Abstract
Pregabalin is an antagonist of voltage gated Ca2+ channels and specifically binds to alpha-2-delta subunit to produce antiepileptic and analgesic actions. It successfully alleviates the symptoms of various types of neuropathic pain and presents itself as a first line therapeutic agent with remarkable safety and efficacy. Preclinical studies in various animal models of neuropathic pain have shown its effectiveness in treating the symptoms like allodynia and hyperalgesia. Clinical studies in different age groups and in different types of neuropathic pain (peripheral diabetic neuropathy, fibromyalgia, post-herpetic neuralgia, cancer chemotherapy-induced neuropathic pain) have projected it as the most effective agent either as monotherapy or in combined regimens in terms of cost effectiveness, tolerability and overall improvement in neuropathic pain states. Preclinical studies employing pregabalin in different neuropathic pain models have explored various molecular targets and the signaling systems including Ca2+ channel-mediated neurotransmitter release, activation of excitatory amino acid transporters (EAATs), potassium channels and inhibition of pathways involving inflammatory mediators. The present review summarizes the important aspects of pregabalin as analgesic in preclinical and clinical studies as well as focuses on the possible mechanisms.
Keywords: Pregabalin, neuropathic pain, diabetic neuropathy, post-herpetic neuralgia.
1. INTRODUCTION
Neuropathic pain as defined by The International Association for the Study of Pain is “pain initiated or caused by a primary lesion in the nervous system”. In other words, neuropathic pain may be defined as the pain originated from the pathology of the nervous system. It involves alterations in the function, chemistry and structure of neurons. Spontaneous pain, hyperalgesia and allodynia are the common symptoms observed in neuropathic pain. Spontaneous pain is characteristically burning or shooting in nature. Hyperalgesia is an increased pain response to supra-threshold noxious stimulus, while allodynia is a sensation pain elicited by a non noxious stimulus (e.g. the gentle touch of clothes, bending of cutaneous hairs by a puff of wind). Spontaneous pain may be simply conceptualized as “stimulus independent” whereas hyperalgesia and allodynia as “stimulus dependent”. Neuropathic pain is a result of various mechanisms operating at the peripheral, spinal cord and supra-spinal levels, which cause alterations in the pain conduction pathway. This may also develop secondary to some other pathological conditions such as diabetes mellitus, cancer, herpes infection, autoimmune diseases and HIV infection etc. [1].
Pregabalin, (S)-3-(aminomethyl)-5-methylhexanoic acid, is a pharmacologically active S-enantiomer of a racemic 3-isobutyl gamma amino butyric acid analogue. It is a well established anticonvulsant and analgesic agent. In fact pregabalin is the first drug to receive an approved labeling from Food and Drug Association (FDA) for the treatment of diabetic neuropathy and post-herpetic neuralgia [2]. Preclinical and clinical studies have shown the effectiveness of pregabalin in managing the neuropathic pain. Animal based studies have helped to describe the mechanisms for its anti-hyperalgesic and anti-allodynic action. Clinical studies have also shown the efficacy and dose dependent effects of pregabalin either as monotherapy or in combination with analgesics in relieving pain and related symptoms [3, 4]. The major advantage of pregabalin is its relative reliability, easy use and high tolerance in patients with neuropathic pain [5]. As a successor of gabapentin, pregabalin has been shown to be effective in several models of neuropathic pain, incisional injury and inflammatory injury. The present review summarizes the preclinical and clinical outcomes of pregabalin with the possible underlying mechanisms.
2. EVIDENCES FOR ITS PAIN ATTENUATING EFFECTS FROM PRECLINICAL STUDIES
There have been a number of studies showing the prominent neuroprotective action of pregabalin in different models (Table 1) [6]. As neuronal injury/damage is an essential component of neuropathic pain therefore, the neuroprotection provided by pregablin may be linked to its neuropathic pain attenuating effects. Accordingly, various preclinical studies have documented the analgesic actions of pregabalin in different pain models including neuropathic pain. Administration of pregabalin (3-30 mg/kg, oral) after 2.5 hrs of carrageenan injection (after full induction of inflammation) has been shown to alleviate the carrageenan- induced thermal hyperalgesia in a dose dependent manner with ED50 value of 6 mg/kg in comparison to gabapentin with ED50 of 19.2 mg/kg [7]. Administration of pregabalin (30 mg/kg or 300 μmol/kg, s.c.) has been shown to significantly block the late-phase (phase-2) nocifensive response in formalin-induced nociception [8-9]. In neuropathic pain models, it has also been reported to significantly attenuate the pain symptoms. In chronic constriction injury (CCI) model, administration of pregabalin (30-100 mg/kg, s.c.) after 10 days of nerve injury (stable allodynia achieved) has been shown to produce a dose-dependent inhibition of punctuate allodynia (assessed with von Frey filaments application) in mice [8]. Even a single dose of pregabalin (30, 100 and 300 μmol/kg, oral) has been shown to reverse cold allodynia in spared nerve injury (SNI) and spinal nerve ligation (SNL) models of neuropathic pain [9]. In weight-drop spinal cord injury model in mice (which mimics the symptoms of neuropathic pain due to spinal cord injury in human), a single dose administration of pregabalin (10 and 30 mg/kg, i.p.) on the 28th day of injury is reported to significantly attenuate mechanical hypersensitivity (allodynia) in a dose-dependent manner [10]. Acute administration of pregabalin (4 mg/kg and 10 mg/kg, i.v. infusion) for 2 h is reported to significantly attenuate the static allodynia (measured in term of paw withdrawal threshold) in a CCI model [11]. A single dose administration of pregabalin (30 mg/kg, i.p) has been shown to be effective in decreasing mechanical sensitivity in the spinal cord contusion (SCC) model of central neuropathic pain in rats [12].
Table 1.
Summarized Data from the Preclinical Reposts Showing the Effectiveness of Pregabalin in Neuropathic as well as in Inflammatory Pain Models
| S.No. | Animal Model | Doses of Pregabalin and Outcomes | References |
|---|---|---|---|
| 1. | Chronic constriction injury model (CCI) | 30-100 mg/kg, s.c./oral/4-10 mg/kg i.v Attenuation of allodynia and spontaneous pain |
[8, 11, 13] |
| 2. | Spinal nerve injury (SNI)/ spinal nerve ligation (SNL)/ spinal cord contusion (SCC)/ weight drop spinal cord injury model | 10-30 mg/kg, i.p./ 10-300 µmol/kg, oral Single dose administration produced significant reduction in mechanical hypersensitivity threshold in a dose dependent manner |
[9, 10, 12] |
| 3. | Cancer Chemotherapy (oxaliplatin/ docetaxel) induced neuropathy model | 10-30 mg/kg, oral/ 2 mg/kg i.v. Amelioration of heat hyperalgesia, cold allodynia, impairment of sciatic nerve, |
[16, 17] |
| 5. | Infraorbital nerve injury model | 10% topical application Reduction in Orofacial pain intensity |
[15] |
| 6. | Carrageenan induced thermal hyperalgesia | 3-30 mg/kg, oral 3 times more potent then gabapentin in hyperalgesia attenuation |
[7] |
| 7. | Formalin test | 30 mg/kg or 300 µmol/kg, s.c. Blockade of phase-2 nocifensive response |
[8, 9] |
A recent study has revealed the effectiveness of pregabalin (30 mg/kg, oral) in reducing the frequency of spontaneous pain behavior (by assessing the abnormal limb movements including lifting/guarding, flinching/shaking and licking in the operated limb using a magnet implanted in injured limb) in a CCI model [13]. Pregabalin has also been shown to attenuate mechanical allodynia in the tibial neuroma transposition (TNT) model [14]. The topical application of pregabalin (10 %) for 4 consecutive days after 7 days of surgical nerve injury is reported to reduce neuropathic orofacial pain intensity in an infraorbital nerve injury model in rats [15]. Administration of pregabalin (2 mg/kg, i.v.) has also been reported to attenuate chemotherapy (oxaliplatin, 6 mg/kg i.p)-induced neuropathic pain symptoms especially cold allodynia [16]. Pregabalin (10 mg/kg and 30 mg/kg, oral) administration for 8 consecutive days has been shown to significantly attenuate docetaxel-induced neuropathy (heat hyperalgesia, cold allodynia) along with reduction in substance P and calcitonin gene-related peptide (CGRP) release suggesting pregabalin as potential agent against chemotherapy-induced neuropathy [17].
Apart from the studies showing its effectiveness as monotherapy, there have also been reports suggesting its synergistic actions with other pain relieving drugs. Its synergism with tapentadol (a μ-opioid receptor agonist and noradrenaline reuptake inhibitor) for analgesic actions (in terms of ED50 value) has been demonstrated. The ED50 value of the combination of pregabalin and tapentadol (in the ratio of 2.5:1) was reported to be 0.83 mg/kg in comparison to ED50 value of 4.21 mg/kg (for pregabalin) and 1.65 mg/kg (for tapentadol) assessed using ipsilateral paw withdrawal threshold with electronic von Frey filaments [18]. The synergistic interaction may be possibly due to enhancement of pregablin-mediated descending pain inhibitory signals (involving the spinal mechanisms) in the presence of increased noradrenaline in the brain (due to decreased noradrenaline re-uptake) by tapentadol. Administration of pregabalin and naproxen (NSAID) in 10:1 ratio is shown to act synergistically to reverse thermal hyperalgesia in the inflamed hind paw. The synergistic actions may be due to potentiation of pregablin-mediated inhibition of glutamate release via Ca2+ channel inhibition by naproxen to inhibit the release of glutamate through inhibition of PGE2 synthesis in the dorsal horn neurons of the spinal cord. The reduced glutamate release-induced reduction in the post-synaptic excitation of dorsal horn neurons may ultimately result in decreased central sensitization to alleviate hyperalgesia [7]. The combination of pregabalin with synthetic cannabinoid WIN 55212-2 mesylate at a fixed ratio of 1:1 has also been shown to exert synergistic interaction in a mouse model of acute thermal pain by unknown mechanisms [19].
