Abstract
Aim:
The aim of this study is to evaluate the enamel remineralization after treatment with three different remineralizing agents using surface microhardness assessment.
Materials and Methods:
This in vitro study involves 50 enamel samples divided into five groups of 10 samples each. The positive control group consisted of intact enamel and a negative control group consisted of demineralized enamel samples. All groups excluding the positive control group were subjected to demineralization following which three of these groups were remineralized using remineralizing agents (casein phosphopeptide amorphous calcium phosphate [CPP-ACP] [GC tooth mousse], casein phosphopeptide amorphous calcium phosphate with fluoride [CPP-ACPF] [GC tooth mousse plus], sodium fluoride [phos-flur]). The groups treated with remineralizing agents were subjected to pH cycling over a period of 28 days. This was followed with assessment of surface microhardness (Micro Vickers Hardness tester, Matsuzawa Co., Ltd, Toshima, Japan).
Statistical Analysis:
One-way analysis of variance test and posthoc Tukey test were conducted for multiple group comparison.
Results:
There was an improved enamel remineralization in the group, remineralized using CPP-ACPF in comparison with the other groups.
Conclusion:
Casein phosphopeptide with fluoride is a promising material for remineralization of enamel subsurface lesions.
Keywords: Casein phosphopeptide, pH cycling, surface microhardness
INTRODUCTION
Enamel subsurface lesions need to be diagnosed as early as possible for the best possible remineralization of enamel and restoration as well as a function of the tooth. There is evidence based on the remineralization potential of agents such as fluoride, casein phosphopeptide amorphous calcium phosphate (CPP-ACP), casein phosphopeptide amorphous calcium phosphate with fluoride (CPP-ACPF). It has been observed that CPP-ACP remineralized initial enamel lesions and has shown increased remineralization potential when used along with fluoridated toothpaste.[1] APF gel forms more fluoride in enamel than neutral gel and it is more efficient in reducing enamel demineralization of enamel blocks submitted to cariogenic challenge than the neutral one. CPP-ACPF is proven to produce significantly greater mean percentage of remineralization in comparison with CPP-ACP.[2] Mineral deposition throughout the lesion has been proven in relation to use of both CPP-ACP and CPP-ACPF.[2] It has been observed that gums comprising of CPP-ACP nano complexes have a marked remineralization potential.[3]
The CPP-ACP has also been shown to remineralize enamel subsurface lesions in situ when delivered through oral care products. The probable anticariogenic mechanism of CPP-ACP is its ability to localize ACP at the tooth surface, which brings about buffering of calcium and phosphate free ion activities, thereby helping to maintain a state of super saturation with respect to tooth enamel negating demineralization and enhancing remineralization. The CPP-ACP nanocomplexes have shown to localize at the tooth surface. CPP-ACPF due to its added fluoride content has shown improved ability to remineralize initial caries.[4,5,6] In incipient enamel caries low concentrations of fluoride are able to penetrate deeper into the body of the lesion and bring about remineralization, hence sodium fluoride (NaF) (200 ppm/0.044%) has been opted for use in this study.[7]
The study aimed at quantitatively evaluating the enamel remineralizing potential of CPP-ACP, CPP-ACPF and NaF using surface microhardness analysis (Vickers hardness test).
MATERIALS AND METHODS
Infection control protocol
The teeth were cleansed of visible blood and gross debris and were maintained in a hydrated state during storage. Extracted teeth were placed in sodium hypochlorite solution diluted with saline in a ratio of 1:10 in the container with a secure lid to prevent leaking and labeled with the biohazard symbol.
Elimination of microbial growth was achieved by using an autoclave cycle for 40 min. Teeth that do not contain amalgam restorations were preferred because they can be safely autoclaved. However, Extracted teeth containing amalgam restorations, were immersed in 10% formalin solution for 2 weeks.[8]
Sample preparation
Fifty freshly extracted human molar teeth were used in this study. Enamel samples (2 mm thickness) were prepared from the buccal and lingual surfaces of the teeth selected, using a double faced diamond disc mounted on a contra-angle hand piece. Following sample preparation windows were created (dimension of 5 mm × 5 mm) using adhesive tape and the sample was made completely resistant to acid attack by coating nail varnish. (Colorama nail varnish, Maybelline).
