Abstract
Objective:
To compare the therapeutic cure rate and adverse reactions in the regimens of the Revised National Tuberculosis Control Program (RNTCP) with directly observed treatment, short-course (DOTS) and without DOTS.
Materials and Methods:
Fifty patients in the DOTS regimen and 50 patients in the non-DOTS regimen were enrolled in the study. All the participants were asked to come regularly for 3 consecutive days for sputum collection, and the sputum samples were examined for acid-fast bacilli. If tuberculosis (TB) was confirmed, the disease status was confirmed through a chest X-ray (PA view). The participants were monitored for adverse events arising from the use of anti-TB drugs for the next 6 months.
Results:
The TB cure rates for RNTCP with DOTS and RNTCP with non-DOTS were 80% and 66%, respectively. The DOTS therapy had a better cure rate for radiologically positive, sputum-positive cases compared with the non-DOTS regimen group. The non-DOTS treatment regimen had significantly increased numbers of adverse events in the hepatic and hematinic systems.
Conclusion:
The DOTS regimen has higher cure rates and a lower incidence of adverse reactions compared with the non-DOTS regimen.
Keywords: Adverse drug reaction, directly observed treatment, short-course, Revised National Tuberculosis Control Program, tuberculosis
INTRODUCTION
Tuberculosis (TB) remains one of the major health problems in our country, and it kills more adults than any other infectious disease. In India about 1.8 million new cases of TB are detected every year, of which one-fifth are extra-pulmonary TB cases.[1,2] TB is treated using the directly observed treatment short-course (DOTS) and Revised National Tuberculosis Control Program (RNTCP).
India has a long history of research and demonstration projects related to TB. Unfortunately, despite the existence of the National Tuberculosis Control Program since 1962, the desired results had not been achieved. In 1982, the RNTCP reviewed the National Tuberculosis Control Program and concluded that it suffered from managerial weakness, inadequate funding, an over-reliance on X-rays, non-standard treatment regimens, low rates of completion of treatment and a lack of systematic information on treatment outcome.[3,4] Following the recommendations of an expect committee, a revised strategy to control TB was tested in 1993, and the RNTCP was started in 1997, and geographic coverage of more than 97% was achieved by the end of 2005.[5] The WHO-recommended treatment strategy for detection and cure of TB is DOTS, which is the most effective strategy available for controlling the TB epidemic today.[6] However, there are no studies available comparing the therapeutic cure rate and adverse reactions in the RNTCP treatment with and without DOTS regimens. Hence, the present study was undertaken to compare the therapeutic cure rate and adverse reactions in RNTCP treatments with the DOTS regimen and without it.
MATERIALS AND METHODS
The study design was aimed at comparing the outcomes (therapeutic cure rate and adverse reactions) in patients with pulmonary TB on the DOTS regimen versus patients with pulmonary TB on the non-DOTS regimen. The study was carried out on patients afflicted with TB at the Department of TB and Chest Diseases, Raja Muthiah Medical College Hospital, Chidambaram. The study was carried out over 8 months between June 2003 and February 2006. The study design and protocol and the informed consent form were approved by the Institute Ethics Committee (Human) of Raja Muthiah Medical College. Written informed consent was obtained from each participant, and the study was conducted according to the recommendations of the Declaration of Helsinki.