Pregabalin has emerged as the most effective drug to manage various neuropathic pain states in comparison to its predecessor analgesic agents. According to preclinical data, pregabalin has turned out to be 3- to 10-fold more potent than gabapentin as an antiepileptic [20] and 2- to 4-fold more potent as an analgesic in treating neuropathic pain [21]. Hurley and co-workers also demonstrated that pregabalin reduces hyperalgesia to an equivalent extent at one third dose as compared to gabapentin [7]. The anti-nociceptive testing in oxaliplatin-induced neuropathic pain model for various drugs (assessed using the rat tail immersion test in cold water) ranked pregabalin (2 mg/kg) as the most efficacious drug as compared to lidocaine (3 mg/kg) and morphine (4 mg/kg) on the basis of the ratio of the pharmacological effect (versus time) to dose [16].
3. EVIDENCES FROM CLINICAL STUDIES
3.1. Diabetic Neuropathic Pain
The various animal experimental studies suggesting the efficacy and safety of pregabalin as monotherapy or as combination have encouraged further research in the clinical settings (Table 2). There have been a number of clinical studies showing its effectiveness in diabetic neuropathic pain [22-25]. Pregabalin at fixed dosages of 300 and 600 mg/day (three times daily), is reported to be superior to placebo in relieving pain and improving pain-related sleep interference in three randomized, double-blind, multicentre studies of 5-8 weeks duration in patients with painful diabetic peripheral neuropathy (pDPN) [26-27]. The efficacy results from a 6-week multicenter study have indicated that 600 mg/day pregabalin significantly decreases mean pain score and increases the proportion of patients with ≥50% decrease from baseline pain (39% vs. 15% for placebo) [28]. The retrospective analyses of daily mean pain scores from nine controlled trials of pregabalin at 150, 300, or 600 mg/day also reported a significant reduction in pain during the first 2 weeks of treatment of painful diabetic peripheral neuropathy [29]. The pooled analysis of seven studies has shown that pregabalin (150, 300, and 600 mg/day TID) significantly reduces pain and pain-related sleep interference associated with pDPN with the highest efficacy at the dose of 600 mg/kg BID [30]. The higher doses of pregabalin produce a faster reduction in pain scores as compared to lower doses, and the most rapid pain reduction is generally observed in patients receiving pregabalin 600 mg/day, divided into 2 or 3 doses [31]. The pooled data from 11 double-blind, randomized clinical studies of pregabalin (75-600 mg/day) in older patients (age ≥65 years) with pDPN or PHN (Post-herpetic neuralgia) shows significant reduction in neuropathic pain and improvement in pain compared to those observed in younger patients [32]. The results obtained from a 12-week randomized, double-blind, multicentre, placebo-controlled, parallel-group study further support the efficacy, tolerability, and safety of pregabalin in flexible- or fixed-dose regimen with its ease of application in clinical settings and project it as a better treatment option in elderly patient, who represent a large proportion of the populations with neuropathic pain syndromes [5].
Table 2.
Clinical Reports of Pregabalin in Various Forms of Neuropathic Pain
| S.No. | Type of Pain | Dose, Duration and Salient Outcomes | Reference |
|---|---|---|---|
| 1. | Diabetic painful neuropathy | 150-600 mg/kg, 4-14 weeks Improvement in pain and related symptoms within 2 weeks of treatment with sustained and long lasting effects throughout study Mild to moderate side effects like dizziness, somnolence edema, weight gain, motor incoordination etc. Exacerbation of CVS dysfunctions particularly in previously cardiac compromised patients |
[5, 22, 27, 30, 33, 37, 38, 39, 40] |
| 2. | Cancer chemotherapy induced neuropathic pain | 75-300 mg/day, 2-8 weeks Effective in oxaliplatin, docetaxel, fluorouracil/cisplatin refractory pain in cancer patients of all age groups as monotherapy as well as in combination to other analgesics. |
[3, 4, 41, 42, 43, 44, 45, 46] |
| 3. | Post-herpetic neuralgia | 150-600 mg/day, 8-13 weeks Highly significant pain reduction with mild to moderate side effects 6 times more potent than gabapentin Mild to moderate dizziness |
[47, 48, 49, 50, 51,52] |
| 4. | Fibromyalgia | 150-600 mg/day, 4-12 weeks Significant and long lasting pain relief with improvement in other global parameters. Cost effective and good tolerability Common side effects like dizziness, somnolence, edema, dry mouth etc. |
[54, 55, 56, 57, 58, 59, 60] |
| 5. | Trigeminal neuralgia | 150-600 mg/kg, 8 week Long lasting pain relief |
[61] |
| 6. | Post-operative pain | 300-600 mg/day, pre-operatively/post-operatively, Attenuation of acute pain Reduced the consumption of opiods |
[62, 63, 64, 65, 66] |
Clinical trials for comparative efficacy and safety analyses of pregabalin and amitriptyline have projected pregabalin as a better treatment option in diabetic neuropathy due to lesser proportion of side effects (25%) than amitriptyline (65.4%) [33]. The comparative study of pregabalin (75-300 mg/day), duloxetine (20-120 mg/day) and gabapentin (300-1800 mg/day) in the treatment of diabetic neuropathy for 12 weeks shows that monotherapy with pregabalin is superior in terms of onset of pain reduction [34]. In a 6 week study, pregabalin is shown to be equally effective as gabapentin for reduction of pain intensity and improvement in sleep quality with painful diabetic neuropathy [35]. A study by Saldaña and co-workers reported that pregabalin produces relief in terms of pain perception, disability level, anxiety, depression, sleep quality and quality of life in gabapentin refractory neuropathy suggesting pregabalin as a valid treatment alternative (either as monotherapy or in combination with other analgesics) for the management of patients with gabapentin-refractory peripheral neuropathic pain [36]. Apart from better pain relieving actions of pregabalin, its cost-effectiveness as monotherapy or as add-on therapy in comparison to existing treatment (antidepressants, opioids, anticonvulsants different than pregabalin and/or analgesics) in the management of refractory diabetic neuropathy has also been demonstrated [37]. The cost effectiveness of pregabalin in treating various types of neuropathic pain has also been supported by various other studies [38-40].
3.2. Cancer and Chemotherapy-induced Neuropathic Pain
There have been studies suggesting the usefulness of pregabalin in cancer-induced pain as well as in anti-cancer agents (chemotherapy)-induced neuropathic pain. A retrospective investigation of Japanese patients revealed the effectiveness of pregabalin (> 300 mg/day) in attenuating cancer-induced neuropathic pain [41]. Pregabalin in combination with oxycodone successfully attenuated neuropathic pain after chemoradiation therapy in patients with non-small cell lung cancer in comparison to a combination of the transdermal fentanyl patch, oxycodone and gabapentin [3]. Shibahara and co-workers further demonstrated the efficacy of pregabalin as adjuvant (75-300 mg/day) with oxycodone in 5-FU/CDDP (5-flourouracil + cisplatin)-induced neuropathic pain, not responding to lornoxicam (NSAID) and oxycodone (semisynthetic opioid) suggesting its efficacy as compared to other commonly used analgesics [4]. Administration of pregabalin (150 mg/tid) for 2-6 weeks has been shown to produce significant improvement in oxaliplatin-induced sensory neuropathy (from grade 2 and 3 to grade 1 and 2) in about 48 % patients [42]. Administration of pregabalin (75-300 mg/day) for 8 weeks has also been shown to produce a significant and long lasting pain relief in chemotherapy-induced neuropathic pain in majority of pediatric oncological patients (86%) with infrequent and transient side effects [43]. Administration of pregabalin (75-150 mg/day) has been shown to significantly reduce paclitaxel (of gemcitabine/paclitaxel regimen)-induced sensory neurotoxicity in patients with left renal pelvic cancer [44].
Amongst the different analgesics, the relative efficacy of pregabalin (in comparison to amitriptyline and gabapentin) for the management of cancer-induced neuropathic pain was demonstrated in 120 patients having severe cancer-associated neuropathic pain. Pregabalin treated patients were shown to exhibit maximum improvement in terms of pain evaluation parameters (pain score and secondary outcome measures such as intensity of lancinating, dysesthesia and burning) along with clinically significant morphine sparing effect [45]. The post hoc analysis of pregabalin vs. non-pregabalin-treated cancer pain patients (n=273) in a 2-month prospective, multicentre study also reported that pregabalin treatment produces more satisfactory improvement to usual treatment (92.6% vs. 78.9%) in terms of decreased pain intensity, interference with normal daily activities and sleep quality [46].
3.3. Post-herpetic Neuralgia (PHN)
An 8-week, multicenter, parallel-group, double-blind, placebo-controlled, randomized clinical trial in 173 PHN patients has shown significant pain reduction in pregabalin-treated (300 and 600 mg/day) patients (starting from the first day of treatment and sustained throughout the study) assessed using the McGill Pain Questionnaire to calculate total (sensory and affective) pain scores. The greater proportion of patients with >or=30% and >or=50% decrease in mean pain scores were observed in pregabalin treated as compared to the placebo group (63% vs 25% and 50% vs 20%, p = 0.001) along with significant improvements in other factors associated with neuropathic pain such as sleep, quality of life, mood and overall global improvement [47]. Two multicentred, double blind, placebo-controlled trials in 370 and 238 in PHN patients for 13 weeks and 8 weeks respectively, reported the efficacy of different doses of pregabalin (150, 300, 600 mg/day) within 1 week of treatment with mild to moderate side effects including dizziness, somnolence, peripheral edema, headache, dry mouth and ataxia [48-49]. A meta-analysis using MEDLINE and AMBASE databases has also shown the efficacy of pregabalin in reducing pain intensity in patients with PHN associated neuropathic pain [50]. An 8 week open randomized comparative study of 50 PHN (thoracic, cervical, trigeminal and lumbosacral types) patients reported satisfactory improvements of pain perception (>75%) with 75 mg twice daily pregabalin with dizziness as the most common side effect which was found within tolerable levels as none of the patient stopped treatment due to adverse reaction [51]. In comparison to gabapentin, pregabalin was shown to produce similar analgesic effects at 1/6th dose of gabapentin with exacerbation of side effects with an increase in its dose [52].
