Abstract
The purines ATP and adenosine are widely recognized for their neuromodulatory effects. They have been shown to have effects on neurons via various receptors and interactions with glial cells. In particular, long-term potentiation (LTP) in hippocampal slice preparations has been found to be modulated by ATP and adenosine. This review gives an overview of purinergic signaling in relation to hippocampal LTP and memory formation. The data supports the hypothesis that adenosine mediates a tonic suppression of synaptic transmission. Thus, low adenosine levels appear to increase basal synaptic activity via a decreased activation of the inhibitor A1 receptor, consequently making it more difficult to induce LTP because of lower contrast. During high stimulation, the inhibition of neighboring pathways by adenosine, in combination with an A2a receptor activation, appears to increase contrast of excited pathways against a nonexcited background. This would enable amplification of specific signaling while suppressing non-specific events. Although a clear role for purinergic signaling in LTP is evident, more studies are needed to scrutinize the modulatory role of ATP and adenosine and their receptors in synaptic plasticity and memory.
Keywords: ATP, adenosine, LTP, hippocampus, memory, synaptic plasticity.
PURINERGIC SIGNALING
The term purinergic signaling refers to effects exerted by adenosine or its phosphoric esters, mainly adenosine triphosphate (ATP). On basis of their actions adenosine and phosphoric esters have been recognized as a neuromodulator and neurotransmitter, respectively [1]. Neurons releasing ATP as a co-transmitter are widely spread throughout the nervous system. For example, there are sympathetic neurons co-releasing ATP and noradrenalin. There are also reports of the co-storage of ATP together with acetylcholine in motor nerves [2]. In the central nervous system (CNS), co-release of ATP with glutamate in the hippocampus has been reported [3]. Adenosine is either released directly by nucleoside transporters and channels or produced through extracellular degradation of nucleotides. Besides vesicular storage and release of ATP, other mechanisms involving connexin- and pannexin hemichannels have also been described [4-6].
In addition, it is accepted that ATP mediates astrocytic calcium waves, by way of astrocyte excitability [4,7]. ATP concentrations in the brain range from about 2 mmol/kg in the cortex up to 4mmol/kg in putamen and hippocampus [8]. Basal extracellular adenosine concentrations as measured with microdialysis in the CNS are estimated to lie between 50 nM and 200 nM [9].
RECEPTORS
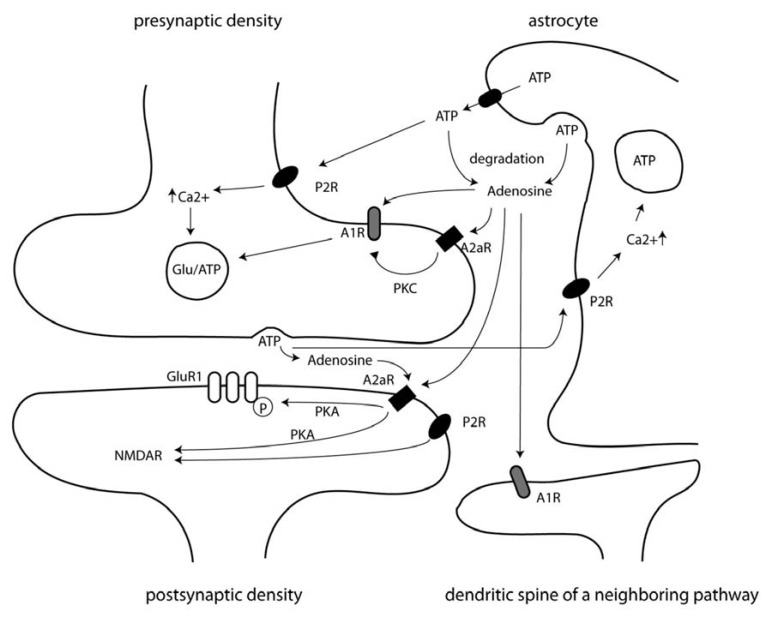
ATP and adenosine exert their effects through distinct receptors [1,2]. Purinergic receptors are divided into two main classes, depending on their main agonist. P1 receptors are activated by adenosine, whereas P2 receptors mainly respond to ATP. However, they are also activated by adenosine diphosphate (ADP) to some extent, depending on the subtype [10]. P1 receptors are further classified into A1, A2a, A2b, and A3 receptors. All adenosine receptors are heptahelical G-protein coupled transmembrane proteins. A2a and A2b receptors increase intracellular cAMP levels via Gs protein activation of adenylate cyclase and stimulate neuronal action. A1 and A3 receptors act via inhibitory Gi proteins and have been shown to activate potassium channels and inhibit Ca2+ channels, both of which inhibit neuronal actions [11,12]. A1 receptors are the most abundant adenosine receptors in the brain. P2 receptors are classified into ligand-gated ionotropic P2x receptors and metabotropic G-protein coupled P2y receptors. There are seven different subtypes of P2x and eight subtypes of P2y receptors expressed in mammalian cells [2]. In the hippocampus, all P2x and several P2y receptor subtypes are expressed [13]. Fig. (1) provides a schematic diagram of the location of the receptors and the purinergic signaling pathways.
