmc.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 167.Kapkova P, Alptuzun V, Frey P, Erciyas E, Holzgrabe U. Search for dual function inhibitors for Alzheimer's disease: synthesis and biological activity of acetylcholinesterase inhibitors of pyridinium-type and their Abeta fibril formation inhibition capacity. Bioorg. Med. Chem. 2006;14(2):472–478. doi: 10.1016/j.bmc.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 168.Xie Q, Wang H, Xia Z, Lu M, Zhang W, Wang X, Fu W, Tang Y, Sheng W, Li W, Zhou W, Zhu X, Qiu Z, Chen H. Bis-(-)-nor-meptazinols as novel nanomolar cholinesterase inhibitors with high inhibitory potency on amyloid-beta aggregation. J. Med. Chem. 2008;51(7):2027–2036. doi: 10.1021/jm070154q. [DOI] [PubMed] [Google Scholar]
- 169.Grill JD, Cummings JL. Current therapeutic targets for the treatment of Alzheimer's disease. Expert Rev. Neurother. 2010;10(5):711–728. doi: 10.1586/ern.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Van der Zee EA, Platt B, Riedel G. Acetylcholine: future research and perspectives. Behav. Brain Res. 2011;221(2):583–586. doi: 10.1016/j.bbr.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 171.Cai Z, Ratka A. Opioid system and Alzheimer's disease. Neuromol. Med. 2012;14(2):91–111. doi: 10.1007/s12017-012-8180-3. [DOI] [PubMed] [Google Scholar]
- 172.Motel WC, Coop A, Cunningham CW. Cholinergic Modulation by Opioid Receptor Ligands: Potential Application to Alzheimer's Disease. Mini Rev. Med. Chem. 2012;13(3):456–466. doi: 10.2174/138955713804999784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Santangelo RM, Acker TM, Zimmerman SS, Katzman BM, Strong KL, Traynelis SF, Liotta DC. Novel NMDA receptor modulators: an update. Expert Opin. Ther. Pat. 2012;22(11):1337–1352. doi: 10.1517/13543776.2012.728587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Toda N, Kaneko T, Kogen H. Development of an efficient therapeutic agent for Alzheimer's disease: design and synthesis of dual inhibitors of acetylcholinesterase and serotonin transporter. Chem. Pharm. Bull (Tokyo) 2010;58(3):273–287. doi: 10.1248/cpb.58.273. [DOI] [PubMed] [Google Scholar]
- 175.Brioni JD, Esbenshade TA, Garrison TR, Bitner SR, Cowart MD. Discovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer's disease. J. Pharmacol. Exp. Ther. 2011;336(1):38–46. doi: 10.1124/jpet.110.166876. [DOI] [PubMed] [Google Scholar]
- 176.Hieke M, Ness J, Steri R, Dittrich M, Greiner C, Werz O, Baumann K, Schubert-Zsilavecz M, Weggen S, Zettl H. Design synthesis and biological evaluation of a novel class of gamma-secretase modulators with PPARgamma activity. J. Med. Chem. 2010;53(12):4691–4700. doi: 10.1021/jm1003073. [DOI] [PubMed] [Google Scholar]
- 177.Sheng M, Sabatini BL, Sudhof TC. Synapses and Alzheimer's disease. Cold Spring Harb Perspect. Biol. 2012;4(5):a005777. doi: 10.1101/cshperspect.a005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nistico R, Pignatelli M, Piccinin S, Mercuri NB, Collingridge G. Targeting Synaptic Dysfunction in Alzheimer's Disease Therapy. Mol. Neurobiol. 2012;46(3):572–587. doi: 10.1007/s12035-012-8324-3. [DOI] [PubMed] [Google Scholar]
- 179.Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer's disease the relationship between pharmacological effects and clinical efficacy. Drugs & Aging. 2004;21(7):453–478. doi: 10.2165/00002512-200421070-00004. [DOI] [PubMed] [Google Scholar]
- 180.Bitner RS, Markosyan S, Nikkel AL, Brioni JD. In-vivo histamine H3 receptor antagonism activates cellular signaling suggestive of symptomatic and disease modifying efficacy in Alzheimer's disease. Neuropharmacology. 2011;60(2-3):460–466. doi: 10.1016/j.neuropharm.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 181.Day T, Greenfield SA. A non-cholinergic trophic action of acetylcholinesterase on hippocampal neurones in vitro: molecular mechanisms. Neuroscience. 2002;111(3):649–656. doi: 10.1016/s0306-4522(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 182.Hicks DA, Makova NZ, Nalivaeva NN, Turner AJ. Characterisation of acetylcholinesterase release from neuronal cells. Chem. Biol. Interact. 2013;203(1):302–308. doi: 10.1016/j.cbi.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 183.Blusztajn JK, Berse B. The cholinergic neuronal phenotype in Alzheimer's disease. Metab. Brain Dis. 2000;15(1):45–64. doi: 10.1007/BF02680013. [DOI] [PubMed] [Google Scholar]
- 184.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 185.Forlenza OV, Spink JM, Dayanandan R, Anderton BH, Olesen OF, Lovestone S. Muscarinic agonists reduce tau phosphorylation in non-neuronal cells via GSK-3beta inhibition and in neurons. J. Neural Transm. 2000;107(10):1201–1212. doi: 10.1007/s007020070034. [DOI] [PubMed] [Google Scholar]
- 186.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003;4(2):131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 187.Schenk D, Basi GS, Pangalos MN. Treatment Strategies Targeting Amyloid beta-Protein. Cold Spring Harb Perspect. Med. 2012;2(9):a006387. doi: 10.1101/cshperspect.a006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brunden KR, Trojanowski JQ, Lee VMY. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat. Rev. Drug Discov. 2009;8(10):783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ansari N, Khodagholi F. Molecular mechanism aspect of ER stress in Alzheimer's disease: Current approaches and future strategies. Curr. Drug Targets. 2012;1(1):114–122. doi: 10.2174/138945013804806532. [DOI] [PubMed] [Google Scholar]
- 190.Siegenthaler BM, Rajendran L. Retromers in Alzheimer's disease. Neurodegener Dis. 2012;10(1-4):116–121. doi: 10.1159/000335910. [DOI] [PubMed] [Google Scholar]
- 191.Frydman-Marom A, Shaltiel-Karyo R, Moshe S, Gazit E. The generic amyloid formation inhibition effect of a designed small aromatic beta-breaking peptide. Amyloid. 2011;18(3):119–127. doi: 10.3109/13506129.2011.582902. [DOI] [PubMed] [Google Scholar]
- 192.Boddapati S, Levites Y, Sierks MR. Inhibiting beta-secretase activity in Alzheimer's disease cell models with single-chain antibodies specifically targeting APP. J. Mol. Biol. 2011;405(2):436–447. doi: 10.1016/j.jmb.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 193.Weggen S, Rogers M, Eriksen J. NSAIDs: small molecules for prevention of Alzheimer's disease or precursors for future drug development?. Trends Pharmacol. Sci. 2007;28(10):536–543. doi: 10.1016/j.tips.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 194.Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314(5799):664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 195.Borgegard T, Jureus A, Olsson F, Rosqvist S, Sabirsh A, Rotticci D, Paulsen K, Klintenberg R, Yan H, Waldman M, Stromberg K, Nord J, Johansson J, Regner A, Parpal S, Malinowsky D, Radesater AC, Li T, Singh R, Eriksson H, Lundkvist J. First and second generation gamma-secretase modulators (GSMs) modulate amyloid-beta (Abeta) peptide production through different mechanisms. J. Biol. Chem. 2012;287(15):11810–11819. doi: 10.1074/jbc.M111.305227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Jeon SY, Kwon SH, Seong YH, Bae K, Hur JM, Lee YY, Suh DY, Song KS. Beta-secretase (BACE1)-inhibiting stilbenoids from Smilax Rhizoma. Phytomedicine. 2007;14(6):403–408. doi: 10.1016/j.phymed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 197.Chang J, Rimando A, Pallas M, Camins A, Porquet D, Reeves J, Shukitt-Hale B, Smith MA, Joseph JA, Casadesus G. Low-dose pterostilbene but not resveratrol is a potent neuro- modulator in aging and Alzheimer's disease. Neurobiol. Aging. 2012;33(9):2062–2071. doi: 10.1016/j.neurobiolaging.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 198.Kojro E, Fahrenholz F. The non-amyloidogenic pathway: structure and function of alpha-secretases. Subcell Biochem. 2005;38:105–127. doi: 10.1007/0-387-23226-5_5. [DOI] [PubMed] [Google Scholar]
- 199.Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene. ADAM10. Cell. 2010;142(2):320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 200.Braidy N, Jayasena T, Poljak A, Sachdev PS. Sirtuins in cognitive ageing and Alzheimer's disease. Curr. Opin. Psychiatry. 2012;25(3):226–230. doi: 10.1097/YCO.0b013e32835112c1. [DOI] [PubMed] [Google Scholar]
- 201.Li F, Gong Q, Dong H, Shi J. Resveratrol a neuroprotective supplement for Alzheimer's disease. Curr. Pharm. Des. 2012;18(1):27–33. doi: 10.2174/138161212798919075. [DOI] [PubMed] [Google Scholar]
- 202.Feng Y, Wang X-p, Yang S-g, Wang Y-j, Zhang X, Du X-t, Sun X-x, Zhao M, Huang L, Liu R-t. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology. 2009;30(6):986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 203.Huang H-C, O’Brien WT, Klein PS. Targeting glycogen synthase kinase-3 in Alzheimer's disease. Drug Discov. Today: Ther. Strategies. 2006;3(4):613–619. [Google Scholar]
- 204.Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer's disease: pathophysiological and therapeutic significance. Cell Mol. Life Sci. 2006;63(11):1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schirmer RH, Adler H, Pickhardt M, Mandelkow E. Lest we forget you--methylene blue. Neurobiol. Aging. 2011;32(12):2325 e2327–2316. doi: 10.1016/j.neurobiolaging.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 206.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR): amyloid-beta and Tau effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chiosis G, Dickey CA, Johnson JL. A global view of Hsp90 functions. Nat. Struct.Mol.Biol. 2013;20(1):1–4. doi: 10.1038/nsmb.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Opattova A, Filipcik P, Cente M, Novak M. Intracellular degradation of misfolded tau protein induced by geldanamycin is associated with activation of proteasome. J. Alzheimers Di. 2013;33(2):339–348. doi: 10.3233/JAD-2012-121072. [DOI] [PubMed] [Google Scholar]