3.4. Fibromyalgia
Fibromyalgia is characterized by chronic widespread musculoskeletal pain due to lowered pain threshold along with disordered sleep and fatigue [53]. Pregabalin has shown efficacy in treating fibromyalgia with rapid and clinically significant improvements in several outcome measures related to core symptoms of the syndrome in patients with long-standing fibromyalgia [54]. Eight-week, multicentred, double-blind, randomized clinical trial demonstrated the efficacy of different doses of pregabalin (150, 300, and 450 mg/day) in pain alleviation in 529 patients with fibromyalgia. Pregabalin the doses 300 and 450 mg/day was shown to significantly reduce the average severity of pain (more patients had >/=50% improvement in pain at the end point, i.e., 29%, versus 13% in the placebo group) and improve the health related quality of life with tolerable mild or moderate side effects among which dizziness and somnolence were the most frequent [55]. The meta-analysis of classical trials reports from Pfizer (four randomized trials including 2754 patients) has shown significant benefit in terms of pain relief with pregabalin (300, 450 and 600 mg/day) for the treatment of fibromyalgia. The maximum response rate with pregablin was observed after 4-6 weeks and remained constant thereafter [56]. A post hoc analysis of data from a multicenter, double-blind, placebo-controlled, randomized trial designed to evaluate the efficacy of pregabalin monotherapy in fibromyalgia pain (based on the pain and Patient Global Impression of Change criteria) demonstrated significant and long lasting clinical improvement in pain and other associated global parameters [57]. The pooled data from 3 open-label extension studies of pivotal randomized clinical trial including 1206 patients (mostly female, 92.4%) also showed that pregabalin (75-300 mg BID) is safe and well tolerated among fibromyalgia patients with dizziness, somnolence, headache, peripheral edema, and increased weight as common side effects of mild to moderate intensity [58]. A 4 week randomized, double-blind, placebo-controlled, 2-period crossover polysomno-graphic (PSG) study to assess the effect of pregabalin on sleep, tiredness, and pain in fibromyalgia patients revealed that pregabalin at 300-450 mg/day significantly improves these symptoms with good tolerability [59]. A decision-analytic model demonstrated pregabalin (225 mg BID) as cost saving and more effective as compared to duloxetine, tramadol, milnacipran, and gabapentin for the treatment of severe fibromyalgia [60].
3.5. Trigeminal Neuralgia
A one year follow-up, open label study to check the efficacy of pregabalin treatment in patients suffering from trigeminal neuralgia with or without concomitant facial pain revealed that pregabalin (150-600 mg/day) significantly reduces pain intensity and frequency (>50%) after 8 weeks. The pain relief was long lasting and sustained even after 1 year, thus suggesting pregabalin as the drug of choice for management of trigeminal neuralgia [61].
3.6. Pre-/post-operative Pain
A randomized controlled trial including 91 female patients scheduled for laparoscopic hysterectomy demonstrated that administration of maximally recommended dose of pregabalin (600 mg/day) pre-operatively was more effective than diazepam (10 mg), and reduced the consumption of opioids (oxycodone) postoperatively for reduction of pain [62]. Another randomized, double-blinded, placebo-controlled trial (including 99 patients) aimed to investigate the efficacy and safety of pregabalin in reducing both acute post-operative pain (up to 48 hr after surgery) and chronic pain (measured as hypoesthesia in the anterior chest after 3 months of surgery) in patients after robot-assisted endoscopic thyroidectomy showed that pre-operative (1 hr before surgery) administration of pregabalin (initial dose 150 mg, followed by same dose twice a day) significantly improves the pain scores and reduces the need for additional analgesics during 48 hr after the surgery. However, there was no significant difference between two groups related to chronic pain and chest hypoesthesia at 3 months after surgery showing that pregabalin was mainly effective in reducing early postoperative pain, but not chronic pain [63]. Patient outcome with different treatments after lumbar discectomy for radicular low back pain is variable and the benefit is inconsistent and many patients continue to experience pain even after 3 months of surgery. On the contrary, administration of pregabalin (300 mg at 90 minutes preoperatively and 150 mg at 12 and 24 hours postoperatively) in lumbar discectomy for radicular low back has demonstrated a greater decrease in pain intensity after 3 months in pregabalin receiving patients with lesser disability score and greater pain tolerance [64]. Another randomized, placebo-controlled prospective trial in 32 patients (18-65 years) with radicular low back pain undergoing elective lumbar discectomy revealed that a single pre-operative pregabalin administration at 300 mg dose 1 hour before surgery did not reduce the intensity of pain, but decreases the morphine consumption [65]. A randomized controlled trial including 108 patients (who underwent lumbar spinal surgery) conferred superior analgesic benefits with combined administration of pregabalin (150 mg twice daily) and dexamethasone (16 mg) for 4 days starting from 1 hour before the induction of anesthesia on postoperative pain [66].
3.7. Other Study Reports
In a prospective randomized trial including 36 patients with 4-week treatment suggested that the combination of celecoxib (cyclooxygenase-2 inhibitor) and pregabalin is more effective than monotherapy for the treatment of chronic low-back pain assessed by using a visual analogue scale (VAS, 0-100 mm) and the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale [67]. A prospective analysis of 683 patients (aged >or=18 years) with a 6-month history of chronic refractory low back pain showed higher reduction in severity of pain with pregabalin as compared to usual care (61.6% vs. 37.3%) with significant reduction in overall cost [68]. A randomized, double-blind, placebo-controlled trial including 64 patients with chronic pancreatitis-associated intense abdominal pain showed positive effects on secondary outcome measures (change in individual dermatomal thresholds) with pregabalin (75-300 mg BID) after three weeks treatment as compared to primary outcome measure (change in the sum of thresholds). It suggests that pregabalin is more effective in reducing skin sensitization as reflected by electric thresholds as compared to deep tissue sensitization (reflected by pressure thresholds) [69]. However, a randomized, double-blind, placebo-controlled, parallel-group trial has shown that pregabalin (150-600 mg/day BID) is well tolerated in HIV patients, but exhibits no superiority to placebo in the treatment of painful HIV neuropathy measured in terms of mean Numeric Pain Rating Scale (NPRS) score, Patient Global Impression of Change (PGIC) and sleep measurements [70].
4. SIDE EFFECTS
The majority of clinical studies involving administration of pregabalin for the treatment of various types of neuropathic pain states have shown dizziness, somnolence, headache, dry mouth, peripheral edema, weight gain, blurred vision, motor in coordination and ataxia as the most common side effects (occurred in 1-10% patients) at lower doses. The intensity of these side effects is generally increased with an increase in dose and at highest prescribed dose (600 mg/day), the incidences of side effects increase with higher frequency of dizziness (70%), blurred vision (63%) and headache (31%). Therefore, administration of pregabalin needs careful monitoring to take care of these side effects at higher doses [62]. There is clinical report documenting that patient withdrawal from the clinical trial is very insignificant despite the presence of some side effects, which may be possibly due to high efficacy of pregabalin [71]. The occurrence of some of these adverse effects may be attributed directly to its primary mechanism of action. It inhibits the various types of calcium channels viz., P, Q, and N- type located in the different brain areas to decrease depolarization-dependent neurotransmitter release [72]. The highest level of expression of these channels has been found in the cerebellum and in the hippocampus, and their dysfunction/decreased activity may affect the vestibulocerebellar/brainstem structures and higher cortical functions leading to dizziness, blurred vision, ataxia [73, 74] and cognitive impairment [75]. Blum and co-workers reported the development of hyponatremia, confusion and disorientation in a 74 year old patient (suffering from cardiovascular problems) treated for diabetic neuropathic pain with pregabalin and these symptoms were resolved within 2 days after discontinuation of drug [76].
Apart from these common side effects, a few number of patients may also develop cardiovascular abnormalities (<1%) with pregabalin usage, particularly in patients with previous history of cardiovascular dysfunction. Pregabalin treatment has been shown to cause chronic heart failure possibly due to calcium channel blockade mediated deleterious effect on myopathic ventricles, especially in patients with left ventricular systolic dysfunction [77]. A 92 year old patient with a history of paraoxystic fibrillation was shown to develop sinusal tachycardia followed by atrial fibrillation and congestive heart failure due to pregabalin [78]. Erdoğan and co-workers reported a case report profile of a 54 year old patient who developed peripheral edema after 2 weeks of pregabalin treatment (150 mg/day for 1st week and 300mg/day for 2nd week). After discontinuation of pregabalin, peripheral edema reduced suggesting a direct casual relationship. This fluid retention may be possibly attributed to inhibition of calcium channel which causes peripheral vasodilation and fluid leakage into the interstitial area. It may be further linked to the unexplained weight gain and may contribute to exacerbate congestive heart failure [79]. Aksakal and coworkers have recently reported that pregabalin (300 mg/day) administration for 8 months in a patient with diabetic neuropathic pain results in complete atrioventricular blockade, which is resolved within 4 days after pregabalin discontinuation. The cardiovascular side
effects of pregabalin have been attributed to its inhibitory effects on L-type calcium channels, predominantly located on the myocardium [80]. These cardiovascular events may be linked to the mechanisms through which other calcium channel blockers such as nifedipine produce increased mortality in susceptible cases, i.e., through coronary steal phenomenon associated with vasodilation and reflex increase in sympathetic activation [81]. In the heart, Ca2+ is essential for electrical activity and is a direct activator of the myofilaments in contraction. Therefore, long term pregabalin administration for the management of neuropathic pain may lead to various cardiac disorders such as arrhythmias or contractile dysfunction by blocking L-type Ca2+ channels, especially in patients with a history of CVS dysfunctions [82].