Fig. (1).
Schematic diagram of the various actions of ATP and adenosine on neuronal activity.
ATP can act on P2 receptors at astrocytes leading to ATP release from vesicles, via transporters, or via connexin hemichannels. ATP has a presynaptic and a postsynaptic site of action. At the pre-synapse, it promotes transmitter release by elevating intracellular Ca2+ levels via P2x and P2y receptors. There is also a direct depolarizing effect of ionotropic P2x receptors at the postsynapse. However, this is only mild, and effects on neurotransmission by these currents are thought to have long term neuromodulatory effects via a so far unknown mechanism, rather than contribute to fast synaptic transmission, as they have beenfound to affect NMDA receptor currents.
Adenosine was shown to inhibit synaptic potentiation via presynaptic A1 receptors. This is thought to occur tonically in states of low frequency firing. Upon activation, ATP which has become set free from nerve-terminals is degraded and leads to an adenosine concentration which preferentially activates A2a receptors. A presynaptic set of A2a receptors was found to decrease inhibitory input of A1 receptors by a PKC mediated mechanism. In some synapses, postsynaptic A2a receptors increase the amount of AMPA receptors in the plasma membrane via PKA mediated GluR1 phosphorylation. At the postsynaptic site of mossy fiber - CA3 pyramidal neuron synapses A2a receptors potentiate NMDA receptor currents. In addition to localized events at the synapse, adenosine was found to inhibit neighboring pathways via A1 receptors.
(P2R, P2 receptors; A1, adenosine type 1 receptors; A2R, adenosine type 2 receptors; PKA, protein kinase A; PKC, protein kinase C).
ATP-INDUCED LTP
Hippocampal long-term potentiation (LTP) is a form of synaptic plasticity observed after electrical stimulation, and is thought to be the neurophysiological correlate of learning and memory processes [14]. Over the past years evidence has accumulated for a role of ATP and adenosine in synaptic modulation. There is a strong impact of adenosine on ischemic conditions, seizure-induced brain injury, and other deficits and diseases [15]. Furthermore, it has been shown that there are effects on hippocampal LTP [16].
There are several studies that have investigated the role of ATP on hippocampal LTP. Interestingly, the application of ATP to hippocampal slice preparations was found to induce a stable potentiation of CA1 neurons to electric stimuli, which by itself would not induce LTP [17]. Furthermore, in some studies, ATP was capable of inducing LTP-like states without electrical stimulation [17,18]. This latter form of LTP is NMDA receptor dependent, since blockade of NMDA receptors by (2R)-amino-5-phosphonopentanoate (AP5) abolishes this LTP facilitation [16-18]. The ATP-induced potentiation was established during and after washout of ATP. Only ATP and its slowly hydrolysable analogue ATP-γ-S exerted these effects. The non-hydrolysable analogue adenylimidiphosphate, other ATP analogues (2-methylthioadenosine triphosphate and α-betamethyleneadenosine 5’-triphosphate), and the purinergic antagonist cibacron blue 3G, showed no such agonistic effects. On basis of these findings, it was concluded that hydrolyses of ATP seem to be necessary [16]. Furthermore, in other studies, it has been shown that application of the ecto–protein kinase inhibitor K-252b blocked the establishment of ATP-induced LTP [17]. Fujii claimed that ATP-induced LTP is based on phosphorylation of the extracellular domains of membrane proteins in the synapse only, since application of basilen blue or pyridoxalphosphate-6-azophenyl-2',4'-disulfonic acid (PPADS) to block P2y or P2x receptors, respectively, had no effect on LTP induction [17]. However, data are not conclusive since others found that the P2 receptor antagonist suramin and PPADS both prevented ATP-induced LTP [18]. Effects of these P2 receptor inhibitors were only seen when applied prior to establishment of the LTP but had no effect on LTP maintenance. These findings strongly suggest that P2 receptors play an important role in the induction of LTP.