After drying, the adhesive tape was removed from the enamel using a sharp tipped instrument exhibiting a rectangular area on the enamel surface.
Groups
A total of 50 enamel slabs were randomly divided into five groups of 10 samples in each based on the type of remineralizing agent to be used [Table 1].
Table 1.
The division of groups

Lesion preparation
Each of the enamel samples were then immersed in 40 ml of demineralizing solution (acetate 0.1 Mol/L, calcium 0.1 Mol/L, phosphate 0.1 Mol/L, fluoride 0.1 mg/L, pH 5.0)[9] for a period of 4 days at a constant temperature of 37°C, in an incubator to induce artificial caries formation, simulating an active area of demineralization.
Slurry preparation of CPP-ACP, CPP-ACPF and NaF
Slurry of CPP-ACP and CPP-ACP along with fluoride were prepared by agitating the above mentioned preparations in deionized water in the ratio of 1:3.[10]
pH cycling model
pH cycling model was adopted to simulate the dynamic process of demineralization and remineralization that occurs in the oral cavity. Each of the enamel samples were treated with the respective remineralizing agents for a period of 2 min, following which the samples were individually immersed in 20 ml of demineralizing solution (calcium 2.0 mMol/L, phosphate 2.0 mMol/L, acetic acid 75.0 mMol/L, pH 4.4)[10] for a period of 3 h. This was followed-up with treatment of the samples again with slurries of the respective remineralizing agents for 2 min. All the enamel samples were individually immersed in 30 ml of remineralizing solution (1.5 mM calcium, 0.9 mM phosphate, 0.15 M KCl in 0.1 m Tris buffer, pH 7) for a period of 17 h.[10] The remineralizing solution was replaced every 48 h and the demineralizing agent replaced every 5 days. The pH cycling was carried out for a period of 28 days. After the completion of the process of pH cycling, all the groups of enamel samples were assessed for surface microhardness using Vickers hardness test (Micro Vickers Hardness tester, Matsuzawa Co., Ltd, Toshima, Japan).
Surface microhardness
The surface microhardness of the specimens was determined using digital microhardness tester (MATSUZAWA Co., Ltd. Model - MMT X7, Japan) with a Vickers elongated diamond pyramid indenter and a ×40 objective lens. A load of 100 g was applied to the surface for 10 s.[11] Five indentations were placed on the surface and the average value was considered. Precision microscopes of magnification of ×400 were used to measure the indentations. The diagonal length of the indentation was measured by built in scaled microscope and Vickers values were converted to microhardness values.
Statistical analysis
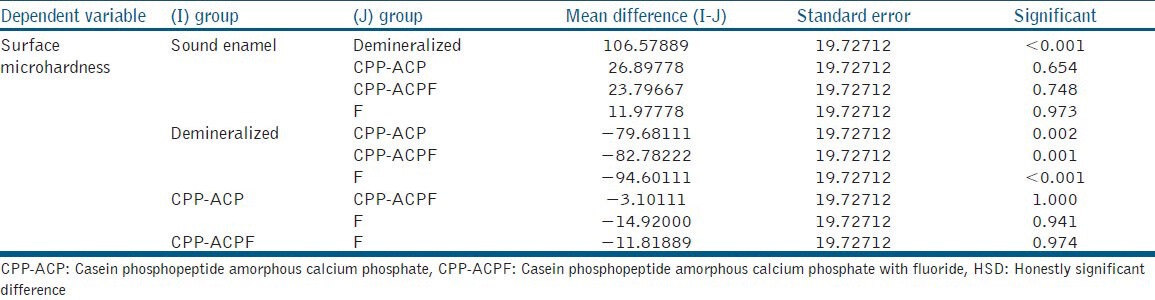
The results were analyzed by one-way analysis of variance (ANOVA). Multiple comparisons between groups were performed by posthoc Tukey test [Tables 2 and 3].