Pregabalin has an abuse liability because it produces the disturbances in central nervous system [83] and therefore, it needs careful administration in patients with alcohol and benzodiazepine dependence [84]. Administration of pregabalin with alcohol or benzodiazepines may synergistically depress neuronal conduction in the CNS possibly due to the enhancement of GABA mediated actions and inhibition of Ca2+ mediated glutamate release at synapses [85]. In preclinical studies, pregabalin has been shown to induce bone marrow changes, hepatic hypoxia, endothelial cell proliferation and hemangiosarcoma in mice [86-88]. The combination of hypoxia and sustained increase in endothelial cell proliferation, angiogenic growth factors, dysregulated erythropoiesis, and macrophage activation has been hypo-thesized as the key event for the hemangiosarcoma formation in mice [89]. However, there has not been any such correlation in humans for such incidents.
5. MECHANISM OF ACTION
5.1. Voltage Gated Calcium Channels
There have been a number of studies showing an up-regulation of voltage gated calcium channel (VGCC) in dorsal root ganglion and dorsal horn in neuropathic pain. These VGCC are composed of different subunits including α1 (pore forming trans-membrane subunit), α2-δ, β and γ (auxiliary subunits) [90]. The α2δ-subunit is composed of extracellular (α2) and membrane-spanning (δ) components that remain covalently associated with a disulfide bond and are required for proper cell-surface expression of VGCC [91]. The β subunit binds to the α1 subunit and controls the VDI (voltage-dependent inactivation), CDI (calcium-dependent inactivation) and CDF (calcium-dependent facilitation) of VGCC [92]. The γ-subunit is mainly associated with skeletal-muscle calcium channels, but its general importance of other channel types is not clear [93]. CaV α2-δ-1 subunits are very critical for the effective functioning of VGCC. These subunits associate directly with pore forming CaV α1 subunit in 1:1 ratio [94]. The expression of CaV α2-δ along with CaV α1 enhances the localization of VGCC to the plasma membrane [95]. Studies have shown that expression of CaV α2-δ, rather than CaV α1, is the rate limiting factor in modulating synaptic function. An important role of highly conserved MIDAS motif of CaV α2-δ has been described in controlling the synaptic VGCC function. The forward
trafficking of VGCC from cytosol to the plasma membrane of presynaptic terminal is controlled by α2-δ, but in MIDAS independent manner. The second local step for the proper VGCC function and coupling to exocytosis is MIDAS dependent. The expression of α2-δ-1 with an intact MIDAS motif leads to an 80% increase in release probability of neurotransmitter and its mutation leads to a corresponding decrease in exocytosis [96]. The experimental nerve injury models have shown an increased level of α2-δ-1 mRNA in the damaged sensory neurons (DRGs) as shown by in situ hybridization [97], microarray analysis [98] and quantitative PCR [99]. There is a corresponding increase in α2-δ-1 protein in DRGs and spinal cord, as determined by Western blot [100] and immunohistochemistry [99]. Furthermore, α2-δ-1 over-expressing mice showed a neuropathic phenotype of hyperalgesia and tactile allodynia even in the absence of nerve injury [101], indicating that α2-δ-1 causes excitability of DRG neurons and the expression of neuropathy.
Pregabalin is a calcium channel antagonist which shows specific binding affinity for the α2-δ (α2-δ-1 and α2-δ-2) auxiliary subunits of voltage-dependent calcium channels particularly P/Q, N and L-type [82,101-103]. The primary evidence regarding α2-δ as the primary target of pregabalin may be deduced from studies employing transgenic mice with mutant CaV α2-δ gene (R217A mutant mice). The autoradiographic study also revealed that the binding affinity of pregabalin was significantly decreased in the cortex (84 %), hippocampus (80 %), caudate putamen (66 %), lumbar dorsal horn (70 %) and cerebellum (37 %) of R217A mice. The analgesic effect of pregabalin was also found to be abolished in these mutant mice (in both CCI and formalin test) without any effect on the analgesic activity of morphine and amitriptyline, showing that pregabalin binding to α2-δ-1 subunit of VDCC is important for its analgesic activity [101]. It has been shown in in vitro studies (in synaptosomes) that pregabalin reduces artificially-stimulated calcium influx and reduces the neurotransmitter release within 10-30 min of application [104].
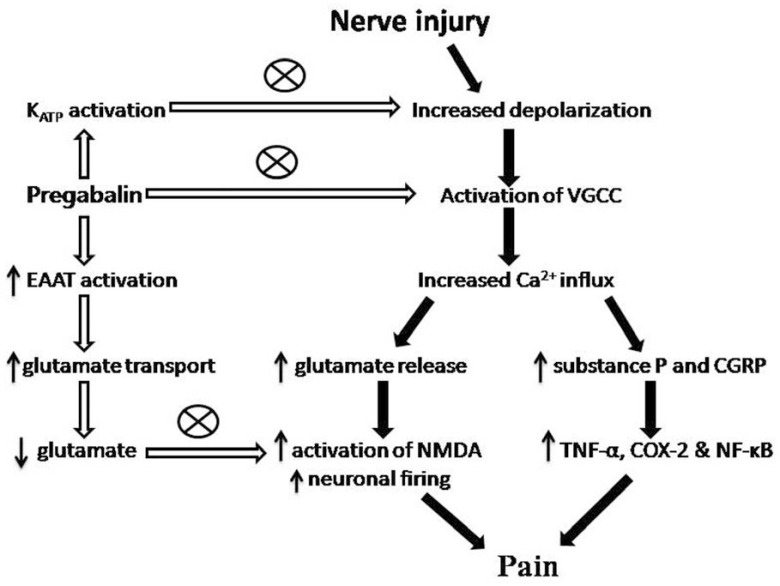
Bauer et al demonstrated the importance of increased trafficking of the CaV α2-δ-1 subunit from the dorsal root ganglia to the dorsal horn in the development of neuropathic pain. Furthermore, the elevated α2-δ-1 protein in the dorsal horn, but not in the DRGs was significantly reduced by chronic treatment with pregabalin for 8 days following spinal nerve ligation, indicating that pregabalin interferes with transport of α2-δ-1 to its terminal zones [99]. Administration of pregabalin is also shown to partially reverse the up-regulated CaV α2-δ-1 at the pre-synaptic nerve terminals in the dorsal horn of the spinal cord [104]. Hence, impaired anterograde trafficking and synaptic expression of VDCC mediated through auxiliary α2-δ subunits present an important mechanism for analgesic action of pregabalin by inhibition of synaptic transmission, decrease in neuro-transmitter release and reduction of spinal sensitization [99]. This may be concluded that pregabalin provides its analgesic action by binding to the α2-δ subunit of VDCC and inhibits its functional expression (membrane and anterograde trafficking) with concomitant inhibition of Ca2+ mediated excitatory neurotransmitter glutamate release at neuronal synapse (Fig. 1).
Fig. (1).
The mechanism of action for pain alleviation by pregabalin: Pregabalin blocks the VGCC and hence decrease glutamate and sensory neuropeptides (substance P and CGRP) release at synapse by decreasing Ca2+ influx. EAATs (excitatory amino acid transporters) activity is increased by pregabalin which caused more decrease in synaptic availability of glutamate. Decreased glutamate levels further inhibited the activation of NMDA and decreased the neuronal firing. Additionally pregabalin also activates the KATP channels, which also contributes to inhibition of neuronal excitation. Pregabalin through all these pathways ultimately provides significant pain relief in various neuropathic pain states.
5.2. Glutamate Transporter
L-Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system and is stored in the synaptic vesicles. Excitatory amino acid transporters (EAATs), located on the plasma membrane of the neurons and glial cells, rapidly terminate the action of glutamate and maintain its extracellular concentration below excitotoxic levels [105, 106]. Amongst the five Na+-dependent glutamate transporters (EAATs 1-5) [107], EAAT3 has been documented as a target of pregabalin. It is present mainly in the cytosol and only 20% of its expression is detected at the plasma membrane in normal state [108, 109]. A recent study has demonstrated that pregabalin increases the trafficking of EAAT3 from cytosol to the plasma membrane (as shown by an increase in Vmax) in a concentration dependent manner. Pregabalin mediated enhanced expression of EAAT3 at the plasma membrane of the neurons and the glial cells may significantly decrease the functional response of excitatory neurotransmitter (glutamate). Furthermore, pregabalin mediated enhancement in EAAT3 activity was abolished by PKC and P13K inhibitors suggesting that its action on EAAT3 may involve these signaling molecules [110]. Earlier studies had shown that EAAT3 activity and expression are highly regulated by these intracellular signaling pathways including PKC and PI3K [111, 112] (Fig. 1).
5.3. Potassium Channels
Pregabalin is shown to modulate different potassium channels including KATP channels which may be another mechanism responsible for its analgesic effect. It has been reported that KATP channel opening produces anti-nociceptive effects by reducing the neuronal excitability and inhibiting the release of different neurotransmitters including substance P in the spinal cord [113]. Kweon et al demonstrated the anti-nociceptive effect of pregabalin in the formalin test in rats. Intrathecal administration of pregabalin, through catheterization of spinal subarachnoid space, was shown to decrease formalin-induced frequency of flinching in a dose dependent manner. However, administration of glibenclamide reversed the analgesic effect of pregabalin suggesting that the anti-nociceptive effect of pregabalin in the rat formalin test may be associated with activation of KATP channels [114]. Earlier studies had shown that application of pregabalin to the intracellular surface of the excised patches activates KATP channels in the differentiated hippocampal neuron-derived H19-7 cells, suggesting that its binding side could be primarily on the intracellular site [115].
