Abstract
Objective:
The aim of this in vitro study was to evaluate and compare the effect of 10% sodium ascorbate, 6.5% proanthocyanidin, and 5% lycopene on the bond strength of composite resin to bleached enamel.
Materials and Methods:
Labial enamel surfaces of 100 extracted human maxillary central incisors were used in this study. Twenty teeth served as group I (control) and received no bleaching treatment. The remaining 80 teeth were randomly divided into four groups of 20 teeth each, based on the antioxidant used as follows: group II- bleaching with 35% carbamide peroxide gel for 30 min without the use of an antioxidant, group III- bleaching followed by use of 10% sodium ascorbate solution, group IV- bleaching followed by use of 6.5% proanthocyanidin, and group V- bleaching followed by use of 5% lycopene. These groups were further subdivided into two subgroups of 10 teeth each, based on whether composite buildup was done immediately (subgroup A) or after a delay of 2 weeks (subgroup B) post bleaching. Shear bond strength of the specimens was tested under universal testing machine. The data were tabulated and statistically analyzed.
Results:
Significantly higher shear bond strength values were observed in teeth treated with control group prior to bonding, followed by sodium ascorbate group.
Conclusion:
Within the limitations of this study, it can be concluded all the antioxidants used in this study increased the bond strength of bleached enamel. Among the antioxidant groups, sodium ascorbate showed significantly higher bond strength compared to proanthocyanidin and lycopene.
Keywords: Antioxidant, bleaching, lycopene, proanthocyanidin, shear bond strength, sodium ascorbate
INTRODUCTION
Increase in the demand for esthetic dentistry has resulted in widespread practice of vital bleaching.[1,2] Vital bleaching is considered a safe, popular, conservative, well-accepted treatment modality for discolored teeth.[3] Bleaching agents in varying concentrations have been used to achieve rapid esthetic results. Hydrogen peroxide and carbamide peroxide have been used successfully for many years to achieve lighter and more desirable tooth color.[1,4,5]
Complications of bleaching may vary from postoperative sensitivity to pulpal irritation to tooth structure alterations or microleakage of existing restorations. Another important complication following bleaching procedure is decreased bond strength of composite resin to enamel.[6] This decreased bond strength is due to the presence of oxygen ions which interfere with resin polymerization.[7,8] This decrease in bond strength of composite resin to enamel can be improved by delaying its placement after 1-3 weeks following the bleaching procedure. Several other techniques have also been proposed to remove the oxygen radical from the surface enamel.
Barghi and Godwin treated bleached enamel with alcohol before restoration. Cvitko et al. proposed removal of the superficial layer of enamel, while Kalili et al. and Sung et al. suggested the use of adhesives containing organic solvent.[1,6,9] Lai et al. and Vidhya et al. suggested that reduced bond strength can be reversed by the use of antioxidants such as sodium ascorbate and proanthocyanidin.[1,2,6] Lai et al. have shown that following treatment with hydrogen peroxide or sodium hypochlorite, reversal of reduction in bond strength of resin to enamel was achieved within 1 h with the use of sodium ascorbate as an antioxidant. Turkun and Kaya shortened the application period of sodium ascorbate to 10 min, and demonstrated that even 10 min application was enough for reversing the reduced bond strength. Therefore, restorative procedures are possible immediately after bleaching, with the use of antioxidants, which shortens the overall time needed for esthetic procedures.[1,2,3,4,5,6]
Proanthocyanidin (OPC), a grape seed extract, is a natural antioxidant that has greater potential to scavenge oxygen free radicals.[1,10,11] Its antioxidant potency has been shown to be 20 times more than that of sodium ascorbate.[1] Another natural antioxidant, lycopene, a carotenoid found in tomato extract, is known to have free radical scavenging ability.[12,13] But the effect of lycopene on bleached enamel has not been investigated so far. Comparative evaluation of these antioxidants, namely, sodium ascorbate, proanthocyanidin, and lycopene, is yet to be done. Hence, the aim of this in vitro study was to evaluate and compare the effect of 10% sodium ascorbate, 6.5% proanthocyanidin, and 5% lycopene on the bond strength of composite resin to bleached enamel.