Because of the high ATP concentrations used in these approaches (500 nM – 10 µM), Pankratov et al. argued that the effects observed are more artificial, or might reflect pathological conditions [19]. In their opinion, the use of receptor antagonists and knock-out (KO) animals as discussed below should be considered as more relevant tools to evaluate the physiological role of ATP or adenosine.
Here we will give an overview of the different purinergic receptors and their involvement in LTP.
A1 AND A2 RECEPTOR (ANT) AGONISTS
Approaches using adenosine receptor antagonists to examine the effects of endogenous adenosine showed an increased hippocampal LTP upon blocking of the A1 receptor or stimulation of the A2 receptor respectively [20-21]. Using DPCPX to block A1 receptors, LTP was facilitated. The use of nitrobenzylthioinosine to block adenosine transporters had inhibitory effects. This is in line with other findings showing that equilibrative nucleoside transporters are involved in reuptake rather than in release of adenosine [22]. Using CGS21680 to stimulate A2a receptors also facilitated LTP. In contrast, an A2 receptor antagonist decreased LTP [21]. Based on these findings, it has been suggested that A1 agonists and A2a antagonists could be relevant targets for cognition enhancement [23].
A1 RECEPTOR KO MICE
Adenosine A1 receptor KO mice show only mild phenotypes. This is surprising, since A1 receptors are not only abundant in the brain but also in the heart and in other tissues [24]. In contrast to in vitro studies using A1 receptor antagonists, in which LTP is enhanced, A1 receptor KO mice show no alterations in LTP [25]. Differences in LTP were seen neither by induction through high frequency stimulation (HFS) nor theta bursts between homozygous (-/-), heterozygous (-/+), and wild type (+/+) animals. However paired-pulse facilitation (PPF), a measure of presynaptic plasticity, is impaired in adenosine KO animals compared to controls. To assess spatial memory abilities and working memory, 9-month-old mice were tested in a spatial water maze without observing any differences between A1-R -/-, -/+, or +/+ animals. When tested in a spatial 6-arm radial maze 10 month later, KO mice showed deficits, which according to the authors were not due to memory deficits, but due to decreased habituation to the test environment' [25]. These findings are consistent with results from another study that investigated the behavior of A1 receptor KO mice. These mice showed normal spatial abilities but emotional instability [26].
Since KO mice lack the receptor in all tissues, specific effects are sometimes difficult to distinguish from systemic changes. Therefore, Scammel et al. created a site specific deletion mutant [27]. Using the Cre-LoxP system, mice were generated with an impaired expression of A1 receptors in hippocampal areas CA1 or CA3 respectively. Absence of A1 receptors in the CA1 region showed no general alteration in hippocampal LTP, whereas absence of A1-receptors in CA3 neurons diminished adenosine mediated inhibition of LTP [27].
A2A RECEPTORS
As already mentioned, A2a receptors have been found to facilitate LTP [20,21]. Interestingly, Lopes et al. found that A2a receptors reduce the inhibitory effect of tonic A1 receptor activation [28]. In their studies they excluded the possibility that the facilitatory effects were mediated via inhibition of GABAergic transmission. This was shown by using GABAa and GABAb receptor inhibitors in their systems. Since the A2a receptor agonist CGS21680 was able to facilitate field-EPSP (fEPSP) slope in the presence of GABA receptor inhibitors, it could be concluded that there was a direct facilitatory effect of A2a receptors. Measurements of PPF after CGS21680 application suggested a presynaptic site of action, i.e. via release of glutamate. This effect was blocked by the A2a receptor antagonist ZM241385. In contrast to the results obtained from PPF, they were not able to demonstrate an enhanced release of glutamate from hippocampal synaptosomes.
A previous study found that inhibitory A1 receptor responses were decreased by A2a receptor agonists [28]. On the basis of these findings, it was hypothesized that free adenosine acting via A1 receptors is necessary for synaptic transmission. To further demonstrate that adenosine is required for the facilitatory effect, they used adenosine deaminase (ADA) to remove endogenous adenosine from slice preparations. To provoke responses, adenosine was replaced by A1R agonist N6-cyclopentyladenosine (CPA). Superfusion of ADA increased the fEPSP slope. Additional application of CGS21680 had no additive effect on the fEPSP slope, while CPA reduced fEPSP amplitude. Application of CGS21680 in the presence of ADA and CPA was able to increase fEPSP slope levels. Supporting these data, simultaneously measured paired pulse facilitation (PPF) showed a mirror image. The effects observed could be explained by direct binding of the A2a agonist CGS21680 to A1 receptors. This explanation was excluded when CGS21680 failed to displace tritium-labeled DPCPX in brain slices. Additionally, no cross reactions of CGS21680 with A1 receptors were observed by binding studies in stably transfected CHO cells [28]. Thus, Lopes et al. suggested that A2a receptors affect presynaptic release of glutamate by attenuating tonic inhibitory influence of A1 receptors.