However, application of pregabalin either inside or outside DRG neurons has been shown to produce enhance-ment of K+ currents suggesting that the actions of pregabalin may involve both extracellular and intracellular target sites [116]. Pregabalin mediated enhanced K+ current may decrease neuronal excitability by decreasing norepinephrine and glutamate release in the rat neocortical tissue [117]. Studies have shown that administration of cAMP analogue prevents pregabalin-induced increase in K+ current suggesting that the intracellular response of pregabalin may be associated with activation of protein kinase A [116]. Other studies have also shown that inhibition of Ca2+ currents by gabapentinoid drugs is sensitive to cAMP analogues capable of activating/inhibiting PKA (Fig. 1) [118].
5.4. GABA
Although pregabalin is structurally related to GABA, it was not found to be metabolized to GABA and it also does not exhibit direct binding affinity for GABA receptors [119]. Pregabalin does not appear to mimic GABA or pharmacologically enhance its actions suggesting that direct or indirect effects on GABA receptors do not contribute significantly to its pharmacological actions. Furthermore, radioligand binding studies have shown that pregabalin has no affinity for GABA-A or GABA-B receptors [120]. Pretreatment with bicuculline (GABA-A antagonist) did not affect the antiallodynic effect of pregabalin suggesting that its therapeutic action is not mediated through GABA-A receptors [121]. Furthermore, pregabalin and gabapentin did not inhibit GABA transport in in-vitro settings [122]. The above findings suggest that there is no significant correlation between GABA and pregabalin action in alleviation of neuropathic pain. On the contrary, some reports suggest that the higher doses of pregabalin might increase the GABA content in the neuronal tissues through enhanced glutamic acid decarboxylase activity, which results in decreased neuronal excitability and hence, may be an alternative mechanism by which pregabalin alleviates neuropathic pain [123, 124].
5.5. Inflammation
Pregabalin modulates the release of sensory neuropeptides like substance P and CGRP in inflammation induced spinal cord sensitization [125]. Pregabalin inhibits lipid peroxidation, microglial cells and attenuates cellular apoptosis especially of oligodendrocytes suggesting that its neuroprotective action is mediated through anti-inflammatory effect [126]. Pregabalin suppresses substance P and other inflammatory neuropeptides induced NF-kB activation in both neuronal and glial cell lines non-specifically by inhibiting p65 nuclear localization. Further-more, pregabalin also suppresses NF-kB-regulated gene products and COX-2 expression and thus, inhibits substance P-induced cytokine synthesis in the different neurological disorders [127]. Studies from our laboratory have shown that pregabalin mediated beneficial effects in vincristine and CCI-induced neuropathic pain is associated with decreased TNF-α expression and myeloperoxidase activity in the sciatic nerve of rats. It suggests that pregabalin mediated anti-inflammatory actions including decreased release of pro-inflammatory cytokines play a key role in mediating its anti-nociceptive actions in different models of peripheral neuropathic pain (Fig. 1) [128, 129].
5.6. Central Versus Peripheral Action
Both systemic (intraperitoneal; i.p.) and local (intra-cerebroventricular or intrathecal; i.c.v. or i.t.) administration of pregabalin reduces thermal and mechanical hyper-sensitivity in partial ligation of the sciatic nerve (the Seltzer model) in mice, suggesting that pregabalin acts at both supraspinal and spinal loci. The depletion of spinal noradrenaline (NA) or pharmacological blockade of spinal α2-adrenoceptors with yohimbine (i.p. or i.t.) has been shown to reduce the analgesic effects of pregabalin (i.p. or i.c.v.) in thermal and mechanical hypersensitivity based tests. It has been proposed that pregabalin acts supraspinally to activate the descending noradrenergic pain inhibitory system coupled with spinal α2-adrenoceptors to ameliorate neuropathic pain [130]. An increase in CaV α2δ-1 expression at the supraspinal location in response to adrenergic system stimulation is abolished in the presence (i.c.v administration) suggesting the possibility of its supra-spinal actions [10].
Electrophysiological studies in intact and spinalized rats have shown that pregabalin significantly inhibits C-fiber-mediated spinal nociceptive specific (NS) neurons with no significant inhibitory effects on A-δ fiber-mediated early responses. However, the pain inhibitory effects of pregabalin are abolished in the spinalized animals as compared to intact rats suggesting that pregabalin-induced selective anti-nociceptive effect involves the supraspinal centers [131]. Martinez and co-workers compared the central and peripheral contributions of pregabalin in treating neuropathic pain in two models including streptozotocin-induced diabetic peripheral neuropathy and traumatic injury (spinal nerve ligation). The sufficient doses of pregabalin provided through intranasal or intrathecal methods were shown to ameliorate tactile allodynia and thermal hypersensitivity in both spinal nerve ligation and diabetic peripheral neuropathy models. In contrast, administration of pregabalin at the level of the peripheral nerve did not attenuate pain behavior in either of the models again suggesting that the site of action of pregabalin is central, not peripheral [132].
6. CONCLUSION
Preclinical as well as clinical studies project pregabalin as a very effective agent for treating neuropathic pain with a very good safety profile. The inhibition of voltage gated Ca2+ channel is the most likely target for pregabalin action, which contributes to the reduction of excitatory neurotransmitters release and inhibition of synaptic transmission. The modulation of K+ channel conductance, excitatory amino acid transporter and inflammation are other possible mechanisms responsible for its analgesic actions. However, studies are still needed to elucidate its mechanism in a more detailed manner.
ACKNOWLEDGEMENTS
The authors are grateful to the Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala, India, for supporting this study and providing technical facilities for the work.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
REFERENCES
- 1.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Tassone DM, Boyce E, Guyer J, Nuzum D. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures and anxiety disorders. Clin. Ther. 2007;29:26–48. doi: 10.1016/j.clinthera.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 3.Shibahara H, Ando A, Suzuki S, Uematsu N, Nishimura D. Oxycodone and pregabalin using transdermal fentanyl patch provided relief of symptoms forpostherpetic neuropathic pain in a patient with non-small cell lung cancer. Gan To Kagaku Ryoho. 2011;38:1675–7. [PubMed] [Google Scholar]
- 4.Shibahara H, Okubo K, Takeshita N, Nishimura D. [Medical treatment including pregabalin and radiation therapy provided remarkable relief for neuropathic pain by brachial plexus invasion in a patient with esophageal cancer]. Gan To Kagaku Ryoho. 2012;39:277–80. [PubMed] [Google Scholar]
- 5.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week randomised double-blind multicentre placebo-controlled trial of flexible- and fixed-dose regimens. Pain. 2005;115:254–63. doi: 10.1016/j.pain.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Andre V, Rigoulot MA, Koning E. Long-term pregabalin treatment protects basal cortices and delays the occurrence of spontaneous seizures in the lithium-pilocarpine model in the rat. Epilepsia. 2003;44:893–903. doi: 10.1046/j.1528-1157.2003.61802.x. [DOI] [PubMed] [Google Scholar]
- 7.Hurley RW, Chatterjea D, Rose FM, Taylor CP. Gabapentin and pregabalin can interact synergistically with naproxen to produce antihyperalgesia. Anesthesiology. 2002;97:1263–73. doi: 10.1097/00000542-200211000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Field MJ, Cox PJ, Stott E, Melrose H, Offord J, Su TZ, Bramwell SZ, Corradini L, England S, Winks J, Kinloch RA, Hendrich J, Dolphin AC, Webb T, Williams D. Identification of the alpha2-delta-1 subunit of voltage-dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103:17537–42. doi: 10.1073/pnas.0409066103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gustafsson H, Sandin J. Oral pregabalin reverses cold allodynia in two distinct models of peripheral neuropathic pain. Eur J Pharmacol. 2009;605:103–8. doi: 10.1016/j.ejphar.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe M, Takasu K, Takeuchi Y, Ono H. Pain relief by gabapentin and pregabalin via supraspinal mechanisms after peripheral nerve injury. J Neurosci Res. 2008;86:3258–3264. doi: 10.1002/jnr.21786. [DOI] [PubMed] [Google Scholar]
- 11.Bender G, Florian JAJr, Bramwell S, Field MJ, Tan KK, Marshall S, DeJongn J, Bies RR, Danhof M. Pharmacokinetic-pharmacodynamic analysis of the static allodynia response to pregabalin and sildenafil in a rat model of neuropathic pain. J Pharmacol Exp Ther. 2010;334:599–608. doi: 10.1124/jpet.110.166074. [DOI] [PubMed] [Google Scholar]