- 209.Sephton CF, Cenik B, Cenik BK, Herz J, Yu G. TDP-43 in central nervous system development and function: clues to TDP-43-associated neurodegeneration. Biol. Chem. 2012;393(7):589–594. doi: 10.1515/hsz-2012-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.LaFerla FM. Preclinical success against Alzheimer's disease with an old drug. N. Engl. J. Med. 2012;367(6):570–572. doi: 10.1056/NEJMcibr1204890. [DOI] [PubMed] [Google Scholar]
- 211.Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, Casali BT, Restivo JL, Goebel WD, James MJ, Brunden KR, Wilson DA, Landreth GE. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen R, Zhang J, Wu Y, Wang D, Feng G, Tang YP, Teng Z, Chen C. Monoacylglycerol Lipase Is a Therapeutic Target for Alzheimer's Disease. Cell Rep. 2012;2(5):1329–1339. doi: 10.1016/j.celrep.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Boothby LA, Doering PL. Vitamin C and vitamin E for Alzheimer's disease. Ann. Pharmacother. 2005;39(12):2073–2080. doi: 10.1345/aph.1E495. [DOI] [PubMed] [Google Scholar]
- 214.Lerner AJ, Gustaw-Rothenberg K, Smyth S, Casadesus G. Retinoids for treatment of Alzheimer's disease. Biofactors. 2012;38(2):84–89. doi: 10.1002/biof.196. [DOI] [PubMed] [Google Scholar]
- 215.Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, Engel J, Münch G. Lipoic acid as a novel treatment for Alzheimer's disease and related dementias. Pharmacol. Therap. 2007;113(1):154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 216.Jimenez-Del-Rio M, Velez-Pardo C. The bad the good and the ugly about oxidative stress. Oxid. Med. Cell Longev. 2012;2012(163913) doi: 10.1155/2012/163913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mohsenzadegan M, Mirshafiey A. The immunopathogenic role of reactive oxygen species in Alzheimer disease. Iran J Allergy Asthma Immunol. 2012;11(3):203–216. [PubMed] [Google Scholar]
- 218.Forlenza OV, Schaeffer EL, Gattaz WF. The role of phospholipase A2 in neuronal homeostasis and memory formation: implications for the pathogenesis of Alzheimer's disease. J. Neural Transm. 2007;114(2):231–238. doi: 10.1007/s00702-006-0597-0. [DOI] [PubMed] [Google Scholar]
- 219.Schaeffer EL, Gattaz WF. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacology (Berl) 2008;198(1):1–27. doi: 10.1007/s00213-008-1092-0. [DOI] [PubMed] [Google Scholar]
- 220.Cordeiro Q, Noguti R, Bottino CMC, Vallada H. Study of association between genetic polymorphisms of phospholipase A2 enzymes and Alzheimer's disease. Arquivos De Neuro-Psiquiatria. 2010;68(2):189–193. doi: 10.1590/s0004-282x2010000200007. [DOI] [PubMed] [Google Scholar]
- 221.Schaeffer EL, Skaf HD, Novaes BD, da Silva ER, Martins BA, Joaquim HDG, Gattaz WF. Inhibition of phospholipase A(2) in rat brain modifies different membrane fluidity parameters in opposite ways. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35(7):1612–1617. doi: 10.1016/j.pnpbp.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 222.Schaeffer EL, Forlenza OV, Gattaz WF. Phospholipase A2 activation as a therapeutic approach for cognitive enhancement in early-stage Alzheimer disease. Psychopharmacology (Berl) 2009;202(1-3):37–51. doi: 10.1007/s00213-008-1351-0. [DOI] [PubMed] [Google Scholar]
- 223.Forlenza OV, Mendes CT, Marie SK, Gattaz WF. Inhibition of phospholipase A2 reduces neurite outgrowth and neuronal viability. Prostaglandins Leukot Essent Fatty Acids. 2007;76(1):47–55. doi: 10.1016/j.plefa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 224.Schaeffer EL, De-Paula VJ, da Silva ER, Novaes BD, Skaf HD, Forlenza OV, Gattaz WF. Inhibition of phospholipase A(2) in rat brain decreases the levels of total Tau protein. J. Neural Transm. 2011;118(9):1273–1279. doi: 10.1007/s00702-011-0619-4. [DOI] [PubMed] [Google Scholar]
- 225.Sun AY, Wang Q, Simonyi A, Sun GY. Botanical phenolics and brain health. Neuromol. Med. 2008;10(4):259–274. doi: 10.1007/s12017-008-8052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zandi PP, Breitner JC. Do NSAIDs prevent Alzheimer's disease?.And if so why?. The epidemiological evidence. Neurobiol. Aging. 2001;22(6):811–817. doi: 10.1016/s0197-4580(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 227.Khoury JE, Luster AD. Mechanisms of microglia accumulation in Alzheimer’s disease: therapeutic implications. Trends Pharmacol. Sci. 2008;29(12):626–632. doi: 10.1016/j.tips.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 228.Ankarcrona M, Mangialasche F, Winblad B. Rethinking Alzheimer's disease therapy: are mitochondria the key?. J. Alzheimers Dis. 2010;20( Suppl 2 ):S579–590. doi: 10.3233/JAD-2010-100327. [DOI] [PubMed] [Google Scholar]
- 229.Silva DF, Santana I, Esteves AR, Baldeiras I, Arduino DM, Oliveira CR, Cardoso SM. Prodromal Metabolic Phenotype in MCI Cybrids: Implications for Alzheimers Disease. Curr. Alzheimer Res. 2013;10(2):180–190. doi: 10.2174/1567205011310020008. [DOI] [PubMed] [Google Scholar]
- 230.Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Mol. Cell Neurosci. 2012;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 231.Rossi L, Mazzitelli S, Arciello M, Capo CR, Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochem. Res. 2008;33(12):2390–2400. doi: 10.1007/s11064-008-9696-7. [DOI] [PubMed] [Google Scholar]
- 232.Reddy PH. Role of mitochondria in neurodegenerative diseases: mitochondria as a therapeutic target in Alzheimer's disease. CNS Spectr discussion 16-18. 2009;14(8) Suppl 7 :8–13. doi: 10.1017/s1092852900024901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lin AL, Poteet E, Du F, Gourav RC, Liu R, Wen Y, Bresnen A, Huang S, Fox PT, Yang SH, Duong TQ. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS One. 2012;7(10):e46585. doi: 10.1371/journal.pone.0046585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16(1):46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- 235.Chong ZZ, Shang YC, Zhang L, Wang S, Maiese K. Mammalian target of rapamycin: hitting the bull's-eye for neurological disorders. Oxid. Med. Cell Longev. 2010;3(6):374–391. doi: 10.4161/oxim.3.6.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ma T, Hoeffer CA, Capetillo-Zarate E, Yu F, Wong H, Lin MT, Tampellini D, Klann E, Blitzer RD, Gouras GK. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5(9):e12845. doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Cai Z, Zhao B, Li K, Zhang L, Li C, Quazi SH, Tan Y. Mammalian target of rapamycin: a valid therapeutic target through the autophagy pathway for Alzheimer's disease?. J. Neurosci. Res. 2012;90(6):1105–1118. doi: 10.1002/jnr.23011. [DOI] [PubMed] [Google Scholar]
- 239.Paccalin M, Pain-Barc S, Pluchon C, Paul C, Besson MN, Carret-Rebillat AS, Rioux-Bilan A, Gil R, Hugon J. Activated mTOR and PKR kinases in lymphocytes correlate with memory and cognitive decline in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 2006;22(4):320–326. doi: 10.1159/000095562. [DOI] [PubMed] [Google Scholar]
- 240.Tian Y, Bustos V, Flajolet M, Greengard P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011;25(6):1934. doi: 10.1096/fj.10-175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Li WP, Chan WY, Lai HW, Yew DT. Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J. Mol. Neurosci. 1997;8(2):75–82. doi: 10.1007/BF02736774. [DOI] [PubMed] [Google Scholar]
- 242.Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer's disease. Neuropathol Appl Neurobiol. 2002;28(3):238–251. doi: 10.1046/j.1365-2990.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- 243.Zatta P, Drago D, Bolognin S, Sensi SL. Alzheimer's disease metal ions and metal homeostatic therapy. Trends Pharmacol. Sci. 2009;30(7):346–355. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 244.Deane RJ. Is RAGE still a therapeutic target for Alzheimer's disease?. Future Med. Chem. 2012;4(7):915–925. doi: 10.4155/fmc.12.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Diaz P, Phatak SS, Xu J, Fronczek FR, Astruc-Diaz F, Thompson CM, Cavasotto CN, Naguib M. 2 3-Dihydro-1-benzofuran derivatives as a series of potent selective cannabinoid receptor 2 agonists: design synthesis and binding mode prediction through ligand-steered modeling. Chemmedchem. 2009;4(10):1615–1629. doi: 10.1002/cmdc.200900226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Carro E. Gelsolin as therapeutic target in Alzheimer's disease. Expert Opin. Ther. Targets. 2010;14(6):585–592. doi: 10.1517/14728222.2010.488222. [DOI] [PubMed] [Google Scholar]
- 247.Andersen F, Viitanen M, Halvorsen DS, Straume B, Engstad TA. Co-morbidity and drug treatment in Alzheimer's disease.A cross sectional study of participants in the dementia study in northern Norway. BMC Geriatr. 2011 doi: 10.1186/1471-2318-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Alvira-Botero X, Perez-Gonzalez R, Spuch C, Vargas T, Antequera D, Garzon M, Bermejo-Pareja F, Carro E. Megalin interacts with APP and the intracellular adapter protein FE65 in neurons. Mol. Cell Neurosci. 2010;45(3):306–315. doi: 10.1016/j.mcn.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 249.Luchicchi A, Pistis M. Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol. Neurobiol. 2012;46(2):374–392. doi: 10.1007/s12035-012-8299-0. [DOI] [PubMed] [Google Scholar]
- 250.Labar G, Wouters J, Lambert DM. A review on the monoacylglycerol lipase: at the interface between fat and endocannabinoid signalling. Curr. Med. Chem. 2010;17(24):2588–2607. doi: 10.2174/092986710791859414. [DOI] [PubMed] [Google Scholar]
- 251.Steele JW, Ju S, Lachenmayer ML, Liken J, Stock A, Kim SH, Delgado LM, Alfaro IE, Bernales S, Verdile G, Bharadwaj P, Gupta V, Barr R, Friss A, Dolios G, Wang R, Ringe D, Protter AA, Martins RN, Ehrlich ME, Yue Z, Petsko GA, Gandy S. Latrepirdine stimulates autophagy and reduces accumulation of alpha-synuclein in cells and in mouse brain. Mol. Psychiatry. 2013;18(8):882–888. doi: 10.1038/mp.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Checler F, Dunys J. p53 a pivotal effector of a functional cross-talk linking presenilins and Pen-2. Neurodegener. Dis. 2012;10(1-4):52–55. doi: 10.1159/000332935. [DOI] [PubMed] [Google Scholar]