More recently, it has been found that A2a receptor-coupled protein kinase A (PKA) activity facilitates AMPA-evoked excitatory postsynaptic currents [29]. It is partly responsible for endogenous AMPA receptor GluR1 phosphorylation which affects synaptic plasticity [28]. A2a receptors are also involved in LTP of NMDA excitatory postsynaptic currents (NMDA-EPSCs) at hippocampal mossy fiber CA3 synapses [30].
Furthermore, A2a receptors are necessary for the LTP facilitatory effects of BDNF in the hippocampus [31]. Fontinha et al. found that LTP facilitation by BDNF was absent under conditions in which either A2a receptors were blocked by SCH58261, or when adenosine was depleted by ADA. Stimulation of A2a receptors by CGS21680 restored the BDNF mediated LTP facilitation. These effects are presumably based on PKA activation, since the adenylate cyclase inhibitor H-89 prevented CGS21680 mediated restoration of BDNF effects, while the adenylate cyclase activator forsokolin was able to induce BDNF effects in the absence of CGS21680 in an ADA treated background. This showed that A2a receptor activation via a cAMP pathway is essential for BDNF to facilitate hippocampal LTP [31]. Likewise, Tebano et al. detected decreased BDNF levels in the hippocampus of A2a receptor deficient mice by ELISA measurements [32]. These findings were supported recently by Jeon et al., who demonstrated that A2a receptor stimulation increases BDNF expression in cultured rat cortical neurons via the Akt/GSK-3β signaling pathway [33].
Behavioral studies with genetically modified mice showed that animals lacking A2a receptors performed better than wild type animals in a working memory task [34]. A more detailed analysis of behavior showed that the deficits were related to the short delays. At the longer delays, no differences between groups were found. Furthermore, rats overexpressing the A2a receptor in various brain areas showed impairments in various models of learning and memory, including working memory [35]. These findings with transgenic animals do not logically follow from the pharmacological studies. A2a stimulation with pharmacological agents leads to enhanced neuroplasticity, whereas the absence or upregulation of receptors in the transgenic mice suggests the opposite.
ADENOSINE RECEPTORS, PLASTICITY AND MEMORY
Several explanations can be offered for these apparently contrasting findings between in vitro studies, pharmacological models and genetic models. Obviously, in knock out models, receptor profiles are modified from conception onwards and adaptation may have been taken place. This may lead to a different regulation of purinergic signaling in these knock out models. This can be circumvented by using inducible knockout models. Another issue that should be mentioned in this respect is that different animal models have been used for the knockout and the pharmacological studies. Studies with knockout models usually used mice whereas rat slices were usually used in the pharmacological studies.
Furthermore, although A1 agonists have been found to improve cognitive functions, no clear effects on learning and memory performance were found in A1 knockout animals. On the other hand, animals lacking the A2a receptor showed improved performance and animals overexpressing the A2a receptor showed memory impairment. These data are in line with studies showing memory enhancing effects of A2a antagonists. These data may hint at a complex interaction between A1 and A2a receptors in memory and neuronal plasticity. Clearly, these findings need to be scrutinized in more detail in further studies.
P2-RECEPTORS IN HIPPOCAMPAL LTP
In 2004, Wang et al. examined the effects of ATP on HFS-induced hippocampal LTP [36]. As expected, HFS induced LTP but superfusion of brain slices with ATP increased LTP to a greater degree, after a slow onset. ATP-γ-S showed a significant increase in LTP as compared to controls, although the increase was smaller when using ATP itself. Moreover, Wang et al. examined P2 receptor involvement by inhibition of P2 receptors through suramin (100µM), or pyridoxalphosphate-6-azophenyl-2', 4'-disulfonic acid (PPADS) (30µM), neither of which differentiate between P2x and P2y. When applied to the medium of the slice preparation, both inhibitors showed a significant decrease in LTP. It was shown that ATP by itself, or the combination of ATP plus suramin, had an effect on PPF when compared to control conditions. This finding suggested that P2x receptors do not affect presynaptic functions. In the presence of N-ethylmaleimide (NEM), ATP did not enhance LTP after HSF, but rather induced paired pulse depression (PPD). It is assumed that NEM increases glutamate release and depletes the glutamate pool. Therefore, normal feedback activation of presynaptic P2y receptors, a physiological process to balance synaptic transmission, could be abolished by NEM. Wang et al. concluded that ATP mainly acts via the postsynaptic (P2x), rather than presynaptic (P2y), receptors.