- 12.Baastrup C, Jensen TS, Finnerup NB. Pregabalin attenuates place escape/avoidance behavior in a rat model of spinal cord injury. Brain Res. 2011;1370:129–35. doi: 10.1016/j.brainres.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki-Yatsugi S, Nagakura Y, Ogino S, Sekizawa T, Kiso T, Takahashi M, Ishikawa G, Ito H, Shimizu Y. Automated measurement of spontaneous pain-associated limb movement and drug efficacy evaluation in a rat model of neuropathic pain. Eur J Pain. 2012;6(10):1426–36. doi: 10.1002/j.1532-2149.2012.00142.x. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki R, Yamamoto T. The Efficacy of Morphine, Pregabalin Gabapentin and Duloxetine on Mechanical Allodynia Is Different from That on Neuroma Pain in the Rat Neuropathic Pain Model. Anesth. Analg. 2012;115(1):182–8. doi: 10.1213/ANE.0b013e31824f94ca. [DOI] [PubMed] [Google Scholar]
- 15.Plaza-Villegas F, Heir G, Markman S, Khan J, Noma N, Benoliel R, Patel J, Eliav E. Topical pregabalin and diclofenac for the treatment of neuropathic orofacial pain in rats. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2012;114:449–56. doi: 10.1016/j.oooo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Ling B, Coudoré F, Decalonne L, Eschalier A, Authier A. Comparative antiallodynic activity of morphine, pregabalin and lidocaine in a rat model of neuropathic pain produced by one oxaliplatin injection. Neuropharmacology. 2008;55:724–8. doi: 10.1016/j.neuropharm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Peng P, Xi Q, Xia S, Zhuang L, Gui Q, Chen Y, Huang Y, Zou M, Rao J, Yu S. Pregabalin attenuates docetaxel-induced neuropathy in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2012;32:586–90. doi: 10.1007/s11596-012-1001-y. [DOI] [PubMed] [Google Scholar]
- 18.Christoph T, De Vry J, Schiene K, Tallarida RJ, Tzschentke TM. Synergistic antihypersensitive effects of pregabalin and tapentadol in a rat model of neuropathic pain. Eur. J. Pharmacol. 2011;666:72–9. doi: 10.1016/j.ejphar.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 19.Luszczki JJ, Florek-Luszczki M. Synergistic interaction of pregabalin with the synthetic cannabinoid WIN 55, 212-2 mesylate in the hot-plate test in mice: an isobolographic analysis. Pharmacol. Rep. 2012;64:723–32. doi: 10.1016/s1734-1140(12)70867-8. [DOI] [PubMed] [Google Scholar]
- 20.Lauria-Horner BA, Pohl RB. Pregabalin: a new anxiolytic. Expert Opin. Investig. Drugs. 2003;12:663–672. doi: 10.1517/13543784.12.4.663. [DOI] [PubMed] [Google Scholar]
- 21.Bryans JS, Wustrow DJ. 3-substituted GABA analogs with central nervous system activity: a review. Med. Res. Rev. 1999;19:149–77. doi: 10.1002/(sici)1098-1128(199903)19:2<149::aid-med3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 22.Moon DE, Lee DI, Lee SC, Song SO, Yoon DM, Yoon MH, Kim HK, Lee YW, Kim C, Lee PB. Efficacy and tolerability of pregabalin using a flexible, optimized dose schedule in Korean patients with peripheral neuropathic pain: a 10-week, randomized, double-blind, placebo-controlled, multicenter study. Clin. Ther. 2010;32:2370–85. doi: 10.1016/j.clinthera.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Satoh J, Yagihashi S, Baba M, Suzuki M, Arakawa A, Yoshiyama T, Shoji S. Ef?cacy and safety of pregabalin for treating neuropathic pain associated with diabetic peripheral neuropathy: a 14 week randomized double-blind placebo-controlled trial. Diabet. Med. 2011;28:109–116. doi: 10.1111/j.1464-5491.2010.03152.x. [DOI] [PubMed] [Google Scholar]
- 24.Gilron I, Wajsbrot D, Therrien F, Lemay J. Pregabalin for peripheral neuropathic pain: a multicenter, enriched enrollment randomized withdrawal placebo-controlled trial. Clin. J. Pain. 2011;27:185–93. doi: 10.1097/AJP.0b013e3181fe13f6. [DOI] [PubMed] [Google Scholar]
- 25.Jensen MP, Gammaitoni AR, Bolognese JA, Alon A, Smugar SS, Galer BS, Hewitt DJ. The pain quality response profile of pregabalin in the treatment of neuropathic pain. Clin. J. Pain. 2012;28:683–6. doi: 10.1097/AJP.0b013e31823f9e64. [DOI] [PubMed] [Google Scholar]
- 26.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. Pain. 2004;110:628–38. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Frampton JE, Scott LJ. Pregabalin: in the treatment of painful diabetic peripheral neuropathy. Drugs. 2004;64:2813–20. doi: 10.2165/00003495-200464240-00006. [DOI] [PubMed] [Google Scholar]
- 28.Richter RW, Portenoy R, Sharma U, Lamoreaux L, Bockbrader H, Knapp LE. Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J. Pain. 2005;6:253–60. doi: 10.1016/j.jpain.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Sharma U, Griesing T, Emir B, Young JP Jr. Time to onset of neuropathic pain reduction: A retrospective analysis of data from nine controlled trials of pregabalin for painful diabetic peripheral neuropathy and postherpetic neuralgia. Am. J. Ther. 2010;17:577–85. doi: 10.1097/MJT.0b013e3181d5e4f3. [DOI] [PubMed] [Google Scholar]
- 30.Freeman R, Durso-Decruz E, Emir B. Efficacy safety and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008;31:1448–54. doi: 10.2337/dc07-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers LC, Armstrong DG. Pregabalin for painful diabetic peripheral neuropathy. Nat. Clin. Pract. Endocrinol. Metab. 2009;5:14–5. doi: 10.1038/ncpendmet1019. [DOI] [PubMed] [Google Scholar]
- 32.Semel D, Murphy TK, Zlateva G, Cheung R, Emir B. Evaluation of the safety and efficacy of pregabalin in older patients with neuropathic pain: results from a pooled analysis of 11 clinical studies. BMC Fam. Pract. 2010;11:85. doi: 10.1186/1471-2296-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal D, Bhansali A, Hota D, Chakrabarti A, Dutta P. Amitriptyline vs.pregabalin in painful diabetic neuropathy: a randomized double blind clinical trial. Diabet. Med. 2009;26:1019–1026. doi: 10.1111/j.1464-5491.2009.02806.x. [DOI] [PubMed] [Google Scholar]
- 34.Devi P, Madhu K, Ganapathy B, Sarma G, John L, Kulkarni C. Evaluation of efficacy and safety of gabapentin duloxetine and pregabalin in patients with painful diabetic peripheral neuropathy. Indian J. Pharmacol. 2012;44:51–56. doi: 10.4103/0253-7613.91867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biyik Z, Solak Y, Atalay H, Gaipov A, Guney F, Turk S. Gabapentin versus pregabalin in improving sleep quality and depression in hemodialysis patients with peripheral neuropathy: a randomized prospective crossover trial. Int. Urol. Nephrol. 2012;45(3):831–7. doi: 10.1007/s11255-012-0193-1. [DOI] [PubMed] [Google Scholar]
- 36.Saldaña MT, Pérez C, Navarro A, Masramón X, Rejas J. Pain alleviation and patient-reported health outcomes following switching to pregabalin in individuals with gabapentin-refractory neuropathic pain in routine medical practice. Clin. Drug Investig. 2012;32:401–12. doi: 10.2165/11599400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.de Salas-Cansado M, Pérez C, Saldaña MT, Navarro A, Rejas J. A cost-effectiveness analysis of the effect of pregabalin versus usual care in the treatment of refractory neuropathic pain in routine medical practice in Spain. Pain Med. 2012;13:699–710. doi: 10.1111/j.1526-4637.2012.01375.x. [DOI] [PubMed] [Google Scholar]
- 38.Navarro A, Saldaña MT, Pérez C, Torrades S, Rejas J. A cost-consequences analysis of the effect of pregabalin in the treatment of peripheral neuropathic pain in routine medical practice in primary care settings. BMC Neurol. 2011;11:7. doi: 10.1186/1471-2377-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogawa S, Satoh J, Arakawa A, Yoshiyama T, Suzuki M. Pregabalin treatment for peripheral neuropathic pain: a review of safety data from randomized controlled trials conducted in Japan and in the west. Drug Saf. 2012;35:793–806. doi: 10.2165/11632660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Prettyjohns M, Sandelin R, Lister S, Norrefalk JR. A cost-utility study of the use of pregabalin added to usual care in refractory neuropathic pain patients in a Swedish setting. J. Med. Econ. 2012;15(6):1097–109. doi: 10.3111/13696998.2012.704458. [DOI] [PubMed] [Google Scholar]
- 41.Baba M, Gomyo I. Retrospective evaluation of pregabalin for cancer-related neuropathic pain. Masui. 2012;61:147–54. [PubMed] [Google Scholar]
- 42.Saif MW, Syrigos K, Kaley K, Isufi I. Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res. 2010;30:2927–33. [PubMed] [Google Scholar]
- 43.Vondracek P, Oslejskova H, Kepak T, Mazanek P, Sterba J, Rysava M, Gal P. Efficacy of pregabalin in neuropathic pain in paediatric oncological patients. Eur. J. Paediatr. Neurol. 2009;13:332–6. doi: 10.1016/j.ejpn.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima T, Kiba T, Ogawa Y, Hosokawa A, Shintani H, Okada Y, Taniguchi T, Shigeta M, Kozawa K. A case of Paclitaxel-induced peripheral neuropathy successfully treated with pregabalin. Gan To Kagaku Ryoho. 2012;39:1443–5. [PubMed] [Google Scholar]
- 45.Mishra S, Bhatnagar S, Goyal GN, Rana SP, Upadhya SP. A comparative efficacy of amitriptyline gabapentin and pregabalin in neuropathic cancer pain: a prospective randomized double-blind placebo-controlled study. Am. J. Hosp. Palliat. Care. 2012;29:177–82. doi: 10.1177/1049909111412539. [DOI] [PubMed] [Google Scholar]
- 46.Manas A, Ciria JP, Fernández MC, Gonzálvez ML, Morillo V, Pérez M, Masramon X, López-Gómez V. TENOR collaborative study group.Post hoc analysis of pregabalin vs. non-pregabalin treatment in patients with cancer-related neuropathic pain better pain relief sleep and physical health. Clin. Transl. Oncol. 2011;13(9):656–63. doi: 10.1007/s12094-011-0711-0. [DOI] [PubMed] [Google Scholar]
- 47.Dworkin RH, Corbin AE, Young JPJr, Sharma U, LaMoreaux L, Bockbrade H, Garofalo EA, Poole RM. Pregabalin for the treatment of postherpetic neuralgia A randomized, placebo-controlled trial. Neurology. 2003;60:1274–1283. doi: 10.1212/01.wnl.0000055433.55136.55. [DOI] [PubMed] [Google Scholar]