The RNTCP's, DOTS regimen has a choice of three different categories of treatment: Category-I (2H3R3Z3E3 + 4H3R3), Category-II (2H3R3Z3 + 4H3R3) and Category-III (2H3R3Z3E3S3 + 1H3R3Z3E3 + 5H3R3E3). The non-DOTS regimen provides conventional chemotherapy (2SHT + 10HT/2SH + 1H2S2/18HT/12HT) or short-course chemotherapy (2RHSZ + 4HR/2RHSZ + 4H2S2/2RHZ + 4HR/2RHSZ + 4S2H2Z2/2RHSZ + 6TH/6R3H3Z3S3).[7,8]
Sixty patients affected with pulmonary TB and receiving the RNTCP's DOTS regimen (group-I) and 65 patients with pulmonary TB receiving the RNTCP's non-DOTS regimen (group-II) were interviewed. Fifty participants from each group who met the inclusion criteria were enrolled in the study. Adult patients who were spectrum-positive for acid-fast bacilli (AFB) were included in the study. Patients with diabetes mellitus, pregnant women, children below 11 years of age, patients infected with HIV, patients with extra-pulmonary TB, and patients with bronchial asthma associated with TB were excluded from the study. All the patients of group-I and group-II were asked to come regularly for 3 consecutive days for sputum collection. The sputum samples were examined for AFB, and TB was confirmed using the Ziehl-Nelsen staining technique. Chest X-rays (PA view) were taken, and infiltration of the lung fields with AFB was confirmed. After enrolment, the patients were monitored continually for adverse events related to anti-TB drugs over the next 6 months. Gastrointestinal toxicity (nausea, vomiting, and diarrhea), hepatic toxicity (estimation of SGOT, SGPT, alkaline phosphatase, bilirubin, and albumin-globulin ratio), hematological parameters (estimation of hemoglobin level, total count, differential count, and erythrocyte sedimentation rate), dermatological reactions (pruritus, rashes, and exfoliative dermatitis), optic neuritis, ototoxicity and renal toxicity (estimation of levels of blood urea, serum creatinine, uric acid, and electrolytes) were monitored during the study period. At the end of the study, the therapeutic cure rate and adverse drug reactions in group-I and group-II were analyzed. Causality and severity assessments were carried out according to the WHO probability scale and Hartwig's severity assessment scale, respectively.[9,10]
Statistical analysis
The values were expressed as actual numbers and the corresponding percentages. The significant differences between groups were determined using the Chi-square test. A P value less than 0.05 was considered to be significant.
RESULTS
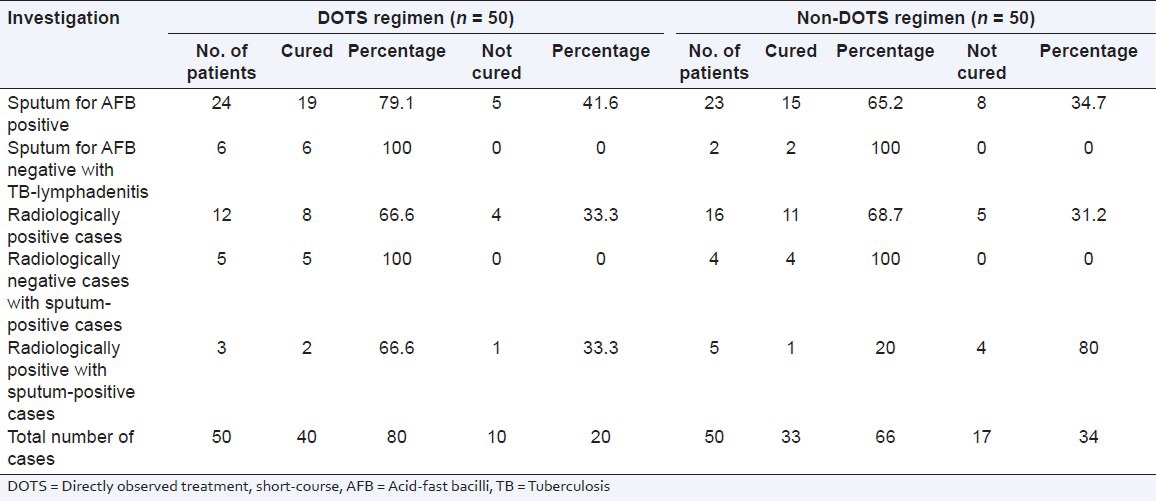
A total of 100 patients were randomly selected to compare the DOTS and non-DOTS regimens of RNTCP. Subjects were enrolled on the basis of their treatment. The TB cure rates for group-1 and group-II were 80% and 66%, respectively. The body mass index of subjects in group-I and group-II were 19.91 ± 0.58 and 20.31 ± 0.43 kg/m2 respectively. The age distribution of the patients and the TB cure rates are presented in Tables 1 and 2. The TB cure rate was assessed using the TB skin test, Mantoux test and sputum culture test (with the sputum samples being collected early in the morning). In group-1, a total of 8 patients showed adverse drug reactions, whereas in group-II a total of 16 cases showed adverse drug reactions [Table 3]. The DOTS regimen had a better cure rate with radiologically positive, sputum-positive cases compared to non-DOTS regimen group [Table 4].
Table 1.