MATERIALS AND METHODS
Preparation of the antioxidant solution
Ten grams of sodium ascorbate in the form of powder was dissolved in 100 ml of distilled water to obtain 10% sodium ascorbate. Also, 6.5 g of grape seed extract in the form of powder was collected from capsule and dissolved in 100 ml of distilled water to obtain 6.5% proanthocyanidin solution. Five grams of tomato extract in the form of powder was dissolved in 100 ml of distilled water to make 5% lycopene solution.
Specimen preparation
One hundred human, single-rooted, caries-free, maxillary central incisor teeth, extracted for periodontal reasons, were taken for the study. Exclusion criteria include fractured, cracked, and dried teeth. The roots were embedded in self-cure acrylic resin block till cemento-enamel junction, keeping only the coronal portion exposed. Labial surfaces were flattened with 600-grit silicon carbide paper.
Twenty teeth served as the control group and did not receive any bleaching procedure. The remaining 80 specimens were bleached with Opalescence (35% carbamide peroxide gel, Ultradent, inc, 505 west 10200 south, south Jordan, UT84095) for 30 min, according to manufacturer's instructions. The bleaching gel was completely rinsed off with water. Then, the specimens were randomly divided into four groups of 20 teeth each, depending on the type of antioxidant used [Table 1].
Table 1.
The distribution of specimens and study groups
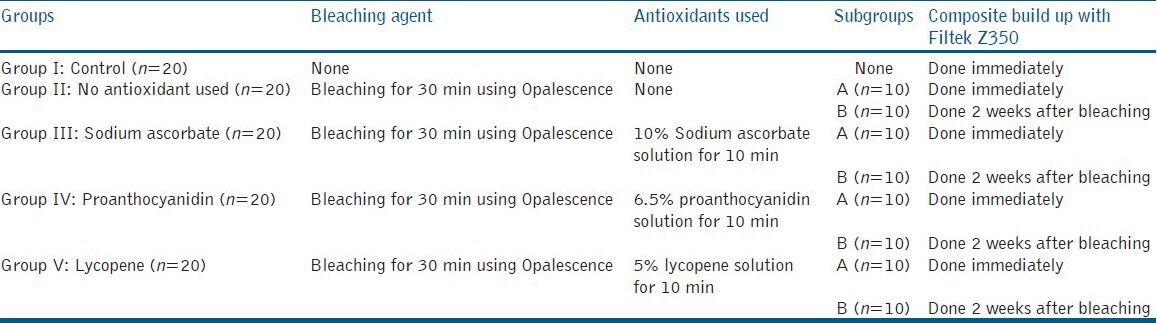
Group I (control group): No bleaching. The samples were etched with 37% phosphoric acid for 15 sec, rinsed with water for 20 sec, and bonded, followed by composite build-up (3 mm diameter and 5mm height using Teflon mold).
Group II: Bleaching only (no antioxidants)
Group III: Bleaching followed by treatment with 10% sodium ascorbate for 10 min
Group IV: Bleaching followed by treatment with 6.5% proanthocyanidin for 10 min
Group V: Bleaching followed by treatment with 5% lycopene for 10 min
The groups II-IV were further subdivided into subgroup A (immediate) and subgroup B (delay of 2 weeks), based on the storage period prior to composite build-up as performed for group I.
Testing procedure
Each specimen was loaded in universal testing machine for shear bond strength testing. The long axis of the specimen was perpendicular to the direction of the applied forces. The knife edge was loaded at the interface between the composite and dentin surface. The shear bond strength was measured in shear mode at a crosshead speed of 0.5 mm/min until fracture occurred. The statistical methods used in this study is one way ANOVA and Turkeys kramer multiple comparison test
RESULTS
The results are presented in Table 2 and Figure 1.
Table 2.
Comparison of mean bond strengths of different study groups
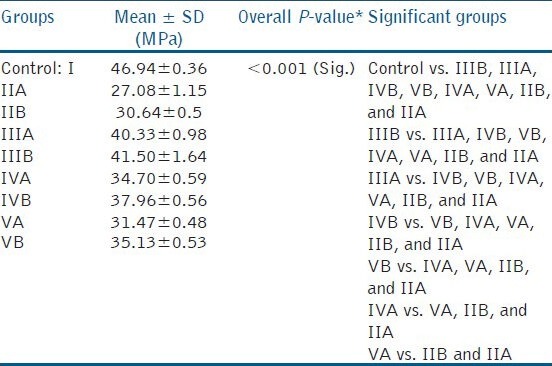
Figure 1.