In contrast, other data have shown an increase in LTP induced by HFS upon P2x receptor blockade by PPADS [37]. This effect was particularly prominent when electrical stimuli were applied which alone did not induce stable LTP. However, the same stimulation parameters, in combination with the P2x receptor antagonist PPADS, were able to induce a stable LTP [36]. Unfortunately, neither Pankratov et al. [37] nor Wang et al. [36] examined effects of adenosine receptor activation in their assays. This would have been interesting, since the effects observed could also be mediated via adenosine receptors, as shown previously (see above).
A study that examined the involvement of both P2 and adenosine receptors was performed by Almeida et al. [38]. In their study, the ATP analog α,β-methyleneATP (α,β-meATP) decreased theta burst induced LTP, an effect prevented by A1 receptor blockade. Furthermore, in the presence of 50 nM DPCPX, a selective A1 receptor antagonist, α,β-meATP and β-γ-ImidoATP (β-γ-ImATP) facilitated LTP. This facilitation was prevented by PPADS or suramin, supporting the idea of P2 receptor involvement. The facilitating effect was also prevented by A2a receptor antagonists. Direct facilitation of LTP by binding of ATP analogues to A2a receptors was therefore excluded as a possible explanation because they were not able to replace the A2a selective agonist CGS21680 (30 nM), even when applied in high concentrations. Almeida et al. further investigated cellular signal transduction pathways by inhibiting either protein kinase C (PKC) or protein kinase A (PKA) [38]. Inhibition of PKC prevented the facilitatory effects of α,β-meATP, while inhibition of PKA had no effect. This suggests that activation of PKC via A2a receptors is required for the induction of LTP by ATP. To investigate the source of adenosine, they measured the outflow of tritium-labeled adenosine from slice preparations. β-γ-ImATP significantly increased adenosine release but the combination with the P2 receptor inhibitor PPADS did not increase adenosine outflow levels. The same effect was seen when β-γ-ImATP was combined with adenosine transporter blocking agents. Interestingly, it was discovered that ATP-triggered adenosine release from cultured hippocampal neurons was lower than that found in slices, whereas levels obtained from astrocytes nearly resembled those found in slice preparations (see also the role of astrocytes in section below). They concluded that activation of P2 receptors triggers adenosine release, which in turn activates facilitatory A2a receptors [38]. These findings corroborate the previous findings of O'Kane and Stone [18].
In general, examining P2 receptors is difficult because of the lack of subtype specific antagonists. The generation of mice lacking P2 receptors of a specific subunit composition would be an interesting approach that could be used to analyze subunit specific effects. A P2x4 KO mouse has shown reduced LTP. However, these changes were mild and LTP was still inducible, implying that other P2x receptors are also involved [39]. Mice lacking P2x7 receptors displayed impaired spatial memory in a hippocampus-dependent task, while memory was unaffected in object recognition [40]. A detailed review on P2x receptors on LTP has been provided by Pankratov et al. [19].
ASTROCYTES AND PURINERGIC SIGNALING
An important aspect of how astrocyte-derived adenosine could depress synaptic activity was discovered by Pascual et al. [22]. Based on the finding that exocytosis of gliotransmitters is SNARE-dependent [41], Pascual et al. generated transgenic mice which express the cytosolic portion of synaptobrevin 2 selectively in astrocytes, resulting in deficient SNARE dependent transmitter release from these cells (dn-SNARE mice). This expression can be prevented by doxycycline treatment, restoring normal function. Using these transgenic animals, they observed that fEPSP slope was significantly larger but LTP was weaker in dn-SNARE mice as compared to wild-type littermates, and compared to dn-SNARE mice maintained on doxycycline. There was no significant difference between wild-type and dn-SNARE mice maintained on doxycycline. Further analysis revealed that the change in LTP was related to adenosine. This conclusion could be drawn because the A1 receptor antagonist DPCPX had no effect on fEPSP slope in dn-SNARE mice, but augmented fEPSP slope in wild-type mice. Furthermore, the observed effects in dn-SNARE mice could be restored by application of an A1 receptor agonist. The adenosine was generated by hydrolysis of ATP. An involvement of P2 receptors or other known gliotransmitters like D-serine was excluded because antagonizing their effects did not change fEPSP slope. Furthermore, they found that stimulation of one pathway leads to inhibition of neighboring pathways. The inhibition was also mediated by adenosine [21]. Since the effects described were restricted to the transgenic dn-SNARE mice which are impaired in transmitter release from atrocytes, but not from neurons, astrocyte-derived ATP has to be the source for adenosine mediating inhibitory effects.