- 48.Sabatowski R, Gálvez R, Cherry DA, Jacquot F, Vincent E, Maisonobe P, Versavel M. 1008-045 Study Group.Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised placebo-controlled clinical trial.. Pain. 2004;109:26–35. doi: 10.1016/j.pain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Seventer RV, Feister HA, Young JP, Stoker M, Versavel M, Rigaudy L. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13week, randomized trial. Curr. Med. Res. Opin. 2006;22:375–384. doi: 10.1185/030079906x80404. [DOI] [PubMed] [Google Scholar]
- 50.Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy safety and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann. Pharmacother. 2011;45:1483–90. doi: 10.1345/aph.1P777. [DOI] [PubMed] [Google Scholar]
- 51.Achar A, Chakraborty PP, Bisai S, Biswas A, Guharay T. Comparative study of clinical efficacy of amitriptyline and pregabalin in postherpetic neuralgia. Acta Dermatovenerol. Croat. 2012;20:89–94. [PubMed] [Google Scholar]
- 52.Ifuku M, Iseki M, Hidaka I, Morita Y, Komatus S, Inada E. Replacement of gabapentin with pregabalin in postherpetic neuralgia therapy. Pain Med. 2011;12:1112–6. doi: 10.1111/j.1526-4637.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 54.Lyseng-Williamson KA, Siddiqui MA. Pregabalin: a review of its use in fibromyalgia. Drugs. 2008;68:2205–23. doi: 10.2165/00003495-200868150-00009. [DOI] [PubMed] [Google Scholar]
- 55.Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, Young JP Jr, LaMoreaux LK, Martin SA, Sharma U. Pregabalin 1008-105 Study Group.Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized double-blind placebo-controlled trial. Arthritis Rheum. 2005;52:1264–73. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 56.Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta-analysis of efficacy and safety from company clinical trial reports. Rheumatology (Oxford) 2010;49:706–15. doi: 10.1093/rheumatology/kep432. [DOI] [PubMed] [Google Scholar]
- 57.Pauer L, Atkinson G, Murphy TK, Petersel D, Zeiher B. Long-term Maintenance of Response Across Multiple Fibromyalgia Symptom Domains in a Randomized Withdrawal Study of Pregabalin. Clin. J. Pain. 2012;28:609–14. doi: 10.1097/AJP.0b013e31823dd315. [DOI] [PubMed] [Google Scholar]
- 58.Arnold LM, Emir B, Murphy TK, Zeiher BG, Pauer L, Scott G, Petersel D. Safety profile and tolerability of up to 1 year of pregabalin treatment in 3 open-label extension studies in patients with fibromyalgia. Clin. Ther. 2012;34:1092–102. doi: 10.1016/j.clinthera.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res. (Hoboken) 2012; 64:597–606. doi: 10.1002/acr.21595. [DOI] [PubMed] [Google Scholar]
- 60.Lloyd A, Boomershine CS, Choy EH, Chandran A, Zlateva G. The cost-effectiveness of pregabalin in the treatment of fibromyalgia. US perspective. J. Med. Econ. 2012;15(3):481–92. doi: 10.3111/13696998.2012.660254. [DOI] [PubMed] [Google Scholar]
- 61.Obermann M, Yoon MS, Sensen K, Maschke M, Diener HC, Katsarava Z. Efficacy of pregabalin in the treatment of trigeminal neuralgia. Cephalalgia. 2008;28(2):174–81. doi: 10.1111/j.1468-2982.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- 62.Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134:106–12. doi: 10.1016/j.pain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Kim SY, Jeong JJ, Chung WY, Kim HJ, Nam KH, Shim YH. Perioperative administration of pregabalin for pain after robot-assisted endoscopic thyroidectomy: a randomized clinical trial. Surg. Endosc. 2010;24:2776–81. doi: 10.1007/s00464-010-1045-7. [DOI] [PubMed] [Google Scholar]
- 64.Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth. Analg. 2010;110:1180–5. doi: 10.1213/ANE.0b013e3181cf949a. [DOI] [PubMed] [Google Scholar]
- 65.Hegarty DA, Shorten GD. A Randomised, Placebo-controlled Trial of the Effects of Preoperative Pregabalin on Pain Intensity and Opioid Consumption following Lumbar Discectomy. Korean J .Pain. 2011;24:22–30. doi: 10.3344/kjp.2011.24.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Choi YS, Shim JK, Song JW, Kim JKC, Yoo YC, Kwak YL. Combination of Pregabalin and Dexamethasone for Postoperative Pain and Functional Outcome in Patients Undergoing Lumbar Spinal Surgery: A Randomized Placebo-controlled Trial. Clin. J. Pain. 2012 doi: 10.1097/AJP.0b013e318246d1a9. [DOI] [PubMed] [Google Scholar]
- 67.Romanò CL, Romanò D, Bonora C, Mineo G. Pregabalin celecoxib and their combination for treatment of chronic low-back pain. J. Orthop. Traumatol. 2009;10:185–91. doi: 10.1007/s10195-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morera-Domínguez C, Ceberio-Balda F, Flórez-García M, Masramón X, López-Gómez V. A cost-onsequence analysis of pregabalin versus usual care in the symptomatic treatment of refractory low back pain: sub-analysis of observational trial data from orthopaedic surgery and rehabilitation clinics. Clin. Drug Investig. 2010;30: 517–31. doi: 10.2165/11536280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 69.Bouwense SA, Olesen SS, Drewes AM, Poley JW, van Goor H, Wilder-Smith OH. Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized controlled trial. PLoS One. 2012;7:e4209601. doi: 10.1371/journal.pone.0042096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R. 1066 HIV Neuropathy Study Group.Pregabalin for painful HIV neuropathy. A randomized double-blind placebo-controlled trial. Neurology. 2010;74:413–420. doi: 10.1212/WNL.0b013e3181ccc6ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zaccara , Perucca P, Gangemi PF. The adverse event profile of pregabalin across different disorders: a meta-analysis. Eur. J. Clin. Pharmacol. 2012;68:903–12. doi: 10.1007/s00228-012-1213-x. [DOI] [PubMed] [Google Scholar]
- 72.Ben-Menachem E. Pregabalin pharmacology and its relevance to clinical practice. Epilepsia. 2004;45 ( suppl 6):13–18. doi: 10.1111/j.0013-9580.2004.455003.x. [DOI] [PubMed] [Google Scholar]
- 73.Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat. Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 74.Liao YJ, Safa P, Chen YR, Sobel RA, Boyden ES, Tsien RW. Anti-Ca2+ channel antibody attenuates Ca2+ currents and mimics cerebellar ataxia in vivo. Proc .Natl. Acad. Sci. US.A. 2008;105:2705–2710. doi: 10.1073/pnas.0710771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakagawasai O, Onogi H, Mitazaki S, Sato A, Watanabe K, Saito H, Murai S, Nakaya K. Murakami M, Takahashi E, Tan-No K, Tadano T. Behavioral and neurochemical characterization of mice deficient in the N-type Ca2+ channel alpha1B subunit. Behav. Brain Res. 2010;208:224–230. doi: 10.1016/j.bbr.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 76.Blum A, Simsolo C, Tatour I. Hyponatremia and confusion caused by pregabalin. Isr. Med. Assoc. J. 2009;11(11):699–700. [PubMed] [Google Scholar]
- 77.Murphy N, Mockler M, Ryder M, Ledwidge M, McDonald K. Decompensation of chronic heart failure associated with pregabalin in patients with neuropathic pain. J. Card Fail. 2007;13(3):227–9. doi: 10.1016/j.cardfail.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 78.Laville MA, de laGastine B, Husson B, Le Boisselier R, Mosquet B, Coquerel A. Should we care about pregabalin for elderly patients with a history of cardiac dysrhythmia?. Rev. Med. Interne. 2008;29(2):152–4. doi: 10.1016/j.revmed.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Erdogan G, Ceyhan D, Güleç S. Possible heart failure associated with pregabalin use. Case report. Agri. 2011;23(2):80–83. [PubMed] [Google Scholar]
- 80.Aksakal E, Bakirci EM, Emet M, Uzkeser M. Complete atrioventricular block due to overdose of pregabalin. Am. J. Emerg. Med. 2012;30(9):2101. e1–4. doi: 10.1016/j.ajem.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Furberg CD, Psaty BM, Meyer JV. Nifedipine.Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92(5):1326–31. doi: 10.1161/01.cir.92.5.1326. [DOI] [PubMed] [Google Scholar]
- 82.Yada H, Murata M, Shimoda K. Dominant negative suppression of Rad leads to QT prolongation and causes ventricular arrhythmias via modulation of L-type Ca2+ channels in the heart. Circ. Res. 2007;101(1):69–77. doi: 10.1161/CIRCRESAHA.106.146399. [DOI] [PubMed] [Google Scholar]
- 83.Skopp G, Zimmer G. Pregabalin--a drug with abuse potential?. Arch. Kriminol. 2012;229:44–54. [PubMed] [Google Scholar]
- 84.Oulis P, Konstantakopoulos G. Efficacy and safety of pregabalin in the treatment of alcohol and benzodiazepine dependence. Expert Opin. Investig. Drugs. 2012;21:1019–29. doi: 10.1517/13543784.2012.685651. [DOI] [PubMed] [Google Scholar]
- 85.Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol. Rev. 1997;7(1):1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- 86.Criswell KA, Cook JC, Morse D, Lawton M, Somps C, Obert L, Roy M, Sokolowski S, Koza-Taylor P, Colangelo J, Navetta K, Brady J, Pegg D, Wojcinski Z, Rahbari R, Duddy S, Anderson T. Pregabalin Induces Hepatic Hypoxia and Increases Endothelial Cell Proliferation in Mice a Process Inhibited by Dietary Vitamin E Supplementation. Toxicol. Sci. 2012;128:42–56. doi: 10.1093/toxsci/kfs148. [DOI] [PubMed] [Google Scholar]
- 87.Criswell KA, Cook JC, Wojcinski Z, Pegg D, Herman J, Wesche D, Giddings J, Brady JT, Anderson T. Mode of action associated with development of hemangiosarcoma in mice given pregabalin and assessment of human relevance. Toxicol. Sci. 2012;128:57–71. doi: 10.1093/toxsci/kfs149. [DOI] [PubMed] [Google Scholar]
- 88.Criswell KA, Wojcinski Z, Pegg D, et al. Key components of the mode of action for hemangiosarcoma induction in pregabalin-treated mice evidence of increased bicarbonate dysregulated erythropoiesis macrophage activation and increased angiogenic growth factors in mice but not in rats. Toxicol. Sci. 2012;128:22–41. doi: 10.1093/toxsci/kfs147. [DOI] [PubMed] [Google Scholar]