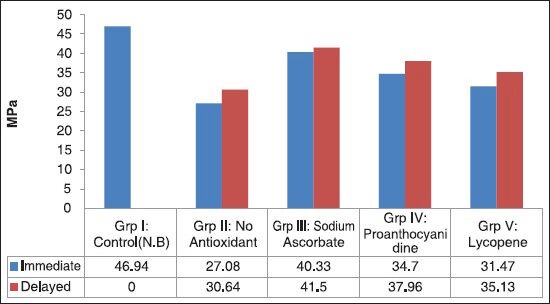
Bar diagram showing the comparison of mean shear bond strength (Mpa) among the groups
Mean shear bond strength of the control group (46.94 ± 0.36) was significantly higher than all other groups (P < 0.001).
Group IIA (no antioxidant used, immediate bonding) had least shear bond strength value (27.08 ± 1.15) of all the groups.
Among the experimental groups, group IIIB (5% sodium ascorbate with delayed composite restoration) showed significantly higher shear bond strength.
DISCUSSION
Tooth discoloration has always been a factor of utmost concern as more emphasis is being placed on esthetics. With the growing awareness of esthetic options, there is a great demand for various modalities in treating discolored teeth. Tooth bleaching has been an option since late 1870s.[14] It permits a successful esthetic outcome at minimal expense while conserving the tooth structure.
Carbamide peroxide, a biological oxidant, is still the most commonly used bleaching agent which releases free radicals in the form of nascent oxygen and hydroxyl or perhydroxyl ions, when applied to the dental structure. Free radical is any molecule that has one unpaired electron, providing its high reactivity that actualizes the bleaching process by oxidizing the macromolecules of stains quickly and breaks them into linear fragments, consequently diffusing across dental hard tissues.[2]
In 1994, Dishman and colleagues reported that a high concentration of oxygen remains among the enamel prisms and in the dentin following dissociation of the bleaching agent.[8] The dentin and dentinal fluid can act as peroxide and oxygen free radical reservoir and could persist until removed by pulpal microcirculation or be released later through surface diffusion (Titley, et al).[7] In this perspective, this property could be deleterious during bonding of the composite resin, as higher levels of peroxide or oxygen may be present in the bonding surface, inhibiting the polymerization and, thus, reducing the bond strength.
Since these oxygen products will persist until removed by pulpal microcirculation, clinicians must allow some time to elapse between the bleaching procedure and restorative treatment to achieve desirable bond strength. Nevertheless, there appears to be no consensus regarding the necessary waiting period, though studies suggest that elimination of residual peroxide degradation product is time dependant.[2] Optical microscopic studies have shown that resin tags in bleached enamel were short, sparse, poorly defined, structurally incomplete, or completely absent, when restored immediately (Dishman and Covey, 1994).[2]
It has been shown that during bleaching with hydrogen peroxide, hydroxyl radicals in the apatite lattice are substituted by peroxide ions, resulting in the formation of peroxide apatite. After 2 weeks storage period, the peroxide ions decompose and the substituted hydroxyl radicals re-enter the apatite lattice, resulting in the elimination of the structural changes caused by incorporation of the peroxide ions.[1,8,15] Hence, delay in bonding by 1-3 weeks following the bleaching procedure was recommended.[16] But this rendered immediate reestablishment of further esthetic procedures impossible.[1,6]
Lai et al. stated that inclusion process of peroxide ions could be reversed by the use of antioxidants.[1,6,15] Sodium ascorbate is a derivate of ascorbic acid with neutral pH. It is a potent antioxidant capable of quenching reactive free radicals in biological systems.[17,18] Since vitamin C and its salts are non-toxic and widely used in food industry as antioxidants, it can be used on the dental hard tissues without creating any adverse biological effect or clinical hazards.[2] The application of 10% sodium ascorbate was effective in reversing the compromised bonding to the oxidized enamel and dentin.[1,6,17] Hence, 10% sodium ascorbate was used in this study.