Astrocytic heterosynaptic inhibition of excitatory neurons by ATP and adenosine was previously reported by others in cultured hippocampal neurons and slice preparations. Mechanical stimulation of astrocytes evoked Ca2+ waves which in turn were mediated by ATP release and P2 receptor activation. These effects inhibited excitatory glutamate transmission in primary hippocampal neurons which was ATP-dependent [42]. Others showed that tonic suppression of glutamatergic synapses by endogenous ATP in cultured hippocampal neurons depends on the presence of co-cultered astrocytes. This suppression was mediated by presynaptic P2y receptors. In contrast, in slice preparations, inhibitory effects were dependent on A1 receptors [43].
ACKNOWLEDGEMENTS
Declared none.
SUMMARY/CONCLUSIONS
From the various studies that have investigated purinergic signaling, it is clear that ATP and adenosine affect hippocampal LTP. Adenosine acts via A1 receptors to suppress synaptic activity, while it has facilitating effects via A2a receptors. While these general metabotropic receptor-mediated effects are well described [12,44], the mechanisms by which facilitatory A2a receptors mediate their effects are not yet known. They probably reduce inhibitory input generated by A1 receptors [28]. The main source of adenosine is unclear and could be site specific. There is evidence for a direct release, either alone or as a co-transmitter, as well as generation through enzymatic breakdown of ATP [10]. Adenosine provided by astrocytes sets a general inhibitory tonus suppressing neighboring pathways [22]. On basis of the pharmacological studies, it could be suggested that the role of adenosine on learning and memory is related to fine tuning. A1 receptor KO mice show a relatively mild phenotype contrasting the abundant expression in many tissues. In hippocampal slice preparation, LTP was not significantly altered. Only a mild cognitive deficit was observed in spatial memory tasks [25]. A2a receptor KO mice outperformed wild type mice in a working memory task [34]. On the other hand, rats with overexpression of the A2a receptor were impaired in various models of learning and memory [35]. As mentioned before, these data were surprising considering the pharmacological effects of A2 a(nta)gonists. Of note, other systemic changes in metabolism could have affected performance of KO mice and rats in these tasks (i.e., side effects), or compensatory processes in KO mice could have masked the effects of the adenosine receptor deletion. To determine effects specifically related to brain A1 and A2 receptors, there is a need for site directed (inducible) KO animals.
ATP, which operates through metabotropic P2y and ionotropic P2x receptors, seems to have a more direct influence on synaptic transmission via LTP induction, as shown by P2 receptor inhibitors [37]. Including astrocytes into models of LTP induction, in particular P2 receptors, could play an important role, because ATP is well known as an inductor of glial calcium waves, and the involvement of P2 receptors in this process has already been described [7,45].
The fact that LTP was attenuated in dn-SNARE mice, which lack ATP release from astrocytes [22], is at first sight contradictory with the observed augmentation of LTP by adenosine A1 antagonists. However, it actually fits quite well with the hypothesis that adenosine mediates a tonic suppression of synaptic transmission in vivo. According to this hypothesis, the application of A1 receptor antagonists would lead to a sudden loss of inhibitory input resulting in LTP facilitation. In contrast, the tonic absence of adenosine would increase basal synaptic activity levels, making it more difficult to induce LTP because of lower contrast. Additionally, the inhibition of neighboring pathways by adenosine, in combination with an A2a receptor activation at high stimuli, would increase contrast of excited pathways against non-excited background. This would enable amplification of specific signaling while suppressing non-specific events.