- 89.Pegg D, Bleavins M, Herman J, Wojcinski Z, Graziano M, Henck J, Criswell KA, Anderson T, Duddy S. Hemangiosarcoma in mice administered pregabalin: analysis of genotoxicity tumor incidence and tumor genetics. Toxicol. Sci. 2012;128:9–21. doi: 10.1093/toxsci/kfs146. [DOI] [PubMed] [Google Scholar]
- 90.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 91.Canti C, Davies A, Dolphin AC. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of a2d subunits is key to trafficking voltage-gated Ca2+ channels. Curr. Neuropharmacol. 2003;1:209–217. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci. STKE. 2006;(318):er10. doi: 10.1126/stke.3182006er1. [DOI] [PubMed] [Google Scholar]
- 93.Kang MG, Campbell KP. Gamma subunit of voltage-activated calcium channels. J. Biol. Chem. 2003;278(24):21315–8. doi: 10.1074/jbc.R300004200. [DOI] [PubMed] [Google Scholar]
- 94.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007;28:220–8. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 95.Cao YQ. Voltage-gated calcium channels and pain. Pain. 2006;126:5–9. doi: 10.1016/j.pain.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 96.Hoppa MB, Lana B, Margas W, Dolphin AC, Ryan TA. a2d expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486(7401):122–5. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Newton RA, Bingham S, Case PC, Sanger GJ, Lawson SN. Dorsal root ganglion neurons show increased expression of the calcium channel alpha2delta-1 subunit following partial sciatic nerve injury. Brain Res. Mol. Brain Res. 2001;95:1–8. doi: 10.1016/s0169-328x(01)00188-7. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Sun H, Della P K, Benz RJ, Xu J, Gerhold DL, Holder DJ, Koblan KS. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–546. doi: 10.1016/s0306-4522(02)00341-x. [DOI] [PubMed] [Google Scholar]
- 99.Bauer CS, Nieto-Rostro M, Rahman W , Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri R Y, Fernandez-Alacid L, Millar NS, Dickenson AH, Lujan R, Dolphin AC. The increased trafficking of the calcium channel subunit a2d-1 to presynaptic terminals in neuropathic pain is inhibited by the a2d ligand pregabalin. J. Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, Yaksh TL. Upregulation of dorsal root ganglion a2d calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J. Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha(2)delta(1) subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marais E, Klugbauer N, Hofmann F. Calcium channel2+ subunits—structure and gabapentin binding. Mol. Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- 103.Gong HC, Hang J, Kohler W, Li L, Su TZ. Tissue-speci?c expression and gabapentin binding properties of calcium channel2+ subunit subtypes. J. Mem. Biol. 2001;184:35–43. doi: 10.1007/s00232-001-0072-7. [DOI] [PubMed] [Google Scholar]
- 104.Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J. Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakamura Y, Iga K, Shibata T, Shudo M, Kataoka K. Glial plasmalemmal vesicles: a subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia. 1993;9:48–56. doi: 10.1002/glia.440090107. [DOI] [PubMed] [Google Scholar]
- 106.Danbolt NC. The high af?nity uptake system for excitatory aminoacids in the brain. Prog. Neurobiol. 1994;44:377–96. doi: 10.1016/0301-0082(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 107.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters.EAATs and VGLUTs. . Brain Res. Brain Res. Rev. 2004;45:250–65. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Kugler P, Schmitt A. Glutamate transporter EAAC1 is expressed in neurons and glial cells in the rat nervous system. Glia. 1999;27:129–42. doi: 10.1002/(sici)1098-1136(199908)27:2<129::aid-glia3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 109.Danbolt NC. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 110.Ryu JH, Lee PB, Kim JH, Do SH, Kim CS. Effects of pregabalin on the activity of glutamate transporter type 3. Br. J. Anaesth. 2012;109:234–9. doi: 10.1093/bja/aes120. [DOI] [PubMed] [Google Scholar]
- 111.Davis KE, Straff DJ, Weinstein EA. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J. Neurosci. 1998;18:2475–85. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krizman-Genda E, Gonzalez MI, Zelenaia O, Robinson MB. Evidence that Akt mediates platelet-derived growth factor-dependent increases in activity and surface expression of the neuronal glutamate transporter, EAAC1. Neuropharmacology. 2005;49:872–82. doi: 10.1016/j.neuropharm.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 113.Soares AC, Leite R, Tatsuo MA, Duarte ID. Activation of ATP-sensitive K+ channels: mechanism of peripheral anti- nociceptive action of the nitric oxide donor sodium nitroprusside. Eur. J. Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 114.Kweon TD, Kim JY, Kwon IW, Choi JB, Lee YW. Participation of K(ATP) Channels in the Antinociceptive Effect of Pregabalin in Rat Formalin Test. Korean J. Pain. 2011;24:131–6. doi: 10.3344/kjp.2011.24.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang CW, Huang CC, Wu SN. The opening effect of pregabalin on ATP-sensitive potassium channels in differentiated hippocampal neuron-derived H19-7 cells. Epilepsia. 2006;47:720–6. doi: 10.1111/j.1528-1167.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 116.McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electro-physiological properties of cultured DRG neurons from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dooley DJ, Donovan CM, Pugsley TA. Stimulus-dependent modulation of [3H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J. Pharmacol. Exp. Ther. 2000;295:1086–1093. [PubMed] [Google Scholar]
- 118.Martin DJ, McClelland D, Herd MB, Sutton KG, Hall MD, Lee K. Pinnock, R.D. Scott RH. Gabapentin-mediated inhibition of voltage-activated Ca2+ channel currents in cultured sensory neurones is dependent on culture conditions and channel sub-unit expression. Neuropharmacology. 2002;42:353–66. doi: 10.1016/s0028-3908(01)00181-2. [DOI] [PubMed] [Google Scholar]
- 119.Lanneau C, Green A, Hirst WD, Wise A, Brown JT, Donnier E, Charles KJ, Wood M, Davies CH, Pangalos MN. Gabapentin is not a GABAB receptor agonist. Neuropharmacology. 2001;41:965–75. doi: 10.1016/s0028-3908(01)00140-x. [DOI] [PubMed] [Google Scholar]
- 120.Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2-delta (alpha2-delta) subunit as a target for antiepileptic drug discovery. Epilepsy Res. 2007;73:137–150. doi: 10.1016/j.eplepsyres.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 121.Eutamene H, Coelho AM, Theodorou V, Toulouse M, Chovet M, Doherty A, Fioramonti J, Bueno L. Antinociceptive effect of pregabalin in septic shock-induced rectal hypersensitivity in rats. J. Pharmacol. Exp. Ther. 2000;295:162–7. [PubMed] [Google Scholar]
- 122.Su TZ, Feng MR, Weber ML. Mediation of highly concentrative uptake of pregabalin by L-type amino acid transport in Chinese hamster ovary and Caco-2 cells. J. Pharmacol. Exp. Ther. 2005;313:1406–1415. doi: 10.1124/jpet.104.082255. [DOI] [PubMed] [Google Scholar]
- 123.Taylor CP, Vartanian MG, Andruszkiewicz R, Silverman RB. 3-alkyl GABA and 3-alkylglutamic acid analogues: two new classes of anticonvulsant agents. Epilepsy Res. 1992;11:103–110. doi: 10.1016/0920-1211(92)90044-t. [DOI] [PubMed] [Google Scholar]
- 124.Bialer M, Johannessen SI, Kupferberg HJ. Progress report on new antiepileptic drugs: a summary of the Seventh Eilat Conference (EILAT VII). Epilepsy Res. 2004;61:1–48. doi: 10.1016/j.eplepsyres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 125.Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–41. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 126.Ha KY, Kim YH, Rhyu KW, Kwon SE. Pregabalin as a neuroprotector after spinal cord injury in rats. Eur. Spine J. 2008;17:864–72. doi: 10.1007/s00586-008-0653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Park S, Ahn ES, Han DW, Lee JH, Min KT, Kim H, Hong YW. Pregabalin and gabapentin inhibit substance P-induced NF-kappaB activation in neuroblastoma and glioma cells. J. Cell Biochem. 2008;105:414–23. doi: 10.1002/jcb.21837. [DOI] [PubMed] [Google Scholar]
- 128.Muthuraman A, Singh N, Jaggi AS. Protective effect of Acorus calamus L in rat model of vincristine induced painful neuropathy: an evidence of anti-inflammatory and anti-oxidative activity. Food Chem. Toxicol. 2011;49:2557–63. doi: 10.1016/j.fct.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 129.Muthuraman A, Singh N. Attenuating effect of Acorus calamus extract in chronic constriction injury induced neuropathic pain in rats: an evidence of anti-oxidative anti-inflammatory neuro- protective and calcium inhibitory effects. BMC Complement. Altern. Med. 2011;11:24. doi: 10.1186/1472-6882-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takeuchi Y, Takasu K, Ono H, Tanabe M. Pregabalin S-(+)-3-isobutylgaba activates the descending noradrenergic system to alleviate neuropathic pain in the mouse partial sciatic nerve ligation model. Neuropharmacology. 2007;53:842–53. doi: 10.1016/j.neuropharm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 131.You HJ, Lei J, Arendt-Nielsen L. Selective inhibitory effects of pregabalin on peripheral C but not A-delta fibers mediated nociception in intact and spinalized rats. Neuroscience. 2009;164:1845–1853. doi: 10.1016/j.neuroscience.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 132.Martinez JA, Kasamatsu M, Rosales-Hernandez A, Hanson LR, Frey WH, Toth CC. Comparison of central versus peripheral delivery of pregabalin in neuropathic pain states. Mol. Pain. 2012;8:3. doi: 10.1186/1744-8069-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]