Proanthocyanidins are high-molecular-weight polymers comprising the monomeric flavan-3-ol (+)-catechin and (−)-epicathechins. Proanthocyanidins are a group of polyphenolic bioflavonoids found in high concentrations in natural sources such as grape seed extract, pine bark extract, cranberries, lemon tree bark, and hazelnut tree leaves. Grape seed extract was chosen for this study since it yields a 10% higher concentration of proanthocyanidins, with a greater degree of oxygen free radical scavenging potential. In vitro studies have confirmed that OPCs are 50 times more effective than vitamin E and 20 times more powerful than vitamin C.[1]
Lycopene, a carotenoid compound, is a natural pigment synthesized by plants. It is a tetraterpene assembled from eight isoprene units composed entirely of carbon and hydrogen, containing 11 conjugated and 2 non-conjugated carbon — carbon double bonds (c = c). Lycopene exerts potent anti-inflammatory effects through its action as an antioxidant and free radical scavenger. The scavenging rate increased linearly with the concentration of lycopene, and reached 60-80% when the concentration was more than 0.75 mg/ml when used systemically.[19] Lycopene is naturally accumulated in ripe tomatoes, watermelons, red chillies, and guavas, giving them their characteristic red color. Tomato and tomato-based foods account for more than 85% of lycopene. Due to its potent antioxidant potential, lycopene obtained from tomato extract was taken up for the study.[13] Since it was used on the external surface of the hard tissues, a minimum concentration of 5% lycopene extract was chosen for this study.
Therefore, the present study was designed to evaluate the neutralizing effects of various antioxidants on the shear bond strength of composite resins to bleached enamel. The shear, rather than tensile mode of testing, was selected for this study, as it would more accurately reflect the type of forces generated on veneer restorations in the tooth.[20]
According to the results of this study, group I [control (no bleaching): 46.94 ± 0.36] showed the highest bond strength compared to the other groups. Bond strength was the lowest for group II (bleached and not treated with antioxidants: 27.08 ± 1.15) compared to the control and the antioxidant groups. This reduced bond strength could be attributed to the residual oxygen free radicals on the enamel surface. This liberation of oxygen could have interfered with the resin infiltration to the etched enamel and/or inhibited polymerization of the resin that cured via free radical mechanism.[21] Moreover, in 1996, Rostein et al. reported that loss of calcium, sulfate, phosphorus, and potassium occurs owing to pH of the bleaching agents. In 1999, Hedeges et al. reported that in addition to the reduction in mineral content of the bleached enamel, such losses lead to structural alterations in the enamel, reducing the bond strength of the composite resin to the tooth.[8] A similar result supporting this claim has been obtained in this study.
In group IIB (delayed, no antioxidant: 30.64 ± 0.5), the bond strength was higher compared to group IIA (immediate, no antioxidant: 27.08 ± 1.15). This could be attributed to the partial loss of oxygen diffusion layer at the tooth and composite interface.
The antioxidant groups III, IV, and V showed increased bond strengths than group II (antioxidant not used), but the values were less than in group I (control group). Group III (sodium ascorbate) showed the highest bond strength among the antioxidant treated groups; both when bonding was performed immediately (40.33 ± 0.98) as well as after 2 weeks (41.50 ± 1.64). These results concur with those of Turkun et al. (2004) who reported that reduced bond strength is reversed by use of 10% sodium ascorbate solution.[1,6] The higher bond strength could be because of their potent antioxidant capacity by quenching free radicals in biological systems.[7] Sodium ascorbate, being a reducing agent, is capable of donating two high-energy electrons to scavenge the free radicals by the mechanism called passive detoxification. The results of this study are in accordance with previous investigations done by Lai et al.
Although the surface treatment with group IV (proanthocyanidin) showed increased bond strength (37.96 ± 0.56) compared to group V (lycopene) (35.13 ± 0.53), it had lesser bond strength than group III (sodium ascorbate), which is contrary to the result of the study done by Vidhya et al.