Distribution of subjects
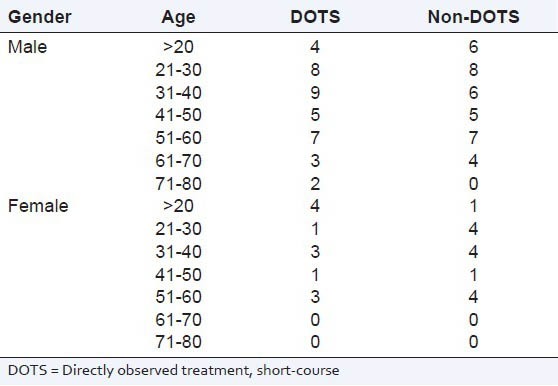
Table 2.
Therapeutic cure rates
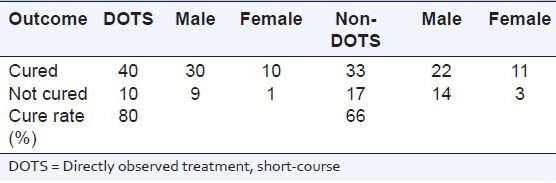
Table 3.
Indications of adverse drug reactions
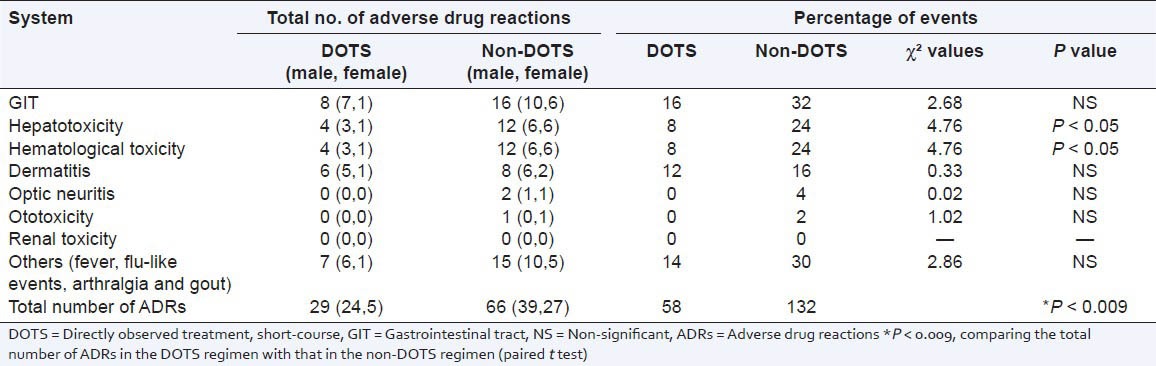
Table 4.
Therapeutic cure rates based on investigation
The DOTS regimen had a smaller number (P < 0.0091) of adverse events compared to the non-DOTS regimen. The non-DOTS regimen showed significantly increased numbers of adverse events in hepatic (24%) and hematological (24%) systems. Ototoxicity and optic neuritis were not noted in the DOTS regimen while a minor percentage of patients suffered from optic neuritis (4%) and ototoxicity (2%) in the non-DOTS regimen. Renal toxicity was not observed in either the DOTS group or the non-DOTS group.
Causality assessment was carried out according to the WHO probability scale. In the DOTS regimen group, the majority of reactions (11, 37.93%) were found to be “probable;” 8 were “unlikely” (27.69%), 7 were “possible” (24.14%), and 3 were “certain” (10.34%). In the non-DOTS regimen group, 24 events (36.36%) were “possible;” 20 were “probable” (30.30%), 16 were “unlikely” (24.24%), 3 were “certain” (4.55%), and 3 were “unassessable” (4.55%).
Severity assessment was carried out according to the Hartwig's severity assessment scale. In DOTS regimen group, the majority of reactions (20, 68.97%) were found to be “mild” and 9 were “moderate” (31.03%). In the non-DOTS regimen group, 46 event (69.70%) were “mild,” and 20 were moderate (30.30%).