Table 2.
One-way ANOVA
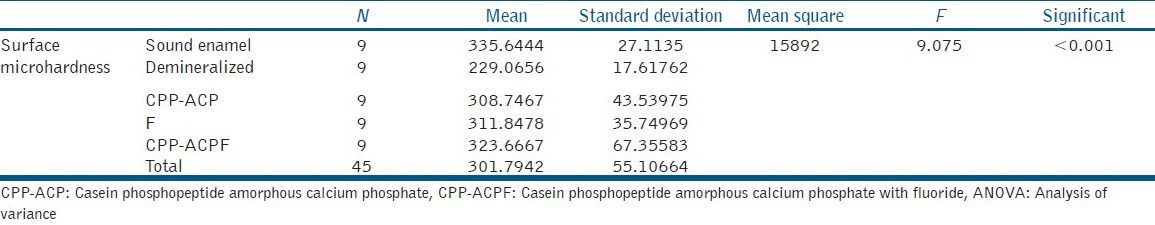
Table 3.
Posthoc Tukey test
DISCUSSION
In spite of the enamel remineralizing potential of saliva, by itself it fails to initiate the process of increasing the levels of calcium and phosphate.[12]
Calcium and phosphate ions must first penetrate the surface layer of enamel, to bring about deposition of minerals through the body of the lesion, which confirms the reason for the CPP supported metastable calcium phosphate solutions of being such efficient remineralizing solutions.[13] Various compositions of demineralizing solutions are available in the vast literature available. Majority of them are composed of calcium and phosphate with acetic acid or lactate. The main variation lies in concentrations of each component, which influences the final pH and the sample exposure time. The pH employed varies from 3.5 to 5 and the time differs from 2 h to 21 days.[14] In this study demineralization similar to enamel subsurface lesion was sought. An intermediate pH 5.0 was therefore employed for 4 days. The composition similar to the one employed by Featherstone in 1992.[9]
Various studies have used different methods to assess the process of enamel remineralization. The commonly used microhardness tests for evaluating enamel remineralization are Vickers microhardness test and Knoop microhardness test.[11] As this study put the focus on evaluation of surface microhardness of enamel, Vickers surface microhardness test was used.
The values of surface microhardness indicate that remineralization of enamel is more in samples of group III (CPP-ACPF) followed by group IV (NaF) and group II (CPP-ACP). This may be because of the presence of Fluoride in CPP-ACP makes it more capable to remineralize the enamel. The results are in accordance with other studies.[15] However, group IV (F) showed a marginal increase in remineralization compared with group II (CPP-ACP). One-way ANOVA shows that there is a significant difference between all the five groups. However, multiple group posthoc Tukey test showed a significant difference only between groups V with the other groups. No other group showed significance when they were inter-compared.
Adopting the usage of low fluoride concentration as present in CPP-ACPF (0.2%), there is a complex localization of free calcium phosphate and fluoride ion activities, which aid in maintaining a state of super saturation by suppressing demineralization.[16] Even though Fluoride is present in tooth pastes manufactured in India and china, their efficacy is not at par with multinational dentrifices.[17] Research has also proven that APF forms more fluoride and is more efficient in reducing enamel demineralization when compared with neutral gel.[18] Sugar free chewing gums containing CPP-ACP and citric acid result in higher remineralisation when compared with chewing gums without CPP-ACP and citric acid and gums with citric acid alone.[19] Research suggest that fluoride dentifrices in combination with daily applications of Acidulated Phosphate Fluoride (APF) topical thrice and four times a day were the only treatments able to increase the surface microhardness of enamel.[20] The use of fluoride and the CPP-ACP in recent years have been the best possible method in prevention of enamel caries and also in halting the progress of the existent enamel lesions.[21,22] CPP-ACP containing remineralising paste has shown remineralization effect within the first three month evaluation period.[23] Conversely, research also suggest that ACP-CPP cream isn’t as effective as fluoride in remineralizing early enamel caries at surface level. Combination of fluoride and ACP-CPP does not provide any additive remineralization potential compared to fluoride alone.[24,25] However, It's important to note that compliance of patient in oral hygiene maintenance and in home fluoride use is of utmost importance in prevention of enamel caries.[26] The results of this study showed that CPP-ACPF remineralized enamel subsurface lesion better in comparison with NaF and CPP-ACP. NaF shown to be better than CPP-ACP in enamel remineralization although the comparison between these two agents was insignificant statistically. With this basis we can confirm that CPP-ACPF is an excellent material for remineralization of white spot or initial enamel caries.