- 253.Glebov K, Walter J. Statins in unconventional secretion of insulin-degrading enzyme and degradation of the amyloid-beta peptide. Neurodegener. Dis. 2012;10(1-4):309–312. doi: 10.1159/000332595. [DOI] [PubMed] [Google Scholar]
- 254.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends. Neurosci. 2011;34(5):269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Shea TB, Rogers E, Remington R. Nutrition and dementia: are we asking the right questions?. J. Alzheimers Dis. 2012;30(1):27–33. doi: 10.3233/JAD-2012-112231. [DOI] [PubMed] [Google Scholar]
- 256.Fernandez-Fernandez L, Comes G, Bolea I, Valente T, Ruiz J, Murtra P, Ramirez B, Angles N, Reguant J, Morello JR, Boada M, Hidalgo J, Escorihuela RM, Unzeta M. LMN diet rich in polyphenols and polyunsaturated fatty acids improves mouse cognitive decline associated with aging and Alzheimer's disease. Behav. Brain Res. 2012;228(2):261–271. doi: 10.1016/j.bbr.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 257.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348(9025):429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 258.Carroll JC, Rosario ER. The potential use of hormone- based therapeutics for the treatment of Alzheimer's disease. Curr. Alzheimer Res. 2012;9(1):18–34. doi: 10.2174/156720512799015109. [DOI] [PubMed] [Google Scholar]
- 259.Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodelling. Nature. 2007;447(7141):178–182. doi: 10.1038/nature05772. [DOI] [PubMed] [Google Scholar]
- 260.Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, Brune K, Paul S, Zhou Y, Liu F, Ni B. Lithium a common drug for bipolar disorder treatment regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43(22):6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- 261.Ricobaraza A, Cuadrado-Tejedor M, Perez-Mediavilla A, Frechilla D, Del Rio J, Garcia-Osta A. Phenylbutyrate ameliorates cognitive deficit and reduces tau pathology in an Alzheimer's disease mouse model. Neuropsychopharmacology. 2009;34(7):1721–1732. doi: 10.1038/npp.2008.229. [DOI] [PubMed] [Google Scholar]
- 262.Park LK, Friso S, Choi SW. Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 2012;71(1):75–83. doi: 10.1017/S0029665111003302. [DOI] [PubMed] [Google Scholar]
- 263.Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, Calamandrei G, Scarpa S. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol. Cell Neurosci. 2008;37(4):731–746. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 264.Pham TX, Lee J. Dietary regulation of histone acetylases and deacetylases for the prevention of metabolic diseases. Nutrients. 2012;4(12):1868–1886. doi: 10.3390/nu4121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hamouda AK, Kimm T, Cohen JB. Physostigmine and galanthamine bind in the presence of agonist at the canonical and noncanonical subunit interfaces of a nicotinic acetylcholine receptor. J. Neurosci. 2013;33(2):485–494. doi: 10.1523/JNEUROSCI.3483-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lilja AM, Luo Y, Yu QS, Rojdner J, Li Y, Marini AM, Marutle A, Nordberg A, Greig NH. Neurotrophic and neuro- protective actions of (-)- and (+)-phenserine candidate drugs for Alzheimer's disease. PLoS One. 2013;8(1):e54887. doi: 10.1371/journal.pone.0054887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK. An overview of phenserine tartrate a novel acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Curr. Alzheimer Res. 2005;2(3):281–290. doi: 10.2174/1567205054367829. [DOI] [PubMed] [Google Scholar]
- 268.Kadir A, Andreasen N, Almkvist O, Wall A, Forsberg A, Engler H, Hagman G, Larksater M, Winblad B, Zetterberg H, Blennow K, Langstrom B, Nordberg A. Effect of phenserine treatment on brain functional activity and amyloid in Alzheimer's disease. Ann. Neurol. 2008;63(5):621–631. doi: 10.1002/ana.21345. [DOI] [PubMed] [Google Scholar]
- 269.Pandya A, Yakel JL. Allosteric modulators of the alpha4beta2 subtype of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2011;82(8):952–958. doi: 10.1016/j.bcp.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Coleman BR, Ratcliffe RH, Oguntayo SA, Shi X, Doctor BP, Gordon RK, Nambiar MP. [+]-Huperzine A treatment protects against N-methyl-D-aspartate-induced seizure/status epilepticus in rats. Chem. Biol. Interact. 2008;175(1-3):387–395. doi: 10.1016/j.cbi.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 271.Perry E, Howes MJ. Medicinal plants and dementia therapy: herbal hopes for brain aging?. CNS Neurosci. Ther. 2011;17(6):683–698. doi: 10.1111/j.1755-5949.2010.00202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ji HF, Shen L. Berberine: a potential multipotent natural product to combat Alzheimer's disease. Molecules. 2011;16(8):6732–6740. doi: 10.3390/molecules16086732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Jia L, Liu J, Song Z, Pan X, Chen L, Cui X, Wang M. Berberine suppresses amyloid-beta-induced inflammatory response in microglia by inhibiting nuclear factor-kappaB and mitogen-activated protein kinase signalling pathways. J. Pharm. Pharmacol. 2012;64(10):1510–1521. doi: 10.1111/j.2042-7158.2012.01529.x. [DOI] [PubMed] [Google Scholar]
- 274.Jung HA, Min BS, Yokozawa T, Lee JH, Kim YS, Choi JS. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009;32(8):1433–1438. doi: 10.1248/bpb.32.1433. [DOI] [PubMed] [Google Scholar]
- 275.Howes MJ, Perry E. The role of phytochemicals in the treatment and prevention of dementia. Drugs Aging. 2011;28(6):439–468. doi: 10.2165/11591310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 276.Hamann M, Alonso D, Martin-Aparicio E, Fuertes A, Perez-Puerto MJ, Castro A, Morales S, Navarro ML, Del Monte-Millan M, Medina M, Pennaka H, Balaiah A, Peng J, Cook J, Wahyuono S, Martinez A. Glycogen synthase kinase-3 (GSK-3) inhibitory activity and structure-activity relationship (SAR) studies of the manzamine alkaloids.Potential for Alzheimer's disease. J. Nat. Prod. 2007;70(9):1397–1405. doi: 10.1021/np060092r. [DOI] [PubMed] [Google Scholar]
- 277.Frost D, Meechoovet B, Wang T, Gately S, Giorgetti M, Shcherbakova I, Dunckley T. beta-carboline compounds, including harmine inhibit DYRK1A and tau phosphorylation at multiple Alzheimer's disease-related sites. PLoS One. 2011;6(5):e19264. doi: 10.1371/journal.pone.0019264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Krskova Z, Martin J, Dusek J. The inhibition activity of selected beta-carboline alkaloids on enzymes of acetylcholinesterase and butyrylcholinesterase. Ceska Slov Farm. 2011;60(3):125–131. [PubMed] [Google Scholar]
- 279.Geissler T, Brandt W, Porzel A, Schlenzig D, Kehlen A, Wessjohann L, Arnold N. Acetylcholinesterase inhibitors from the toadstool Cortinarius infractus. Bioorg. Med. Chem. 2010;18(6):2173–2177. doi: 10.1016/j.bmc.2010.01.074. [DOI] [PubMed] [Google Scholar]
- 280.Satheeshkumar N, Mukherjee PK, Bhadra S, Saha BP. Acetylcholinesterase enzyme inhibitory potential of standardized extract of Trigonella foenum graecum L and its constituents. Phytomedicine. 2010;17(3-4):292–295. doi: 10.1016/j.phymed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 281.Tohda C, Kuboyama T, Komatsu K. Search for natural products related to regeneration of the neuronal network. Neurosignals. 2005;14(1-2):34–45. doi: 10.1159/000085384. [DOI] [PubMed] [Google Scholar]
- 282.Pecic S, McAnuff MA, Harding WW. Nantenine as an acetylcholinesterase inhibitor: SAR, enzyme kinetics and molecular modeling investigations. J. Enzyme Inhib. Med. Chem. 2011;26(1):46–55. doi: 10.3109/14756361003671078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Rojsanga P, Boonyarat C, Utsintong M, Nemecz A, Yamauchi JG, Talley TT, Olson AJ, Matsumoto K, Vajragupta O. The effect of crebanine on memory and cognition impairment via the alpha-7 nicotinic acetylcholine receptor. Life Sci. 2012;91(3-4):107–114. doi: 10.1016/j.lfs.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 284.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5(3):247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Gao ZG, Jacobson KA. Emerging adenosine receptor agonists: an update. Expert Opin. Emerg. Drugs. 2011;16(4):597–602. doi: 10.1517/14728214.2011.644786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, Filla L, Zanatta D, Rios Romenets S, Altman R, Chuang R, Shah B. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79(7):651–658. doi: 10.1212/WNL.0b013e318263570d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Rouleau J, Iorga BI, Guillou C. New potent human acetylcholinesterase inhibitors in the tetracyclic triterpene series with inhibitory potency on amyloid beta aggregation. Eur. J. Med. Chem. 2011;46(6): 0. doi: 10.1016/j.ejmech.2011.02.073. [DOI] [PubMed] [Google Scholar]
- 288.Conforti F, Rigano D, Formisano C, Bruno M, Loizzo MR, Menichini F, Senatore F. Metabolite profile and in vitro activities of Phagnalon saxatile (L. Cass. relevant to treatment of Alzheimer's disease. J. Enzyme Inhib. Med. Chem. 2010;25(1):97–104. doi: 10.3109/14756360903018260. [DOI] [PubMed] [Google Scholar]
- 289.Zhou F, Chen S, Xiong J, Li Y, Qu L. Luteolin reduces zinc-induced tau phosphorylation at Ser262/356 in an ROS-dependent manner in SH-SY5Y cells. Biol Trace Elem Res. 2012;149(2):273–279. doi: 10.1007/s12011-012-9411-z. [DOI] [PubMed] [Google Scholar]
- 290.Dragicevic N, Smith A, Lin XY, Yuan F, Copes N, Delic V, Tan J, Cao CH, Shytle RD, Bradshaw PC. Green Tea Epigallocatechin-3-Gallate (EGCG) and Other Flavonoids Reduce Alzheimer's Amyloid-Induced Mitochondrial Dysfunction. J. Alzheimers Dis. 2011;26(3):507–521. doi: 10.3233/JAD-2011-101629. [DOI] [PubMed] [Google Scholar]