Molecular weight of the materials also seems to have played a role. Hydrogen peroxide, being a low-molecular-weight substance, permeates into the dental hard tissues and breaks down into free radicals,[1,6] so surface treatment done with any antioxidant to remove these free radicals should have low molecular weight for efficient scavenging action. According to Lipinski's rule, molecular weight of the drug should be less than 500g/mol for its bioavailability.[22] Proanthocyanidin belongs to the category known as condensed tannins. Tannins have a molecular weight of 500-3000 and are highly hydroxylated structures that can form insoluble complexes with proteins and carbohydrates.[23] Sodium ascorbate has a molecular weight of 198.11 g/mol[1] and is water soluble.[24] This could have enabled sodium ascorbate to penetrate better than proanthocyanidin. Probably, this may be the reason for better bond strength with sodium ascorbate compared to proanthocyanidin. Although the molecular weight of lycopene (536.87 g/mol[1])[25] is less than proanthocyanidin, its bond strength is comparatively low since it is insoluble in water. On the other hand, proanthocyanidin has the specificity for hydroxyl free radicals and it possesses multiple donor sites, which enables it to trap superoxide radicals.[1]
Though the bond strength values obtained for group V (lycopene) were the least among the antioxidant groups, its values were not statistically significant from group IV (proanthocyanidin). Lycopene, a carotenoid compound, is most likely involved in the scavenging of two reactive oxygen species, namely, singlet oxygen and peroxide radicals. Further, they are effective deactivators of electronically excited sensitizer molecules which are involved in the generation of radicals and singlet oxygen.
Singlet oxygen quenching by carotenoid occurs via physical quenching (i.e. the carotenoid remains intact and can undergo further cycles of singlet oxygen quenching).[26] This supports the results obtained in this study. However, bond strength with immediate and delayed surface treatments in all the groups was not statistically significant.
CONCLUSION
Within the limitations of the study, sodium ascorbate has proven to be superior compared to proanthocyanidin and lycopene when used as an antioxidant in reversing the bond strength of composite resin to bleached enamel.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Vidhya S, Srinivasulu S, Sujatha M, Mahalaxmi S. Effect of grape seed extract on the bond strength of bleached enamel. Oper Dent. 2011;36:433–8. doi: 10.2341/10-228-L. [DOI] [PubMed] [Google Scholar]
- 2.Carlos RG, Kand FA, Alessandra BB. The effects of antioxidant agents as neutralizers of bleaching agents on enamel bond strength. Braz J Oral Sci. 2006;5:971–6. [Google Scholar]
- 3.Kimyai S, Oskoee SS, Rafighi A, Valizadeh H, Ajami AA, Helali ZN. Comparison of the effect of hydrogel and solution forms of sodium ascorbate on orthodontic bracket enamel shear bond strength immediately after bleaching: An in vitro study. Indian J Dent Res. 2010;21:54–8. doi: 10.4103/0970-9290.62818. [DOI] [PubMed] [Google Scholar]
- 4.Bulut H, Kaya AD, Turkun M. Tensile bond strength of brackets after antioxidant treatment on bleached teeth. Eur J Orthod. 2005;27:466–71. doi: 10.1093/ejo/cji044. [DOI] [PubMed] [Google Scholar]
- 5.Türkün M, Celik EU, Kaya AD, Arici M. Can the hydrogel form of sodium ascorbate be used to reverse compromised bond strength after bleaching? J Adhes Dent. 2009;11:35–40. [PubMed] [Google Scholar]
- 6.Gökçe B, Cömlekoğlu ME, Ozpinar B, Türkün M, Kaya AD. Effect of antioxidant treatment on bond strength of a luting resin to bleached enamel. J Deny. 2008;36:780–5. doi: 10.1016/j.jdent.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Titley KC, Torneck CD, Smith DC, Chernecky R, Adibfar A. Scanning electron microscopy observations on the penetration and structure of resin tags in bleached and unbleached bovine enamel. J Endod. 1991;17:72–5. doi: 10.1016/S0099-2399(06)81611-0. [DOI] [PubMed] [Google Scholar]