CONCLUSION
Under the conditions of this study, these conclusions can be derived.
CPP-ACP effectively remineralizes initial enamel caries, but to a lesser extent in comparison to CPP-ACPF and NaF.
The addition of Fluoride to CPP-ACP shows improved remineralization of initial enamel caries when compared with CPP-ACP and NaF.
ACKNOWLEDGMENT
We would like to thank the faculty of the Department of Mechanical Engineering, Manipal Institute of Technology for their enormous contribution to this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: An in vitro study. Aust Dent J. 2008;53:34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 2.Cochrane NJ, Saranathan S, Cai F, Cross KJ, Reynolds EC. Enamel subsurface lesion remineralisation with casein phosphopeptide stabilised solutions of calcium, phosphate and fluoride. Caries Res. 2008;42:88–97. doi: 10.1159/000113161. [DOI] [PubMed] [Google Scholar]
- 3.Manton DJ, Walker GD, Cai F, Cochrane NJ, Shen P, Reynolds EC. Remineralization of enamel subsurface lesions in situ by the use of three commercially available sugar-free gums. Int J Paediatr Dent. 2008;18:284–90. doi: 10.1111/j.1365-263X.2008.00920.x. [DOI] [PubMed] [Google Scholar]
- 4.Hegde MN, Shetty S, Pardal D. Remineralization of enamel subsurface lesion using CPP-ACP - A quantitative energy dispersive X-ray analysis. J Conserv Dent. 2007;10:19–25. doi: 10.4103/0972-0707.92609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds EC, del Rio A. Effect of casein and whey-protein solutions on caries experience and feeding patterns of the rat. Arch Oral Biol. 1984;29:927–33. doi: 10.1016/0003-9969(84)90093-1. [DOI] [PubMed] [Google Scholar]
- 6.Jayarajan J, Janardhanam P, Jayakumar P, Deepika Efficacy of CPP-ACP and CPP-ACPF on enamel remineralization - An in vitro study using scanning electron microscope and DIAGNOdent. Indian J Dent Res. 2011;22:77–82. doi: 10.4103/0970-9290.80001. [DOI] [PubMed] [Google Scholar]
- 7.Mellberg JR, Mallon DE. Acceleration of remineralization in vitro by sodium monofluorophosphate and sodium fluoride. J Dent Res. 1984;63:1130–5. doi: 10.1177/00220345840630090701. [DOI] [PubMed] [Google Scholar]
- 8.Pantera EA, Jr, Schuster GS. Sterilization of extracted human teeth. J Dent Educ. 1990;54:283–5. [PubMed] [Google Scholar]
- 9.Featherstone JD, Zero DT. An in situ model for simultaneous assessment of inhibition of demineralization and enhancement of remineralization. J Dent Res. 1992;71(Spec No):804–10. doi: 10.1177/002203459207100S02. [DOI] [PubMed] [Google Scholar]
- 10.Stookey GK, Featherstone JD, Rapozo-Hilo M, Schemehorn BR, Williams RA, Baker RA, et al. The Featherstone laboratory pH cycling model: A prospective, multi-site validation exercise. Am J Dent. 2011;24:322–8. [PubMed] [Google Scholar]
- 11.Davari AR, Kazemi AD, Ataei E, Vatanpour M, Abdollahi H. Effects of bleaching and remineralizing agents on the surface hardness of enamel. J Dentistry. 2012;13:156–63. [Google Scholar]
- 12.Amaechi BT, Higham SM. In vitro remineralisation of eroded enamel lesions by saliva. J Dent. 2001;29:371–6. doi: 10.1016/s0300-5712(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds EC. Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res. 1997;76:1587–95. doi: 10.1177/00220345970760091101. [DOI] [PubMed] [Google Scholar]