- 291.Ono K, Li L, Takamura Y, Yoshiike Y, Zhu L, Han F, Mao X, Ikeda T, Takasaki J, Nishijo H, Takashima A, Teplow DB, Zagorski MG, Yamada M. Phenolic compounds prevent amyloid beta-protein oligomerization and synaptic dysfunction by 10.074/jbc.M111.325456 site-specific binding. J. Biol. Chem. 2012;287(18):14631–14643. doi: 10.1074/jbc.M111.325456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shimmyo Y, Kihara T, Akaike A, Niidome T, Sugimoto H. Three distinct neuroprotective functions of myricetin against glutamate-induced neuronal cell death: Involvement of direct inhibition of caspase-3. J. Neurosci. Res. 2008;86(8):1836–1845. doi: 10.1002/jnr.21629. [DOI] [PubMed] [Google Scholar]
- 293.Hamaguchi T, Ono K, Murase A, Yamada M. Phenolic compounds prevent Alzheimer's pathology through different effects on the amyloid-beta aggregation pathway. Am. J. Pathol. 2009;175(6):2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Anand P, Singh B, Singh N. A review on coumarins as acetylcholinesterase inhibitors for Alzheimer's disease. Bioorg. Med. Chem. 2012;20(3):1175–1180. doi: 10.1016/j.bmc.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 295.Matos MJ, Vina D, Vazquez-Rodriguez S, Uriarte E, Santana L. Focusing on new monoamine oxidase inhibitors: differently substituted coumarins as an interesting scaffold. Curr Top Med Chem. 2012;12(20):2210–2239. doi: 10.2174/156802612805220002. [DOI] [PubMed] [Google Scholar]
- 296.Patil PO, Bari SB, Firke SD, Deshmukh PK, Donda ST, Patil DA. A comprehensive review on synthesis and designing aspects of coumarin derivatives as monoamine oxidase inhibitors for depression and Alzheimer's disease. Bioorg. Med. Chem. 2013;21(9):2434–2450. doi: 10.1016/j.bmc.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 297.Teismann P, Ferger B. Effects of ensaculin on dopamine metabolite levels and K(+)-induced glutamate release. Eur. J. Pharmacol. 2000;398(2):247–250. doi: 10.1016/s0014-2999(00)00290-9. [DOI] [PubMed] [Google Scholar]
- 298.Hoerr R, Noeldner M. Ensaculin (KA-672 HCl): a multitransmitter approach to dementia treatment. CNS Drug Rev. 2002;8(2):143–158. doi: 10.1111/j.1527-3458.2002.tb00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Jeon S-Y, Bae K, Seong Y-H, Song K-S. Green tea catechins as a BACE1 (ß-Secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003;13(22):3905–3908. doi: 10.1016/j.bmcl.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 300.Cheng XR, Zhou JW, Zhou Y, Cheng JP, Yang RF, Zhou WX, Zhang YX, Yun LH. The green tea polyphenol (2)-epigallocatechin-3-gallate (EGCG) is not a beta-secretase inhibitor. Bioorg. Med. Chem. Lett. 2012;22(3):1408–1414. doi: 10.1016/j.bmcl.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 301.Bieschke J, Russ J, Friedrich RP, Ehrnhoefer DE, Wobst H, Neugebauer K, Wanker EE. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci U S A. 2010;107(17):7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Mandel SA, Amit T, Weinreb O, Reznichenko L, Youdim MB. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 2008;14(4):352–365. doi: 10.1111/j.1755-5949.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Smith A, Giunta B, Bickford PC, Fountain M, Tan J, Shytle RD. Nanolipidic particles improve the bioavailability and alpha-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer's disease. Int. J. Pharm. 2010;389(1-2):207–212. doi: 10.1016/j.ijpharm.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T. Ferulic Acid Is a Nutraceutical beta-Secretase Modulator That Improves Behavioral Impairment and Alzheimer-like Pathology in Transgenic Mice. PLoS One. 2013;8(2):e55774. doi: 10.1371/journal.pone.0055774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yan JJ, Jung JS, Kim TK, Hasan A, Hong CW, Nam JS, Song DK. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013;36(1):140–143. doi: 10.1248/bpb.b12-00798. [DOI] [PubMed] [Google Scholar]
- 306.Jiang H, Wang X, Huang L, Luo Z, Su T, Ding K, Li X. Benzenediol-berberine hybrids: Multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. 2011;19(23):7228–7235. doi: 10.1016/j.bmc.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 307.Iuvone T. The Spice Sage and Its Active Ingredient Rosmarinic Acid Protect PC12 Cells from Amyloid-beta Peptide-Induced Neurotoxicity. J. Pharmacol. Exper. Therap. 2006;317(3):1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 308.Hamaguchi T, Ono K, Yamada M. REVIEW: Curcumin and Alzheimer's disease. CNS Neurosci. Ther. 2010;16(5):285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Narasingappa RB, Javagal MR, Pullabhatla S, Htoo HH, Rao JK, Hernandez JF, Govitrapong P, Vincent B. Activation of alpha-secretase by curcumin-aminoacid conjugates. Biochem. Biophys. Res. Commun. 2012;424(4):691–696. doi: 10.1016/j.bbrc.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 310.Villaflores OB, Chen YJ, Chen CP, Yeh JM, Wu TY. Curcuminoids and resveratrol as anti-Alzheimer agents. Taiwan J. Obstet. Gynecol. 2012;51(4):515–525. doi: 10.1016/j.tjog.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 311.Mutsuga M, Chambers JK, Uchida K, Tei M, Makibuchi T, Mizorogi T, Takashima A, Nakayama H. Binding of curcumin to senile plaques and cerebral amyloid angiopathy in the aged brain of various animals and to neurofibrillary tangles in Alzheimer's brain. J. Vet. Med. Sci. 2012;74(1):51–57. doi: 10.1292/jvms.11-0307. [DOI] [PubMed] [Google Scholar]
- 312.Jaques JA, Rezer JF, Carvalho FB, da Rosa MM, Gutierres JM, Goncalves JF, Schmatz R, de Bairros AV, Mazzanti CM, Rubin MA, Schetinger MR, Leal DB. Curcumin protects against cigarette smoke-induced cognitive impairment and increased acetylcholinesterase activity in rats. Physiol. Behav. 2012;106(5):664–669. doi: 10.1016/j.physbeh.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 313.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models. J. Alzheimers Dis. 2004;6(4):367–377. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- 314.Feng X, Liang N, Zhu D, Gao Q, Peng L, Dong H, Yue Q, Liu H, Bao L, Zhang J, Hao J, Gao Y, Yu X, Sun J. Resveratrol Inhibits beta-Amyloid-Induced Neuronal Apoptosis through Regulation of SIRT1-ROCK1 Signaling Pathway. PLoS One. 2013;8(3):e59888. doi: 10.1371/journal.pone.0059888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Choi CW, Choi YH, Cha MR, Kim YS, Yon GH, Hong KS, Park WK, Kim YH, Ryu SY. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med. 2011;77(4):374–376. doi: 10.1055/s-0030-1250370. [DOI] [PubMed] [Google Scholar]
- 316.Frozza RL, Bernardi A, Hoppe JB, Meneghetti AB, Matte A, Battastini AM, Pohlmann AR, Guterres SS, Salbego C. Neuroprotective Effects of Resveratrol Against Abeta Administration in Rats are Improved by Lipid-Core Nanocapsules. Mol. Neurobiol. 2013;47(3):1066–1080. doi: 10.1007/s12035-013-8401-2. [DOI] [PubMed] [Google Scholar]
- 317.Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer's disease. BMC Neurosci. 2008;9( Suppl 2 ):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Anekonda TS. Resveratrol--a boon for treating Alzheimer's disease?. Brain Res. Rev. 2006;52(2):316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 319.Jang MH, Piao XL, Kim JM, Kwon SW, Park JH. Inhibition of cholinesterase and amyloid-beta aggregation by resveratrol oligomers from Vitis amurensis. Phytother. Res. 2008;22(4):544–549. doi: 10.1002/ptr.2406. [DOI] [PubMed] [Google Scholar]
- 320.Zhu X, Ye L, Ge H, Chen L, Jiang N, Qian L, Li L, Liu R, Ji S, Zhang S, Jin J, Guan D, Fang W, Tan R, Xu Y. Hopeahainol A attenuates memory deficits by targeting beta-amyloid in APP/PS1 transgenic mice. Aging Cell. 2013;12(1):85–92. doi: 10.1111/acel.12022. [DOI] [PubMed] [Google Scholar]
- 321.Ge HM, Zhu CH, Shi da H, Zhang LD, Xie DQ, Yang J, Ng SW, Tan RX. Hopeahainol A: an acetylcholinesterase inhibitor from Hopea hainanensis. Chemistry. 2008;14(1):376–381. doi: 10.1002/chem.200700960. [DOI] [PubMed] [Google Scholar]
- 322.Hoi CP, Ho YP, Baum L, Chow AH. Neuroprotective effect of honokiol and magnolol, compounds from Magnolia officinalis on beta-amyloid-induced toxicity in PC12 cells. Phytother. Res. 2010;24(10):1538–1542. doi: 10.1002/ptr.3178. [DOI] [PubMed] [Google Scholar]
- 323.Eldeen IM, Van Heerden FR, Van Staden J. In vitro biological activities of niloticane a new bioactive cassane diterpene from the bark of Acacia nilotica subsp.kraussiana. J. Ethnopharmacol. 2010;128(3):555–560. doi: 10.1016/j.jep.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 324.Lee B, Jung K, Kim DH. Timosaponin AIII a saponin isolated from Anemarrhena asphodeloides ameliorates learning and memory deficits in mice. Pharmacol. Biochem. Behav. 2009;93(2):121–127. doi: 10.1016/j.pbb.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 325.Huang JF, Shang L, Liu P, Zhang MQ, Chen S, Chen D, Fan CL, Wang H, Xiong K. Timosaponin-BII inhibits the up-regulation of BACE1 induced by ferric chloride in rat retina. BMC Complement. Altern. Med. 2012 doi: 10.1186/1472-6882-12-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Grover A, Shandilya A, Agrawal V, Bisaria VS, Sundar D. Computational evidence to inhibition of human acetyl cholinesterase by withanolide a for Alzheimer treatment. J. Biomol. Struct. Dyn. 2012;29(4):651–662. doi: 10.1080/07391102.2012.10507408. [DOI] [PubMed] [Google Scholar]
- 327.Sehgal N, Gupta A, Valli RK, Joshi SD, Mills JT, Hamel E, Khanna P, Jain SC, Thakur SS, Ravindranath V. Withania somnifera reverses Alzheimer's disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. U. S. A. 2012;109(9):3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Kumar S, Seal CJ, Howes MJ, Kite GC, Okello EJ. In vitro protective effects of Withania somnifera (L. dunal root extract against hydrogen peroxide and beta-amyloid(1-42)-induced cytotoxicity in differentiated PC12 cells. Phytother. Res. 2010;24(10):1567–1574. doi: 10.1002/ptr.3261. [DOI] [PubMed] [Google Scholar]