- 8.Bittencourt ME, Trentin MS, Linden MS, de Oliveira Lima Arsati YB, França FM, Flório FM, et al. Influence of in situ post bleaching times on shear bond strength of resin based composite restoration. J Am Dent Assoc. 2010;41:300–6. doi: 10.14219/jada.archive.2010.0164. [DOI] [PubMed] [Google Scholar]
- 9.Barghi N, Godwin JM. Reducing the adverse effect of bleaching on composite enamel bond. J Esthet Dent. 1994;6:157–61. doi: 10.1111/j.1708-8240.1994.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 10.Fine AM. Oligomeric Proanthocyanidin Complexes: History, Structure, and Phytopharmaceutical applications. Altern Med Rev. 2010;5:144–51. [PubMed] [Google Scholar]
- 11.Kim SY, Jeong SM, Park WP, Nam KC, Ahn DU, Lee SC. Effect of heating conditions of grape seeds on the antioxidant activity of grape seed extracts. Food Chem. 2006;97:472–9. [Google Scholar]
- 12.Pennathur S, Maitra D, Byun J, Sliskovic I, Abdulhamid I, Saed GM, et al. Potent antioxidative activity of lycopene: A potential role in scavenging Hypochlorous acid. Free Radic Bio Med. 2010;49:205–13. doi: 10.1016/j.freeradbiomed.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R, Dan H, Wu R, Meng W, Liu N, Jin X, et al. Lycopene: Features and potential significance in the oral cancer and precancerous lesions. J Oral Pathol Med. 2011;40:361–8. doi: 10.1111/j.1600-0714.2010.00991.x. [DOI] [PubMed] [Google Scholar]
- 14.Sharafeddin F, Jamalipour G. Effect of 35% carbamide peroxide gel on surface roughness and hardness of composite resins. J Dent (Tehran) 2010;7:6–12. [PMC free article] [PubMed] [Google Scholar]
- 15.Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SH, et al. Reversal of compromised bonding in bleached enamel. J Dent Res. 2002;81:477–81. doi: 10.1177/154405910208100709. [DOI] [PubMed] [Google Scholar]
- 16.Thapa A, Vivekananda PA, Thomas MS. Evaluation and comparison of bond strength to 10% carbamide peroxide bleached enamel following the application of 10% and 25% sodium ascorbate and alfa tocopherol solution: An in vitro study. J Conserv Dent. 2013;16:111–5. doi: 10.4103/0972-0707.108184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul P, Hannah Rosaline H, Balagopal S. The effect of hydrogel and solution of sodium ascorbate on the bond strength of bleached enamel. J Conserv Dent. 2007;10:43–7. [Google Scholar]
- 18.Dabas D, Patil AC, Uppin VM. Evaluation of the effect of concentration and duration of application of sodium ascorbate hydrogel on bond strength of composite resin to bleached enamel. J Conserv Dent. 2011;14:356–60. doi: 10.4103/0972-0707.87197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu W, Zhao Y, Xue Z, Jin H, Wang D. The antioxidant properties of lycopene concentrate extracted from tomato paste. J Am Oil Chem Soc. 2001;78:697–701. [Google Scholar]
- 20.Titley KC, Torneck CD, Ruse ND. Effect of carbamide peroxide gel on the shear bond strength of a microfill resin to bovine enamel. J Dent Res. 1992;71:20–4. doi: 10.1177/00220345920710010301. [DOI] [PubMed] [Google Scholar]
- 21.Kaya AD, Turkun M. Reversal of dentin bonding to bleached enamel. Oper Dent. 2003;28:825–9. [PubMed] [Google Scholar]
- 22.Chikhi A, Bensegueni A. In silico study of the selective inhibition of bacterial peptide deformylases by several drugs. J Proteomics Bioinform. 2010;3:61–5. [Google Scholar]
- 23.Porter WM, Hemingway LJ. Molecular weight profile of proanthocyanidin polymer. Phytochemistry. 1983;22:569–72. [Google Scholar]
- 24.Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Dekant W, et al. Use of lycopene as a food colour scientific opinion of the panel on food additives, flavourings, processing aids and materials in contact with food. Eur Food Saf Authority J. 2008;674:1–66. [Google Scholar]
- 25.Aguilar F, Dusemund B, Galtier P, Gilbert J, Gott DM, Grilli S, et al. Scientific Opinion on the use of sodium ascorbate as a food additive in vitamin D preparations intended to be used in formulae and weaning food for infants and young children. Eur Food Saf Authority J. 2010;8:1–13. [Google Scholar]
- 26.Stahl W, Sies H. Bioactivity and protective effects of natural carotenoids. Biochim Biophys Acta. 2005;1740:101–7. doi: 10.1016/j.bbadis.2004.12.006. [DOI] [PubMed] [Google Scholar]