- 14.Puig-Silla M, Monteil-Company JM, Almerich-Silla JM. Comparison of the remineralizing effect of a sodium fluoride mouth rinse versus a sodium monofluorophosphate and calcium mouth rinse. An in vitro study. J Clin Exp Dent. 2009;1:e31–6. [PubMed] [Google Scholar]
- 15.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344–8. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 16.Holler BE, Friedl KH, Jung H, Hiller KA, Schmalz G. Fluoride uptake and distribution in enamel and dentin after application of different fluoride solutions. Clin Oral Investig. 2002;6:137–44. doi: 10.1007/s00784-002-0164-5. [DOI] [PubMed] [Google Scholar]
- 17.Itthagarun A, Wei SH, Wefel JS. The effect of different commercial dentifrices on enamel lesion progression: An in vitro pH-cycling study. Int Dent J. 2000;50:21–8. doi: 10.1111/j.1875-595x.2000.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 18.Delbem AC, Cury JA. Effect of application time of APF and NaF gels on microhardness and fluoride uptake of in vitro enamel caries. Am J Dent. 2002;15:169–72. [PubMed] [Google Scholar]
- 19.Cai F, Manton DJ, Shen P, Walker GD, Cross KJ, Yuan Y, et al. Effect of addition of citric acid and casein phosphopeptide-amorphous calcium phosphate to a sugar-free chewing gum on enamel remineralization in situ. Caries Res. 2007;41:377–83. doi: 10.1159/000104796. [DOI] [PubMed] [Google Scholar]
- 20.Jardim JJ, Pagot MA, Maltz M. Artificial enamel dental caries treated with different topical fluoride regimes: An in situ study. J Dent. 2008;36:396–401. doi: 10.1016/j.jdent.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Llena Puy C, Forner Navarro L. Evidence concerning the medical management of caries. Med Oral Patol Oral Cir Bucal. 2008;13:E325–30. [PubMed] [Google Scholar]
- 22.Somasundaram P, Vimala N, Mandke LG. Protective potential of casein phosphopeptide amorphous calcium phosphate containing paste on enamel surfaces. J Conserv Dent. 2013;16:152–6. doi: 10.4103/0972-0707.108199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vashisht R, Indira R, Ramachandran S, Kumar A, Srinivasan MR. Role of casein phosphopeptide amorphous calcium phosphate in remineralization of white spot lesions and inhibition of Streptococcus mutans. J Conserv Dent. 2013;16:342–6. doi: 10.4103/0972-0707.114370. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Lata S, Varghese NO, Varughese JM. Remineralization potential of fluoride and amorphous calcium phosphate-casein phospho peptide on enamel lesions: An in vitro comparative evaluation. J Conserv Dent. 2010;13:42–6. doi: 10.4103/0972-0707.62634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chedid SJ, Cury JA. Effect of 0.02% NaF solution on enamel demineralization and fluoride uptake by deciduous teeth in vitro. Braz Oral Res. 2004;18:18–22. doi: 10.1590/s1806-83242004000100004. [DOI] [PubMed] [Google Scholar]
- 26.Hu W, Featherstone JD. Prevention of enamel demineralization: An in-vitro study using light-cured filled sealant. Am J Orthod Dentofacial Orthop. 2005;128:592–600. doi: 10.1016/j.ajodo.2004.07.046. [DOI] [PubMed] [Google Scholar]