- 329.Patil SP, Maki S, Khedkar SA, Rigby AC, Chan C. Withanolide A and asiatic acid modulate multiple targets associated with amyloid-beta precursor protein processing and amyloid-beta protein clearance. J. Nat. Prod. 2010;73(7):1196–1202. doi: 10.1021/np900633j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nasir MN, Abdullah J, Habsah M, Ghani RI, Rammes G. Inhibitory effect of asiatic acid on acetylcholinesterase, excitatory post synaptic potential and locomotor activity. Phytomedicine. 2012;19(3-4):311–316. doi: 10.1016/j.phymed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 331.Zhang X, Wu J, Dou Y, Xia B, Rong W, Rimbach G, Lou Y. Asiatic acid protects primary neurons against C2-ceramide-induced apoptosis. Eur. J. Pharmacol. 2012;679(1-3):51–59. doi: 10.1016/j.ejphar.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 332.Yi P, Schrott L, Castor TP, Alexander JS. Bryostatin-1 vs.TPPB dose-dependent APP processing and PKC-alpha -delta and -epsilon isoform activation in SH-SY5Y neuronal cells. J. Mol. Neurosci. 2012;48(1):234–244. doi: 10.1007/s12031-012-9816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB3rd, Lee VM, Brunden KR. The microtubule-stabilizing agent epothilone D, reduces axonal dysfunction, neurotoxicity cognitive deficits and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. J. Neurosci. 2012;32(11):3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, Oddo S. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies. Aging Cell. 2013;12(3):370–380. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Heo HJ, Hong SC, Cho HY, Hong B, Kim HK, Kim EK, Shin DH. Inhibitory effect of zeatin isolated from Fiatoua villosa on acetylcholinesterase activity from PC12 cells. Mol. Cells. 2002;13(1):113–117. [PubMed] [Google Scholar]
- 336.Choi SJ, Jeong CH, Choi SG, Chun JY, Kim YJ, Lee J, Shin DH, Heo HJ. Zeatin prevents amyloid beta-induced neurotoxicity and scopolamine-induced cognitive deficits. J Med Food. 2009;12(2):271–277. doi: 10.1089/jmf.2007.0678. [DOI] [PubMed] [Google Scholar]
- 337.Peng Y, Hu Y, Xu S, Li P, Li J, Lu L, Yang H, Feng N, Wang L, Wang X. L-3-n-butylphthalide reduces tau phosphorylation and improves cognitive deficits in AbetaPP/PS1-Alzheimer's transgenic mice. J. Alzheimers Dis. 2012;29(2):379–391. doi: 10.3233/JAD-2011-111577. [DOI] [PubMed] [Google Scholar]
- 338.Garrido-Mesa N, Zarzuelo A, Galvez J. What is behind the non-antibiotic properties of minocycline?. Pharmacol. Res. 2013;67(1):18–30. doi: 10.1016/j.phrs.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 339.Biscaro B, Lindvall O, Tesco G, Ekdahl CT, Nitsch RM. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer's disease. Neurodegener. Dis. 2012;9(4):187–198. doi: 10.1159/000330363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Chen SD, Yin JH, Hwang CS, Tang CM, Yang DI. Anti-apoptotic and anti-oxidative mechanisms of minocycline against sphingomyelinase/ceramide neurotoxicity: implication in Alzheimer's disease and cerebral ischemia. Free Radic Res. 2012;46(8):940–950. doi: 10.3109/10715762.2012.674640. [DOI] [PubMed] [Google Scholar]
- 341.Ferretti MT, Allard S, Partridge V, Ducatenzeiler A, Cuello AC. Minocycline corrects early, pre-plaque neuroinflammation and inhibits BACE-1 in a transgenic model of Alzheimer's disease-like amyloid pathology. J. Neuroinflamm. 2012 doi: 10.1186/1742-2094-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Yu Y, Xie ZL, Gao H, Ma WW, Dai Y, Wang Y, Zhong Y, Yao XS. Bioactive iridoid glucosides from the fruit of Gardenia jasminoides. J. Nat. Prod. 2009;72(8):1459–1464. doi: 10.1021/np900176q. [DOI] [PubMed] [Google Scholar]
- 343.Liu H, Chen YF, Li F, Zhang HY. Fructus Gardenia (Gardenia jasminoides J Ellis) phytochemistry pharmacology of cardiovascular and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2013;15(1):94–110. doi: 10.1080/10286020.2012.723203. [DOI] [PubMed] [Google Scholar]
- 344.Park SJ, Jung HJ, Son MS, Jung JM, Kim DH, Jung IH, Cho YB, Lee EH, Ryu JH. Neuroprotective effects of INM-176 against lipopolysaccharide-induced neuronal injury. Pharmacol. Biochem. Behav. 2012;101(3):427–433. doi: 10.1016/j.pbb.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 345.Park SJ, Jung JM, Lee HE, Lee YW, Kim DH, Kim JM, Hong JG, Lee CH, Jung IH, Cho YB, Jang DS, Ryu JH. The memory ameliorating effects of INM-176, an ethanolic extract of Angelica gigas, against scopolamine- or Abeta(1-42)-induced cognitive dysfunction in mice. J. Ethnopharmacol. 2012;143(2):611–620. doi: 10.1016/j.jep.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 346.Piazzi L, Rampa A, Bisi A, Gobbi S, Belluti F, Cavalli A, Bartolini M, Andrisano V, Valenti P, Recanatini M. 3-(4-[[Benzyl(methyl)amino]methyl]phenyl)-6 7-dimethoxy-2H-2-chromenone (AP2238) inhibits both acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation: a dual function lead for Alzheimer's disease therapy. J. Med. Chem. 2003;46(12):2279–2282. doi: 10.1021/jm0340602. [DOI] [PubMed] [Google Scholar]
- 347.Fernandez-Bachiller MI, Perez C, Gonzalez-Munoz GC, Conde S, Lopez MG, Villarroya M, Garcia AG, Rodriguez-Franco MI. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer's disease with neuroprotective cholinergic antioxidant and copper-complexing properties. J. Med. Chem. 2010;53(13):4927–4937. doi: 10.1021/jm100329q. [DOI] [PubMed] [Google Scholar]
- 348.Camps P, Formosa X, Galdeano C, Gomez T, Munoz-Torrero D, Scarpellini M, Viayna E, Badia A, Clos MV, Camins A, Pallas M, Bartolini M, Mancini F, Andrisano V, Estelrich J, Lizondo M, Bidon-Chanal A, Luque FJ. Novel donepezil-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. J. Med. Chem. 2008;51(12):3588–3598. doi: 10.1021/jm8001313. [DOI] [PubMed] [Google Scholar]
- 349.Camps P, Formosa X, Galdeano C, Munoz-Torrero D, Ramirez L, Gomez E, Isambert N, Lavilla R, Badia A, Clos MV, Bartolini M, Mancini F, Andrisano V, Arce MP, Rodriguez-Franco MI, Huertas O, Dafni T, Luque FJ. Pyrano[3 2-c]quinoline-6-chlorotacrine hybrids as a novel family of acetylcholinesterase- and beta-amyloid-directed anti-Alzheimer compounds. J. Med. Chem. 2009;52(17):5365–5379. doi: 10.1021/jm900859q. [DOI] [PubMed] [Google Scholar]
- 350.Munoz-Ruiz P, Rubio L, Garcia-Palomero E, Dorronsoro I, del Monte-Millan M, Valenzuela R, Usan P, de Austria C, Bartolini M, Andrisano V, Bidon-Chanal A, Orozco M, Luque FJ, Medina M, Martinez A. Design synthesis and biological evaluation of dual binding site acetylcholinesterase inhibitors: new disease-modifying agents for Alzheimer's disease. J. Med. Chem. 2005;48(23):7223–7233. doi: 10.1021/jm0503289. [DOI] [PubMed] [Google Scholar]
- 351.del Monte-Millan M, Garcia-Palomero E, Valenzuela R, Usan P, de Austria C, Munoz-Ruiz P, Rubio L, Dorronsoro I, Martinez A, Medina M. Dual binding site acetylcholinesterase inhibitors: potential new disease-modifying agents for AD. J. Mol. Neurosci. 2006;30(1-2):85–88. doi: 10.1385/JMN:30:1:85. [DOI] [PubMed] [Google Scholar]
- 352.Garcia-Palomero E, Munoz P, Usan P, Garcia P, Delgado E, De Austria C, Valenzuela R, Rubio L, Medina M, Martinez A. Potent beta-amyloid modulators. Neurodegener. Dis. 2008;5(3-4):153–156. doi: 10.1159/000113688. [DOI] [PubMed] [Google Scholar]
- 353.Bolognesi ML, Bartolini M, Mancini F, Chiriano G, Ceccarini L, Rosini M, Milelli A, Tumiatti V, Andrisano V, Melchiorre C. Bis(7)-tacrine derivatives as multitarget-directed ligands: Focus on anticholinesterase and antiamyloid activities. Chemmedchem. 2010;5(8):1215–1220. doi: 10.1002/cmdc.201000086. [DOI] [PubMed] [Google Scholar]
- 354.Rizzo S, Bisi A, Bartolini M, Mancini F, Belluti F, Gobbi S, Andrisano V, Rampa A. Multi-target strategy to address Alzheimer's disease: design synthesis and biological evaluation of new tacrine-based dimers. Eur. J. Med. Chem. 2011;46(9) doi: 10.1016/j.ejmech.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 355.Ouberai M, Brannstrom K, Vestling M, Olofsson A, Dumy P, Chierici S, Garcia J. Clicked tacrine conjugates as acetylcholinesterase and beta-amyloid directed compounds. Org. Biomol. Chem. 2011;9(4):1140–1147. doi: 10.1039/c0ob00393j. [DOI] [PubMed] [Google Scholar]
- 356.Galdeano C, Viayna E, Sola I, Formosa X, Camps P, Badia A, Clos MV, Relat J, Ratia M, Bartolini M, Mancini F, Andrisano V, Salmona M, Minguillon C, Gonzalez-Munoz GC, Rodriguez-Franco MI, Bidon-Chanal A, Luque FJ, Munoz-Torrero D. Huprine-tacrine heterodimers as anti-amyloidogenic compounds of potential interest against Alzheimer's and prion diseases. J. Med. Chem. 2012;55(2):661–669. doi: 10.1021/jm200840c. [DOI] [PubMed] [Google Scholar]
- 357.Munoz-Torrero D, Pera M, Relat J, Ratia M, Galdeano C, Viayna E, Sola I, Formosa X, Camps P, Badia A, Clos MV. Expanding the multipotent profile of huprine-tacrine heterodimers as disease-modifying anti-Alzheimer agents. Neurodegener. Dis. 2012;10(1-4):96–99. doi: 10.1159/000333225. [DOI] [PubMed] [Google Scholar]
- 358.Viayna E, Gomez T, Galdeano C, Ramirez L, Ratia M, Badia A, Clos MV, Verdaguer E, Junyent F, Camins A, Pallas M, Bartolini M, Mancini F, Andrisano V, Arce MP, Rodriguez-Franco MI, Bidon-Chanal A, Luque FJ, Camps P, Munoz-Torrero D. Novel huprine derivatives with inhibitory activity toward beta-amyloid aggregation and formation as disease-modifying anti-Alzheimer drug candidates. Chemmedchem. 2010;5(11):1855–1870. doi: 10.1002/cmdc.201000322. [DOI] [PubMed] [Google Scholar]
- 359.Rizzo S, Bartolini M, Ceccarini L, Piazzi L, Gobbi S, Cavalli A, Recanatini M, Andrisano V, Rampa A. Targeting Alzheimer's disease: Novel indanone hybrids bearing a pharmacophoric fragment of AP2238. Bioorg. Med. Chem. 2010;18(5):1749–1760. doi: 10.1016/j.bmc.2010.01.071. [DOI] [PubMed] [Google Scholar]
- 360.Rodriguez-Franco MI, Fernandez-Bachiller MI, Perez C, Castro A, Martinez A. Design and synthesis of N-benzylpiperidine-purine derivatives as new dual inhibitors of acetyl- and butyrylcholinesterase. Bioorg. Med. Chem. 2005;13(24):6795–6802. doi: 10.1016/j.bmc.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 361.Shan WJ, Huang L, Zhou Q, Meng FC, Li XS. Synthesis biological evaluation of 9-N-substituted berberine derivatives as multi-functional agents of antioxidant, inhibitors of acetylcholinesterase butyrylcholinesterase and amyloid-beta aggregation. Eur. J. Med. Chem. 2011;46(12):5885–5893. doi: 10.1016/j.ejmech.2011.09.051. [DOI] [PubMed] [Google Scholar]