DISCUSSION
So far there have been no reports comparing the two categories of the RNTCP treatment regimen. Our results show that both drug regimens have a cure rate greater than 50% cure rate, but that the RNTCP DOTS regimen has a cure rate better than about 80%. The occurrence of adverse drug reactions is almost equal in male and female patients. The same was observed with DOTS therapy at the Regional Tuberculosis Center, Western Nepal.[11] There were more adverse events with the non-DOTS regime. This may have been due to the drug combinations and frequency of drug administration. The better cure rates and lower incidence of adverse effects in the DOTS regimen could be due to a combination of potent first-line drugs in therapeutic and sub-toxic doses. Gastrointestinal toxicity was observed commonly in the DOTS and non-DOTS regimens, and this may be due to first-line anti-TB agents. The hepatotoxicities caused by first-line anti-TB agents are well known. In 2012, Shinde et al. reported 12.65% of gastrointestinal upset cases and 6.27% of hepatotoxicity cases were caused by first-line anti-TB agents.[12] In the present study, we observed that the percentage of gastrointestinal events was 16% and that of hepatotoxicity was 8% in DOTS therapy, whereas in non-DOTS therapy the percentage of gastrointestinal events was 32% and that of hepatoxicity was 24%. Khalili et al. reported that adverse drug reactions including hepatotoxicity can be one of the main reasons for poor adherence and it will interruption and change in the treatment.[13] We observed adverse drug reactions 5 weeks after the initiation of therapy, which is in agreement with previous studies.[13,14] The non-DOTS regimen had twice the number of adverse events compared with the DOTS regimen. This may be due to the combinations of the drugs in the therapy.
CONCLUSION
In conclusion, the DOTS regimen is far superior in terms of a higher cure rats and a lower incidence of adverse reactions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.TB's History. [Accessed 2012 Dec 26]. Available from: http://www.library.thinkquest.org/C0126375/tb_in_the_world.htm .
- 2.Krishnamoorthy S, Gopalakrishnan G. Surgical management of renal tuberculosis. Indian J Urol. 2008;24:369–75. doi: 10.4103/0970-1591.42620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma R, Khanna P, Mehta B. Revised national tuberculosis control program in India: The need to strengthen. Int J Prev Med. 2013;4:1–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Khatri GR. The revised national tuberculosis control programme: A status report on first 1,00,000 patients. Indian J Tuberc. 1999;46:157–66. [Google Scholar]
- 5.About RNTCP. [Accessed 2012 Dec 26]. Available from: http://www.tbcindia.nic.in/RNTCP.html .
- 6.Pandit S, Dey A, Chaudhuri AD, Saha M, Sengupta A, Kundu S, et al. Five-years experiences of the Revised National Tuberculosis Control Programme in northern part of Kolkata, India. Lung India. 2009;26:109–13. doi: 10.4103/0970-2113.56343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unit 5, part 1: The DOTS strategy for controlling TB. [Last accessed 2012 Dec 25]. Available from: http://www.who.int/tb/publications/manual_for_participants_pp51_98.pdf .
- 8.Dhingra VK, Rajpal S, Aggarwal N, Aggarwal JK. Treatment of tuberculous pleural effusion patients and their satisfaction with DOTS: 1½ year follow up. Ind J Tuberc. 2004;51:209–12. [Google Scholar]
- 9.The use of the WHO-UMC system for standardised case causality assessment. [Last accessed 2013 Mar 28]. Available from: http://www.who-umc.org/Graphics/24734.pdf .
- 10.Srinivasan R, Ramya G. Adverse drug reaction-causality assessment. Int J Res Pharm Chem. 2011;1:606–12. [Google Scholar]
- 11.Chhetri AK, Saha A, Verma SC, Palaian S, Mishra P, Shankar PR. Study of adverse drug reactions caused by first line anti-tubercular drugs used in directly observed treatment, short course (DOTS) therapy in Western Nepal, Pokhara. J Pak Med Assoc. 2008;58:531–6. [PubMed] [Google Scholar]
- 12.Shinde KM, Pore SM, Bapat TR. Adverse reactions to first-line anti-tuberculous agents in hospitalised patients: Pattern, causality, severity and risk factors. Ind J Med Spec. 2013;4:16–21. [Google Scholar]
- 13.Khalili H, Dashti-Khavidaki S, Rasoolinejad M, Rezaie L, Etminani M. Anti-tuberculosis drugs related hepatotoxicity: Incidence, risk factors, pattern of changes in liver enzymes and outcome. DARU J Pharm Sci. 2009;17:163–7. [Google Scholar]
- 14.Tak DK, Acharya LD, Gowrinath K, Rao Padma GM, Subish P. Safety evaluation of antitubercular therapy under Revised National Tuberculosis Control Programme in India. J Clin Diagn Res. 2009;3:1395–401. [Google Scholar]