To summarize, purinergic signaling plays an important role in mechanisms involved in synaptic plasticity. It appears that purinergic signaling has modulatory characteristics, yet more studies are needed to examine the role of ATP and adenosine and their receptors in synaptic plasticity.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Khakh B S, North R A. Neuromodulation by extracellular ATP and P2X receptors in the CNS. Neuron. 2012;76:51–69. doi: 10.1016/j.neuron.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnstock G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007;64(12):1471–83. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori M, Heuss C, Gähwiler B H, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J. Physiol. 2001;15:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowser D N, Khakh B S. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J. Gen. Physiol. 2007;129:485–491. doi: 10.1085/jgp.200709780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pankratov Y, Lalo U, Verkhratsky A, North R A. Vesicular release of ATP at central synapses. Pflügers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 6.Scemes E, Suadicani S O, Dahl G, Spray D C. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koizumi S. Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J. 2010;277:286–292. doi: 10.1111/j.1742-4658.2009.07438.x. [DOI] [PubMed] [Google Scholar]
- 8.Kogure K, Alonso O F. A pictoral representation of endogenous brain ATP by a bioluminescent method. Brain Res. 1978;154:273–284. doi: 10.1016/0006-8993(78)90700-x. [DOI] [PubMed] [Google Scholar]
- 9.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J. Neurochem. 2001;79: 463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 11.Butt A. ATP A ubiquitous gliotransmitter integrating neuron-glial networks. Sem. Cell Develop. Biol. 2011;22:205–213. doi: 10.1016/j.semcdb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Dunwiddie T V, Masino S A. The role and regulation of adenosine in the central nervous system. Annu. Rev. Neurosci PMID: 11283304. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1 P2X2/3 and P2X3 and inhibitory P2Y1 P2Y2 and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bliss T V P, Collingridge G L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G. Purinergic signalling in the CNS. Open Neurosci. J ISSN: 1874-0820. 2010;4:24–30. [Google Scholar]
- 16.Wieraszko A, Ehrlich Y H. On the role of extracellular ATP in the induction of long-term potentiation in the hippocampus. J. Neurochem. 1994;63:1731–1738. doi: 10.1046/j.1471-4159.1994.63051731.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujii S. ATP- and adenosine-mediated signaling in the central nervous system: The role of extracellular ATP in hippocampal long-term potentiation. J. Pharmacol. Sci. 2004;94:103–106. doi: 10.1254/jphs.94.103. [DOI] [PubMed] [Google Scholar]
- 18.O'Kane E M, Stone T W. Characterisation of ATP-induced facilitation of transmission in rat hippocampus. Eur. J. Pharmacol. 2000;409:159–166. doi: 10.1016/s0014-2999(00)00785-8. [DOI] [PubMed] [Google Scholar]
- 19.Pankratov Y, Lalo U, Krishtal O A, Verkhratsky A. P2x receptors and synaptic plasticity. Neuroscience . 2009;158:137–148. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 20.Forghani R, Krnjevic K. Adenosine antagonists have differential effects on induction of long-term potention in hippocampal slices. Hippocampus. 1995;5:71–77. doi: 10.1002/hipo.450050109. [DOI] [PubMed] [Google Scholar]
- 21.de Mendonca A, Ribeiro J A. Endogenous adenosine modulates long term potentiation in the hippocampus. Neuroscience. 1994;62:385–390. doi: 10.1016/0306-4522(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 22.Pascual O, Casper K B, Kubera C, Zhang J, Revilla-Sanchez R, Sul J Y, Takano H, Moss S J, McCarthy J, Haydon P G. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front. Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- 24.Fredholm B B, Chen J F, Masino S A, Vaugeois J M. Actions of adenosine and its receptors in the CNS: insights from knockouts and drugs. Annu. Rev. Pharmacol. Toxicol. 2005; 45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 25.Gimenez-Llort L, Masino S A, Diao L, Fernandez-Teruel A, Tobena A, Halldner L, Fredholm B B. Mice lacking the adenosine A1 receptor have normal spatial learning and synaptic plasticity in the CA1 region of the hippocampus, but they habituate more slowly. Synapse. 2005;57:8–16. doi: 10.1002/syn.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang U E, Lang F, Richter K, Vallon V, Lipp H P, Schnermann J, Wolfer D P. Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav. Brain Res. 2003;145:179–188. doi: 10.1016/s0166-4328(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 27.Scammell T E, Arrigoni E, Thompson M A, Ronan P J, Saper C B, Green R W. Focal deletion of the adenosine A1 receptor in adult mice using an adeno-associated viral vector. PMID 12843280 J. Neurosci. 2003;23:5762–5770. doi: 10.1523/JNEUROSCI.23-13-05762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopes L V, Cunha R A, Kull B, Fredholm B B, Ribeiro J A. Adenosine A2a receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience. 2002;112:319–329. doi: 10.1016/s0306-4522(02)00080-5. [DOI] [PubMed] [Google Scholar]