- 362.Shi A, Huang L, Lu C, He F, Li X. Synthesis biological evaluation and molecular modeling of novel triazole-containing berberine derivatives as acetylcholinesterase and beta-amyloid aggregation inhibitors. Bioorg. Med. Chem. 2011;19(7):2298–2305. doi: 10.1016/j.bmc.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 363.Yan JW, Li YP, Ye WJ, Chen SB, Hou JQ, Tan JH, Ou TM, Li D, Gu LQ, Huang ZS. Design synthesis and evaluation of isaindigotone derivatives as dual inhibitors for acetylcholinesterase and amyloid beta aggregation. Bioorg Med Chem. 2012;20(8):2527–2534. doi: 10.1016/j.bmc.2012.02.061. [DOI] [PubMed] [Google Scholar]
- 364.Tang H, Zhao HT, Zhong SM, Wang ZY, Chen ZF, Liang H. Novel oxoisoaporphine-based inhibitors of acetyl- and butyrylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Bioorg. Med. Chem. Lett. 2012;22(6):2257–2261. doi: 10.1016/j.bmcl.2012.01.090. [DOI] [PubMed] [Google Scholar]
- 365.Tang H, Wei YB, Zhang C, Ning FX, Qiao W, Huang SL, Ma L, Huang ZS, Gu LQ. Synthesis biological evaluation and molecular modeling of oxoisoaporphine and oxoaporphine derivatives as new dual inhibitors of acetylcholinesterase/ butyrylcholinesterase. Eur. J. Med. Chem. 2009;44(6):2523–2532. doi: 10.1016/j.ejmech.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 366.Tang H, Zhao LZ, Zhao HT, Huang SL, Zhong SM, Qin JK, Chen ZF, Huang ZS, Liang H. Hybrids of oxoisoaporphine-tacrine congeners: novel acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation inhibitors. Eur. J. Med. Chem. 2011;46(10):4970–4979. doi: 10.1016/j.ejmech.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 367.Luo W, Li YP, He Y, Huang SL, Tan JH, Ou TM, Li D, Gu LQ, Huang ZS. Design synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as dual inhibitors for cholinesterases and amyloid beta aggregation. Bioorg Med Chem. 2011;19(2):763–770. doi: 10.1016/j.bmc.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 368.Mohamed T, Zhao X, Habib LK, Yang J, Rao PP. Design synthesis and structure-activity relationship (SAR) studies of 2,4-disubstituted pyrimidine derivatives: dual activity as cholinesterase and Abeta-aggregation inhibitors. Bioorg. Med. Chem. 2011;19(7):2269–2281. doi: 10.1016/j.bmc.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Mohamed T, Yeung JC, Rao PP. Development of 2-substituted-N-(naphth-1-ylmethyl) and N-benzhydrylpyrimidin-4-amines as dual cholinesterase and Abeta-aggregation inhibitors: Synthesis and biological evaluation. Bioorg. Med. Chem. Lett. 2011;21(19):5881–5887. doi: 10.1016/j.bmcl.2011.07.091. [DOI] [PubMed] [Google Scholar]
- 370.Kwon YE, Park JY, No KT, Shin JH, Lee SK, Eun JS, Yang JH, Shin TY, Kim DK, Chae BS, Leem JY, Kim KH. Synthesis in vitro assay and molecular modeling of new piperidine derivatives having dual inhibitory potency against acetylcholinesterase and Abeta1-42 aggregation for Alzheimer's disease therapeutics. Bioorg. Med. Chem. 2007;15(20):6596–6607. doi: 10.1016/j.bmc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 371.Arce MP, Rodriguez-Franco MI, Gonzalez-Munoz GC, Perez C, Lopez B, Villarroya M, Lopez MG, Garcia AG, Conde S. Neuroprotective and cholinergic properties of multifunctional glutamic acid derivatives for the treatment of Alzheimer's disease. J. Med. Chem. 2009;52(22):7249–7257. doi: 10.1021/jm900628z. [DOI] [PubMed] [Google Scholar]
- 372.Rizzo S, Tarozzi A, Bartolini M, Da Costa G, Bisi A, Gobbi S, Belluti F, Ligresti A, Allara M, Monti JP, Andrisano V, Di Marzo V, Hrelia P, Rampa A. 2-Arylbenzofuran-based molecules as multipotent Alzheimer's disease modifying agents. Eur. J. Med. Chem. 2012;58C:519–532. doi: 10.1016/j.ejmech.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 373.Belluti F, Bartolini M, Bottegoni G, Bisi A, Cavalli A, Andrisano V, Rampa A. Benzophenone-based derivatives: a novel series of potent and selective dual inhibitors of acetylcholinesterase and acetylcholinesterase-induced beta-amyloid aggregation. Eur. J. Med. Chem. 2011;46(5):1682–1693. doi: 10.1016/j.ejmech.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 374.Rosini M, Andrisano V, Bartolini M, Bolognesi ML, Hrelia P, Minarini A, Tarozzi A, Melchiorre C. Rational approach to discover multipotent anti-Alzheimer drugs. J. Med. Chem. 2005;48(2):360–363. doi: 10.1021/jm049112h. [DOI] [PubMed] [Google Scholar]
- 375.Cavalli A, Bolognesi ML, Capsoni S, Andrisano V, Bartolini M, Margotti E, Cattaneo A, Recanatini M, Melchiorre C. A small molecule targeting the multifactorial nature of Alzheimer's disease. Angew Chem. Int. Ed. Engl. 2007;46(20):3689–3692. doi: 10.1002/anie.200700256. [DOI] [PubMed] [Google Scholar]
- 376.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratman NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature. 1999;402(6761):533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 377.Bolognesi ML, Chiriano G, Bartolini M, Mancini F, Bottegoni G, Maestri V, Czvitkovich S, Windisch M, Cavalli A, Minarini A, Rosini M, Tumiatti V, Andrisano V, Melchiorre C. Synthesis of monomeric derivatives to probe memoquin's bivalent interactions. J. Med. Chem. 2011;54(24):8299–8304. doi: 10.1021/jm200691d. [DOI] [PubMed] [Google Scholar]
- 378.Capurro V, Busquet P, Lopes JP, Bertorelli R, Tarozzo G, Bolognesi ML, Piomelli D, Reggiani A, Cavalli A. Pharmacological characterization of memoquin, a multi-target compound for the treatment of Alzheimer's disease. PLoS One. 2013;8(2):e56870. doi: 10.1371/journal.pone.0056870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Bolognesi ML, Banzi R, Bartolini M, Cavalli A, Tarozzi A, Andrisano V, Minarini A, Rosini M, Tumiatti V, Bergamini C, Fato R, Lenaz G, Hrelia P, Cattaneo A, Recanatini M, Melchiorre C. Novel class of quinone-bearing polyamines as multi-target-directed ligands to combat Alzheimer's disease. J. Med. Chem. 2007;50(20):4882–4897. doi: 10.1021/jm070559a. [DOI] [PubMed] [Google Scholar]
- 380.Bolognesi ML, Bartolini M, Tarozzi A, Morroni F, Lizzi F, Milelli A, Minarini A, Rosini M, Hrelia P, Andrisano V, Melchiorre C. Multitargeted drugs discovery: Balancing anti-amyloid and anticholinesterase capacity in a single chemical entity. Bioorg. Med. Chem. Lett. 2011;21(9):2655–2658. doi: 10.1016/j.bmcl.2010.12.093. [DOI] [PubMed] [Google Scholar]
- 381.Rodriguez-Franco MI, Fernandez-Bachiller MI, Perez C, Hernandez-Ledesma B, Bartolome B. Novel tacrine-melatonin hybrids as dual-acting drugs for Alzheimer disease with improved acetylcholinesterase inhibitory and antioxidant properties. J. Med. Chem. 2006;49(2):459–462. doi: 10.1021/jm050746d. [DOI] [PubMed] [Google Scholar]
- 382.Fernandez-Bachiller MI, Perez C, Campillo NE, Paez JA, Gonzalez-Munoz GC, Usan P, Garcia-Palomero E, Lopez MG, Villarroya M, Garcia AG, Martinez A, Rodriguez-Franco MI. Tacrine-melatonin hybrids as multifunctional agents for Alzheimer's disease with cholinergic antioxidant and neuroprotective properties. Chemmedchem. 2009;4(5):828–841. doi: 10.1002/cmdc.200800414. [DOI] [PubMed] [Google Scholar]
- 383.Fang L, Kraus B, Lehmann J, Heilmann J, Zhang Y, Decker M. Design and synthesis of tacrine-ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008;18(9):2905–2909. doi: 10.1016/j.bmcl.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 384.Minarini A, Milelli A, Tumiatti V, Rosini M, Simoni E, Bolognesi ML, Andrisano V, Bartolini M, Motori E, Angeloni C, Hrelia S. Cystamine-tacrine dimer: a new multi-target-directed ligand as potential therapeutic agent for Alzheimer's disease treatment. Neuropharmacology. 2012;62(2):997–1003. doi: 10.1016/j.neuropharm.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 385.Gonzalez-Munoz GC, Arce MP, Lopez B, Perez C, Villarroya M, Lopez MG, Garcia AG, Conde S, Rodriguez-Franco MI. Old phenothiazine and dibenzothiadiazepine derivatives for tomorrow's neuroprotective therapies against neuro- degenerative diseases. Eur. J. Med. Chem. 2010;45(12):6152–6158. doi: 10.1016/j.ejmech.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 386.Gonzalez-Munoz GC, Arce MP, Lopez B, Perez C, Romero A, del Barrio L, Martin-de-Saavedra MD, Egea J, Leon R, Villarroya M, Lopez MG, Garcia AG, Conde S, Rodriguez-Franco MI. N-acylaminophenothiazines: neuroprotective agents displaying multifunctional activities for a potential treatment of Alzheimer's disease. Eur. J. Med. Chem. 2011;46(6):2224–2235. doi: 10.1016/j.ejmech.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 387.Zheng H, Weiner LM, Bar-Am O, Epsztejn S, Cabantchik ZI, Warshawsky A, Youdim MB, Fridkin M. Design synthesis and evaluation of nov.l bifunctional iron-chelators as potential agents for neuroprotection in Alzheimer's Parkinson's and other neurodegenerative diseases. Bioorg. Med. Chem. 2005;13(3):773–783. doi: 10.1016/j.bmc.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 388.Zheng H, Youdim MB, Fridkin M. Site-activated multi- functional chelator with acetylcholinesterase and neuroprotective-neurorestorative moieties for Alzheimer's therapy. J. Med. Chem. 2009;52(14):4095–4098. doi: 10.1021/jm900504c. [DOI] [PubMed] [Google Scholar]
- 389.Meng FC, Mao F, Shan WJ, Qin F, Huang L, Li XS. Design synthesis and evaluation of indanone derivatives as acetylcholinesterase inhibitors and metal-chelating agents. Bioorg. Med. Chem. Lett. 2012;22(13):4462–4466. doi: 10.1016/j.bmcl.2012.04.029. [DOI] [PubMed] [Google Scholar]
- 390.Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, Harrison J, Lannfelt L, Blennow K, Zetterberg H, Ingelsson M, Masters CL, Tanzi RE, Cummings JL, Herd CM, Bush AI. PBT2 rapidly improves cognition in Alzheimer's Disease: additional phase II analyses. J. Alzheimers Dis. 2010;20(2):509–516. doi: 10.3233/JAD-2010-1390. [DOI] [PubMed] [Google Scholar]
- 391.Piazzi L, Cavalli A, Colizzi F, Belluti F, Bartolini M, Mancini F, Recanatini M, Andrisano V, Rampa A. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-Alzheimer compounds. Bioorg. Med. Chem. Lett. 2008;18(1):423–426. doi: 10.1016/j.bmcl.2007.09.100. [DOI] [PubMed] [Google Scholar]
- 392.Li W, Mak M, Jiang H, Wang Q, Pang Y, Chen K, Han Y. Novel anti-Alzheimer's dimer Bis(7)-cognitin: cellular and molecular mechanisms of neuroprotection through multiple targets. Neurotherapeutics. 2009;6(1):187–201. doi: 10.1016/j.nurt.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Pang YP, Quiram P, Jelacic T, Hong F, Brimijoin S. Highly potent selective and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase.Steps toward novel drugs for treating Alzheimer's disease. J. Biol. Chem. 1996;271(39):23646–23649. doi: 10.1074/jbc.271.39.23646. [DOI] [PubMed] [Google Scholar]
- 394.Fernandez-Bachiller MI, Perez C, Monjas L, Rademann J, Rodriguez-Franco MI. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer's disease with cholinergic, antioxidant, and beta-amyloid-reducing properties. J. Med. Chem. 2012;55(3):1303–1317. doi: 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- 395.Zhu Y, Xiao K, Ma L, Xiong B, Fu Y, Yu H, Wang W, Wang X, Hu D, Peng H, Li J, Gong Q, Chai Q, Tang X, Zhang H, Li J, Shen J. Design synthesis and biological evaluation of novel dual inhibitors of acetylcholinesterase and beta-secretase. Bioorg. Med. Chem. 2009;17(4):1600–1613. doi: 10.1016/j.bmc.2008.12.067. [DOI] [PubMed] [Google Scholar]
- 396.Huang W, Tang L, Shi Y, Huang S, Xu L, Sheng R, Wu P, Li J, Zhou N, Hu Y. Searching for the Multi-Target-Directed Ligands against Alzheimer's disease: discovery of quinoxaline-based hybrid compounds with AChE, H(3)R and BACE 1 inhibitory activities. Bioorg. Med. Chem. 2011;19(23):7158–7167. doi: 10.1016/j.bmc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 397.Youdim MB. Multi target neuroprotective and neurorestorative anti-Parkinson and anti-Alzheimer drugs ladostigil and m30 derived from rasagiline. Exp. Neurobiol. 2013;22(1):1–10. doi: 10.5607/en.2013.22.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Kupershmidt L, Amit T, Bar-Am O, Weinreb O, Youdim MB. Multi-target neuroprotective and neurorestorative M30 improves cognitive impairment and reduces Alzheimer's-like neuropathology and age-related alterations in mice. Mol. Neurobiol. 2012;46(1):217–220. doi: 10.1007/s12035-012-8304-7. [DOI] [PubMed] [Google Scholar]
- 399.Bolea I, Juarez-Jimenez J, de Los Rios C, Chioua M, Pouplana R, Luque FJ, Unzeta M, Marco-Contelles J, Samadi A. Synthesis biological evaluation, and molecular modeling of donepezil and N-[(5-(benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids as new multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer's disease. J. Med. Chem. 2011;54(24):8251–8270. doi: 10.1021/jm200853t. [DOI] [PubMed] [Google Scholar]
- 400.Melchiorre C, Andrisano V, Bolognesi ML, Budriesi R, Cavalli A, Cavrini V, Rosini M, Tumiatti V, Recanatini M. Acetylcholinesterase noncovalent inhibitors based on a polyamine backbone for potential use against Alzheimer's disease. J. Med. Chem. 1998;41(22):4186–4189. doi: 10.1021/jm9810452. [DOI] [PubMed] [Google Scholar]
- 401.Roman S, Vivas NM, Badia A, Clos MV. Interaction of a new potent anticholinesterasic compound (+/-)huprine X with muscarinic receptors in rat brain. Neurosci. Lett. 2002;325(2):103–106. doi: 10.1016/s0304-3940(02)00245-8. [DOI] [PubMed] [Google Scholar]
- 402.Dvir H, Wong DM, Harel M, Barril X, Orozco M, Luque FJ, Munoz-Torrero D, Camps P, Rosenberry TL, Silman I, Sussman JL. 3D structure of Torpedo californica acetylcholinesterase complexed with huprine X at 2. A resolution: kinetic and molecular dynamic correlates. Biochemistry. 2002;41(9):2970–2981. doi: 10.1021/bi011652i. [DOI] [PubMed] [Google Scholar]
- 403.Cui W, Cui GZ, Li W, Zhang Z, Hu S, Mak S, Zhang H, Carlier PR, Choi CL, Wong YT, Lee SM, Han Y. Bis(12)-hupyridone a novel multifunctional dimer promotes neuronal differentiation more potently than its monomeric natural analog huperzine.A possibly through alpha7 nAChR. Brain Res. 2011;1401:10–17. doi: 10.1016/j.brainres.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 404.Borroni E, Damsma G, Giovacchini C, Mutel V, Jakob-Rotne R, Da Prada M. A novel acetylcholinesterase inhibitor, Ro 46-5934 which interacts with muscarinic M2 receptors. Biochem. Soc. Trans. 1994;22(3):755–758. doi: 10.1042/bst0220755. [DOI] [PubMed] [Google Scholar]
- 405.Petroianu G, Arafat K, Sasse BC, Stark H. Multiple enzyme inhibitions by histamine H3 receptor antagonists as potential procognitive agents. Pharmazie. 2006;61(3):179–182. [PubMed] [Google Scholar]
- 406.Incerti M, Flammini L, Saccani F, Morini G, Comini M, Coruzzi M, Barocelli E, Ballabeni V, Bertoni S. Dual-acting drugs: an in vitro study of nonimidazole histamine H3 receptor antagonists combining anticholinesterase activity. Chemmedchem. 2010;5(7):1143–1149. doi: 10.1002/cmdc.201000008. [DOI] [PubMed] [Google Scholar]
- 407.Rosini M, Simoni E, Bartolini M, Cavalli A, Ceccarini L, Pascu N, McClymont DW, Tarozzi A, Bolognesi ML, Minarini A, Tumiatti V, Andrisano V, Mellor IR, Melchiorre C. Inhibition of acetylcholinesterase beta-amyloid aggregation and NMDA receptors in Alzheimer's disease: a promising direction for the multi-target-directed ligands gold rush. J. Med. Chem. 2008;51(15):4381–4384. doi: 10.1021/jm800577j. [DOI] [PubMed] [Google Scholar]
- 408.Li W, Pi R, Chan HH, Fu H, Lee NT, Tsang HW, Pu Y, Chang DC, Li C, Luo J, Xiong K, Li Z, Xue H, Carlier PR, Pang Y, Tsim KW, Li M, Han Y. Novel dimeric acetylcholinesterase inhibitor bis7-tacrine but not donepezil prevents glutamate-induced neuronal apoptosis by blocking N-methyl-D-aspartate receptors. J. Biol. Chem. 2005;280(18):18179–18188. doi: 10.1074/jbc.M411085200. [DOI] [PubMed] [Google Scholar]
- 409.Rook Y, Schmidtke K-U, Gaube F, Schepmann D, Wunsch B, Heilmann Jr, Lehmann J, Winckler T. Bivalent ß-Carbolines as Potential Multitarget Anti-Alzheimer Agents. J. Med. Chem. 2010;53(9):3611–3617. doi: 10.1021/jm1000024. [DOI] [PubMed] [Google Scholar]
- 410.Steele JW, Gandy S. Latrepirdine (Dimebon ((R)) ): a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse model. Autophagy. 2013;9(4):617–618. doi: 10.4161/auto.23487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Sabbagh MN, Shill HA. Latrepirdine a potential novel treatment for Alzheimer's disease and Huntington's chorea. Curr. Opin. Investig. Drugs. 2010;11(1):80–91. [PMC free article] [PubMed] [Google Scholar]
- 412.Ivachtchenko AV, Frolov EB, Mitkin OD, Kysil VM, Khvat AV, Okun IM, Tkachenko SE. Synthesis and biological evaluation of novel gamma-carboline analogues of Dimebon as potent 5-HT6 receptor antagonists. Bioorg. Med. Chem. Lett. 2009;19(12):3183–3187. doi: 10.1016/j.bmcl.2009.04.128. [DOI] [PubMed] [Google Scholar]
- 413.Sachdeva D, Burns A. Dimebolin in dementia. CNS Neurosci. Ther. 2011;17(3):199–205. doi: 10.1111/j.1755-5949.2010.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Cappelli A, Gallelli A, Manini M, Anzini M, Mennuni L, Makovec F, Menziani MC, Alcaro S, Ortuso F, Vomero S. Further studies on the interaction of the 5-hydroxytryptamine3 (5-HT3) receptor with arylpiperazine ligands.development of a new 5-HT3 receptor ligand showing potent acetylcholinesterase inhibitory properties. J. Med. Chem. 2005;48(10):3564–3575. doi: 10.1021/jm0493461. [DOI] [PubMed] [Google Scholar]
- 415.Lange JHM, Coolen HKAC, van der Neut MAW, Borst AJM, Stork B, Verveer PC, Kruse CG. Design Synthesis Biological Properties and Molecular Modeling Investigations of Novel Tacrine Derivatives with a Combination of Acetylcholinesterase Inhibition and Cannabinoid CB1Receptor Antagonism. J. Med. Chem. 2010;53(3):1338–1346. doi: 10.1021/jm901614b. [DOI] [PubMed] [Google Scholar]
- 416.Lecanu L, Tillement L, McCourty A, Rammouz G, Yao W, Greeson J, Papadopoulos V. Dimethyl-carbamic acid 2 3-Bis-Dimethylcarbamoyloxy-6-(4-Ethyl-Piperazine-1-Carbonyl)-phenyl ester: a novel multi-target therapeutic approach to neuroprotection. Med. Chem. 2010;6(3):123–140. doi: 10.2174/1573406411006030123. [DOI] [PubMed] [Google Scholar]
- 417.Orozco C, de Los Rios C, Arias E, Leon R, Garcia AG, Marco JL, Villarroya M, Lopez MG. ITH4012 (ethyl 5-amino-6 7 8 9-tetrahydro-2-methyl-4-phenylbenzol[1 8]naphthyridine-3-carboxylat e): a novel acetylcholinesterase inhibitor with "calcium promotor" and neuroprotective properties. J. Pharmacol. Exp. Ther. 2004;310(3):987–994. doi: 10.1124/jpet.104.068189. [DOI] [PubMed] [Google Scholar]
- 418.Gao X, Zheng CY, Yang L, Tang XC, Zhang HY. Huperzine A protects isolated rat brain mitochondria against beta-amyloid peptide. Free Radic. Biol. Med. 2009;46(11):1454–1462. doi: 10.1016/j.freeradbiomed.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 419.Marco-Contelles J, Leon R, de los Rios C, Samadi A, Bartolini M, Andrisano V, Huertas O, Barril X, Luque FJ, Rodriguez-Franco MI, Lopez B, Lopez MG, Garcia AG, Carreiras Mdo C, Villarroya M. Tacripyrines the first tacrine-dihydropyridine hybrids as multitarget-directed ligands for the treatment of Alzheimer's disease. J. Med. Chem. 2009;52(9):2724–2732. doi: 10.1021/jm801292b. [DOI] [PubMed] [Google Scholar]
- 420.Bartolini M, Pistolozzi M, Andrisano V, Egea J, Lopez MG, Iriepa I, Moraleda I, Galvez E, Marco-Contelles J, Samadi A. Chemical and pharmacological studies on enantiomerically pure p-methoxytacripyrines, promising multi-target-directed ligands for the treatment of Alzheimer's disease. Chemmedchem. 2011;6(11):1990–1997. doi: 10.1002/cmdc.201100239. [DOI] [PubMed] [Google Scholar]