- 29.Dias R B, Ribeiro J A, Sebastiao A M. Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A(2A) receptors. Hippocampus. 2012;22:276–291. doi: 10.1002/hipo.20894. [DOI] [PubMed] [Google Scholar]
- 30.Rebola N, Lujan R, Cunha R A, Mulle C. Adenosine A2A receptors are essential for long-term potentiation of NMDA-EPSCs at hippocampal mossy fiber synapses. Neuron. 2008;57:121–134. doi: 10.1016/j.neuron.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Fontinha B M, Diógenes M J, Ribeiro J A, Sebastião A M. Enhancement of long-term potentiation by brain-derived neurotrophic factor requires adenosine A2A receptor activation by endogenous adenosine. Neuropharmacology . 2008;54:924–933. doi: 10.1016/j.neuropharm.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Tebano M T, Martire A, Potenza R L, Gro C, Pepponi R, Armida M, Domenici M R, Schwarzschild M A, Chen J F, Popoli P. Adenosine A(2A) receptors are required for normal BDNF levels and BDNF-induced potentiation of synaptic transmission in the mouse hippocampus. J. Neurochem. 2008;104:279–286. doi: 10.1111/j.1471-4159.2007.05046.x. [DOI] [PubMed] [Google Scholar]
- 33.Jeon S J, Rhee S Y, Ryu J H, Cheong J H, Kwon K, Yang S I, Park S H, Lee J, Kim H Y, Han S H, Ko K H, Shin C Y. Activation of adenosine A2A receptor up-regulates BDNF expression in rat primary cortical neurons. Neurochem Res. 2011;36:2259–2269. doi: 10.1007/s11064-011-0550-y. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S J, Zhu M E, Shu D, Du X P, Song X H, Wang X T, Zheng R Y, Cai X H, Chen J F, He J C. Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res. 2009;1303:74–83. doi: 10.1016/j.brainres.2009.09.082. [DOI] [PubMed] [Google Scholar]
- 35.Gimenez-Llort L, Schiffmann S N, Shmidt T, Canela L, Camon L, Wassholm M, Canals M, Terasmaa A, Fernandez-Teruel A, Tobena A, Popova E, Ferre S, Agnati L, Ciruela F, Martinez E, Scheel-Kruger J, Lluis C, Franco R, Fuxe K, Bader M. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol. Learn. Mem. 2007;87:42–56. doi: 10.1016/j.nlm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Haughey N J, Mattson M P, Furukawa K. Dual effects of ATP on rat hippocampal synaptic plasticity. Neuro Report. 2004;15:633–636. doi: 10.1097/00001756-200403220-00012. [DOI] [PubMed] [Google Scholar]
- 37.Pankratov Y, Ulyana V, Lalo U, Krishtal O A. Role for P2x receptors in long term potentiation. J. Neurosci PMID: 12351710. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almeida T, Rodrigues R J, de Mendonça A, Ribeiro J A, Cunha RA. Purinergic P2 receptors trigger adenosine release leading to adenosine A2A receptor activation and facilitation of long-term potentiation in rat hippocampal slices. Neuroscience 00523-2. 2003;122:111–121. doi: 10.1016/s0306-4522(03)00523-2. [DOI] [PubMed] [Google Scholar]
- 39.Sim J A, Chaumont S, Jo J, Ulmann L, Young M T, Cho K, Buell G, North R A, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J. Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labrousse V F, Costes L, Aubert A, Darnaudéry M, Ferreira G, Amédée T, Layé S. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS One. 2009;4:e6006. doi: 10.1371/journal.pone.0006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Araque A, Li N, Doyle R T, Haydon P G. SNARE protein-dependent glutamate release from astrocytes. J. Neurosci PMID: 10632596. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koizumi S, Fujishita K, Tsuda M, Shigemoto-Mogami Y, Inoue K. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc Natl Acad Sci U S A. 2003;100:11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J M, Wang H K, Ye C Q, Ge W, Chen Y, Jiang Z L, Wu C P, Poo M M, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 44.Costenla A R, Cunha R A, de Mendonca A. Caffeine adenosine receptors and synaptic plasticity. J. Alzheimers Dis. 2010;20:525–534. doi: 10.3233/JAD-2010-091384. [DOI] [PubMed] [Google Scholar]
- 45.James G, Butt A M. P2y and P2x purinoceptor mediated Ca²+ signalling in glial cell pathology in the central nervous system. Eur J Pharmacol. 2002;447:247–260. doi: 10.1016/s0014-2999(02)01756-9. [DOI] [PubMed] [Google Scholar]