- 421.Leon R, de los Rios C, Marco-Contelles J, Huertas O, Barril X, Luque FJ, Lopez MG, Garcia AG, Villarroya M. New tacrine-dihydropyridine hybrids that inhibit acetylcholinesterase calcium entry and exhibit neuroprotection properties. Bioorg. Med. Chem. 2008;16(16):7759–7769. doi: 10.1016/j.bmc.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 422.Tomassoli I, Ismaili L, Pudlo M, de los Ríos C, Soriano E, Colmena I, Gandía L, Rivas L, Samadi A, Marco-Contelles J, Refouvelet B. Synthesis biological assessment and molecular modeling of new dihydroquinoline-3-carboxamides and dihydroquinoline-3-carbohydrazide derivatives as cholinesterase inhibitors, and Ca channel antagonists. Eur. J. Med. Chem. 2011;46(1):1–10. doi: 10.1016/j.ejmech.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 423.Li J, Hu J, Shao B, Zhou W, Cui Y, Dong C, Ezoulin J-MM, Zhu X, Ding W, Heymans F, Chen H. Protection of PMS777 a New AChE Inhibitor with PAF Antagonism, Against Amyloid-ß-Induced Neuronal Apoptosis and Neuroinflammation. Cell. Mol. Neurobiol. 2009;29(4):589–595. doi: 10.1007/s10571-009-9351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Miezan Ezoulin JM, Shao BY, Xia Z, Xie Q, Li J, Cui YY, Wang H, Dong CZ, Zhao YX, Massicot F, Qiu ZB, Heymans F, Chen HZ. Novel piperazine derivative PMS1339 exhibits tri-functional properties and cognitive improvement in mice. Int. J. Neuropsychopharmacol. 2009;12(10):1409–1419. doi: 10.1017/S1461145709000455. [DOI] [PubMed] [Google Scholar]
- 425.Avramovich-Tirosh Y, Amit T, Bar-Am O, Zheng H, Fridkin M, Youdim MB. Therapeutic targets and potential of the novel brain- permeable multifunctional iron chelator-monoamine oxidase inhibitor drug, M-30 for the treatment of Alzheimer's disease. J. Neurochem. 2007;100(2):490–502. doi: 10.1111/j.1471-4159.2006.04258.x. [DOI] [PubMed] [Google Scholar]
- 426.Weinreb O, Mandel S, Bar-Am O, Yogev-Falach M, Avramovich-Tirosh Y, Amit T, Youdim MB. Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer's disease drugs. Neurotherapeutics. 2009;6(1):163–174. doi: 10.1016/j.nurt.2008.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Weinreb O, Mandel S, Bar-Am O, Amit T. Iron-chelating backbone coupled with monoamine oxidase inhibitory moiety as novel pluripotential therapeutic agents for Alzheimer's disease: a tribute to Moussa Youdim. J. Neural Transm. 2011;118(3):479–492. doi: 10.1007/s00702-011-0597-6. [DOI] [PubMed] [Google Scholar]
- 428.Petzer JP, Steyn S, Castagnoli KP, Chen JF, Schwarzschild MA, Van der Schyf CJ, Castagnoli N. Inhibition of monoamine oxidase B by selective adenosine A2A receptor antagonists. Bioorg. Med. Chem. 2003;11(7):1299–1310. doi: 10.1016/s0968-0896(02)00648-x. [DOI] [PubMed] [Google Scholar]
- 429.van den Berg D, Zoellner KR, Ogunrombi MO, Malan SF, Terre'Blanche G, Castagnoli N Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase B by selected benzimidazole and caffeine analogues. Bioorg. Med. Chem. 2007;15(11):3692–3702. doi: 10.1016/j.bmc.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 430.Pretorius J, Malan SF, Castagnoli N Jr, Bergh JJ, Petzer JP. Dual inhibition of monoamine oxidase B and antagonism of the adenosine A(2A) receptor by (E E)-8-(4-phenylbutadien-1-yl)caffeine analogues. Bioorg. Med .Chem. 2008;16(18):8676–8684. doi: 10.1016/j.bmc.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 431.Binda C, Aldeco M, Geldenhuys WJ, Tortorici M, Mattevi A, Edmondson DE. Molecular Insights into Human Monoamine Oxidase B Inhibition by the Glitazone Anti-Diabetes Drugs. ACS Med. Chem. Lett. 2011;3(1):39–42. doi: 10.1021/ml200196p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Mendgen T, Steuer C, Klein CD. Privileged scaffolds or promiscuous binders: a comparative study on rhodanines and related heterocycles in medicinal chemistry. J. Med. Chem. 2012;55(2):743–753. doi: 10.1021/jm201243p. [DOI] [PubMed] [Google Scholar]
- 433.Geldenhuys WJ, Darvesh AS, Funk MO, Van der Schyf CJ, Carroll RT. Identification of novel monoamine oxidase B inhibitors by structure-based virtual screening. Bioorg. Med. Chem. Lett. 2010;20(17):5295–5298. doi: 10.1016/j.bmcl.2010.06.128. [DOI] [PubMed] [Google Scholar]
- 434.Hey JA, Aslanian RG. Use of dual H3/M2 antagonists in the treatment of cognition deficit disorders. U S Patent 6 906 081 june 14. 2005 [Google Scholar]
- 435.Lockman JA, Geldenhuys WJ, Jones-Higgins MR, Patrick JD, Allen DD, Van der Schyf CJ. NGP1-01 a multi-targeted polycyclic cage amine attenuates brain endothelial cell death in iron overload conditions. Brain Res. 2012;1489:133–139. doi: 10.1016/j.brainres.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 436.Geldenhuys WJ, Malan SF, Bloomquist JR, Van der Schyf CJ. Structure-activity relationships of pentacycloundecylamines at the N-methyl-d-aspartate receptor. Bioorg. Med. Chem. 2007;15(3):1525–1532. doi: 10.1016/j.bmc.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 437.Danysz W, Parsons CG. Alzheimer's disease beta-amyloid glutamate NMDA receptors and memantine--searching for the connections. Br. J. Pharmacol. 2012;167(2):324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Atri A, Molinuevo JL, Lemming O, Wirth Y, Pulte I, Wilkinson D. Memantine in patients with Alzheimer's disease receiving donepezil: new analyses of efficacy and safety for combination therapy. Alzheimers Res. Ther. 2013 doi: 10.1186/alzrt160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Kamat PK, Rai S, Swarnkar S, Shukla R, Ali S, Najmi AK, Nath C. Okadaic acid-induced Tau phosphorylation in rat brain: Role of NMDA receptor. Neuroscience. 2013;238:97–113. doi: 10.1016/j.neuroscience.2013.01.075. [DOI] [PubMed] [Google Scholar]
- 440.Marcade M, Bourdin J, Loiseau N, Peillon H, Rayer A, Drouin D, Schweighoffer F, Desire L. Etazolate a neuroprotective drug linking GABA(A) receptor pharmacology to amyloid precursor protein processing. J. Neurochem. 2008;106(1):392–404. doi: 10.1111/j.1471-4159.2008.05396.x. [DOI] [PubMed] [Google Scholar]
- 441.Huang W, Lv D, Yu H, Sheng R, Kim SC, Wu P, Luo K, Li J, Hu Y. Dual-target-directed 1 3-diphenylurea derivatives: BACE 1 inhibitor and metal chelator against Alzheimer's disease. Bioorg. Med. Chem. 2010;18(15):5610–5615. doi: 10.1016/j.bmc.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 442.Jiaranaikulwanitch J, Boonyarat C, Fokin VV, Vajragupta O. Triazolyl tryptoline derivatives as beta-secretase inhibitors. Bioorg Med. Chem. Lett. 2010;20(22):6572–6576. doi: 10.1016/j.bmcl.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Jiaranaikulwanitch J, Govitrapong P, Fokin VV, Vajragupta O. From BACE1 inhibitor to multifunctionality of tryptoline and tryptamine triazole derivatives for Alzheimer's disease. Molecules. 2012;17(7):8312–8333. doi: 10.3390/molecules17078312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Hieke M, Ness J, Steri R, Greiner C, Werz O, Schubert-Zsilavecz M, Weggen S, Zettl H. SAR studies of acidic dual gamma-secretase/PPARgamma modulators. Bioorg. Med. Chem. 2011;19(18):5372–5382. doi: 10.1016/j.bmc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 445.Paterniti I, Mazzon E, Gil C, Impellizzeri D, Palomo V, Redondo M, Perez DI, Esposito E, Martinez A, Cuzzocrea S. PDE 7 inhibitors: new potential drugs for the therapy of spinal cord injury. PLoS One. 2011;6(1):e15937. doi: 10.1371/journal.pone.0015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Kozikowski AP, Chen Y, Subhasish T, Lewin NE, Blumberg PM, Zhong Z, D'Annibale MA, Wang WL, Shen Y, Langley B. Searching for disease modifiers-PKC activation and HDAC inhibition - a dual drug approach to Alzheimer's disease that decreases Abeta production while blocking oxidative stress. Chemmedchem. 2009;4(7):1095–1105. doi: 10.1002/cmdc.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Flaherty DP, Kiyota T, Dong Y, Ikezu T, Vennerstrom JL. Phenolic bis-styrylbenzenes as beta-amyloid binding ligands and free radical scavengers. J. Med. Chem. 2010;53(22):7992–7999. doi: 10.1021/jm1006929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Lenhart JA, Ling X, Gandhi R, Guo TL, Gerk PM, Brunzell DH, Zhang S. "Clicked" bivalent ligands containing curcumin and cholesterol as multifunctional abeta oligomerization inhibitors: design synthesis and biological characterization. J. Med. Chem. 2010;53(16):6198–6209. doi: 10.1021/jm100601q. [DOI] [PubMed] [Google Scholar]
- 449.Dedeoglu A, Cormier K, Payton S, Tseitlin KA, Kremsky JN, Lai L, Li X, Moir RD, Tanzi RE, Bush AI, Kowall NW, Rogers JT, Huang X. Preliminary studies of a novel bifunctional metal chelator targeting Alzheimer's amyloidogenesis. Exp. Gerontol. 2004;39(11-12):1641–1649. doi: 10.1016/j.exger.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 450.Ji HF, Zhang HY. A new strategy to combat Alzheimer's disease.Combining radical-scavenging potential with metal-protein-attenuating ability in one molecule. Bioorg. Med. Chem. Lett. 2005;15(1):21–24. doi: 10.1016/j.bmcl.2004.10.047. [DOI] [PubMed] [Google Scholar]
- 451.Bebbington D, Monck NJ, Gaur S, Palmer AM, Benwell K, Harvey V, Malcolm CS, Porter RH. 3 5-Disubstituted-4-hydroxyphenyls linked to 3-hydroxy-2-methyl-4(1H)-pyridinone: potent inhibitors of lipid peroxidation and cell toxicity. J. Med. Chem. 2000;43(15):2779–2782. doi: 10.1021/jm990945v. [DOI] [PubMed] [Google Scholar]
- 452.Schugar H, Green DE, Bowen ML, Scott LE, Storr T, Bohmerle K, Thomas F, Allen DD, Lockman PR, Merkel M, Thompson KH, Orvig C. Combating Alzheimer's disease with multifunctional molecules designed for metal passivation. Angew Chem. Int. Ed. Engl. 2007;46(10):1716–1718. doi: 10.1002/anie.200603866. [DOI] [PubMed] [Google Scholar]
- 453.Storr T, Merkel M, Song-Zhao GX, Scott LE, Green DE, Bowen ML, Thompson KH, Patrick BO, Schugar HJ, Orvig C. Synthesis characterization and metal coordinating ability of multifunctional carbohydrate-containing compounds for Alzheimer's therapy. J. Am. Chem. Soc. 2007;129(23):7453–7463. doi: 10.1021/ja068965r. [DOI] [PubMed] [Google Scholar]